Abstract
OBJECTIVE
This study’s primary aim was to compare the growth (daily weight gain) of infants consuming a new (Test) amino acid-based formula (AAF) or a commercially available AAF (Control).
METHODS
Healthy infants were randomized to Test or Control from 14 to 112 days of age. Anthropometric measurements were taken at 14, 28, 56, 84, and 112 days of age. Tolerance records were completed prior to each visit. Serum albumin and plasma amino acids were ascertained in a subset of infants at 84 days of age.
RESULTS
A total of 119 subjects completed the study per protocol. Mean daily weight gains were 27.26 ± 4.92 g/day for Control and 27.42 ± 6.37 g/day for Test (P = 0.8812). There were no significant differences between groups in formula intake, adverse events, flatulence, spit-up/vomiting, mood, or sleep. Albumin and plasma amino acids were within normal limits for both groups.
CONCLUSIONS
Infants fed the new AAF had similar daily weight gains as infants fed a commercially available AAF.
Keywords: infant formula, amino acid-based formula, growth
Introduction
It is widely accepted that breast-feeding is the gold standard to promote optimal growth and development of newborns. Cow’s milk-based infant formulas are often used to supplement human breast milk or used exclusively in infants whose mothers are not able to provide a human milk diet. However, some infants may require infant formulas with protein sources that are not based on intact cow’s milk.
Approximately 2%–3% of infants are allergic or intolerant to cow’s milk formula (CMF) and require an alternative formula.1–3 The manifestations of cow’s milk protein allergy (CMPA) can include immunoglobulin E (IgE)-mediated symptoms, such as angioedema, urticaria, wheezing, rhinitis, vomiting, and eczema, and/or non-IgE-mediated conditions, such as pulmonary hemosiderosis, malabsorption with villous atrophy, eosinophilic proctocolitis, enterocolitis, and esophagitis.4 The American Academy of Pediatrics (AAP) recommends extensively hydrolyzed formula (EHF) for the dietary management of infants who are allergic or intolerant to intact CMF.4 If the infant does not tolerate an EHF, an amino acid-based infant formula (AAF) would then be recommended.4 Reactions to EHF in infants and children with CMPA have been documented,5–8 and it has been estimated that ~10% of children with CMPA may react to EHF. An AAF would be necessary for this population4 and can be successfully used for the dietary management of CMPA.8,9 AAFs may also be indicated for infants with multiple food allergies, severe malabsorption, short bowel syndrome, or eosinophilic esophagitis.
A new amino acid-based infant formula with 43% of its fat from medium-chain triglycerides (MCTs) has been developed and shown to be hypoallergenic and appropriate for use in CMPA.10 This would provide another option for infants who require the use of AAF in addition to those already commercially available. The formula was given to infants and children with documented CMPA in a double-blind, placebo-controlled food challenge, no allergic reactions occurred to the new AAF,10 and the criteria set forth by the AAP were met.4 The purpose of this study was to determine whether this new formula supports normal growth of healthy, exclusively formula-fed full-term infants. The primary aim of this study was to compare growth (expressed as weight gain in grams/day) in infants consuming a new AAF (Test) or a commercially available AAF (Control). Secondary objectives included the comparison of length and head circumference, formula tolerance, and adverse events.
Methods
This study was a randomized, controlled, double-blind, multicenter clinical trial of two formula groups in parallel conducted at 17 sites throughout the United States. Subjects were healthy, full-term (>37 weeks gestation), exclusively formula-fed infants with birth weights ranging from 2500 to 4500 g whose caregivers had given informed consent to participate in the study. Subjects were 0–17 days of age at enrollment. A study visit was required at 14 ± 3 days to obtain baseline anthropometric measurements. Therefore, if a subject was enrolled at 10 days of age or younger, s/he was required to return to the study site at 14 ± 3 days of age. Exclusion criteria were: congenital illness or malformations that affected infant feeding and/or normal growth, suspected or known allergy to cow’s milk protein, significant prenatal and/or postnatal disease, any readmission to hospital (except for hyperbilirubinemia) prior to enrollment, receiving prescription medication (with the exception of topical antibiotics and/or treatment for thrush), or frequent use of over the counter medications except vitamin and mineral supplements.
Subjects were randomized to either a new AAF with 43% of its fat source from MCT (Test; Nestlé Health Science) or a commercially available AAF with 33% of its fat source from MCT (Control; Nutricia, North America). Both formulas contained all the vitamins and minerals known to be essential for the healthy growth and development of infants. A comparison of the formulas is given in Table 1. The products had identical labels except for the product code number. The identity of the specific product was unknown to subjects, support staff, the statistician, and the investigator.
Table 1.
Formula macronutrient composition.
| PER 100 kcal | CONTROL FORMULA | TEST FORMULA |
|---|---|---|
| Protein source | Amino acids | Amino acids |
| Protein (g/100 kcal, % kcal) | 3.1/12.4% | 2.8/11.2% |
| Fat source | Refined vegetable oil (medium chain triglycerides (palm kernel and/or coconut oil), high oleic sunflower, soy), ARA, DHA | Medium chain triglycerides, soybean oil, high oleic sunflower oil, esterified palm oil, ARA, DHA |
| Fat (g/100 kcal, % kcal) | 4.5/41% | 5/45% |
| MCT, % of fat | 33% | 43% |
| Carbohydrate source | Corn syrup solids | Corn syrup solids, potato starch |
| Carbohydrate (g/100 kcal, % kcal) | 11.7/47% | 11.12/44% |
| Osmolality (mOsm/kg water) | 340 | 330 |
Upon enrollment subjects received the assigned study formula as their exclusive source of nutrition until 112 days of life. Study visits were scheduled at 14, 28, 56, 84, and 112 days of life where weight, length, and head circumference were measured. These anthropometric measurements were also compared to the standards of World Health Organization (WHO).11 For two days prior to each study visit, caregivers kept a detailed record in English of formula intake, stool characteristics (frequency recorded per day; consistency as hard, firm, soft, or liquid; color as black, brown, green, or yellow), flatulence (none, rarely, sometimes, or often), spit-up (none, occasional, frequent), vomit (frequency), longest stretch of sleep, and mood of the infant (scale of 1–5, with 1 being “happy/content” and 5 being “very irritable”). Adverse events (defined as any untoward occurrence, including any illnesses, signs, or symptoms occurring or worsening, and/or abnormal laboratory findings during the course of the study) were assessed throughout the study. In a subset of 38 infants (n = 18 in Test; n = 20 in Control), a blood sample was taken at 84 days of life for the analysis of serum albumin and plasma amino acids.
The first subject was enrolled in June 2012. The last subject completed the study in July 2013. The approval of institutional review board was given by Copernicus Group IRB, and for sites affiliated with medical schools/universities, their respective institutional review boards gave their approval. The study was conducted within the principle of the Declaration of Helsinki. Subjects were recruited from pediatrician offices and advertisements. Study formula was provided at no charge, and a stipend was given to the caregivers at each study visit. This study was registered with ClinicalTrials.gov (NCT01583673).
Statistical methods
The primary objective of this clinical trial was to assess growth (weight gain in grams per day calculated by dividing the difference in weight between two visits by the number of days between those two visits) in infants fed the Test formula compared to those fed the Control formula.
In each study group, the number of subjects to complete the study protocol was 56. The sample size was chosen according to US Food and Drug Administration contract 223-86-2117, “Clinical Testing of Infant Formulas with respect to Nutritional Suitability for Term Infants.”12 The document states, “The standard deviation of gain in weight on a sex-specific and formula-specific basis for a 3-1/2 month interval beginning during the first month of life is about 4.5 g/day. The number of subjects of a specified sex needed in each of two groups to detect a 3 g/day difference in weight gain (P < 0.05) with a power of 0.8 in a one-tailed test is therefore 28.” Thus, a sample size of 56 per treatment group (with both genders) was required.
All statistical analyses were performed using the SAS statistical software (Version 9.2). Statistical significance was tested at the one-sided 5% level for anthropometric measures. The comparisons for tolerance-related outcomes were made using mixed effects models for repeated measure data from visit 1 to visit 4 and using general linear models for all visits combined, modeled with visit, formula group, and interaction between visit and formula group. In the mixed model, the F-test was performed to test the formula differences, and in the general linear model, F-test of the type III partial sum of squares was performed.
Continuous safety and effectiveness parameters were summarized by presenting the number of subjects, mean, standard deviation, median, minimum, and maximum by formula group (Test or Control). The tabulation of categorical parameters by formula group included counts and percentages. The 95% confidence intervals were provided as appropriate.
All randomized subjects were analyzed using the following analysis populations. The primary outcome was analyzed in the intent-to-treat (ITT) and per protocol (PP) populations. Secondary outcomes including safety endpoints were analyzed in the ITT population.
The ITT population was defined as all randomized subjects who took any amount of the study formula. The PP population was defined as subjects who took study formula continuously over the whole treatment period. A break of no longer than three days was accepted to remain in the PP population. In addition, subjects with the following conditions were excluded from the PP population: hospitalization for more than three days and nonexclusive feeding of assigned formula during the first four months of the study. A nonexclusive formula diet is defined as more than one bottle of another formula per week, being off study formula feeding for ≥3 consecutive days, taking 4 or more teaspoons (ie, 20 g) of complementary foods per day, and/or taking more than 3 oz of juice per day.
Results
Population
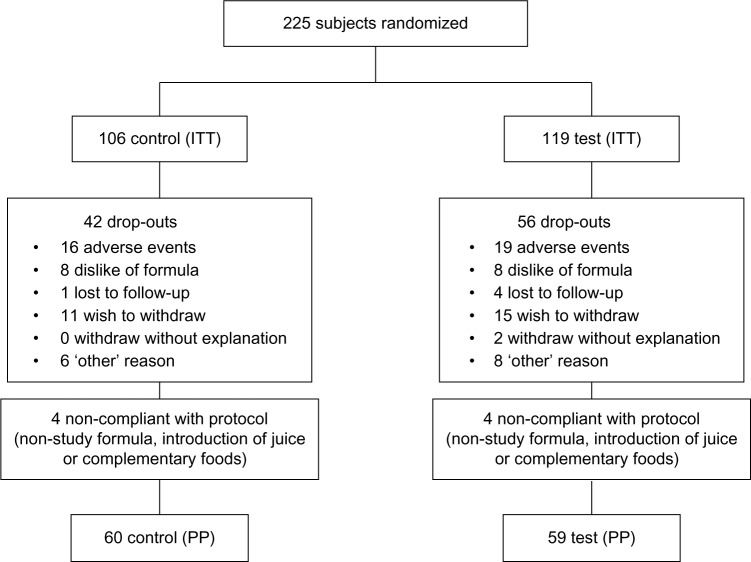
Caregivers of 225 subjects gave informed consent for their infants to participate in the study. A total of 106 subjects were assigned to the Control group and 119 subjects to the Test group. Overall, 98 subjects dropped out of the study (dropout rate, 44%). Sixty-four infants in the Control formula group completed the study, and 42 infants dropped out, resulting in a dropout rate of 40%. Sixty-three infants in the Test formula group completed the study, and 56 infants dropped out, yielding a dropout rate of 47%. There was no significant difference between the number of dropouts between groups. Reasons for dropout included dislike of formula, adverse event, lost to follow up, caregivers wished to withdraw, or for other reasons not specified. Eight subjects did not comply with the protocol; a total of 119 infants completed the study per protocol (60 Control, 59 Test; Fig. 1).
Figure 1.
CONSORT flowchart depicting the number of subjects who withdrew throughout the study.
Demographics of the ITT population are presented in Table 2. A majority of infants were Caucasian males. No differences were seen in race, sex, route of delivery, or gestational age between groups. There were also no differences in maternal education, maternal age at enrollment, and smoking in the household. There were no differences in boys in weight (P = 0.7044), recumbent length (P = 0.8049), or head circumference (P = 0.9686) at birth between formula assignment for all three variables. The corresponding P-values for girls for weight, length, and head circumference are P = 0.0569, P = 0.2248, and P = 0.7444, respectively, with girls in the Test group trending toward significantly lower birth weight than girls in the Control group.
Table 2.
Demographics (mean ± SD) of the ITT population.
| CONTROL n = 106 | TEST n = 119 | ||
|---|---|---|---|
| Race (P = 0.1907) | Black | 7 (7%) | 12 (10%) |
| Caucasian | 86 (81%) | 97 (82%) | |
| Hispanic | 4 (4%) | 4 (3%) | |
| Other | 9 (8%) | 6 (5%) | |
| Sex (P = 0.5307) | Girls | 49 (46%) | 60 (50%) |
| Boys | 57 (54%) | 59 (50%) | |
| Delivery (P = 0.9818) | C- section | 34 (32%) | 38 (32%) |
| Vaginal | 72 (68%) | 81 (68%) | |
| Gestational age (weeks; P = 0.9139) | Girls | 38.86 ± 0.91 | 38.70 ± 1.23 |
| Boys | 38.58 ± 0.91 | 38.75 ± 1.14 | |
| Birth weight (kg; P = 0.3012) | Girls (P = 0.0569) | 3.35 ± 0.39 | 3.20 ± 0.43 |
| Boys (P = 0.7044) | 3.32 ± 0.48 | 3.35 ± 0.41 | |
| Age at Enrollment (days; P = 0.1505) | Girls | 8.96 ± 5.12 | 9.35 ± 4.81 |
| Boys | 7.96 ± 4.73 | 9.41 ± 5.19 |
Growth parameters
Mean daily gains in weight in grams/day were computed from 14 ± 3 days to 112 ± 3 days for each infant. Table 3 presents means and standard deviations of mean daily weight gains in grams/day from visit 0 (14 ± 3 days) to visit 4 (112 ± 3 days) by formula, each sex, and both sexes for the ITT and PP populations. The differences in mean daily weight gain for girls, boys, or both sexes were not statistically significantly different.
Table 3.
Weight gain in grams/day (mean ± SD) for the ITT and PP populations.
| CONTROL | TEST | |||
|---|---|---|---|---|
| ITT n = 72 | PP n = 59 | ITT n = 74 | PP n = 59 | |
| Girls (g/day) | 25.85 ± 16.13 | 25.37 ± 4.15 | 24.94 ± 5.31 | 25.59 ± 5.11 |
| Boys (g/day) | 29.71 ± 5.59 | 28.97 ± 4.99 | 29.80 ± 6.73 | 29.30 ± 7.06 |
| Genders combined (g/day) | 27.99 ± 5.32 | 27.26 ± 4.92 | 27.30 ± 6.51 | 27.42 ± 6.37 |
Note: Daily weight gains for both ITT and PP populations were not significant (P > 0.05) for girls, boys, or both genders.
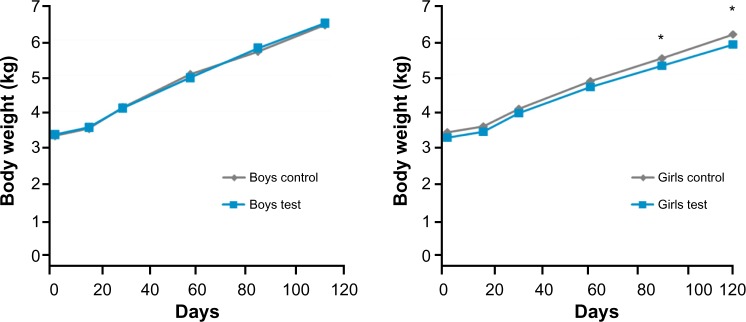
In the ITT population, the Test group had significantly lower weights at 56 (P = 0.0232) and 112 days of age (P = 0.022). There were no differences in boys’ weights at any visit. There was a trend for girls in the Test formula to have lower weights than the Control formula at 14 (P = 0.0671), 28 (P = 0.0588), and 56 days of age (P = 0.0545). This difference became statistically significant at 84 (P = 0.0249) and 112 days of age (P = 0.0003; Fig. 2). When looking at the PP population, there were no significant differences in actual body weights for boys, girls, or when sexes were combined at any time point.
Figure 2.
Boys’ and girls’ body weights by visit. There were no differences in boys’ weights at any visit. In girls, there was a trend for Test girls to have lower body weights at 14, 28, and 56 days (P < 0.07). For girls, there was a statistically significant difference (*P < 0.05) at 84 and 112 days of age.
At birth and all subsequent visits, mean WHO weight-for-age z-scores were negative (<0) for both the Test and Control groups at birth and all subsequent visits, except for the boys from the Control group who had a mean weight-forage z-score at birth that was above zero (0.22). Girls in the ITT Test group had lower weight-for-age z-scores (P < 0.05 at birth and all visits) and weight-for-age percentiles (at birth, baseline, 28 and 112 days; at 56 days, P = 0.0696; at 84 days, P = 0.0364) according to the WHO growth charts. When differences in birth weight were accounted for, there were no significant differences between the groups for girls, boys, or both sexes for weight-for-age z-scores or weight-for-age percentiles. From 14 to 112 days of age, mean weight-for-age percentiles ranged 31st–37th in ITT Test girls and 39th–47th in the ITT Control girls; for boys, the range was 30th–37th in the Test group and 28th–36th in the Control group.
For the PP population, there was a trend for girls in the Test group to have lower weight-for-age percentile at baseline (14 days of age; P = 0.0507), but not at any other time point assessed. There were no differences in weight-for-age percentiles for PP boys or both sexes. For weight-for-age z-scores for the PP population, there were no differences seen in girls, boys, or both sexes.
In both the ITT and PP populations, for actual lengths, no differences were seen in boys at any time. However, girls assigned to the Control formula group were significantly (P < 0.05) longer than girls assigned to the Test formula group at 14, 56, 84, and 112 days with a difference of borderline significance in the same direction at 28 days (P = 0.0530). When the analysis was performed with both sexes, it was found that infants assigned to Control formula were significantly longer at 14, 84, and 112 days than infants assigned to Test formula. Similar results were seen when looking at length-for-age z-scores and percentiles. Length gains from 14 to 112 days of age were not statistically significantly different between the groups for boys, girls, or both sexes. Weight-for-length assessment according to the WHO growth charts indicated no difference between the two groups, although values were numerically higher for the Test group. There were no differences in daily gains in length (1.07 mm/day in Control, 1.04 mm/day in Test; P = 0.398).
In the ITT population, there were no statistically significant differences in head circumference for either sex or both sexes at any visit, except for a trend (P = 0.0653) for girls in the Test group to have lower head circumference-for-age z-scores and significantly lower head circumference-for-age percentiles (50th vs 63rd) at 84 days of age compared to the girls in the Control group. There were no differences seen in the PP population.
Other outcomes
Formula intake was not significantly different between the groups (28.75 ± 11.23 oz/day (850 ± 332 mL/day) for Control vs 29.34 ± 9.68 oz/day (868 ± 286 mL/day) for Test). No differences between groups were seen in flatulence, frequency of spit-up/vomiting, mood, or sleep. The Test group had significantly more frequent stools (2.03 stools/day in Control, 3.36 stools/day in Test; P = 0.001) that were more often yellow (P < 0.0009) and less often black compared to Control group (P < 0.0001; Table 4). There were no significant differences between groups for stool consistency, with the majority of stools reported as being soft (Test 67%, Control 71%) or liquid (Test 27%, Control 25%; Table 4).
Table 4.
Stool characteristics for visits combined (mean ± SD).
| CONTROL | TEST | |
|---|---|---|
| Stool Frequency (#/day; p=0.001) | 2.03 ± 1.56 | 3.36 ± 2.16 |
| Stool Color (% of stools) | ||
| Yellow (P = 0.0009) | 17 ± 24 | 32 ± 31 |
| Green (P = 0.932) | 49 ± 36 | 49 ± 34 |
| Brown (P = 0.8884) | 18 ± 27 | 19 ± 26 |
| Black (P < 0.0001) | 16 ± 27 | 1 ± 6 |
| Stool Consistency (% of stools) | ||
| Hard (P = 0.6075) | 1 ± 3 | 0 ± 3 |
| Firm (P = 0.5218) | 4 ± 12 | 5 ± 12 |
| Soft (P = 0.5275) | 71 ± 30 | 67 ± 29 |
| Liquid (P = 0.65) | 25 ± 29 | 27 ± 30 |
Adverse events were defined as any untoward occurrence during the course of the study, which may or may not have been related to the study formula. This included (but was not limited to) any febrile episodes, colds, unusual stooling habits, or rashes. Serious adverse events were defined as a fatal or life-threatening event causing permanent harm or requiring/extending inpatient treatment at a hospital or which is considered medically relevant. There was no difference in the total number of adverse events (nonserious or serious) reported between the groups. In the ITT population, 11 infants assigned to Control formula and 17 infants assigned to Test formula reported an adverse event of loose stools (P = 0.0019). This difference was not seen in the PP population. In a subset of subjects, serum albumin (4.30 g/day for Control, 4.33 g/day for Test) and plasma amino acids (data not shown) were within normal limits for both groups.
Discussion
Mean daily weight gains from 14 ± 3 days to 112 ± 3 days were 29.71 g/day for boys and 25.85 g/day for girls assigned to Control formula and 29.80 g/day for boys and 24.94 g/day for girls assigned to Test formula in the ITT sample. The mean difference in mean daily weight gains between the Control and Test formula groups for both sexes in both ITT (27.99 g/day vs 27.30 g/day) and PP (27.26 g/day vs 27.42 g/day) samples do fall within the AAP–Committee on Nutrition recommended difference of 3 g/day, used to establish noninferiority between different infant formulas,12 as well as when looking at the sexes separately.
Other infant growth studies of AAFs have been published showing similar results to our current study. One compared growth of infants fed an extensively hydrolyzed casein formula versus an AAF.13 The group fed the AAF gained a mean of 28.2 g/day, which is quite comparable to that observed in the study presented here (27.42 g/day in the PP Test group). In a study of a different AAF, but again compared to an extensively hydrolyzed casein formula, mean weight gains in the AAF group were reported as 29.1 g/day,14 which is within 3 g/day of the reported weight gains in the present study. A study by Harvey et al.15 used the same formula as the Control formula for the present study, with and without the addition of synbiotics. Unfortunately, the data for the study of Harvey et al are presented not as weight gain in grams/day but as a ratio of the absolute values for the growth parameters between the two groups. As a comparison to non-AAFs, a growth study comparing a partially hydrolyzed 100% whey-based formula with or without probiotics reported daily weight gains of 30.7 g/day in the nonprobiotic group compared to 29.6 g/day in the probiotic group.16 In our study, weight gains were reported at 27.99 and 27.3 g/day for the two groups which are numerically lower than that reported in a recent growth study of a non-AAF.
The weight-for-age z-scores according to the WHO growth charts were all below zero for both groups from 14 days of age to 112 days of age, with girls in the Test group being statistically significantly smaller than the Control group at all time points, including birth and 14 days of age, before study formula had even been started. These findings indicate that overall, the subjects included in this study were small according to the WHO reference group and girls in the Test were statistically significantly smaller. When the birth weight of subjects was taken into conside ration in the statistical analysis, no differences were seen between the two groups. Although it cannot be explained why infants, particularly the girls, were smaller in the Test group from the beginning of the study, the differences between the two groups are not seen when birth weight was statistically accounted for. Therefore, differences in weight seen between groups are most likely attributed to the fact that the baby girls randomized to the Test formula were already smaller than those randomized to the Control formula at enrollment. In addition, the values were numerically (but not statistically significant) higher for the Test group for WHO weight-for-length z-scores and percentiles between the two groups. This indicates that although the Test group may have been shorter and lighter, when looking at the weights of the subjects in relation to their lengths, the proportions were similar to that of the Control group.
The dropout rate observed in this study was higher than expected (44% overall) in both groups (Test 47%, Control 40%; P > 0.05). Most likely, these high dropout rates could be attributed to the smell and taste of AAFs as compared to standard infant formulas. Similar numbers of subjects in each group dropped out due to “dislike of the formula.” Subjects in this study were healthy infants, with no clinical indication for an AAF. Therefore, the unusual smell/taste could have prompted caregivers to withdraw their infants from the study. In comparison, the dropout rate for the study by Harvey et al.15 was 39.1% and 39% for the amino acid formula group in the study by Borschel et al.13, comparing to the overall dropout rate observed in our study. Formula intakes were similar between the two groups, indicating comparable acceptance of the two formulas studied.
The study population was comprised of healthy infants who would not typically consume AAFs. Whether the differences in weight noted in this study would also occur in intended populations for these types of products (cow’s milk allergy, multiple food allergies, eosinophilic esophagitis and gastrointestinal malabsorption) remains unknown. No differences in flatulence, vomit, spit-up, duration of sleep, or mood were observed in this healthy, full-term population. As the main objective of this study was growth during the first months of life when most infants have not yet received complementary foods, long-term effects of the study formula cannot be ascertained from this study. Longer term follow-up controlling for the addition of complementary feeding would be needed to determine any long-term effects of the study formula.
The main objective of this study was to compare the growth of healthy infants fed exclusively a new AAF to an already commercially available AAF. This study demonstrated that both AAFs supported growth and the new AAF could be another option for infants requiring an AAF. It also provides more objective data on growth anthropometric measurements in an infant population fed an AAF. Serum albumin and plasma amino acid levels for both groups were within normal limits, suggesting that the two formulas provide adequate protein to infants. Using two AAFs in this study made it possible to blind the study. Comparisons of growth of healthy infants fed more standard, routine, intact infant formulas could help determine whether the broader category of AAFs supports growth in the same manner as intact infant formulas. However, as these AAFs are typically used in infants with particular indications, such a study comparing it to a standard infant formula may be irrelevant. Also, such a study would be difficult to blind, as AAFs have a distinct odor and taste that is not similar to intact protein-based infant formulas. Again, further studies of the new AAF in clinically indicated populations are warranted.
In conclusion, the multicenter four-month trial of Control and Test formula showed similar mean daily weight gain well within 3 g/day of each other. Girls in the Test formula group were smaller than the Control groups, but this difference was seen at birth and baseline before study formula was consumed. When the statistical analysis was performed taking birth weight into consideration, no statistical differences were observed. This study shows that the new AAF supports growth similarly to a commercially available AAF and is another suitable option for infants who may require the use of an AAF.
Footnotes
ACADEMIC EDITOR: Praveen Kumar, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1258 words, excluding any confidential comments to the academic editor.
FUNDING: This study was funded by Nestlé Health Science. LAC, HMS, and JMS are employees of Nestlé Nutrition.
COMPETING INTERESTS: Nestlé Health Science is the developer of the new AAF tested in the research presented here. LC, JS and HS are employees of Nestlé, the sponsor of the study. MC discloses payment from Nestlé Nutrition for general oversight of the study. SS discloses consulting fees from Nestlé Health Science for statistical services to this study.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JMS, HMS, LAC. Analyzed the data: SS. Wrote the first draft of the manuscript: LAC. Contributed to the writing of the manuscript: MC, HMS, SS, LAC, JMS. Agreed with manuscript results and conclusions: MC, HMS, SS, LAC, JMS. Jointly developed the structure and arguments for the paper: MC, HMS, SS, LAC, JMS. Made critical revisions and approved the final version: MC, HMS, SS, LAC, JMS. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Venter C, Pereira B, Grundy J, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–24. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 3.Host A. Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89:33. doi: 10.1016/s1081-1206(10)62120-5. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106:346–9. [PubMed] [Google Scholar]
- 5.Saylor JD, Bahna SL. Anaphylaxis to casein hydrolysate formula. J Pediatr. 1991;118:71–4. doi: 10.1016/s0022-3476(05)81848-7. [DOI] [PubMed] [Google Scholar]
- 6.Hill DJ, Cameron DJ, Francis DE, et al. Challenge confirmation of late-onset reactions to extensively hydrolyzed formulas in infants with multiple food protein intolerance. J Allergy Clin Immunol. 1995;96:386–94. doi: 10.1016/s0091-6749(95)70058-7. [DOI] [PubMed] [Google Scholar]
- 7.de Boissieu D, Matarazzo P, Dupont C. Allergy to extensively hydrolyzed cow milk proteins in infants: identification and treatment with an amino acid-based formula. J Pediatr. 1997;131:744–7. doi: 10.1016/s0022-3476(97)70104-5. [DOI] [PubMed] [Google Scholar]
- 8.de Boissieu D, Dupont C. Allergy to extensively hydrolyzed cow’s milk proteins in infants: safety and duration of amino acid-based formula. J Pediatr. 2002;141:271–3. doi: 10.1067/mpd.2002.126299. [DOI] [PubMed] [Google Scholar]
- 9.Dupont C, Kalach N, Soulaines P, et al. Safety of a new amino acid formula in infants allergic to cow’s milk and intolerant to hydrolysates: a randomized trial. J Pediatr Gastroenterol Nutr. 2015;61(4):456–63. doi: 10.1097/MPG.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 10.Nowak-Węgrzyn A, Czerkies LA, Collins B, et al. Evaluation of hypoallergenicity of a new, amino acid-based formula. Clin Pediatr (Phila) 2015;54:264–72. doi: 10.1177/0009922814557785. [DOI] [PubMed] [Google Scholar]
- 11.WHO Multicentre Growth Reference Study Group WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics . Report on “Clinical Testing of Infant Formulas with Respect to Nutritional Suitability for Term Infants”. American Academy of Pediatrics; Elk Grove Village, IL: 1988. [Google Scholar]
- 13.Borschel MW, Ziegler EE, Wedig RT, et al. Growth of healthy term infants fed an extensively hydrolyzed casein-based or free amino acid-based infant formula: a randomized, double-blind, controlled trial. Clin Pediatr (Phila) 2013;52:910–7. doi: 10.1177/0009922813492883. [DOI] [PubMed] [Google Scholar]
- 14.Burks W, Jones SM, Berseth CL, et al. Hypoallergenicity and effects on growth and tolerance of a new amino acid-based formula with docosahexaenoic acid and arachidonic acid. J Pediatr. 2008;153:266–71. doi: 10.1016/j.jpeds.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 15.Harvey B, Langford JE, Harthoorn LF, et al. Effects on growth and tolerance and hypoallergenicity of an amino acid-based formula with synbiotics. Pediatr Res. 2014;75:343–51. doi: 10.1038/pr.2013.211. [DOI] [PubMed] [Google Scholar]
- 16.Cekola PL, Czerkies LA, Storm HM, et al. Growth and tolerance of term infants fed formula with probiotic Lactobacillus reuteri. Clin Pediatr (Phila) 2015;54:1175–84. doi: 10.1177/0009922815574076. [DOI] [PubMed] [Google Scholar]