Summary
The molecular and cellular mechanisms of memory storage have attracted a great deal of attention. By comparison, little is known about memory allocation, the process that determines which specific neurons in a neural network will store a given memory [1, 2]. Previous studies demonstrated that memory allocation is not random in the amygdala; these studies showed that amygdala neurons with higher levels of the cAMP response element binding protein (CREB) are more likely to be recruited into encoding and storing fear memory [3–6]. To determine whether specific mechanisms also regulate memory allocation in other brain regions, and whether CREB also has a role in this process, we studied insular cortical memory representations for conditioned taste aversion (CTA). In this task, an animal learns to associate a taste (CS) with the experience of malaise (such as that induced by LiCl; US). The insular cortex is required for CTA memory formation and retrieval [7–12]. CTA learning activates a subpopulation of neurons in this structure [13–15], and the insular cortex and the basolateral amygdala (BLA) interact during CTA formation [16, 17]. Here, we used a combination of approaches, including viral vector transfections of insular cortex, arc Fluorescence In Situ Hybridization (FISH) and Designer Receptors Exclusively Activated by Designer Drugs (DREADD) system, to show that CREB levels determine which insular cortical neurons go on to encode a given conditioned taste memory.
Results and Discussion
Although CREB has a critical role in memory allocation in the amygdala, it is unclear whether cortical circuits share similar memory allocation mechanisms. To test the hypothesis that taste memory is preferentially allocated to a specific population of neurons expressing higher levels of CREB, we made a lentivirus vector to overexpress CREB tagged with green fluorescent protein (GFP) in a subpopulation of insular cortex neurons (vCREB neurons). The CREB vector we derived also co-expresses the inducible hM4Di DREADD receptor tagged with HA (also referred to as Gi-DREADD). The UbC promoter in the FG12 plasmid [18] was replaced by a 1.3 kb Ca2+/calmodulin-dependent protein kinase (CaMK2a) promoter to direct expression to excitatory neurons. The CREB and hM4Di genes are expressed under the CaMK2a promoter and cloned on either side of a T2A self-processing viral peptide (CREB virus, Fig. 1A). This allows them to be co-expressed in the same neurons [19]. The hM4Di receptor is specifically activated by CNO, which does not activate endogenous muscarinic acetylcholine receptor [20]. The activation of hM4Di turns on endogenous G protein–coupled inwardly rectifying K+ (GIRK) channels causing membrane hyperpolarization and decreased neuronal excitability. There is abundant expression of GIRK channels in the cortex [21], which allows the activity of infected neurons to be manipulated by systemic injection of CNO. As a control, we used a lentivirus that expresses dTomato tagged GFP instead of EGFP/CREB (Control virus; Fig. 1A).
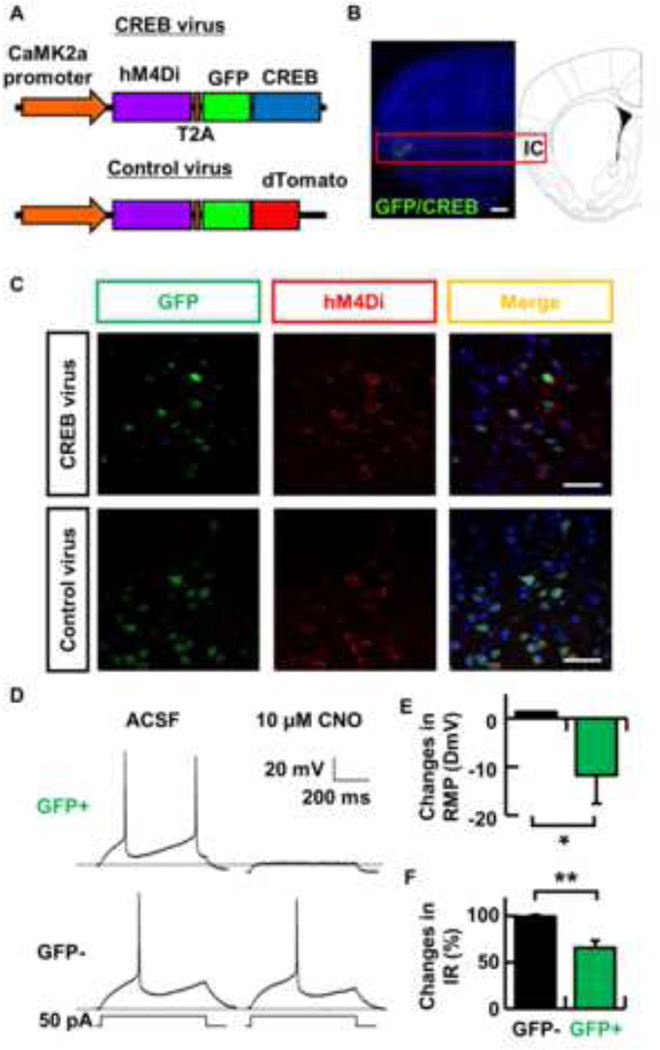
Fig. 1. Selective modulation of a set of genetically tagged insular cortex neurons.
(A) Schematic map of the lentivirus construct designed to express CREB and hM4Di. The hM4Di and GFP/CREB (or GFP/dTomato) genes located on both side of the T2A sequence are under the control of a Camk2a promoter. (B) Representative picture showing localized GFP expression in the insular cortex by CREB virus infection. Scale bar indicates 500 µm. (C) Representative pictures showing co-expression of CREB and hM4Di in the same insular cortical neurons. CREB and hM4Di were detected by immunohistochemical staining using specific antibodies to GFP (green) and HA-tag (red). Merged images show co-localized expression of either GFP/CREB or GFP/dTomato with hM4Di. GFP/CREB and hM4Di proteins show different subcellular localization, as expected. Scale bar indicates 50 µm. (D) Representative traces showing that CNO (10 µM) inactivated transduced (GFP+) neurons in the insular cortex, whereas it had no effect on GFP− neurons. The dashed line represents the resting membrane potential (RMP) before drug administration. The currents injected are shown underneath associated traces. ACSF, artificial cerebrospinal fluid. (E and F) Summary of the selective effect of CNO on (E) changes in resting membrane potential (RMP) and (F) changes in input resistance (IR) of the insular cortex neurons. * p < 0.05 and ** p < 0.01. Data represent mean ± s.e.m. (GFP− cells, black columns; GFP+ cells, green columns).
We bilaterally microinjected these viruses into the insular cortex. Immunohistochemical studies with an antibody against GFP detected the expression of this viral gene in a region about 300 µm from the injection sites in the insular cortex (Fig. 1B). We found that approximately 30 % of the cells in the insular cortex expressed EGFP near the injection sites (CREB virus, 25.0 ± 2.1 %, control virus, 31.2 ± 2.1 %, n = 4 mice per group). The expression of the HA tagged hM4Di receptor was detected using an HA tag antibody. As expected, we found that GFP and hM4Di were co-expressed in the same neurons (Fig. 1C). To test whether CNO could selectively silence insular cortex neurons, we performed whole-cell patch-clamp recordings on both visually identified GFP-positive (hM4Di positive) and GFP-negative neurons in brain slices from mice transfected with the CREB virus. Consistent with previous studies [20, 22, 23], we found that the resting membrane potential (RMP) and input resistance in GFP-positive (GFP+) neurons exposed to CNO were significantly more hyperpolarized and decreased than those in GFP-negative (GFP−) neurons (Fig. 1D, E, F; Change in resting membrane potential (ΔmV), 1.3 ± 1.0, n = 4, GFP−; −11.8 ± 5.8 mV, n = 3, GFP+, p < 0.05; Change in input resistance (%), 99.7 ± 1.8, n = 4, GFP−; 65.4 ± 8.2 mV, n = 3, GFP+, p < 0.01). We also observed that frequency of firing in insular cortex around GFP+ neurons was decreased after CNO injection in an awake mouse, while firing was unchanged in areas of the insular cortex not infected with the virus (Fig. S1).
To first determine whether CREB has a role in memory formation in the mouse insular cortex, we used the lenti viruses described above to test the impact of CREB over expression on memory strength. Previous results showed that over expression of CREB in either the amygdala or hippocampus leads to enhancements in memory tasks that depend on these structures [3, 6, 24]. Accordingly, our results demonstrated that over expression of CREB in the insular cortex results in enhancements in CTA memory, a finding that is consistent with a role for this transcription factor in learning and memory in this structure (Fig. S2A, B and C).
Selective silencing of vCREB neurons impairs CTA memory retrieval
Next, we determined whether silencing the subpopulation of insular cortical neurons that express vCREB disrupts the retrieval of a long-term memory for CTA. If vCREB preferentially recruits the neuronal ensemble encoding CTA in the insular cortex, then silencing these neurons should impair recall when compared to mice transfected with the control virus. To test this hypothesis, CREB and control viruses were bilaterally microinjected into the insular cortex 3 weeks before training. During training, mice were given 150 mM LiCl, which tastes salty (CS) and simultaneously induces malaise (US) [25]. Mice were then administered saline or CNO prior to the test. In this test, mice were presented with two choices: water and 150 mM NaCl. Trained mice show a clear avoidance of NaCl during the test. We utilized this CTA protocol to avoid any stress associated with an i.p. injection during training. To evaluate CTA memory, we used a learning index (LI) along with an aversion index that is commonly used in the CTA literature [7]. The advantage of the learning index is that for each animal tested, it compares avoidance of the salty taste after training with that measured before training (see details in Material and Methods). This comparison is important because not all mice show the same preference for salty tastes before training, and differences between mice could bias performance after training.
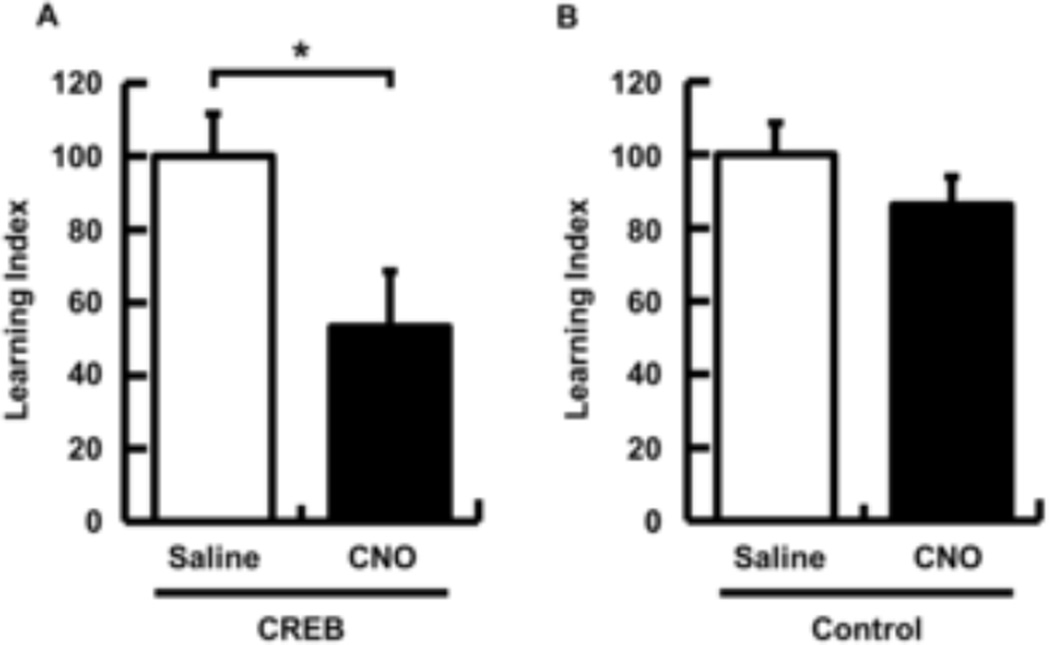
In support of the hypothesis that CREB modulates memory allocation in the insular cortex, memory retrieval was impaired by silencing vCREB positive neurons (Fig. 2A, LI, 100.0 ± 11.8, n = 8, CREB/saline; 53.3 ± 15.3, n = 9, CREB/CNO, p < 0.05, Fig. S2D, Aversion Index, 77.0 ± 6.7, CREB/saline; 57.0 ± 6.2, CREB/CNO, p < 0.05). Importantly, silencing control virus positive neurons in the insular cortex did not result in impairments of memory retrieval (Fig. 2B, n = 9 per group, LI, 100.0 ± 8.7, control/saline; 86.4 ± 7.6, control/CNO, p = 0.25, Fig. S2E, Aversion Index, 73.9 ± 5.3, control/saline; 66.4 ± 4.7, control/CNO, p = 0.30). This result suggests that vCREB positive neurons are preferentially chosen to encode taste memory in the insular cortex since inactivation of a similar population of insular cortical neurons with normal CREB levels did not affect CTA. Taken together, these data suggest that the insular cortex neurons expressing higher levels of CREB are preferentially involved in memory for CTA. Interestingly, Han and colleagues showed that disruption of CREB function in the LA before tone fear training did not impair subsequent memory formation, due to the likely exclusion of those neurons from memory allocation, while enhancing CREB function in a similar population of neurons before tone fear training biases them towards allocation [3, 4]. These findings suggest that CREB regulates memory allocation during training/memory encoding.
Fig. 2. Silencing of CREB+ neurons in the insular cortex impairs CTA memory retrieval.
CREB or control lentiviruses were infused into the insular cortex 3 weeks before CTA training. Saline or CNO was systemically injected 45 min before CTA memory retrieval. (A) CTA memory was significantly impaired by selective silencing of CREB positive neurons by CNO (Saline group, n = 8; CNO group, n = 9). (B) CNO injection did not impair CTA memory retrieval in control mice (Saline group, n = 9; CNO group, n = 9). LI was normalized to 100 for a saline group in each experiment. Data represent mean ± s.e.m. *p < 0.05. (Saline-injected mice, white columns; CNO-injected mice, black columns).
vCREB neurons are preferentially activated during CTA memory retrieval
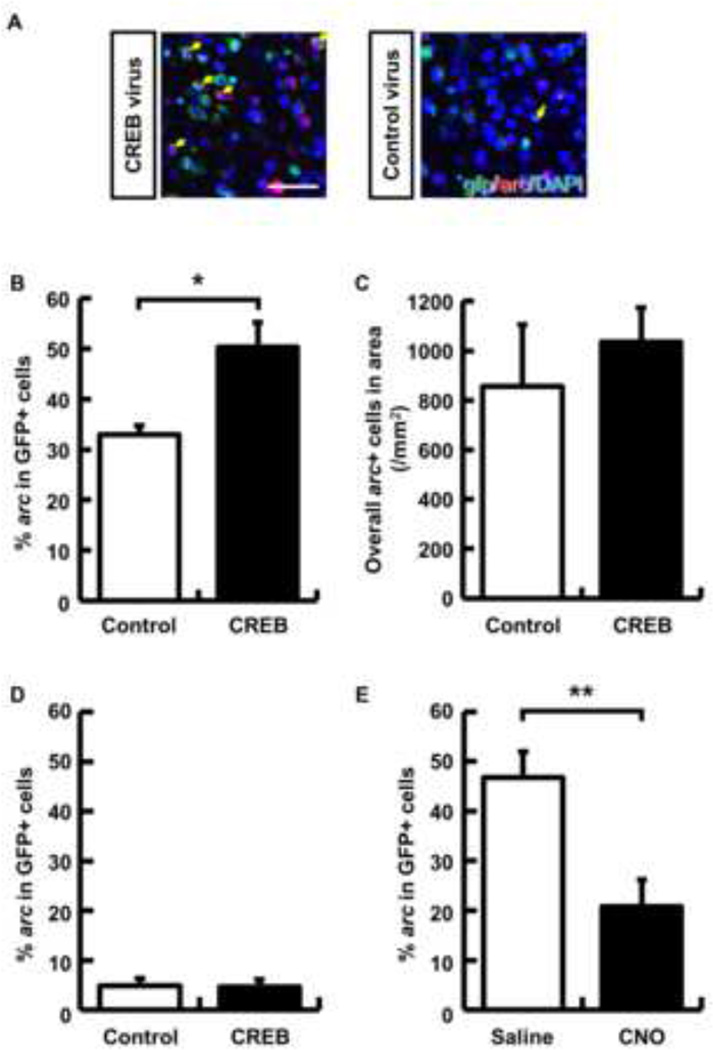
We next imaged the insular cortex following CTA retrieval to further test whether vCREB neurons are preferentially incorporated into neurocircuits encoding CTA. To visualize these neurocircuits, we used the activity-dependent gene arc (activity-regulated cytoskeleton-associated protein; also termed arg3.1) [26]. Neuronal activity induces a rapid but transient increase in arc transcription, such that arc RNA expression serves as a molecular signature of a recently (5 to 15 min) active neuron [26]. CREB and control viruses were bilaterally microinjected into the insular cortex 3 weeks before CTA training. Five minutes after CTA memory retrieval, we harvested the brain and performed fluorescence in situ hybridization (FISH) to analyze co-expression of arc and gfp mRNA (denoting viral transfection) in insular cortex neurons. If increased CREB expression biases memory allocation, then vCREB-positive neurons should be more likely to express arc mRNA than control virus-positive neurons. Consistent with this hypothesis, the probability of arc mRNA expression induced by memory retrieval in vCREB-positive neurons was higher than in control virus-infected neurons (Fig. 3A and B, n = 4 mice per group, control virus, 32.9 ± 1.9 %; CREB virus, 50.3 ± 4.9 %; p < 0.05). There were no differences between the number of arc+ neurons between the mice transfected with either CREB or control viruses (Fig. 3C). To further test whether arc expression is induced by vCREB expression independently of memory retrieval, we also measured the probability of arc expression in vCREB and control neurons in home cage mice. Our results show that there is no difference between these two groups (Fig. 3D, n = 4 mice per group, control virus, 4.9 ± 1.5 %; CREB virus, 4.7 ± 1.5 %; p = 0.92). This result is consistent with a similar previous study in which CREB was overexpressed in the amygdala using the Herpes simplex virus vector [3]. To further test whether vCREB neurons make a critical contribution to memory retrieval, we analyzed arc expression following CTA memory retrieval in vCREB-positive neurons after CNO or saline treatment. Consistent with the behavioral result (Fig. 2A), the probability of arc expression in vCREB neurons during retrieval was significantly decreased by silencing these neurons (Fig. 3E, Saline group, 46.8 ± 5.1 %, n = 6; CNO group, 20.9 ± 5.4 %, n = 7; p < 0.01). These results further support the hypothesis that CREB levels bias which neurons store memory for CTA in the insular cortex.
Fig. 3. Insular cortex neurons with increased CREB level are preferentially recruited into encoding conditioned taste memory.
(A) Representative images showing expression of gfp (green) and arc (red). Yellow arrows indicate double-labeled neurons (gfp+ and arc+). Nuclei were stained using DAPI. Scale bar indicates 50 µm. (B) Probability of cells expressing arc following taste memory retrieval over cells transduced with the CREB or control viruses in the insular cortex (n = 4 mice per group). (C) Overall arc+ cells in the analyzed area (n = 4 mice per group). (D) Probability of cells expressing arc in home cage mice in cells transduced with the CREB or control viruses in the insular cortex (n = 4 mice per group). (E) Probability of cells expressing arc following taste memory retrieval with saline or CNO in vCREB-positive cells (n = 6 saline group and n = 7 CNO group.) Data represent mean ± s.e.m., * p < 0.05 and ** p < 0.01. (B,C and D, control virus group, white columns; CREB virus group, black columns; E, saline group, white columns; CNO group, black columns).
The results presented here demonstrate that specific mechanisms regulate memory allocation outside of the amygdala. They showed that CREB plays a critical role in the allocation of CTA memory in the insular cortex. Since CREB plays a role in memory in two very different structures in the mouse brain, it is reasonable to propose that CREB may have a general role in memory allocation in the mammalian brain. By implication, these results also suggest that memory allocation is a general, highly regulated process in the mammalian brain.
We used two independent strategies to determine whether CREB regulates memory allocation in the insular cortex: With the DREADD neuronal-inactivation system, we showed that CTA memory retrieval was impaired by selectively silencing vCREB positive neurons. Additionally, we used FISH to analyze the expression of the immediate early gene arc, and showed that insular cortical neurons with virally transduced CREB are preferentially activated after memory retrieval. Arc is required for memory formation and arc transcription is turned on in neurons involved in learning and memory [26–28]. All together, these findings indicate that, just as in the amygdala [3–6], taste memory is preferentially allocated into insular cortex neurons with higher levels of CREB. Therefore, these results suggest that CREB is a key component of a general mechanism of memory allocation across brain regions.
Our findings suggest that both the insular cortex and the amygdala use common mechanisms for memory allocation. In the amygdala, the CS and US are thought to converge on the same neurons, where associative memory is stored [29]. The insular cortex receives both taste and visceral information from thalamic areas responding to these different sensory stimuli [30, 31]. However, it is unclear whether this information converges on the same neurons in the insular cortex [29]. Additionally, some studies have shown that the insular cortex is specifically activated by a CS (a novel taste) presentation in the absence of a US (e.g., visceral malaise) [13–15, 32]. These findings suggest that the insular cortex may be more tuned to process CS information. Thus, our results suggest that CREB-dependent mechanisms of memory allocation are not only critical for neurons that encode CS-US associations, they are also critical for memory allocation in circuits that process primarily CS information.
Perhaps, CREB-dependent memory allocation links neuronal representations across brain regions; Since CREB modulates memory allocation for CTA in the amygdala [6] and insular cortex, it is possible that CREB also coordinates memory allocation between these two regions, presumably strengthening the synaptic connection between these subassemblies. It is possible that coordination of memory allocation across brain regions may facilitate memory retrieval.
In summary, the findings presented here demonstrate that specific mechanisms regulate memory allocation outside of the amygdala, and that CREB in the insular cortex plays a critical role in the allocation of CTA memory.
Experimental Procedures
Mice
Adult F1 hybrid (C57Bl/6NTac × 129S6/SvEvTac) mice used in behavioral analysis were group housed (2 per cage) on a 12-h light/dark cycle. 4 – 5 months old mice were used for behavioral analyses. All experimental protocols were approved by the Chancellor's Animal Research Committee of the University of California, Los Angeles, in accordance with US National Institutes of Health guidelines.
Lentivirus production
VSVG-pseudotyped lentiviral vectors were produced by calcium phosphate-mediated transient transfection of HEK-293T cells, as previously described [18]. Lentivirus vectors were titered on HEK-293T cells based on EGFP expression. We used about 5 × 10^5 cells/ml titer of CREB and control viruses in this study.
Surgery
Mice were mounted in a stereotaxic apparatus and anesthetized with Isoflurane (1–2 %). A 2 mm diameter craniotomy was bilaterally performed above the insular cortex. A 1 µl virus solution was bilaterally infused using a Hamilton syringe through a glass micropipettes at the following coordinates; relative to bregma (mm): AP: + 1.3, ML: ± 3.7, DV: − 2.5 from dura mater and AP: + 0.3, ML: ± 3.9, DV: − 2.5 from dura mater, taken from the mouse brain atlas (Paxinos G. and Franklin KB., 2001) at a rate of 0.1 µl/min. A glass capillary was left in place for an additional 5 min. Behavioral tests were implemented 3 weeks after surgery to allow for recovery and sufficient expression of genes.
Conditioned taste aversion (CTA) test
Mice were dehydrated by removal of a water bottle for 24 hours before each test. [Pretest]; The thirsty mice were presented with two choices, water and 50 mM NaCl, for 3 days (for 12 min at day 1 and 2, and 5 min at day 3). On day 1 and 2, mice were supplied water in their home cage for 48 min after the pretest. Baseline preference to NaCl (NaCl consumed [ml] /total amount of drinking [ml]×100) in individual mouse was calculated from pretests. [Training]; On day 3, 150 mM LiCl (presenting US and CS) was presented to a mouse for 10 min after pretest. A water bottle was returned to their home cage 1 hour following training. [Test]; 3 days after training, a two-choice test was implemented to determine the acquired aversion to a salty taste. The mice were presented with two choices, water and 150 mM NaCl, for 12 min. Saline or 1 mg/Kg of CNO (Enzo Life Sciences) was intraperitoneally injected to mice 45 min before this test. Neuronal inactivation by the hM4Di receptor peaks at around 45 – 60 min after systemic injection of CNO [33]. We used a learning index [LI] to assess CTA memory. This index normalizes the individual differences for salty taste preference across mice in this task. LI was defined as [1 − (preference to NaCl in test) / (baseline preference to NaCl]) ×100 and then was normalized to 100 for the mice in the saline group in Fig. 2 and for control virus mice in Fig. S2C. Aversion index was defined as [(water consumed in test) / (water + NaCl consumed in test) × 100][7]. For the FISH experiment, a separate set of mice was used, and 150 mM NaCl was presented to mice for 5 min in a test designed to trigger the retrieval of the CTA memory. Home cage mice were taken directly from their home cages.
Statistical analyses
For comparisons of two groups, we used unpaired two-tailed t tests. Differences were considered to be statistically significant when the probability value was <0.05.
Supplementary Material
Acknowledgments
We would like to thank Sotiris C. Masmanidis, Yong-Seok Lee, and Katie Cai for their assistance. The plasmid containing hM4Di was kindly provided by Dr. Bryan L. Roth (University of North Carolina Chapel Hill Medical School). Supported by grants R37 AG013622, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation to A.J.S., the UEHARA Memorial Foundation of JAPAN to Y.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rogerson T, Cai DJ, Frank A, Sano Y, Shobe J, Lopez-Aranda MF, Silva AJ. Synaptic tagging during memory allocation. Nature reviews. Neuroscience. 2014 doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva AJ, Zhou Y, Rogerson T, Shobe J, Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 2009;326:391–395. doi: 10.1126/science.1174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 4.Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Kwon JT, Kim HS, Josselyn SA, Han JH. Memory recall and modifications by activating neurons with elevated CREB. Nature neuroscience. 2014;17:65–72. doi: 10.1038/nn.3592. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nature neuroscience. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science. 2011;331:1207–1210. doi: 10.1126/science.1200215. [DOI] [PubMed] [Google Scholar]
- 8.Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behavioral and neural biology. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- 9.Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7017–7023. doi: 10.1523/JNEUROSCI.20-18-07017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion. II. Insular (gustatory) cortex. Brain research. 1998;800:40–47. doi: 10.1016/s0006-8993(98)00492-2. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory area in rats and its role in taste discrimination. Journal of neurophysiology. 1980;44:440–455. doi: 10.1152/jn.1980.44.3.440. [DOI] [PubMed] [Google Scholar]
- 12.Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain research. 1999;839:323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- 13.Barot SK, Bernstein IL. Polycose taste pre-exposure fails to influence behavioral and neural indices of taste novelty. Behavioral neuroscience. 2005;119:1640–1647. doi: 10.1037/0735-7044.119.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh MT, Wilkins EE, Bernstein IL. Novel tastes elevate c-fos expression in the central amygdala and insular cortex: implication for taste aversion learning. Behavioral neuroscience. 2003;117:1416–1422. doi: 10.1037/0735-7044.117.6.1416. [DOI] [PubMed] [Google Scholar]
- 15.Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behavioral neuroscience. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Duran LF, Castillo DV, Moguel-Gonzalez M, Escobar ML. Conditioned taste aversion modifies persistently the subsequent induction of neocortical long-term potentiation in vivo. Neurobiology of learning and memory. 2011;95:519–526. doi: 10.1016/j.nlm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira G, Miranda MI, De la Cruz V, Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Basolateral amygdala glutamatergic activation enhances taste aversion through NMDA receptor activation in the insular cortex. The European journal of neuroscience. 2005;22:2596–2604. doi: 10.1111/j.1460-9568.2005.04440.x. [DOI] [PubMed] [Google Scholar]
- 18.Ringpis GE, Shimizu S, Arokium H, Camba-Colon J, Carroll MV, Cortado R, Xie Y, Kim PY, Sahakyan A, Lowe EL, et al. Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice. PloS one. 2012;7:e53492. doi: 10.1371/journal.pone.0053492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey BW, Markoulaki S, Hanna J, Saha K, Gao Q, Mitalipova M, Jaenisch R. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parnaudeau S, O'Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013;77:1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nature neuroscience. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekeres MJ, Neve RL, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learning & memory. 2010;17:280–283. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Zhu Q, Liu T, Yu M, Xiao K, Kong Q, Zhao R, Li GD, Zhou Y. Ghrelin modulates lateral amygdala neuronal firing and blocks acquisition for conditioned taste aversion. PloS one. 2013;8:e65422. doi: 10.1371/journal.pone.0065422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 27.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barot SK, Kyono Y, Clark EW, Bernstein IL. Visualizing stimulus convergence in amygdala neurons during associative learning. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20959–20963. doi: 10.1073/pnas.0808996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly S, Schachtman TR. Conditioned taste aversion : behavioral and neural processes. Oxford ; New York: Oxford University Press; 2009. [Google Scholar]
- 31.Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nature reviews. Neuroscience. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein IL, Koh MT. Molecular signaling during taste aversion learning. Chemical senses. 2007;32:99–103. doi: 10.1093/chemse/bjj032. [DOI] [PubMed] [Google Scholar]
- 33.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.