Abstract
Targeted α-particle therapy (TAT), in which an α-particle emitting radionuclide is specifically directed to a biological target, is gaining more attention to treat cancers as new targets are validated. Bio-vectors such as monoclonal antibodies are able to selectively transport α-particles to destroy targeted cancer cells. TAT has the potential for an improved therapeutic ratio over β-particle targeted conjugate therapy. The short path length and the intense ionization path generated render α-emitters suitable for treatment and management of minimal disease such as micrometastases or residual tumor after surgical debulking. 212Pb is the longer-lived parent radionuclide of 212Bi and serves as an in vivo generator of 212Bi. 212Pb has demonstrated significant utility in both in vitro and in vivo models. Recent evaluation of 212Pb-TCMC-trastuzumab in a Phase I clinical trial has demonstrated the feasibility of 212Pb in TAT for the treatment of ovarian cancer patients. This review highlights progress in radionuclide production, radiolabeling chemistry, molecular mechanisms, and application of 212Pb to targeted pre-clinical and clinical radiation therapy for the management and treatment of cancer.
Keywords: targeted α-therapy (TAT), 212Pb-Radioimmunotherapy (RIT), bio-vector, trastuzumab, cancer
1. Introduction
An obstacle in cancer therapy is the presence of minimal disease such as micrometastases or residual tumor tissue remaining after surgical debulking, and the failure of chemotherapy or low linear energy transfer (LET) radiotherapy to eradicate this residual disease. Furthermore, efficacy of traditional cytotoxic cancer therapy generally is associated with significant normal tissue toxicity, which can limit the success of therapy.
One very promising strategy to overcome this obstacle is to target potent radioactive isotopes specifically to tumors via molecular delivery systems (bio-vectors). Thus, targeted α-particle therapy (TAT) has been designed to directly deliver radionuclides to cancer cells. Unlike low LET radiation, the cytocidal efficacy of high LET α-particle radiation is generally considered indifferent to dose fractionation, dose rate, or hypoxia, and overcomes the resistance to chemotherapeutics. Thus, the intense radiation (100 keV/µm) and considerably shorter path length (50–100 μm) make the resulting DNA damage caused by α-particle much more difficult to repair than the β-particle [1–5].
If significantly differential targeting is achieved by the vector, then the toxic payload on the vector will deliver a lethal dose preferentially to those cells expressing higher concentrations of the target molecule thereby sparing nearby normal cells. This will be governed not only by differences in specificity/antigen-target concentration but also factors like vascularity, antibody/antigen-rate contrasts, affinity etc. Monoclonal antibodies (mAbs) such as trastuzumab (FDA-approved anti-HER2 mAb) are the most commonly used vectors. The mAbs can be the whole immunoglobulin molecule or fragments like F(ab’)2. Other targeting agents include substrate analogs, normally in the form of peptides [2,6,7].
The development of TAT also rests upon the physical (and economical) availability of a radionuclide combined with suitable linker or chelation chemistry that would securely sequester α-emitters along with a biological vector. Choosing appropriate chelating agents that stably sequester the radionuclide to limit dissociation of radionuclide from the vector in vivo is a critical component of TAT [2,4]. Thus, in vivo stability of radioimmunoconjugates is a prerequisite for reaching a high therapeutic ratio in TAT.
Many studies employing TAT have involved intraperitoneal (i.p.) injections. Pre-clinical studies with intraperitoneal (i.p.) TAT have demonstrated that α-particles such as 211At (T1/2 = 7.2 h), 213Bi (T1/2 = 46 m), and 212Pb (T1/2 = 10.64 h) are ideal for the treatment of smaller tumor burdens, micrometastatic disease, and disseminated disease [8–10] within the peritoneal cavity. Although TAT has found success pre-clinically in treatment of microscopic residual diseases, the transition and acceptance of TAT into the clinic has been challenging, because of high cost of the radionuclide, limited availability, the short physical half-lives of the available α-emitters. In addition, mechanistic studies have until recently been restricted to those that explore cell death in vitro, which are not reflective of TAT of tumors in the complex and dynamic in vivo environment. To overcome these limitations, a better understanding of the underlying in vivo mechanisms of action in response to TAT will be required for appropriate clinical applications.
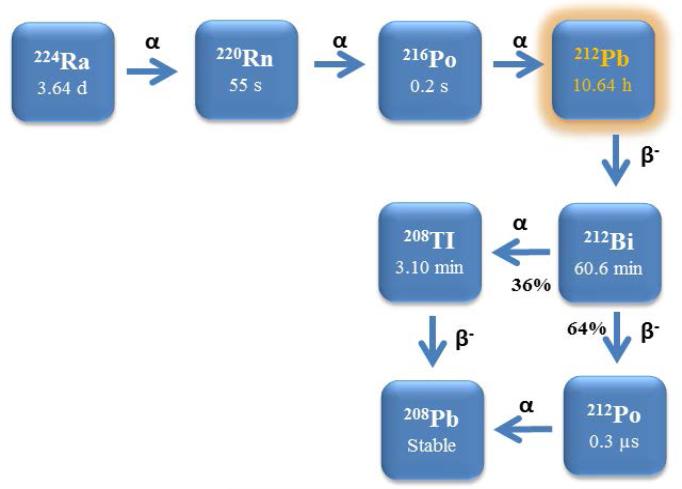
Among the α-particle emitters possible to use in nuclear medicine, considerable interest continues to grow in 212Pb for TAT. 212Pb is the immediate parental nuclide of 212Bi (T1/2 = 61 m). Therefore, one strategy has been proposed to label mAb with 212Pb as an in vivo generator for 212Bi to effectively overcome the problem of short half-life 212Bi. There is a distinct advantage of targeting tumor with 212Pb over 212Bi because 212Pb can deliver more than 10 times the administrated activity of 212Bi while traversing its time constraint, which makes use of 212Pb easier for dose preparation and administration in TAT (Figure 1).
Figure 1.
Decay schemes for the production of 212Pb.
In pre-clinical studies, TAT using 212Pb has shown significant therapeutic efficacy in both in vitro and in vivo model systems [10–12]. A mechanistic study of 212Pb-labeled TAT demonstrated significant impact of catastrophic dsDNA destruction as a result of high-LET 212Pb traversal of the nucleus, with interference with DNA damage repair and perturbation of the cell cycle, leading to significant tumor cell killing [11]. Therefore, a single targeted therapeutic strategy that delivers α-particles from a source such as 212Pb would be an innovative approach to the elusive goal of creating a magic-bullet therapy to target cancer. Recent progress in pre-clinical and mechanistic studies in in vivo tumor models has provided critical information (such as induction of apoptosis, G2/M arrest, blockage of double-strand DNA damage repair, modulation of genes) for clinical transition of 212Pb-labeled TAT [10–12]. With the advent of the first clinical trial that employed 212Pb as the therapeutic agent (targeted by trastuzumab), this radionuclide has finally reached a landmark position in the family of α-emitters validated as suitable for such applications [13]. This review provides a brief update on the recent efforts and progress toward a clinical transition of 212Pb-labeled TAT.
2. Production Route of 212Pb-labeled Immunoconjugate
Among ~ 100 α-emitting radionuclides (atomic number > 82), there are but a few (211At, 212Bi, 213Bi, 225Ac, 223Ra, 212Pb, 227Th, and 149Tb) that are medically relevant and realistically available for potential clinical use on the basis of production availability, cost, emission characteristics, decay chain (acceptable daughter radionuclides), and half-life [14]. Appropriate use of TAT is defined by a combination of the radionuclidic properties, including actual emissions and half-life, the choice of targeting vector, scale of disease, and accessibility of disease by the targeting vector such that the α-emitting radionuclide might be delivered within a realistic time frame and targeted volume or disease presentation. Thus, use of α-emitting radionuclides is envisioned as being exceptionally potent and appropriate for the treatment of small lesions and metastases; locoregional or compartmentalized diseases of similar presentation. Furthermore, because of the limited range of the α-particle, normal tissue toxicity is expected to be quite low when a TAT strategy is used. Finally, although it is generally accepted that there is no effective resistance to α-particle lethality and no oxygen or hypoxia limitations to efficacy, making such therapies extremely potent in the therapeutic. Of radionuclides proposed for applications in TAT, 212Pb and 212Bi are produced from the decay chain of 228Th and either is available from a 224Ra-based generator which provides clinical grade material suitable for administration to patients [4,15–17].
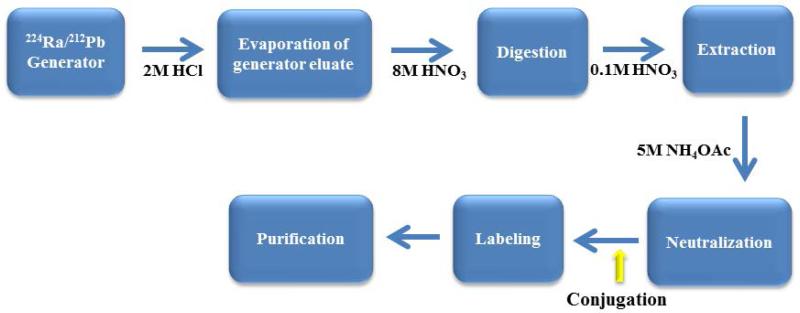
For the chemical and pre-clinical development of 212Pb, considerable evolution and modification in the radiochemical protocols for elution of 212Pb from the 224Ra generator have been undergone. Unfortunately, the original 228Th generator experienced radiolytic damage to the support resin with consequent diminished yield, and was also a serious radiation safety problem. To avoid problems originating from 228Th-based generators, a generator based on 224Ra (t1/2 = 3.6 d) was designed [15–16]. The 224Ra-based generator facilitates on-site production of 212Bi or 212Pb suitable for radiolabeling mAbs, peptides, or other vectors conjugated with appropriate bifunctional chelating reagents.
212Pb is eluted from the 224Ra/212Pb generator with 2 M HCl [17] and after dilution (0.1M HCl), the 212Pb eluate is loaded onto a small AG-50 × 4 resin. The 212Bi can be eluted from the resin with 0.2 M HI. After evaporation of the generator eluate, the residue is digested with 8M HNO3 with 75% – 90% of the 212Pb being extracted into HNO3 (0.1 M).
In general, conjugation of mAbs with the chelator is routinely performed at molar chelate to antibody/peptide ratios of 5–20:1 [9,10]. In the specific case of using TCMC (isothiocyanate conjugation chemistry) for Pb(II) radionuclides, well defined conditions and methods have been published [18] Following the conjugation reaction, free chelating agents is removed from the conjugate solution; for purification of a 212Pb-labeled radioconjugate, a simple desalting column such as a PD-10 column can be used: HPLC methods are viable alternatives. Although it generates high yields of 212Bi and its parent nuclide 212Pb, the 224Ra generator must be replaced after 1–2 weeks because of the short half-life of 224Ra (Figure 2). When the generator is eluted daily, > 90 % of the expected 212Pb activity can be eluted. At the time of injection of purified 212Pb-labeled immunoconjugate, by necessity, 212Pb exists with its daughters 212Bi and 212Po. All decays of the 212Pb and 212Bi lead to α-emissions, either directly, or through their daughter, 212Po (t1/2 = 0.3 μs). The alpha decays of 212Bi and 212Po are the active ones destroying cancer cells over couple of hours. The deposited energy come from the α-decays of 212Bi and 212Po; 21.1 % from the 6.2 MeV 212Bi α-particle emission and 67.3 % from the 8.9 MeV 212Po α-particle emission. Hence, the majority of the dose delivered to the cell (93.4 %) originates from the high LET α-particle emissions [19,20].
Figure 2.
Labeling conjugates with 212Pb.
3. Radiolabeling Chemistry of 212Pb
Continued interest exists in the area of synthesis of bifunctional chelating agents, their conjugation to mAbs, and their subsequent use for sequestering radioactive metal ions for TAT applications. One of the functional requirements is that the conjugation of a radionuclide to a mAb must maintain the affinity/avidity of the mAb for its target antigen. Thus, choosing an appropriate bifunctional chelating agent is a critical factor as it must form an adequately stable complex of the metallic radionuclide of choice.
Among a variety of technologies to conjugate radioisotope to a mAb, two chelating systems have been used successfully to form conjugates for labeling with 212Pb, namely, the macrocyclic agents 2-(4-isothiocyanatobenzyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (C-DOTA) and 2-(4-isothiocyanatobenzyl-1,4,7,10-tetraaza-1,4,7,10-tetra-(2-carbamonylmetyl)-cyclododecane (TCMC). The TCMC chelate was specifically developed for chelating Pb(II) radionuclides while C-DOTA is more multi-purpose and has been used for chelating other metals including 111In, 90Y, 177Lu, and 212Bi. Although DOTA is known to form strong complexes with both Bi and Pb, a significant part of 212Bi (~35%) is released from carrier after the 212Pb/212Bi radioactive decay, resulting in the formation of highly ionized daughter atoms. Loss of 212Pb post-internalization of mAb delivery to cells has been reported as a source of marrow toxicity when using a DOTA conjugate, wherein the dissociated 212Pb was free to be transported to the bone and subsequently decay to 212Bi [21].
In contrast, the differences in susceptibility to acidic conditions by the Pb(II) TCMC complex were well demonstrated by Chappell et al. The Pb(II)[4-NCS-Bz-TCMC] complex, which has been very efficiently radiolabeled with Pb(II) radionuclides, was less labile at pH 3.5 than corresponding DOTA complex, conferring enhanced resistance to acid-catalyzed dissociation with the cell. More recently, the structure of the Pb(II)-TCMC complex revealed that Pb(II) is fully encapsulated by the TCMC with the eight-coordinate sphere saturated by the four ring nitrogen and four amide oxygen atoms, supporting this complex as being extremely stable with decreased acid lability for in vivo sequestration of Pb(II) [22–25]. These combined advantages promote use of TCMC over use of C-DOTA mAb immunoconjugates for TAT when using Pb(II) radionuclides (Figure 3).
Figure 3.
Schematic representation of radioimmunoconjugate with 212Pb-TCMC-trastuzumab targeting HER2.
4. Molecular delivery platform of 212Pb
Greater understanding of the molecular differences between cancer cells and normal cells has led to the development of therapies that specifically target cancer cells, including antibodies targeted to tumor-associated antigens. When a cell becomes cancerous a cascade of a biochemical responses occurs, and as a result, the diseased cells exhibit many biomarkers that are either mutated or over-expressed. Various surface receptors are over-expressed in particular tumor types. Receptors are integral to all aspects of cellular function, such as proliferation, migration and communication, and triggering or blocking receptors with a monoclonal antibody (mAb) can induce either cell growth or death [26,27]. HER2 (epidermal growth factor receptor 2) is a biomarker over-expressed by a variety of epithelial tumors: 25–30% of breast and ovarian cancers, 35–45% of all pancreatic adenocarcinomas, and up to 90% of colorectal carcinomas. Over-expression of this oncogene is associated with poor prognosis and aggressive tumor attributes [28–30]. Thus, over-expression of HER2 in a variety of epithelial cancers makes this an ideal target for TAT.
Since the effectiveness of targeted therapy depends on the molecular target expression in the patient, the choice of targeting vector is crucially important in the development of a successful TAT. Currently, several radionuclides (111Ln, 64Cu, 89Zr, and 212Pb) conjugated to a FDA-approved HER-2 targeting monoclonal antibody, trastuzumab, can be found in clinical trials [31]. Among radionuclides conjugated to trastuzumab, 212Pb-labeled trastuzumab is being developed to improve upon those results obtained with other radionuclides such as 90Y and 177Lu that have been evaluated for intraperitoneal (i.p.) therapy by providing more potent radiation targeted to malignant cells while limiting radiation exposure to normal tissues [32]. A unique advantage of TAT versus monotherapy with trastuzumab is that neither high expression nor homogenous expression of HER2 throughout the tumor is required to affect therapy. In pre-clinical studies using 212Pb-labeled trastuzumab, Brechbiel's group demonstrated the exquisite effectiveness of 212Pb-labeled trastuzumab for the treatment of low HER2-expressing i.p. diseases [33,34]. Recently, a Phase I clinical study demonstrated the feasibility of 212Pb-labeled trastuzumab in this setting for the treatment of ovarian cancer [13]. It must be noted that a completely non-specific antibody would contribute a considerable dose and effect, provided it is retained locally since transmissible biologic effects resulting from the radiation insult are pronounced following high LET radiation, such as α-particle irradiation.
Most popular targeting vectors of choice for TAT are peptides and antibodies. Recent advances in protein engineering have led to range of antibody fragments of varying sizes with corresponding specificity and affinity of parent antibodies that can be used as targeting vectors in TAT. They include single-chain variable fragment (scFv), diabodies, fragment antigen binding (Fab), minibodies, and F(ab’)2 fragments, and others. For instance, the therapeutic efficacy of 211At-labeled MX35 F(ab’)2 in ovarian cancer was evaluated in vivo. MX35 is a murine mAb reactive with a cell surface glycoprotein expressed homogeneously which provides desirable characteristics for use with α-particles because cross-fire irradiation of antigen negative cells will not be high. Additionally, MX35 F(ab’)2 would also be expected to increase the homogeneity of delivery because of its smaller molecular size and high diffusivity versus an intact IgG [8,35,36].
To further address limitations of size, penetration and clearance rate associated toxicity, pre-targeting strategies have been developed. Su et al examined the potential of antibody pre-targeting therapy with 212Pb using an NR-LU-10 antibody-streptavidin conjugate administrated first followed by an N-acetyl-galactosamie-biotin clearing agent [37]. The pre-targeting of 212Pb/212Bi provided good tumor uptake, tumor-to-blood ratio and normal non-target tissue/blood ratio with the exception of kidney, the primary biological deposition site for Bi(III).
Radiolabeled peptides have also been evaluated as potential therapeutic agents for TAT during the last decade. Peptides have very favorable characteristics as diagnostic and therapeutic targeting vectors due to an excellent permeability, lack of antigenicity, minimal side-effects and rapid clearance from the body, and many can be chemically modified and radiolabelled. Miao's group recently evaluated DOTA-Re(Arg11)CCMSH radiolabeled with 203Pb as a matched pair imaging agent for 212Pb-DOTA-Re(Arg11)CCMSH [38].
For another delivery option, 212Pb can be also encapsulated into liposomes as mediators of in vivo targeted radiotherapy. Rosenow et al demonstrated the formation, characterization, stability, and in vivo biodistribution as a function of lipid bilayer. Liposomes incorporating 212Pb remained partially intact in vivo. A unique potential role for 212Pb in TAT has been also proposed by more stably encapsulating the radionuclide inside fullerenes. A 212Pb@C60 derivative was generated by 212Pb@C60 and along with its malonic ester derivatives from the decay of 224Ra. 212Pb was not accumulated in bone after administration as an endohedral fullerene, compared to a conventional polyaminocarboxylate 212Pb complex [39,40].
5. Dosimetry
Alpha-particle emitters are attractive alternatives to traditional radiation therapy or chemotherapy as a systematic cancer treatment. Generally, dosimetry provides useful methods for standardizing and comparing the efficacy of different radiation-based treatments and it is now also needed for optimization of TAT [41,42]. Short half-lives, short range, high LET, and complicated decay pathways of α-particle emitters differentiate their dosimetry from that of β-particle emitters. In TAT, a potential benefit of 212Pb is the combined release of both α- and β-particles. Due to the short range of α-particles, extremely high and uniform uptake is required to ensure that all dividing cells are irradiated. Cross-fire from β--particles may ensure greater uniformity in delivered dose and reduce the likelihood of non-irradiated cells, i.e. local failure.
In addressing dosimetry for α-particle emitters, a number of different parameters have to be accounted for including heterogeneous target expression, antibody avidity, tumor vascularity, and interstitial pressure in the tumor. Two calculating methods for the energy deposited by individual α-particles have been described, Medical Internal Radionuclide Dose (MIRD) and microdosimetry. MIRD has been applied to in vivo experiments for dose determination after α-particle radiation therapy. Microdosimetry is the study of the stochastic nature of energy deposition in small targets as quantitative means of understanding the biophysical and biological interactions of radiation with matter [43–47].
The observed dose in tumors and normal tissues needs to be estimated from pre-clinical studies before initiating treatment studies. To estimate the absorbed dose delivered to the different organs and tumors in TAT with 212Pb, Boudousq et al. determined mean absorbed dosimetry by multiplying Ars by 8.7 MeV, which corresponds to overall energy released by the alpha (7.8 MeV) and beta (0.9 MeV) particles emitted during the decay of 212Pb, 212Po, and 208Tl. The mean absorbed doses were at the highest activity, 35.5 Gy for 212Pb-35A7 and 27.6 Gy for 212Pb-trastuzumab in tumors and 10.9 and 7.2 Gy, respectively in blood [48].
The translation of animal data to the human requires a pharmacokinetic model that links macro- and micro-scale pharmacokinetics thereby enabling the extrapolation of micro-scale kinetics from macroscopic measurements and the converse. In clinical dosimetry for α-particle emitters, pharmacokinetic data such as urine, blood, and peritoneal fluids are required. Andersson et al demonstrated the encouraging Phase I results using i.p. 211At-MX35 F(ab’)2 in ovarian cancer patients. The radioactive flow out of the abdominal cavity can be determined using a radioactive tracer analog, by monitoring the activity concentration in blood over time. Pharmacokinetic data showed that the variation in the absorbed dose in bone marrow can be around 20 % [8]. Recently, Meredith et al reported on dosimetry data in the first human clinical study using α-particle RIT with 212Pb-TCMC-trastuzumab [13]. The measurements included administered 212Pb activity remaining in the whole body, blood serum, peritoneal cavity, and urine for various time points after infusion, where the standard MIRD dosimetry approach was used to calculate organ doses. This Phase1 human study confirmed the predicted dosimetry with low toxicity. These data were closely comparable to dosimetry reported i.p. administration of 211At-MX35 F(ab’)2.
To improve the relevance of small-scale dosimetry studies, new tools are required to provide better image data. Images from γ-camera and single proton emission computerized tomography (SPECT) have some limitations such as low resolution, a poor signal to noise ratio. Recent studies suggested that the α-camera, a quantitative bioimaging technique based on digital autoradiography and the principle of scintillation, is a promising to detect α-particles in tissues ex vivo. This technique was applied for quantitative imaging the activity distribution of 211At-MX35 F(ab’)2 of tumors, kidney and the whole body [49,50].
The dosimetry of α-particle emitters is challenging because of the stochastic nature of energy deposited in small subcellular targets. Average cell survival probabilities derived from macroscopic dosimetry experiments also may not reflect the true cell survival probabilities. Thus, other approaches for α-particle dosimetry such as microdosimetry typically required for the analysis of cell culture experiments involving low concentrations of α-particle emitting nuclide. Microdosimetry should be considered for both targeted and non-targeted tissues. Likewise, relative biological effectiveness (RBE) values should be considered carefully; α-particle irradiation is 3-7 times more therapeutically effective per unit of absorbed dose than protons or electrons. In TAT clinical studies, a value of 5 is normally used to estimate the equivalent absorbed doses [51,52].
6. Mechanistic studies of targeted 212Pb
Cell death is brought about through a number of mechanisms such as apoptosis, autophagy, necrosis, and mitotic catastrophe [53–57]. To insure the maintenance of the integrity of the genome, the cell is endowed with a myriad of redundant DNA repair mechanisms. Failure of these systems from catastrophic cellular injury results in cell death [58,59]. High LET α-particle radiation produces a dense linear ionization track and generates locally multiple damage sites in sensitive targets like DNA. These lesions, produced in close proximity to each other, are poorly repairable, thus making α-particles highly deleterious to cell survival.
To date, the literature on TAT mechanistic studies has been effectively restricted to in vitro studies, which are not immediately reflective or predictive of TAT treatment in a complex in vivo tumor environment. It is also noteworthy that there have been few studies related to the determination of the actual mechanisms involved in TAT cytotoxicity [60,61]. Therefore, elucidating in vivo mechanisms is important because they are more relevant to the actual tumor environment. However, these studies are exceedingly challenging and can be expensive to perform.
A recent study from Yong et al investigated the in vivo tumor response at the cellular level using 212Pb-TCMC-trastuzumab in mice bearing human colon cancer LS-174T intraperitoneal xenografts [11]. In this study, the increase of DNA double strand breaks and down-regulation of Rad51 were observed, indicating delayed DNA ds damage repair as compared to the non-specific controls. The increased killing efficacy of 212Pb-TCMC-trastuzumab was, in part, associated with G2/M arrest, depression of the S phase fraction, and depressed DNA synthesis. Treatment with 212Pb-TCMC-trastuzumab also delayed open chromatin structure and expression of p21, suggesting a correlation between modification of chromatin structure and induction of p21 in response to TAT.
In cancer therapy, more than 50% of therapies incorporate radiation as one of the efficacious forms of therapy [62,63] and combination therapies outperform single modalities. A second study from Yong et al examined the impact of TAT combination therapy wherein gemcitabine (GEM), a standard of care therapeutic for pancreatic cancer and a well-defined radiosensitizer, was administered prior to the 212Pb-TCMC-trastuzumab and compared with non-specific controls [64,65]. In this instance, 212Pb-TCMC-trastuzumab administered after pre-treatment with Gem abrogated G2/M arrest, which was associated with inhibition of Chk1 phosphorylation, leading to increased apoptosis. This combination therapy also resulted in reduced DNA synthesis, accumulation of unrepaired DNA, and modification in the chromatin structure of p21, indicating a strong correlation between the inhibition of Chk1 phosphorylation, interference with DNA damage repair, and chromatin modification.
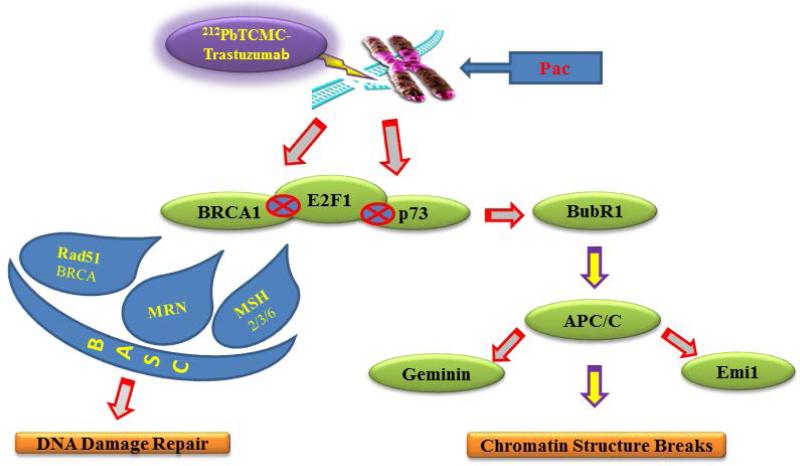
Gemcitabine and paclitaxel (Pac) were the first two chemotherapeutic drugs evaluated in conjunction with 212Pb-TCMC-trastuzumab. A recent study from Yong et al. examined the effect of TAT combined with Pac, a recognized radiosensitizer. The results demonstrated that the combined modality of Pac with 212Pb-TCMC-trastuzumab markedly reduced DNA content in the S-phase of the cell cycle with a concomitant increase observed in the G2/M-phase. This treatment regimen also diminished phosphorylation of histone H3, accompanied by an increase in multi-micronuclei, or mitotic catastrophe in nuclear profiles, and positively stained γH2AX foci, suggesting possible effects on the mitotic spindle checkpoint by the Pac and 212Pb-TCMC-trastuzumab treatment. Consistent with this observation, interference with a functional Aurora B, which reduces the affinity of BubR1 for kinetochores, was observed in response to this treatment regimen. Therefore, the impairment of the mitotic spindle checkpoint may lead to death of tumor cells [66].
Cells cannot function if DNA damage corrupts the integrity and accessibility of essential information in the genome. Exposure of cells to ionizing radiation activates multiple signal transduction pathways, which results in complex alterations in gene expression. Thus, studies of gene expression may lead to identification of radiation responsive genes that could potentially provide biomarkers of radiation exposure. Gene expression qRT-PCR array profiling after exposure to 212Pb-TCMC-trastuzumab was recently reported from Yong et al. In this study, 212Pb-TCMC-trastuzumab treatment differentially regulated genes including genes involved in apoptosis (ABL, GADD45a, GADD45r, PCBP4, and p73), Cell cycle (ATM, DDIT3, GADD45a, GTSE1, MKK6, PCBP4, and SESN1), and damaged DNA binding (DDB) and repair (ATM, and BTG2). Furthermore, the p73/GADD45 signaling pathway mediated by p38 kinase signaling may be involved in the cellular response of tumors exposed to 212Pb-TCMC-trastuzumab [12].
Gene expression profiling provides a potentially powerful approach towards understanding the molecular basis of the cellular response to therapeutic agents or radiation. Irradiation results in major damage to DNA while Pac affects microtubules. Thus, modification in gene expression invoked by Pac/212Pb-TCMC-trastuzumab may derive mainly from perturbation of the microtubule network and DNA damage signaling pathways [67]. The proposed cell killing mode by TAT using 212Pb when combined with Pac is shown in Figure 4. The increased stressful growth arrest conditions induced by Pac/212Pb-trastuzumab treatment suppressed cell proliferation through regulation of genes involved in apoptosis and damaged DNA repair including ssDNA and dsDNA breaks. This combination also caused perturbation of genes related to the mitotic spindle checkpoint and BRCA1-associated genome surveillance complex such as BRCA1, Rad50-MRE11-NBN, MSH2-MSH6, indicating that there is a fine cross-talk between DNA damage repair and the spindle damage response.
Figure 4. Proposed mode of cell killing action by 212Pb-TCMC-trastuzumab with paclitaxel (Pac).
Irradiation results in major damage to DNA while paclitaxel affects microtubules. Perturbation of DNA damage repair and mitotic checkpoint in tumor exposed to paclitaxel and 212Pb-trastuzumab may be responsible for the cell death. Many genes and proteins such as BRCA1, p73, and BubR1 are involved in these pathways. A fine cross talk between DNA damage and the spindle damage response is evident [67].
7. Pre-clinical studies of targeted 212Pb
The treatment and management of cancer ultimately requires a multimodality approach, and development of such treatment regimens remains a priority for investigators. The benefit of treating cancer with TAT continues to be demonstrated in pre-clinical studies. Thus, α-particle radionuclide antibody-conjugate such as 212Pb-TCMC-trastuzumab, which binds to HER2, ultimately may be efficacious and more appropriately employed in a coordinated strategy as shown in Figure 3.
212Pb-labeled trastuzumab for the treatment of disseminated peritoneal disease has been proposed and tested using mice bearing LS-174T i.p. xenografts. Milenic et al. evaluated the therapeutic efficacy of 212Pb-TCMC-trastuzumab [10] finding the maximum tolerate dose (MTD) to range from 0.74–1.48 MBq. The median survival of animals receiving 0.37 MBq was prolonged from 19 to 56 days. A multi-dose regime of 212Pb-TCMC-trastuzumab administrated monthly (for up to 3 months) increased the median survival of mice to 110 days.
The therapeutic regimen combining TAT and chemotherapeutics may have tremendous potential, particularly in the context of microscopic and residual disease post-surgical resection treatment. To enhance the therapeutic efficacy of TAT, treatment using mice bearing i.p. LS-174T xenografts with gemcitabine (GEM) followed 24–30 h later by either 0.19 or 0.37 MBq of 212Pb-TCMC-trastuzumab showed significant improvement of median survival from 31 (without GEM) to 51 (with GEM) days at the 0.19 MBq, and from 45 up to 70 days at the 0.37 MBq, respectively, compared to 16 days for untreated animals. Three weekly treatment of GEM with 212Pb prolonged the median survival to 90 days compared to 21 days of the untreated. Gemcitabine was found to provide the greatest benefit when a multi-cycle treatment regimen was implemented; 2 doses of 212Pb-TCMC-trastuzumab and a total 4 doses of GEM extended the median survival to 196.5 days as compared to 45 days after a single injection of 212Pb-TCMC-trastuzumab [33].
Paclitaxel was the second chemotherapeutic drug evaluated in conjunction with 212Pb-TCMC-trastuzumab in the same animal tumor model system. To examine the optimal order for administration, Pac was given 24 h prior to, concurrent with, or 24 h post administration of 212Pb-TCMC-trastuzumab. Enhanced therapeutic efficacy was found in the group receiving 600 μg of Pac 24 h before the RIT. The median survival increased from 44 to 171 days, compared with the group receiving 212Pb-TCMC-trastuzumab alone. The results showed higher specificity to 212Pb-TCMC-trastuzumab than 212Pb-TCMC-HuIgG, the non-specific control [34]. Thus, application of 212Pb-TCMC-trastuzumab in mono therapy or in combination with chemotherapy, are promising therapeutic options for treatment of carcinomas that are characterized by dissemination of single tumor cells in the peritoneum like ovarian or gastric cancer.
The mechanism of action by the platinum-based drugs differs from both GEM and Pac and as such would add another class of chemotherapeutics to the armamentarium to be combined with radiolabeled antibodies. A recent study of Milenic et al. also examined the potential of platinum chemotherapy to enhance the therapeutic efficacy of both HER2-targeting α-emitting high-LET 213Bi and 212Pb-labeled trastuzumab [68]. The therapeutic efficacy of 212Pb-TCMC-trastuzumab was significantly increased the median survival from 58 to 157 days when the mice were pre-treated with carboplatin 24 h prior. These results demonstrated the necessity of empirically determining the administration sequence when combining therapeutic modalities.
Tan et al. evaluated the therapeutic effects and systemic toxicity of 212Pb-trastuzumab in an orthotopic model of human prostate cancer cells in nude mice [69]. A single intravenous injection of 212Pb-trastuzumab reduced tumor growth by 60–80%, reduced aortic lymph node metastasis, and prolonged survival of tumor-bearing mice. Treatment with 212Pb-trastuzumab did not cause significant changes in body weight, serum glutamic pyruvic transaminase (SGPT), blood urea nitrogen (BUN), hematological profiles, and histological morphology of several major organs of tumor-bearing mice, suggesting that a systemic delivery of 212Pb-trastuzumab could be an effective modality for management of advanced human prostate cancer.
Boudousq et al. compared the contribution of mAb internalization in the efficacy and toxicity of intraperitoneal α-RIT of small volume carcinomatosis using 212Pb-labeled mAbs that targeting HER2 (internalizing) or CEA (non-internalizing) receptors [48]. The median survival was significantly higher in mice injected with 212Pb-35A7 (HER2 targeting) (94-day) than in animals treated with the same activity of 212Pb-PX (CEA targeting) or with NaCl (18-day). Median survival was even not reached after 130 days when follow-up was discontinued in mice treated with 212Pb-trastuzumab. The mean absorbed doses were 35.5 Gy 212Pb-35A7 and 27.6 Gy for 212Pb-trastuzumab. Although the dose absorbed by the tumor was higher for 212Pb-25A7, 212Pb-trastuzumab was more efficient. These results indicate potential advantage of using internalizing anti-HER2 based in geometry compared with non-internalizing anti-CEA 212Pb-labeled mAbs in the therapy of small volume xenograft tumors.
8. Clinical studies of targeted 212Pb
Use of α-emitting radionuclides is envisioned as being exceptionally potent and appropriate for the treatment of small lesions and metastases, and loco-regional disease. However, there have been a very limited number of clinical trials executed to date evaluating TAT because of the many of the overarching obstacles (economic availability, production availability, suitable chemistry, radiation fears, etc.) confronting clinical transition of TAT. Those α-particle emitters used so far in clinical studies include: 211At, 212Bi, 223Ra, 212Pb, and 225Ac.
TAT can deliver high absorbed dose to locally confined disease with minimal irradiation to radiosensitive organs. The advantage of intraperitoneal (i.p.) administration compared to intravenous (i.v.) injection for localizing radiolabeled mAb to microscopic peritoneal tumor disease has been shown in animal models and in human studies [70,71]. RIT with β--emitters such as 131I, 177Lu, or 90Y have been used in i.p. RIT and displaced promising results. However, on the large-scale, i.p. RIT with 90Y-labeled HMFG1 was not successful, likely due to insufficient irradiation of long range of β-emitters on small tumor clusters [72].
Prior experience with i.p. radionuclide conjugate therapy suggested that α-particles with a shorter half-life would allow dose escalation with less toxicity. Therefore, TAT with radio-biologically potent α-particle emitters such as 212Pb and 211At should provide the improved efficacy over β-emitters. For instance, animal studies treated with 211At-labled RIT have shown low risk of adverse events through the highest dose level approximately at 200 MBq/L [73]. In a Phase I clinical trial, i.p. therapy of 211At-MX35 F(ab’)2 has shown promise as an adjuvant therapy for ovarian cancer in patients with no evidence of gross disease. The highest administrated activity concentration at 100 MBq/L was needed for high therapeutic efficacy [74].
Based on the successful 212Pb pre-clinical data published from the Brechbiel group, 212Pb-TCMC-trastuzumab may have potential for an improved therapeutic ratio over β-emitter such as 90Y and 177Lu targeted conjugate therapy and have beneficial activity against patients with HER-2 expression malignancy. Recently, the Phase I clinical trial with i.p. 212Pb-TCMC-trastuzumab at the University of Alabama, Birmingham was designed to determine safety, distribution, pharmacokinetics, and safety of i.p. 212Pb-TCMC-trastuzumab in patients with HER2-expressing malignancy [13]. In this published aspect of the study, 212Pb-TCMC-trastuzumab was delivered i.p. to 3 patients with HER2 expressing cancer who had failed standard therapies. After administration of 7.4 MBq/m2, gamma camera imaging studies showed no distribution of radioactivity out of the peritoneal cavity or normal organ uptake. These data were consistent with pre-clinical studies. Cumulative urinary excretion was < 6 % in 2.3 half-lives. The maximum external exposure rate immediately post-infusion at skin contact over the abdomen averaged 7.67 mR/h and dropped to 0.67 mR/h by 24 h. The data points correlate closely with 212Pb physical decay. Follow-up > 6 months showed no evidence of agent-related toxicity, indicating good tolerance.
In the second study report using 212Pb-TCMC-trastuzumab, Meredith et al determined the safety, pharmacokinetics, immunogenicity, and tumor response of IP 212Pb-TCMC-trastuzumab in patients with human HER2 expressing malignancy [75]. Five dose levels of i.p. 212Pb-TCMC-trastuzumab treatment resulted in little toxicity in patients with ovarian cancer. Limited redistribution of radioactivity out of peritoneal cavity to circulating blood, which cleared via urinary excretion, and no specific uptake in major organs were observed at 24 h. The highest administrated activity concentration at 500 Bq/mL was needed for high therapeutic efficacy without any side effects.
Therefore, the phase study I with intraperitoneal (i.p.) 212Pb-TCMC-trastuzumab confirmed its medical potential and feasibility. The data obtained are consistent with those of prior non-human studies in showing prolonged retention of the 212Pb-TCMC-trastuzumab within the peritoneal cavity and with no evidence for localization to normal organs on planar images. The relatively low radiation dose is also closely comparable to dosimetry data obtained from 211At-MX35 F(ab’)2.
9. Conclusions
Standard radiotherapy procedures fail to treat distant, undetected metastatic or disseminated disease. Targeted radiation therapy with mAbs which bind to tumor associated antigens may be efficacious in an innovative strategy under these circumstances. Thus, the use of α-particle radionuclides has been the subject of intense research over last few years. Their path length is short so that they traverse a few cells from the point of decay. These properties render α-particles ideal for treatment of smaller tumor burdens, micrometastatic disease, and disseminated diseases where α-emitters may provide an irradiation that conforms to small tumor burden.
Given the promise of α-particle TAT in pre-clinical and clinical investigations, there has been recent interest in innovative approaches using 212Pb-labeled TAT for treatment and management for disseminated peritoneal disease. 212Pb-labeled TAT was developed to improve upon results over other radionuclides that had been proposed or evaluated for intraperitoneal therapy such as β-particles, e.g. 177Lu or 90Y, by providing more potent radiation to targeted malignant cells. A potential benefit of 212Pb is the combined release of α- and β-particles. Labeling targeting vectors with 212Pb can be performed using TCMC, currently the most accepted bifunctional chelating agent to obviate a source of toxicity originating from intercellular dissociation of 212Pb from DOTA. As magic bullets, the use of mAbs such as trastuzumab enhances the therapeutic index, due to the selective localization of the cytotoxic agent. Therefore, 212Pb-TCMC-trastuzumab would be an innovative approach to the elusive goal of creating a magic-bullet therapy to treat disseminated intraperitoneal diseases. 212Pb is actually a β-emitter and has been evaluated as an in vivo generator for the production of 212Bi thereby effectively extending the logistically challenging half-life of 212Bi to be ~11 h, which makes 212Pb easier to use for dose preparation and administration, and also extends targeting time. The combination of greater efficacy as compared to 212Bi on the basis μCi vs mCi lowers administered dose, and then also issues related to availability vs. cost. 212Pb internalization could contribute to TAT efficacy and and further reduce toxicity due to geometry. However, the loss of 212Bi is of minimal concern in i.p. pre-clinical applications with 212Pb to treat local metastatic or residual disease.
Although 212Pb-labeled TAT has enormous potential to provide effective therapies for cancer and significant progress has been made, use of 212Pb-labeled TAT has also proven challenging primarily due to economic issues. More efficient conjugation and radiolabeling protocols continue to be developed to facilitate further development of superior delivery systems that have more favorable biodistribution characteristics to optimize therapeutic potentials. In addition, efforts to develop a bispecific tumor targeting antibody for 212Pb application to establish a multimodality regimen for the management of cancer patients with disseminated i.p. disease would be an attractive pursuit. A recent study from Milenic et al demonstrated that 212Pb-cetuximab, which binds to HER1, had a therapeutic efficacy similar to that of 212Pb-trastuzumab, providing the possible option for a second component of a multimodality therapy regimen [76]. Recent developments in recombinant DNA technology have also allowed synthesis of antibody formats, small antibody fragments such as F(ab’)2 to improve tumor penetration and pharmacokinetics. Wong et al recently provided a pre-clinical analysis of a F(ab’)2 fragment of panitumumab for molecular imaging and therapy of HER1 positive cancer [77].
In cancer therapy, one of the most efficacious forms of therapy is radiation. If tumor dissemination is confined to a cavity e.g., the peritoneum, extensive cyto-reductive surgery with i.p. chemotherapy is suggested for selected patients. TAT may be used as an additional boost therapy. Thus, increases in efficacy obtained by combination therapy with 212Pb-TCMC-trastuzumab and other modalities such as chemotherapeutics are becoming more evident. Furthermore, the development of new tools to examine fundamental molecular events that transpire during combination therapy of 212Pb-labeled TAT with chemotherapeutics are expected to aid in making good choices to take advantage of synergistic activities. Toward these purposes, a full mechanistic understanding of the TAT and identifying genes pivotal in patients’ responses to TAT are critical to design of novel strategies for TAT and may also provide direction for optimizing combination therapy, leading to accelerate future clinical translation of TAT with chemotherapy.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and AREVA Med LLC.
Footnotes
Conflict of Interest
All authors declare no conflicts of interest in this paper.
References
- 1.Zalutsky MR, Bigner DD. Radioimmunotherapy with α-particle emitting radioummunoconjugates. Acta Oncol. 1996;35:373–379. doi: 10.3109/02841869609101654. [DOI] [PubMed] [Google Scholar]
- 2.Milenic DE, Brady ED, Brechbiel MW. Antibody targeted radiation cancer therapy. Nat Rev Drug Discov. 2004;3:488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 3.Allen BJ, Raja C, Rizvi S, et al. Targeted alpha therapy for cancer. Phys Med Biol. 2004;49:3703–3712. doi: 10.1088/0031-9155/49/16/016. [DOI] [PubMed] [Google Scholar]
- 4.Brechbiel MW. Targeted α-therapy:past, present, future? Dalton Trans. 2007;43:4918–4928. doi: 10.1039/b704726f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiston SL, Cuccurullo V, Moody TS, et al. Radionuclide antibody-conjugates, α- targeted therapy towards cancer. Curr Radiopharm. 2013;6:57–71. doi: 10.2174/1874471011306020001. [DOI] [PubMed] [Google Scholar]
- 6.McDevitt MR, Sgouros G, Finn RD, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341–1351. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 7.Ramogida CF, Orvig C. Tumour targeting with radiometals for diagnosis and therapy. Chem Comm. 2013;49:4720–4739. doi: 10.1039/c3cc41554f. [DOI] [PubMed] [Google Scholar]
- 8.Elgqvist J, Andersson H, Back T, et al. Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCR-3 cells in nude mice with 211At-labeled monoclonal antibody MX35. J Nucl Med. 2005;46:1907–1915. [PubMed] [Google Scholar]
- 9.Milenic D, Gamestani K, Dadachova E, et al. Radioimmunotherapy of human colon carcinoma xenografts using a 213Bi labeled domain deleted humanized monoclonal antibody. Cancer Biother Radiopharm. 2004;19:135–147. doi: 10.1089/108497804323071904. [DOI] [PubMed] [Google Scholar]
- 10.Milenic DE, Garmestani K, Brady ED, et al. Alpha-particle radioimmunitherapy of disseminated peritoneal diseases using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 11.Yong KJ, Milenic DE, Baidoo KE, et al. 212Pb-radioimmunotherapy induces G2 cell-cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol Cancer Ther. 2012;11:639–648. doi: 10.1158/1535-7163.MCT-11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yong KJ, Milenic DE, Baidoo KE, et al. Gene expression profiling upon 212Pb-TCMC-trastuzumab treatment in the LS-174T i.p. xenograft model. Cancer Med. 2013;2:646–653. doi: 10.1002/cam4.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meredith RF, Torgue J, Azure MT, et al. Pharmacokinetics and imaging of 212Pb-TCMC- trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm. 2014;29:12–17. doi: 10.1089/cbr.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YS, Brechbiel MW. An overview of targeted alpha therapy. Tumor Biol. 2012;33:573–590. doi: 10.1007/s13277-011-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atcher RW, Friedman AM, Hines JJ. An improved generator for the production of 212Pb and 212Bi from 224Ra. Int J Rad Appl Instrum A. 1988;39:283–286. doi: 10.1016/0883-2889(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 16.Atcher RW, Hines JJ, Friedman AM. A remote system for the separation of 228Th and 224Ra. J Radioanal Nucl Chem. 1987;117:155–162. [Google Scholar]
- 17.Baidoo KE, Milenic DE, Brechbiel MW. Methodology for labeling proteins and peptides with lead-212(212Pb). Nucl Med Biol. 2013;40:592–599. doi: 10.1016/j.nucmedbio.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzadeh S, Brechbiel MW, Atcher RW, et al. Radiometal labeling of immunoproteins: covalent linkage of 2-(4-isothiocyanatobenzyl)diethylenetriaminepentaacetic acid ligands to immunoglobulin. Bioconjug Chem. 1990;1:59–65. doi: 10.1021/bc00001a007. [DOI] [PubMed] [Google Scholar]
- 19.Howell RW, Axure MT, Narra VR, et al. Relative biological effectiveness of alpha particle emitters in vivo at low doses. Radiat Res. 1994;137:352–360. [PMC free article] [PubMed] [Google Scholar]
- 20. www.NIST.gov.
- 21.Ruegg CL, Anderson-Berg ET, Brechbiel MW, et al. Improved in vivo stability and tumor targeting of bismuth-labeled antibody. Cancer Res. 1990;50:4221–4226. [PubMed] [Google Scholar]
- 22.Chappell LL, Dadachova E, Milenic DE, et al. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotope 203Pb and 212Pb. Nucl Med Biol. 2000;27:93–100. doi: 10.1016/s0969-8051(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 23.Ruble G, Wu C, Squire RA, et al. The use of 212Pb-labeled monoclonal antibody in the treatment of murine erythroleukemia. Int J Radiat Oncol Biol Phys. 1996;34:609–616. doi: 10.1016/0360-3016(95)02119-1. [DOI] [PubMed] [Google Scholar]
- 24.McMurry TJ, Brechbiel MW, Kumar K, et al. Convenient synthesis of bifunctional tetraaza macrocycles. Bioconjugate Chem. 1992;3:108–117. doi: 10.1021/bc00014a004. [DOI] [PubMed] [Google Scholar]
- 25.Cuenot F, Meyer M, Espinosa E, et al. New insights into the complexation of lead(II) by 1,4,7,10-tetrakis(carbamoylmetyl)-1,4,7,10-tetraazacyclododecane (DOTAM): structural, thermodynamic, and kinetic studies. Eur J Inorg Chem. 2008:267–283. [Google Scholar]
- 26.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 27.Johnson P, Glennie M. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol. 2003;30:3–8. doi: 10.1053/sonc.2003.50025. [DOI] [PubMed] [Google Scholar]
- 28.Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185(HER2/neu) monoclonal antibody plus cisplatin in patients with HER2/neu overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 29.Menard C, Smith IC, Somorjai RL, et al. Magnetic resonance of the malignant prostate gland after radiotherapy: a histopathologic study of diagnostic validity. Int J Radiat Oncol Biol Phys. 2001;50:317–323. doi: 10.1016/s0360-3016(01)01480-8. [DOI] [PubMed] [Google Scholar]
- 30.Kim YS, Konoplev SN, Montemurro F, et al. Her-2/neu overexpression as a poor prognostic factor for patients with metastatic breast cancer undergoing high-dose chemotherapy with autologous stem cell transplantation. Clin Cancer Res. 2001;7:4008–4012. [PubMed] [Google Scholar]
- 31. www.ClinicalTrials.gov.
- 32.Macey DJ, Meredith RF. A strategy to reduce red marrow dose for intraperitoneal radioimmunotherapy. Clin Cancer Res. 1999;5:3044–3047. [PubMed] [Google Scholar]
- 33.Milenic DE, Gamestani K, Brady ED, et al. Potentiation of high-LET radiation by gemcitabine: targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin Cancer Res. 2007;13:1926–1935. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 34.Milenic DE, Gamestani K, Brady ED, et al. Multimodality therapy: Potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2008;14:5108–5115. doi: 10.1158/1078-0432.CCR-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner M, Neri D. Antibody-radionuclide conjugates for cancer therapy: historical considerations and new trends. Clin Cancer Res. 2011;17:6406–6416. doi: 10.1158/1078-0432.CCR-11-0483. [DOI] [PubMed] [Google Scholar]
- 36.Olasfen T, Elgqvist J, Wu AM. Protein targeting constructs in alpha therapy. Curr Radiopharm. 2011;4:197–213. doi: 10.2174/1874471011104030197. [DOI] [PubMed] [Google Scholar]
- 37.Su FM, Beaumier P, Axworthy D, et al. Pre-targeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl Med Biol. 2005;32:741–747. doi: 10.1016/j.nucmedbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Miao Y, Figueroa SD, Fisher DR, et al. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008;49:823–829. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenow MK, Xucchini GL, Bridwell PM, et al. Properties of liposomes containing 212Pb. Int J Nucl Med Biol. 1983;10:189–197. doi: 10.1016/0047-0740(83)90078-5. [DOI] [PubMed] [Google Scholar]
- 40.Diener MD, Alford JM, Kennel SJ, et al. 212Pb@C(60) and its water-soluble derivatives: synthesis, stability, and suitability for radioimmunotherapy. J Am Chem Soc. 2007;129:5131–5138. doi: 10.1021/ja068639b. [DOI] [PubMed] [Google Scholar]
- 41.International commission on radiation doses to body tissues from international contamination to occupational exposure. 1st ed. Pergamon; Oxford: 1968. p. 94p. [Google Scholar]
- 42.Loveinger R, Berman M. A formalism for calculation of absorbed dose from radionuclides. Phys Med Biol. 1968;13:205–217. doi: 10.1088/0031-9155/13/2/306. [DOI] [PubMed] [Google Scholar]
- 43.Bolch WE, Eckerman KF, Sgouros G, et al. MIRD pamphlet no.21: a generalized schema for radiopharmaceutical dosimetry-standardization of nomenclature. J Nucl Med. 2009;50:477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- 44.Sgouros G, Roeske JC, McDevitt MR, et al. MIRD Phamplet No 22: radiobiology and dosimetry of α-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–328. doi: 10.2967/jnumed.108.058651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanzonico PB. Internal radionuclide radiation dosimetry: a review of basic concepts and recent developments. J Nucl Med. 2000;41:297–308. [PubMed] [Google Scholar]
- 46.Buchsbaum DJ, Langmuir VK, Wessels BW. Experimental radioimmunotherapy. Med Phys. 1993;20:551–567. doi: 10.1118/1.597142. [DOI] [PubMed] [Google Scholar]
- 47.Chouin N, Bardies M. Alpha-particle microdosimetry. Curr Radiopharm. 2011;4:266–280. doi: 10.2174/1874471011104030266. [DOI] [PubMed] [Google Scholar]
- 48.Boudousq V, Busson M, Bobyk L, et al. Comparison between internalizing anti-HER2 mAbs and non-internalizing anti-CEA mAbs in α-radioimmunotherapy of small volume peritoneal cacinomatosis using 212Pb. PloS One. 2013;8:e69613. doi: 10.1371/journal.pone.0069613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Back T, Jacobsson L. The alpha-camera: a quantitative digital autoradiography technique using a charge-coupled device for ex vivo high-resolution bio-imaging of alpha-particles. J Nucl Med. 2010;51:1616–1623. doi: 10.2967/jnumed.110.077578. [DOI] [PubMed] [Google Scholar]
- 50.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 51.Sqouros G, Roeske JC, McDevitt MR, et al. MIRD pamphlet no. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–328. doi: 10.2967/jnumed.108.058651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruland OS, Nilsson S, Fisher DR, et al. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006:126250–6257. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 53.Dimri GP. What has senescence got to do with cancer? Cancer Cell. 2005;7:505–512. doi: 10.1016/j.ccr.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 55.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 56.Castedo M, Perfettini JL, Roumier T, et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 57.Chouin N, Lindegren S, Frost SH, et al. Ex vivo activity quantification in micrometastases at the cellular scale using the α-camera technique. J Nucl Med. 2013;54:1347–1353. doi: 10.2967/jnumed.112.113001. [DOI] [PubMed] [Google Scholar]
- 58.Pouget JP, Mather SJ. General aspects of the cellular response to low- and high-LET radiation. Eur J Nucl Med. 2001;28:541–561. doi: 10.1007/s002590100484. [DOI] [PubMed] [Google Scholar]
- 59.Tompson LH. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutation Res. 2012;751:158. doi: 10.1016/j.mrrev.2012.06.002. united. [DOI] [PubMed] [Google Scholar]
- 60.Teshima T, Owen JB, Hanks GE, et al. A Comparison of the structure of radiation oncology in the United States and Japan. Int J Radiat Oncol Biol Phys. 1996;34:243–250. doi: 10.1016/0360-3016(95)02046-2. [DOI] [PubMed] [Google Scholar]
- 61.Physician Characteristics and Distribution in the US Edition. 2008 https://www.astro.org/News-and-Media/Media-Resources/FAQs/Fast-Facts-About-Radiation-Therapy/Index.aspx.
- 62.Friesen C, Glatting G, Koop B, et al. Breaking chemoresistance and radioresistance with 213Bi anti-CD45 antibodies in leukemia cells. Cancer Res. 2007;67:1950–1958. doi: 10.1158/0008-5472.CAN-06-3569. [DOI] [PubMed] [Google Scholar]
- 63.Supiot S, Gouard S, Charrier J, et al. Mechanisms of cell sensitization to α-radioimmunotherapy by doxorubicin or paclitaxel in multiple myeloma cell lines. Clin Cancer Res. 2005;11:7047–7052. doi: 10.1158/1078-0432.CCR-1004-0021. [DOI] [PubMed] [Google Scholar]
- 64.Tochi L, Finocchiaro G, Bartolini S, et al. Role of gemcitabine in cancer therapy. Future Oncol. 2005;1:7–17. doi: 10.1517/14796694.1.1.7. [DOI] [PubMed] [Google Scholar]
- 65.Yong KJ, Milenic DE, Baidoo KE, et al. Sensitization of tumor to 212Pb radioimmunotherapy by gemcitabine involves initial abrogation of G2 arrest and blocked DNA damage repair by interfere with Rad51. Int J Radiat Oncol Biol Phys. 2013;85:1119–1126. doi: 10.1016/j.ijrobp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yong KJ, Milenic DE, Baidoo KE, et al. 212Pb-radioimmunotherapy potentiates paclitaxel-induced cell killing efficacy by perturbing the mitotic spindle checkpoint. Br J Cancer. 2013;108:2013–2020. doi: 10.1038/bjc.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yong KJ, Milenic DE, Baidoo KE, et al. Impact of α-targeted radiation therapy on gene expression in a pre-clinical model for disseminated peritoneal disease when combined with paclitaxel. PloS One. 2014;9:e108511. doi: 10.1371/journal.pone.0108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milenic DE, Baidoo KE, Shin JH, et al. Evaluation of platinum chemotherapy in combination with HER2-targeted a-particle radiation. Cancer Biother Radiopharm. 2013;28:441–449. doi: 10.1089/cbr.2012.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan Z, Chen P, Schneider N, et al. Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against tumors of androgen-independent human prostate cancer in mice. Int J Oncol. 2012;40:1881. doi: 10.3892/ijo.2012.1357. [DOI] [PubMed] [Google Scholar]
- 70.Andersson H, Elgqvist J, Horvath G, et al. Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: an overview of results in an ovarian tumor model. Clin Cancer Res. 2003;9:S3914–3921. [PubMed] [Google Scholar]
- 71.Ward BG, Mather SJ, Hawkins LR, et al. Localization of radioiodine conjugated to the monoclonal antibody HMFG2 in human ovarian carcinoma: assessment of intravenous and intraperitoneal routes of administration. Cancer Res. 1987;47:4719–4723. [PubMed] [Google Scholar]
- 72.Verheijen RH, Massuger LF, Benigno BB, et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24:571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 73.Elgqvist J, Andersson H, Bernhardt P, et al. Adiministrated activity and metastatic cure probability during radioimmunotherapy of ovarian cancer in nude mice with 211At-MX35F(ab')2. Int J Radiat Oncol Biol Phys. 2006;66:1228–1237. doi: 10.1016/j.ijrobp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Andersson H, Cederkranz E, Back T, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)At-MX35F(ab')2- a phase I study. J Nucl Med. 2009;50:1153–1160. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 75.Meredith R, Torgue J, Shen S, et al. Dose escalation and dosimetry of first-in-human α-radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med. 2014:551636–551642. doi: 10.2967/jnumed.114.143842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milenic DE, Baidoo KE, Kim YS, et al. Evaluation of cetuximab as a candidate for targeted a-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. Mabs. 2015;7:255–264. doi: 10.4161/19420862.2014.985160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong KJ, Baidoo KE, Nayak TK, et al. In vitro and in vivo pre-clinical analysis of a F(ab')2 fragment of panitumumab for molecular imaging and therapy of HER1 positive cancers. EJNMMI Res. 2011:1. doi: 10.1186/2191-219X-1-1. doi:10.1186/2191-219x-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]