Abstract
Large herbivores and carnivores (the megafauna) have been in a state of decline and extinction since the Late Pleistocene, both on land and more recently in the oceans. Much has been written on the timing and causes of these declines, but only recently has scientific attention focused on the consequences of these declines for ecosystem function. Here, we review progress in our understanding of how megafauna affect ecosystem physical and trophic structure, species composition, biogeochemistry, and climate, drawing on special features of PNAS and Ecography that have been published as a result of an international workshop on this topic held in Oxford in 2014. Insights emerging from this work have consequences for our understanding of changes in biosphere function since the Late Pleistocene and of the functioning of contemporary ecosystems, as well as offering a rationale and framework for scientifically informed restoration of megafaunal function where possible and appropriate.
Keywords: extinctions, trophic cascades, vegetation structure, biogeochemistry, rewilding
For hundreds of millions of years, an abundance of large animals, the megafauna, was a prominent feature of the land and oceans. However, in the last few tens of thousands of years—a blink of an eye on many evolutionary and biogeochemical timescales—something dramatic happened to Earth’s ecology; megafauna largely disappeared from vast areas, rendered either actually or functionally extinct (1, 2). Only in small parts of the world do megafauna exist at diversities anything close to their previous state, and, in many of these remaining regions, they are in a state of functional decline through population depletion and range contraction. In the oceans, a similar process has occurred over the last few hundred years: although there has been little absolute extinction, there has been a dramatic decline in the abundance of whales and large fish through overharvesting (3). Both on land and in oceans, declines continue today (4–7).
Homo sapiens evolved and dispersed in a world teeming with giant creatures. Our earliest art forms, such as the haunting and mesmerizing Late Pleistocene cave paintings of Lascaux and Altamira, show that megafauna had a profound impact on the psyche and spirituality of our ancestors. To humans past and modern, they indicate resources, danger, power, and charisma, but, beyond these impacts, such large animals have profound and distinct effects on the nature and functioning of the ecosystems they inhabit.
Martin (8) first posited a major human role in past megafaunal disappearances, and, since then, much has been written on their patterns and causes and the relative importance of human effects, climate change, and other factors (8–15). Only recently has work begun to address the environmental consequences of this dramatic transition from a megafaunal to a nonmegafaunal world on Earth’s ecology, as manifested through vegetation cover (16), plant–animal interactions (17), ecosystem structure (16, 18), trophic interactions (7), fire regimes (19), biogeochemical cycling (20), and climate (21, 22).
In this paper, we review evidence for megafaunal impacts on ecosystem function, on timescales ranging from the Late Pleistocene to the present. Understanding the consequences of past extinctions is valuable for a number of reasons: in particular because the loss of megafauna may have an enduring but little-recognized legacy on the functioning of the contemporary biosphere. Much of our current understanding of ecosystem ecology and biogeochemistry has been developed in a world artificially depleted of giants. We explore what lessons can be learned from the impacts of past extinctions and declines for contemporary conservation and explore what role megafauna may have yet to play in maintaining and rebuilding viable and vibrant ecosystems.
Megafauna and the Trophic Structure of Ecosystems
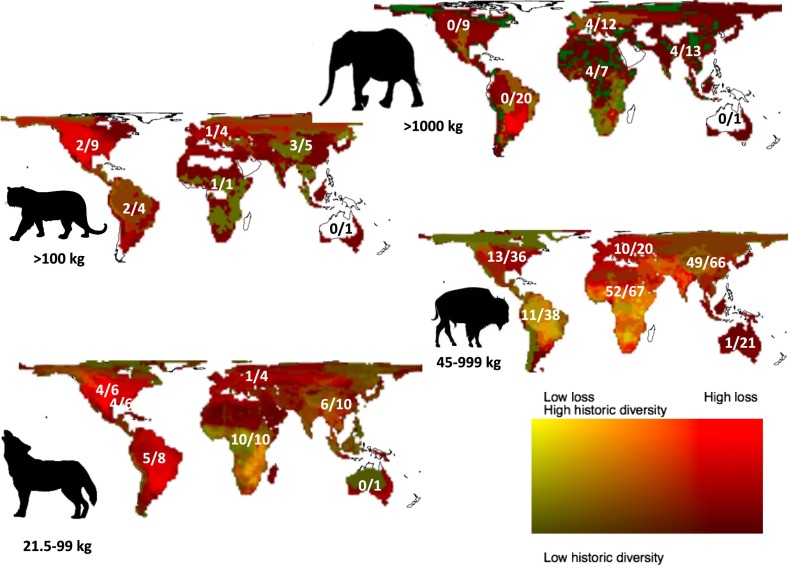
Megafauna are often defined as animals with adults larger than some threshold mass; Martin (23) originally suggested 100 pounds (45.3 kg). An alternative approach is to use a trophic herbivore–carnivore cascade definition (Fig. 1). For herbivores, large size is a generally effective strategy for protection against predators, which is one of the factors that results in a tendency toward large animal size in all ecosystems, subject to resource supply and thermal, mechanical, and demographic constraints (24). Lacking effective predators, megaherbivores (here defined as ≥1,000 kg for land animals) achieve high population biomass and are considered ecological engineers capable of altering vegetation on a landscape scale. Their large body size means that they disrupt ecosystem structure by directly destroying woody vegetation and consuming large amounts of foliage (25). In trophic terms, megaherbivore populations are generally considered to be limited from the “bottom-up” by food availability, on and thereby exert strong “top-down” control on vegetation structure and composition (26).
Fig. 1.
Extant and lost megafauna, divided by continent and into megaherbivores (≥1,000 kg), megacarnivores (≥100 kg), large herbivores (45–999 kg), and large carnivores (21.5–999 kg). Carnivores prey on the guilds below them, and to some extent on juveniles of herbivores above them. Megacarnivores can also limit the activity and abundance of the next-size class of carnivores (21.5–99 kg) by excluding them from prime habitat or killing them outright In each continent. The first number indicates the number of species remaining (often in greatly reduced abundance and restricted range), and the second number indicates how many would have existed in a Late Pleistocene baseline. Data from ref. 44. Background colors indicate prehistoric diversity and relative loss rate in each guild. Yellow/light green shows areas of high intrinsic megafaunal diversity and low loss (e.g., large herbivores in Africa), dark green indicates low historic diversity and low loss (e.g., large herbivores in high latitude North America), red indicates high diversity and high loss (e.g., Americas), and dark brown indicates low diversity and high loss (e.g., high latitude Eurasia).
Carnivores are also an integral part of a complete animal community, and top carnivores are also usually limited by their food supply (26). Megacarnivores (≥100 kg) can influence ecosystems by regulating the abundance and activity of large herbivores that are smaller than megaherbivores (i.e., 45–999 kg), either through direct consumption or by behavioral changes induced by generation and shaping of “landscapes of fear” (27). Therefore, these large herbivores potentially suffer high rates of predation and are frequently limited from the top-down by predation (28, 29) by either megacarnivores or groups of large carnivores (21.5–99 kg). Carnivores smaller than 21.5 kg generally feed on small prey (30). Group behavior can modify these size thresholds. Aggregation of herbivores into groups can limit or satiate local predation pressure (31), leading to reduced levels of mortality. These groups of herbivores are therefore also resource-limited and can exert significant effects on ecosystems through trampling, grazing, or browsing of juvenile woody vegetation.
Because of these thresholds in consumption and predation patterns, we here define megafauna on continents as large herbivores (45–999 kg), megaherbivores (≥1,000 kg), large carnivores (21.5–99 kg), and megacarnivores (≥100 kg) (Fig. 1). On the largest islands (e.g., Madagascar and New Zealand), animal communities were similar, except that threshold body masses for herbivores and carnivores tended to be smaller, probably because of resource limitation. Using this trophic definition, on smaller islands, even giant tortoises and geese can be considered megaherbivores (32) because they are free from predation controls. However, here, we limit our focus to larger megafauna in continental and large island ecosystems. In prehuman times, a complete animal community comprised multiple levels of both food-limited and predator-limited species (33). This situation is the baseline: the structure of nature as it evolved on all of the continents and large islands.
Megafauna Prehistory
With the notable exception of a period following the end-Cretaceous mass extinction, megafauna have been almost continuously abundant in almost all of the landscapes and seascapes on Earth for hundreds of millions of years, including through multiple glacial–interglacial transitions and other periods of climate change. Then, mainly in the last 50,000 y, there has been a rapid decrease in large animal abundance and diversity.
The first hints of abnormal rates of megafaunal loss appear earlier, in the Early Pleistocene in Africa around 1 Mya, where there was a pronounced reduction in African proboscidean diversity (11) and the loss of several carnivore lineages, including sabertooth cats (34), which continued to flourish on other continents. Their extirpation in Africa is likely related to Homo erectus evolution into the carnivore niche space (34, 35), with increased use of fire and an increased component of meat in human diets, possibly associated with the metabolic demands of expanding brain size (36). Although remarkable, these early megafauna extinctions were moderate in strength and speed relative to later extinctions experienced on all other continents and islands, probably because of a longer history in Africa and southern Eurasia of gradual hominid coevolution with other animals.
Outside of these core hominid regions, the timing of megafaunal loss coincides closely with the global expansion of H. sapiens (2, 14, 16): Australia ∼45 kya (37), Europe over 50–7 kya, Japan (∼30 kya), North America over 15–10 kya, South America over 13–7 kya (38), the Caribbean (∼6 kya), the Pacific islands (1–3 kya), Madagascar (∼2 kya), and New Zealand (∼700 y) (2). The overall global pattern has been rapid loss in regions experiencing sudden arrival of H. sapiens (14, 38), with no overall correlation with climatic variation. Approximately 1 billion individual large animals were lost from the Earth’s land surface (39).
Although still much discussed, there is increasingly strong evidence for a causal link between megafaunal extinction and the arrival of H. sapiens (2, 14, 38, 40). The predominant reason is that humans were effective and generalist superpredators that were able to hunt even the largest mammals that had previously faced little predation pressure. Being omnivorous and generalist, humans were able to maintain predation pressure even when prey abundance was low. Large-bodied species, with long generation times and slow reproduction rates, are particularly susceptible to even moderate sustained increases in predation pressure (13). In addition, the accompanying domestic dogs may have competed with endemic carnivores (41), and some domesticates may have introduced diseases. The loss of keystone large herbivores may have triggered cascades of extinctions through ensuing vegetation change, changing fire regimes, and the loss of a prey base for megacarnivores (9, 12).
Much of the controversy around the relative role of humans vs. climate change has focused on North America, where human arrival coincided with near-simultaneous end-Pleistocene climate change, and on Europe, where there is a strong climate signal and a drawn-out extinction event. Two recent studies that take a global perspective find human arrival as the most likely and parsimonious explanation of the pattern and timing of extinctions (14, 15). A new study (38) analyzes direct carbon dates for megafauna declines across the Americas, finding a north-to-south temporal decrease consistent with the dates for the first significant human presence. Furthermore, temporally detailed studies in North America have not found a close link to climate change (40, 42). Still, some role for climate change is often still proposed, as perhaps acting in conjunction with anthropogenic pressures in some cases (2, 12, 43). The particularly high intensity of extinction in the Americas may be explained by the coincidence of human arrival with end-Pleistocene climate change, the two factors together intensifying pressures on megafaunal populations in a way that did not occur in the multiple previous glacial–interglacial transitions (2).
Fig. 1 maps the deficit in current megafaunal diversity compared with what would be expected if Late Pleistocene species had survived to the present (44). Loss has been most dramatic in the megaherbivores and megacarnivores. The Late Pleistocene harbored at least 50 species of terrestrial megaherbivores (≥1,000 kg) in mammal orders including Proboscidea (elephants and relatives: 16 species), Cetartiodactyla (camels, bovids, hippopotamus: 7 species), Perissodactyla (rhinoceros: 9 species), and, specific to the Americas, Cingulata (glyptodonts: 5 species), Pilosa (giant sloths: 8 species), Notoungulata (toxodonts: 3 species), and Liptoterna (1 species), and, in Australia, Diprotodontia (1 species). Of these 50 megaherbivore species, only 9 remain worldwide: 3 elephants (African bush and forest elephants and Asian elephants), 5 rhinoceros, and the hippopotamus, with all of these survivors severely depleted in range. Of the lost species, 10 were larger than the largest remaining species, the African bush elephant, including 8 proboscideans. The loss of megaherbivores was most dramatic in the Americas, with all 27 megaherbivore species lost.
The loss of megacarnivores (≥100 kg) has been equally dramatic. Globally, the Late Pleistocene witnessed 15 species of mammal megacarnivore (9 big cats, 5 bears, and the Australian “marsupial lion,” Thylacoleo carnifex). Of these megacarnivores, only 6 species remain. North America alone hosted 9 species, where none remain today (Fig. 1). Major Pleistocene megacarnivores included several massive sabertooths (200–300 kg), large relatives of the extant lion (Panthera atrox, 430 kg, in North America; Panthera spelaea, 380 kg, in Eurasia and Beringia), and the huge and relatively carnivorous short-faced bear (Arctodus simus, 720 kg) in North America. Dramatic losses are also seen among large herbivores (44–999 kg), especially in North America (23/36 species lost), South America (27/38 lost), and Australia (20/21 lost). The extinction of large carnivores (21.5–99 kg) has been substantial but less dramatic, with some possible benefits of release from megacarnivores. From a trophic perspective, in most land regions, the entire top trophic level (megaherbivores) was lost, and the next trophic level (megacarnivores) was either lost or greatly diminished. The consequences of this trophic simplification likely have ramifications for the structure, composition, and functioning of even wild ecosystems today that we explore in this paper.
Humans have also hunted ocean megafauna for millennia, but only in the last few centuries has the industrialization of whaling and fishing led to massive defaunation and local extirpations of marine megafauna, both mammal and fish (3). Whale densities have declined by 66–99% (3, 45). To date there have been few global extinctions of marine megafauna, with the exception of some coastal aquatic species such as Steller’s sea cow (46).
In all continental areas, extinctions, reductions in abundance, and local extirpations of wild megafauna have continued up to recent times (2, 4, 47) and are still ongoing in most parts of the world (3, 5, 6). Megafauna that have survived to the present are greatly reduced in distribution and abundance, often rendering them functionally extinct when considering large-scale ecosystem structure and processes. In ocean regions, whale populations have recovered somewhat from near extinction, albeit to levels well below preexisting baselines, but large fish continue to face severe depletion (3, 48).
Next, we review some of the major types of impact that megafauna (particularly terrestrial megafauna) have on ecosystem structure, diversity, and function. A range of tools have been applied to tackle this topic, including ecological observations and experimental manipulations in the remaining megafauna-rich regions (18, 49). The impacts of past extinctions have also been explored through paleoecological studies using pollen grains, charcoal particles, and beetle communities as indicators of ecosystem structure (14). Recently, the spores of Sporormiella, a coprophilous fungus, have proved particularly insightful as a key indicator of megafaunal dung abundance (19, 37, 40, 50). Other insights have come from model simulations of biogeochemical processes (20, 48, 51). Observations of the impacts of contemporary change can also yield many insights, both through following ongoing or recent megafaunal decline and from reintroduction experiments or recovery of some species.
Key Impacts of Megafaunal Loss
Ecosystem Physical Structure.
The most immediate and direct environmental effect of terrestrial megafauna is on the physical structure of ecosystems. Bakker et al. (18) review the various ways in which megafauna shape ecosystem structure. Megafaunal browsers, and proboscideans in particular, are shapers of habitat structure through destruction of vegetation, through either their high rates of consumption or through breakage and trampling, and also by reducing productivity through damage to plants. They can thereby alter the competitive balance between herbaceous and woody vegetation.
Some African savannas and woodlands are among the few places where megafauna persist at high abundances. They are a product of a “natural” megafauna regime in which a diverse community of bottom-up– and top-down–regulated herbivores suppress the accumulation of woody vegetation, opening the habitat for grasses and forbs. Exclosure studies and new extensive airborne lidar surveys in African savannas reveal that megafauna, especially elephants, reduce woody species cover by 15–95% (18, 49, 52), the extent depending on body size, feeding mode, and local environmental factors. African bush elephants in Kruger National Park uproot up to 1,500 adult trees per elephant per year (52). Grazing megafauna can have negative effects on juvenile woody plants through trampling and feeding but also can have indirect positive effects on woody vegetation through enhanced nutrient cycling, diminished competition with herbaceous vegetation, and associated reduced rodent densities and fire frequency (52). Hippopotamuses and other large semiaquatic herbivores can also have strong effects on aquatic vascular plant ecosystems (53).
In many ecosystems, megaherbivores act alongside, or in competition with, an abiotic “herbivore,” fire (54). Depending on relative strengths of influences, including rainfall and ecosystem productivity, three possible ecosystem states exist as possible end points: a “green world” of tree cover dominated by a bottom-up resource constraint (water or nutrients), and two consumer-controlled states, “a black world” controlled by fire dynamics and a “brown world” controlled by herbivores (54). The density of megaherbivores (and of carnivores controlling large herbivores) and the relative abundance of grazers and browsers (especially proboscideans) mediate transitions between these alternative states. Strong bottlenecks in tree establishment create a “fire trap” or a “megafauna trap” from which juvenile trees escape only in unusual locations or during unusual events.
The loss of megafauna cascades through the trophic structure of terrestrial ecosystems, converting plant communities from top-down to bottom-up-regulation, with numerous consequences for constituent organisms (55). The exact direction of transition depends on the ecological roles of lost megafauna and also on rainfall seasonality and frequency of fire ignition. In drier systems, or where human activity has greatly increased fire ignition frequency, the loss of grazers can increase grass fuel loads and lead to a shift to a fire-dominated ecosystem (a brown-to-black transition). In wetter systems, loss of browsing and grazing can lead to closed canopy forests (a brown-to-green transition). A number of recent studies demonstrate strong evidence for ecosystem state shifts after Late Pleistocene megafauna decline. During Pleistocene glacials, the relationship between megafauna and vegetation cover may have been exacerbated through low atmospheric CO2 concentrations, which further inhibited woody vegetation growth and made it more susceptible to browsing pressure (56).
Evidence of brown–black transitions comes from periods of megafaunal loss in Australia (∼40–50 kya) and North America (∼12 kya). At Lynch’s Crater in northeast Australia, a decline in megafauna around 40–42 kya, coincident with human arrival but not with any unusual climate variation, was followed by a sharp increase in fire incidence, and a shift of the predominant vegetation type from mixed open woodland to a more uniform, dense forest dominated by fire-adapted sclerophyll trees (19, 37). In eastern North America, megafaunal decline directly preceded and probably caused an enhanced fire regime and the replacement of open and patchy woodlands by forest (40, 42, 57) In southwest Madagascar, the decline of large herbivores around 1,700 y ago was also followed by an increased fire regime (58).
Evidence of brown–green transitions comes from Europe, which hosted a range of megaherbivores, including the straight-tusked elephant (Elephas antiquus). During previous interglacials, northwest European wooded landscapes seem to have been heterogeneous woodlands containing both densely tree-covered parts and areas with an open park-like structure (59). In the postmegafaunal early Holocene, much more of the landscape was covered by relatively dense tree cover (60). As another example, contemporary tree cover in savanna regions is greater in South America relative to Africa, a difference perhaps explainable by the complete loss of megaherbivores in South America (61).
However, brown–green transitions after megafauna loss are not inevitable and seem to be less likely in regions of inherent low productivity, or in regions of inherent low megafaunal density. In the Argentine Pampas, there was no expansion in tree cover after megafaunal loss, probably because of rainfall or soil constraints (62). In southern Patagonia, there does not seem to have been an expansion of tree cover after megafaunal collapse, probably because proboscideans were never present there (62). Similarly, at Caledonia Fen, a cool high-elevation site in temperate southeast Australia, the loss of megafauna did not trigger a change in vegetation type or fire regime, perhaps because cold conditions and low atmospheric CO2 placed severe limitations on tree growth (37).
Even in closed-canopy forests, megafauna may have effects on forest structure and dynamics. In the rainforests of Gabon, Terborgh and coworkers (63, 64) note the near absence of small trees in forests with high densities of forest elephant. This feature suggests the presence of an “elephant trap” that exerts a strong control on the composition of the adult tree community. Through reducing below-ground competition for nutrients, this trap may affect forest carbon dynamics, possibly increasing the longevity of mature forest trees and resulting in higher forest biomass in elephant-rich forests (65).
Another zone of megafaunal control of ecosystem state may exist in high northern latitudes (northern Eurasia and Beringia), determining the distribution of water-logged vegetation vs. a dry “mammoth steppe” that once supported a high biomass of megafauna, including mammoths, horses, and bison (66). Zimov et al. (67) proposed that heavy grazing maintained these steppes by suppressing woody growth, stimulating production by deep-rooted, grazing-resistant grasses, and accelerating nutrient cycling in this cold climate through consumption and egestion. These grasses in turn increased transpiration rates and summer warming, leading to drying out of upper soil layers and avoidance of waterlogging. Mammoth steppes seem to have persisted in previous interglacials but are absent from the Holocene, replaced by a waterlogged landscape of unpalatable low-diversity wet mossy tundra, shrub tundra, and forest.
The spatial and temporal structure of landscapes can also be influenced by megafauna. The strength of herbivore pressure varies across space when it is tied to proximity to water sources or travel routes, to steep slopes, and to avoidance of areas with high predation risk (27, 29). Such spatial variation in pressure can result in landscapes that are a mosaic of grasses, shrubs, and trees and therefore are richer in landscape scale biodiversity (18). Such landscape heterogeneity can be dynamic over time through cyclic succession, as grasslands are colonized by thorny shrubs, within which palatable trees can regenerate and outcompete the shrubs; ultimately, these trees die and herbivory pressure inhibits woody regeneration, leading to a cycling back to grassland (60).
Ecosystem Trophic Structure.
Large herbivores and carnivores both play an important role in shaping the abundance and composition of the whole animal community. Large herbivores have effects through habitat as outlined above but can also suppress smaller herbivore species through competition. Top carnivores play an important role in ecosystem stability by regulating the abundance and behaviors of lesser herbivores (such as deer) and mesopredators (7). These mesopredators are mostly omnivores, which increase disproportionately in the absence of large predators through their ability to consume both plant and animal matter, turning them into superpredators for smaller prey such as songbirds, reptiles, amphibians, and small mammals. Much of the effect of top carnivores comes from behavior change in herbivores because they avoid vulnerable parts of the “landscapes of fear” that carnivores create (27). A well-known example is the reintroduction of wolves into Yellowstone National Park, which seems to have decreased browsing pressure by American elk (Cervus elaphus) on exposed alluvial floodplains, resulting in regrowth of willow tree cover and reduced erosion and river sediment content (68).
In the Late Pleistocene, the abundance and diversity of megacarnivores in most continental regions was much greater than that found even in African protected areas today. Van Valkenburgh et al. (69) argue that this abundance may have had an influence on megaherbivore populations through predation of juveniles and young adults, overturning the expectation that megaherbivore populations are not predation-limited. Although stable isotope studies have not generally supported an important role of megaherbivores in megacarnivore diets (70, 71), a new study by Smith et al. (72) demonstrates significant positive associations between megacarnivores and megaherbivores in Pleistocene Texas, suggesting that these species were interacting through tightly linked predator–prey relationships.
The loss of megafauna can result in an increased abundance of smaller herbivores and predators and can lead to simpler ecosystems with few interspecific interactions, shorter food chains, and less functional redundancy and resilience (73, 74). A new study of the Hall’s Cave site in Texas (72) shows that megafaunal loss led to a fundamental restructuring of the mammal community, with a shift from large grazers to many small frugivores, granivores, and browsers. Changes in the body size distribution at the site suggest a fundamental change in energy flow and ecological interactions. If the species associations and connectivity within the community reflect a measure of ecosystem cohesion, modern communities may be less resilient than those found in the Late Pleistocene.
The loss of keystone species can induce trophic cascades that lead to habitat change, shifting the abundance of other species, and can lead to further extinction. Estes et al. (46) provide a compelling aquatic example of such a cascade with the case of Steller’s sea cow (Hydrodamalis gigas) in the Commander Islands in the mid-1700s. They argue that the cascade was triggered by hunting to virtual extinction of sea otter (Enhydra lutris) for the fur trade, which greatly reduced otter predation on sea urchins, which, in turn, led to increased sea urchin densities and herbivory of kelp forests above a critical threshold. This cascade led to a kelp forest collapse, replacement by urchin barrens, and the loss of the food base for sea cows, resulting in their complete extinction within a few decades, in the absence of any direct hunting pressure. Although similarly detailed knowledge of community interactions is lacking for most past terrestrial extinctions, such trophic cascades associated with the loss of keystone species and ensuing habitat change are likely to be a widespread feature of extinctions past and future (54, 57).
Vegetation Community Composition and Diversity.
The direct effects of megafauna in ecosystem structure as outlined above can be almost immediate. A number of other effects on ecosystem composition and diversity can play out over timescales of centuries or even millennia through alteration in the relative interspecific effectiveness of key ecological processes, such as seed dispersal and plant growth rates. Many megafaunal herbivores are avid consumers of fruit and/or seeds. Janzen and Martin (75) suggested the existence of a “megafauna fruit syndrome”: Several fruit species in contemporary American ecosystems seem too large to be eaten and dispersed by the living frugivores (17) and may have been adapted to megafaunal dispersal.
There is no evidence that any tree species has become extinct due to megafaunal extinction, but distributions, abundance, and population genetic structure may have been affected. The lack of extinction is probably due to the ability of plants to be dispersed by water, small scatter-hoarding rodents, resprouting, and humans (17, 76, 77). By eating and dispersing large seeded fruits, megafauna were able to facilitate long-distance seed dispersal and gene flow, and the restriction of such long-distance flow leaves a signature on the distribution and genetic structure and distribution of remnant plant populations (77). Correlations between seed dispersal syndrome and tree stature and wood density would lead to changes in ecosystem biomass and carbon stocks as a consequence of the loss of megafaunal seed dispersal (78). However, the positive effect of megafauna on seed dispersal is counteracted by the negative effect on juvenile tree survival, leading to ambiguous net effects on ecosystem structure and composition that can vary in space and according to megafaunal species.
In addition to seed dispersal, megafaunal herbivory can affect woody species composition by promoting browsing-tolerant vegetation (18). In African savannas, browsers shift the species composition toward dominance by thorny acacias and chemically defended species (79). In boreal forests, moose and white-tailed deer selectively avoid the hard-needled white spruce, creating a spruce parkland rather than the closed hardwood forest that would otherwise predominate (80). Several plant species have adaptations to deter herbivory by megafauna. Several apparent “fire adaptations” on plants, such as sclerophyllous leaves and thick bark, could also be used to deter large herbivores (54). Plants in Neotropical savannas present adaptations to inhibit megaherbivores, such as long spines on the leaves and trunks (81). In New Zealand and Madagascar, where moas and elephant birds were the main large herbivores, plants independently evolved several adaptations to avoid being eaten, such as wide-angled branches, small leaves, and divaricate growth form, traits that are not present in Africa where the major herbivores are mammals (82).
Ecosystem Biogeochemistry.
Large animals play a disproportionately important role in accelerating ecosystem biogeochemical cycling. Nutrients that would be locked for years in leaves and stems are liberated for use through animal consumption, digestion, defecation, and urination (83). Nutrients potentially locked in recalcitrant woody biomass for decades are moved to the decomposition pool through breakage and plant mortality. These effects are likely to be particularly important on nutrient-poor soils and in low-productivity dry or cold climates, where megafaunal guts can act as giant warm and moist incubating vats that accelerate otherwise slow nutrient cycling. After the extinctions of the megafauna in the cold Pleistocene steppes, nutrients were locked into slowly decomposing plant matter, making the entire ecosystem more nutrient poor (67). In dry conditions such as African savannas, megafauna have similarly been shown to encourage rapid nutrient cycling by breaking down and digesting recalcitrant plant matter (84).
Because of their high food consumption rates, long gut residence times, and large diurnal movement ranges, megafauna can also play a disproportionate role in the lateral movement of nutrients across landscapes through their feces and urine. Animals can diffuse significant quantities of nutrients along concentration gradients even without net mass flow of feces out of the fertile area, merely by eating and defecating back and forth across the nutrient concentration gradient (85). A world with high megaherbivore abundance is likely to have had much more efficient lateral diffusion of nutrients across landscapes. Two recent studies attempted to quantify this nutrient diffusion effect and concluded that larger animals are disproportionally important in transferring nutrients across landscapes, acting as “arteries” that increase nutrient diffusion rates by at least an order of magnitude (20, 51).
In the oceans, a similar megafaunal nutrient transfer occurs, with whales and other marine mammals consuming nutrients in the deep ocean and transferring them to the surface through feces and physical mixing (86). The decline in marine mammal abundance in recent centuries may have reduced this oceanic vertical nutrient pump by around 80% (48). It is possible that these oceanic and continental megafaunal nutrient pumps were connected via nutrient transfers by migratory anadromous fish, such as salmon, and by seabirds. This interconnectivity raises the possibility of a global megafaunal nutrient pump that works against the abiotic entropic flow of nutrients from weathering continents to oceanic sediments, an interlinked system recycling nutrients, with whales moving nutrients from the deep sea to surface waters, anadromous fish and seabirds moving nutrients from the ocean to land, and terrestrial megafauna moving nutrients away from hotspots, such as river floodplains, into the continental interior. Doughty et al. (48) explored the magnitudes of these nutrient fluxes and estimate that the vertical ocean pump has declined by 77%, the sea-to-land pump has reduced by 94%, and the terrestrial diffusion of these nutrients has decreased by 92%.
In these studies, phosphorus has been used as the metric for nutrient transfer. However, a very similar framework could be applied to many other potentially limiting micronutrients, such as sodium on land (87) and iron in the oceans. Sodium is unique among elements because it is needed by animals but often toxic in high concentrations to plants. In the past, megafauna may have diffused sodium into continental interiors and reduced sodium concentration buildup on coasts. The activity of animals of all sizes is often sodium-limited; studies from Amazonia suggest that increases in sodium can greatly increase the rate of litter breakdown by soil arthropods, leading to a cascading influence on the mineralization of nitrogen, phosphorus, and other key nutrients (88).
Regional and Global Climate.
Although it is well-known that animals are influenced by climate, an interesting question is the extent to which animals can themselves influence climate (89). Through consumption and digestion, megafauna can have impacts on biogeochemical cycling, including the release of greenhouse gases. Their massive size can alter vegetation and soil structures and composition through trampling or browsing (18, 90), which can affect soil biogeochemical processes, alter water tables and soil methane emissions, and also affect land surface albedo and evapotranspiration.
Although domestic livestock are recognized as a major source of methane emissions, wild animals also produce substantial methane, and these emissions scale allometrically with body mass (22, 39, 91). Smith et al. (22) predict detectable decreases in the global methane budget related to the extirpation of megaherbivores and estimate the greenhouse gas effect of Pleistocene megafaunal loss to be a global cooling of 0.08–0.20 °C. The computed decrease in methane input is reflected in an abrupt drop in the atmospheric concentration at this time and by a change in the isotopic signature of atmospheric methane from one dominated by herbivores to a system dominated by boreal and tropical wetlands (90). These studies suggest the abrupt end-Pleistocene megafauna extinction could have contributed to the Younger Dryas cold episode. In addition, the shift to increased woody vegetation after megafaunal collapse (18) would also have acted as a sink of atmospheric CO2, further contributing to a greenhouse cooling effect.
However, a more potent impact on climate after the extinctions may have been through the modification of albedo at high latitudes through effects on tree cover. Assessment of the net impact of tree cover on climate requires consideration of carbon, evapotranspiration, and albedo impacts. In regions with abundant winter snow cover, trees tend to warm the surface (92) because they are dark features that peek above highly reflective snow. Two studies have tried to estimate whether the extinction of high latitude megafauna impacted albedo and therefore global temperatures (21, 89) and estimate albedo-related global warming impacts of up to 0.2 °C after the extinctions. Therefore, at a global scale, the greenhouse gas and albedo effects of megafauna work in opposite directions. However, at lower latitudes and local scales, if increased tree cover increases evapotranspiration, surface evaporative cooling, and the formation of reflective clouds, the loss of megafauna may lead to a net cooling.
Large animals can also alter albedo simply through regular trampling and grazing in the absence of tree cover. An experiment where herbivores were introduced to a Siberian ecosystem (Pleistocene Park) (93) shows how abundant herbivores trample the snow in winter, reducing soil temperatures by 15–20 °C. In addition, grazing removes some of the darker brush from these areas, thus exposing bright snow and greatly increasing the albedo. This increased reflectivity cools the surface, helping to keep large reserves of soil carbon from decomposing. Thus, a full accounting of megafauna effects on climate, which has yet to be done, needs to include enteric methane emissions, soil greenhouse gas emissions related to changes in hydrology and temperature, and changes in surface albedo and evapotranspiration related to vegetation structure.
Practical Insights and Applications for the Anthropocene
The ecological role of megafauna, notably via habitat structure and trophic cascades, is increasingly discussed in a conservation context. In much of the world, megafauna defaunation continues. Conservation science focuses primarily on what can be done to avoid these losses, and only secondarily on their likely ecological consequences (3, 5, 6). Studies of the impacts of past megafaunal declines reviewed here provide insights into long-term consequences of the extirpation of large-bodied species from ecosystems and can provide valuable clues into the ecological pressures that emerge as megafauna and other animals disappear from ecosystems. Some of these insights can also be applied to the contemporary decline in smaller animals: For example, a new analysis of the scale and impacts of defaunation in Amazonia (94) argues that the overhunting of seed dispersers, such as large monkeys and tapirs, will lead to a long-term decline in high biomass tree species, and thereby a decline in the carbon stock even in structurally intact forests.
Beyond a pure focus on animal loss, however, an opportunity exists to explore how to rebuild the ecosystem functions provided by large animals. In parts of the world, societal changes have facilitated more or less spontaneous megafauna comebacks: e.g., brown bear (Ursus arctos) and wolf (Canis lupus) (95, 96). Additionally, active restoration of the ecological function of wild megafauna is increasingly being debated and implemented under the concept of rewilding (33, 97, 98). Where and when megafaunal rewilding is appropriate or practical, and with what species, are the subjects of much discussion (33, 98–100). Recent widespread reexpansions of a range of large carnivore species in Europe and North America indicate that modern societies and landscapes may offer greater possibilities for human–megafauna coexistence than often thought (95, 96).
Trophic cascades offer a valuable theoretical framework for rewilding, and trophic (or megafaunal) rewilding is an ecological restoration strategy that uses species introductions to restore top-down trophic interactions to promote self-regulating biodiverse ecosystems (33). Unfortunately, the international scientific literature on rewilding remains dominated by opinion pieces and other nonempirical work. There is a strong need for much more empirical rewilding work, including rigorous experimental design and hypothesis testing. Key priorities for future rewilding research are understanding the role of trophic complexity and its interactions with landscape setting, climate change, and human activities and developing the global scope for rewilding.
As in Late Pleistocene North America, contemporary direct human pressures on megafaunal populations are coinciding with an episode of potentially rapid climate change. Restored megafaunas and associated trophic cascades may provide increased ecological resilience against climate change in some cases (33). For example, restored megafaunas may increase other species’ ability to track climate change by increasing dispersal distances. Conversely, absence or ongoing loss of megafauna may increase extinction risk of such associated species. In some cases, restored megafauna may also confer greater resistance toward ecosystem shifts under climate change. Notably, large herbivores may confer resistance of low-growing vegetation types (grasslands, scrub, tundra) to invasion by taller growing woody invasion: e.g., retarding shrubification of arctic tundra (101, 102) or woody encroachment of natural savannas (49). Conversely, the ongoing decline of African elephant populations in some regions may increase the likelihood of a state shift in savanna ecosystems under high atmospheric CO2. However, it is also likely that high megafauna abundance, as in some parts of South Africa (49), could facilitate regime shifts in other cases: e.g., forest-to-savanna shifts in a drying climate.
Ever-increasing globalization causes invasive species to be a significant threat to biodiversity. Restored megafaunas and associated trophic cascades may provide increased ecological resilience against such invasions: e.g., via top-down control of invasive medium-sized carnivores (41). In a number of cases, restoring native top predators helps suppress invasive mesopredators or invasive herbivores to the benefit of native species (103). More controversially, exotic top predators may provide similar effects, by replacing lost native species. One example is the dingo (Canis lupus dingo), which, by suppressing invasive mesopredators and herbivores, is reported to have positive effects on a range of native species in Australia (103). For dingo-free parts of mainland Australia, reintroduction of the Tasmanian devil (Sarcophilus harrissii) not only could help ensure its own survival, but also could help control invasive predators and herbivores (104).
Conclusion
Wallace was perhaps the first to recognize that megafauna were ubiquitous in near prehistory and that their impacts must have been substantial (1). Nevertheless, the wider environmental impacts of megafaunal abundance and loss are only just making their way into mainstream environmental thinking. In regions of recent or ongoing megafaunal decline, there are often concurrent changes in ecosystem structure, energy and nutrient flow, composition, and genetic structure that cascade from changes in megafaunal abundance. In looking at even wild landscapes where megafauna are long gone, imagining such landscapes as recently teeming with elephants, sabertooths, and other giant herbivores and carnivores can yield fresh perspectives on contemporary ecosystem questions, ranging from the distribution of tropical savannas and grasslands, through the natural ranges and abundance of extant animals, to the response of high latitude systems to climate change. Whether megafaunal rewilding is appropriate, acceptable, practical, or not, such a perspective challenges our thinking about what kind of nature we seek to conserve or create (33, 97, 98).
In recent decades, Earth systems science has emerged as an important new discipline focused on understanding the interactions of the biosphere, atmosphere, and oceans in the context of global change. To date, in this new discipline, nonhuman or nondomestic animals are still largely invisible, assumed to be passive inhabitants and consumers of a world where plants dominate biogeochemical processes, with vegetation distribution and cover determined by abiotic factors (climate, soils, atmospheric CO2) and human decisions on ecosystem use. This viewpoint is a legacy of a world where animal control of ecosystem function has been diminished by massive extinction. Recognition of the view that much terrestrial surface cover is potentially strongly influenced by megafaunal top-down controls allows a more complete understanding of the interactions among humans, megafauna, fire, soils, and climate in determining the geography and structure of the biosphere. There is new appreciation of the significant role of megafauna in regional and global biogeochemical cycling, accompanied by the first attempts to mathematically model these processes, both on land (20, 51) and in the oceans (48, 86). New modeling approaches (105) explicitly allow for a trophic framework for quantitative representation of megafaunal effects in Earth system models. These approaches are still in their infancy, but, if we are to understand ecosystem functioning in the megafaunal past, or how megafaunal changes are one of the agents of contemporary global ecosystem change, or the possible role for megafaunal restoration in future ecosystems, such models can provide an essential quantitative framework that needs to be further developed and tested.
More philosophically, the Pleistocene and early Holocene megafaunal extinctions can stimulate us to reevaluate what is natural in the world and what sort of natures we seek to conserve or restore. If we accept the increasing evidence for a strong human role in these early extinctions, it forces a look inwards and recognition of the deep prehistoric entanglement between humans and environmental change, a realization that some of the most dramatic human-induced changes to the nature of life on Earth and the functioning of the biosphere may have occurred even before the dawn of agriculture.
Acknowledgments
We thank Timothy Kuiper for assistance with manuscript preparation. The conference this paper and the special features were based on was funded by the Oxford Martin School.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Wallace AR. The Geographical Distribution of Animals: With a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface. Harper; New York: 1867. [Google Scholar]
- 2.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306(5693):70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 3.McCauley DJ, et al. Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 4.Ripple WJ, et al. Collapse of the world’s largest herbivores. Sci Adv. 2015;1(4):e1400103. doi: 10.1126/sciadv.1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343(6167):1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 6.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 7.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 8.Martin PS. Prehistoric overkill. In: Martin PS, Wright HE, editors. Pleistocene Extinctions: The Search for a Cause. Yale Univ Press; New Haven, CT: 1967. pp. 354–403. [Google Scholar]
- 9.Guthrie RD. Mosaics, allelochemics and nutrients: An ecological theory of late Pleistocene megafaunal extinctions. In: Martin PS, Klein RG, editors. Quaternary Extinctions: A Prehistoric Revolution. Univ of Arizona Press; Tucson, AZ: 1984. pp. 259–298. [Google Scholar]
- 10.Grayson DK. Deciphering North American Pleistocene extinctions. J Anthropol Res. 2007;63(2):185–213. [Google Scholar]
- 11.Todd NE. Trends in proboscidean diversity in the African Cenozoic. Journal of Mammalian Evolution. 2006;13(2):1–10. [Google Scholar]
- 12.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37(1):215–250. [Google Scholar]
- 13.Zuo W, Smith FA, Charnov EL. A life-history approach to the late Pleistocene megafaunal extinction. Am Nat. 2013;182(4):524–531. doi: 10.1086/671995. [DOI] [PubMed] [Google Scholar]
- 14.Sandom C, Faurby S, Sandel B, Svenning J-C. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc Biol Sci. 2014;281(1787):1–9. doi: 10.1098/rspb.2013.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett LJ, et al. 2015 Robustness despite uncertainty: Regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna. Ecography 39:152–161. [Google Scholar]
- 16.Johnson CN. Ecological consequences of Late Quaternary extinctions of megafauna. Proc Biol Sci. 2009;276(1667):2509–2519. doi: 10.1098/rspb.2008.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guimarães PR, Galetti M, Jordano P. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS One. 2008;3(3):e1745. doi: 10.1371/journal.pone.0001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker ES, et al. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc Natl Acad Sci USA. 2016;113:847–855. doi: 10.1073/pnas.1502545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 20.Doughty CE, Wolf A, Malhi Y. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat Geosci. 2013;6(9):761–764. [Google Scholar]
- 21.Doughty CE, Wolf A, Field CB. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: The first human-induced global warming? Geophys Res Lett. 2010;37(15):1–5. [Google Scholar]
- 22.Smith FA, Elliott SM, Lyons SK. Methane emissions from extinct megafauna. Nat Geosci. 2010;3(6):374–375. [Google Scholar]
- 23.Martin PS. 1989. Prehistoric overkill: The global model. Quaternary Extinctions: A Prehistoric Revolution, eds Martin PS, Klein RG (Univ of Arizona Press, Tucson, AZ), pp 354–403.
- 24.Okie JG, et al. Effects of allometry, productivity and lifestyle on rates and limits of body size evolution. Proc Biol Sci. 2013;280(1764):20131007. doi: 10.1098/rspb.2013.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen-Smith N. Meagaherbivores: The Influence of Very Large Body Size on Ecology. Cambridge Univ Press; Cambridge, UK: 1992. [Google Scholar]
- 26.Hairston NG, Smith FE, Slobodkin LB. Community structure, population control, and competition. Am Nat. 1960;94(879):421–425. [Google Scholar]
- 27.Laundré JW, Hernández L, Ripple WJ. The landscape of fear: Ecological implications of being afraid. Open Ecol J. 2010;3:1–7. [Google Scholar]
- 28.Sinclair ARE, Mduma S, Brashares JS. Patterns of predation in a diverse predator-prey system. Nature. 2003;425(6955):288–290. doi: 10.1038/nature01934. [DOI] [PubMed] [Google Scholar]
- 29.Hopcraft JGC, Olff H, Sinclair AR. Herbivores, resources and risks: Alternating regulation along primary environmental gradients in savannas. Trends Ecol Evol. 2010;25(2):119–128. doi: 10.1016/j.tree.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Carbone C, Mace GM, Roberts SC, Macdonald DW. Energetic constraints on the diet of terrestrial carnivores. Nature. 1999;402(6759):286–288. doi: 10.1038/46266. [DOI] [PubMed] [Google Scholar]
- 31.Fryxell JM, Sinclair AR. Causes and consequences of migration by large herbivores. Trends Ecol Evol. 1988;3(9):237–241. doi: 10.1016/0169-5347(88)90166-8. [DOI] [PubMed] [Google Scholar]
- 32.Hansen DM, Galetti M. Ecology: The forgotten megafauna. Science. 2009;324(5923):42–43. doi: 10.1126/science.1172393. [DOI] [PubMed] [Google Scholar]
- 33.Svenning J-C, et al. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc Natl Acad Sci USA. 2016;113:898–906. doi: 10.1073/pnas.1502556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis ME, Werdelin L. Patterns of change in the Plio-Pleistocene carnivorans of eastern Africa. In: Bobe R, Alemseged Z, Behrensmeyer AK, editors. Hominin Environments in the East African Pliocene: An Assessment of the Faunal Evidence. Springer; Dordrecht, The Netherlands: 2007. pp. 77–105. [Google Scholar]
- 35.Werdelin L, Lewis ME. Temporal change in functional richness and evenness in the eastern African plio-pleistocene carnivoran guild. PLoS One. 2013;8(3):e57944. doi: 10.1371/journal.pone.0057944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard WR, Robertson ML. Comparative primate energetics and hominid evolution. Am J Phys Anthropol. 1997;102(2):265–281. doi: 10.1002/(SICI)1096-8644(199702)102:2<265::AID-AJPA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Johnson CN, et al. 2015 Geographic variation in the ecological effects of extinction of Australia’s Pleistocene megafauna. Ecography 39:109–116. [Google Scholar]
- 38.Surovell TA, Pelton SR, Anderson-Sprecher R, Myers AD. Test of Martin’s overkill hypothesis using radiocarbon dates on extinct megafauna. Proc Natl Acad Sci USA. 2016;113:886–891. doi: 10.1073/pnas.1504020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith FA, Lyons SK, Wagner PJ, Elliott SM. The importance of considering animal body mass in IPCC greenhouse inventories and the underappreciated role of wild herbivores. Glob Change Biol. 2015;21(10):3880–3888. doi: 10.1111/gcb.12973. [DOI] [PubMed] [Google Scholar]
- 40.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 41.Pardi MI, Smith FA. 2015. Biotic responses of canids to the terminal Pleistocene megafauna extinction. Ecography 39:141–151.
- 42.Gill JL, Williams JW, Jackson ST, Donnelly JP, Schellinger GC. Climatic and megaherbivory controls on late-glacial vegetation dynamics: A new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat Sci Rev. 2012;34:66–80. [Google Scholar]
- 43.Villavicencio NA, et al. 2015. Combination of humans, climate, and vegetation change triggered Late Quaternary megafauna extinction in the Última Esperanza region, southern Patagonia, Chile. Ecography 39:125–140.
- 44.Faurby S, Svenning J-C. Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers Distrib. 2015;21:1155–1166. [Google Scholar]
- 45.Christensen L. 2006. Marine Mammal Populations: Reconstructing Historical Abundances at the Global Scale (Fisheries Centre, University of British Columbia, Vancouver), Fisheries Centre Research Reports, Vol 14, No. 9.
- 46.Estes JA, Burdin A, Doak DF. Sea otters, kelp forests, and the extinction of Steller’s sea cow. Proc Natl Acad Sci USA. 2016;113:880–885. doi: 10.1073/pnas.1502552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turvey ST, Fritz SA. The ghosts of mammals past: Biological and geographical patterns of global mammalian extinction across the Holocene. Philos Trans R Soc Lond B Biol Sci. 2011;366(1577):2564–2576. doi: 10.1098/rstb.2011.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doughty CE, et al. Global nutrient transport in a world of giants. Proc Natl Acad Sci USA. 2016;113:868–873. doi: 10.1073/pnas.1502549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asner GP, Vaughn N, Smit IPJ, Levick S. 2015. Ecosystem-scale effects of megafauna in African savannas. Ecography 39:240–252.
- 50.Baker AG, Bhagwat SA, Willis KJ. Do dung fungal spores make a good proxy for past distribution of large herbivores? Quat Sci Rev. 2013;62:21–31. [Google Scholar]
- 51.Wolf A, Doughty CE, Malhi Y. Lateral diffusion of nutrients by mammalian herbivores in terrestrial ecosystems. PLoS One. 2013;8(8):e71352. doi: 10.1371/journal.pone.0071352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keesing F, Young TP. Cascading consequences of the loss of large mammals in an African Savanna. Bioscience. 2014;64(6):487–495. [Google Scholar]
- 53.Bakker ES, Pagès JF, Arthur R, Alcoverro T. 2015. Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39:162–179.
- 54.Bond WJ, Keeley JE. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol Evol. 2005;20(7):387–394. doi: 10.1016/j.tree.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Terborgh J. The trophic cascade on islands. In: Losos JB, Ricklefs RE, editors. The Theory of Island Biogeography Revisited. Princeton Univ Press; Princeton: 2009. pp. 116–142. [Google Scholar]
- 56.Ward JK, et al. Carbon starvation in glacial trees recovered from the La Brea tar pits, southern California. Proc Natl Acad Sci USA. 2005;102(3):690–694. doi: 10.1073/pnas.0408315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson GS, Burney LP, Burney DA. Landscape paleoecology and megafaunal extinction in Southeastern New York State. Ecol Monogr. 2005;75(3):295–315. [Google Scholar]
- 58.Burney DA, Robinson GS, Burney LP. Sporormiella and the late Holocene extinctions in Madagascar. Proc Natl Acad Sci USA. 2003;100(19):10800–10805. doi: 10.1073/pnas.1534700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svenning J-C. A review of natural vegetation openness in north-western Europe. Biol Conserv. 2002;104(2):133–148. [Google Scholar]
- 60.Vera FWM. Grazing Ecology and Forest History. CABI; Wallingford, UK: 2000. [Google Scholar]
- 61.Doughty CE, Faurby S, Svenning J-C. 2015. The impact of the megafauna extinctions on savanna woody cover in South America. Ecography 39:213–222.
- 62.Barnosky AD, et al. Variable impact of late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proc Natl Acad Sci USA. 2016;113:856–861. doi: 10.1073/pnas.1505295112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terborgh J, Davenport LC. 2015. Megafaunal influences on tree recruitment in African equatorial forests. Ecography (Cop), 10.1111/ecog.01641.
- 64.Terborgh J, et al. 2015. The African rainforest: Odd man out or megafaunal landscape? African and Amazonian forests compared. Ecography 39:187–193.
- 65.Poulsen JR, Clark CJ, Palmer TM. Ecological erosion of an Afrotropical forest and potential consequences for tree recruitment and forest biomass. Biol Conserv. 2013;163:122–130. [Google Scholar]
- 66.Zimov SA, Zimov NS, Tikhonov N, Chapin FS. Mammoth steppe: A high-productivity phenomenon. Quat Sci Rev. 2012;57:26–45. [Google Scholar]
- 67.Zimov SA, et al. Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am Nat. 1995;146(5):765–794. [Google Scholar]
- 68.Ripple WJ, Beschta RL. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol Conserv. 2012;145(1):205–213. [Google Scholar]
- 69.Van Valkenburgh B, Hayward MW, Ripple WJ, Meloro C, Roth VL. The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc Natl Acad Sci USA. 2016;113:862–867. doi: 10.1073/pnas.1502554112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bocherens H. Isotopic tracking of large carnivore palaeoecology in the mammoth steppe. Quat Sci Rev. 2015;117:42–71. [Google Scholar]
- 71.Coltrain JB, et al. Rancho la Brea stable isotope biogeochemistry and its implications for the palaeoecology of late Pleistocene, coastal southern California. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;205(3-4):199–219. [Google Scholar]
- 72.Smith FA, et al. 2015. Unraveling the consequences of the terminal Pleistocene megafauna extinction on mammal community assembly. Ecography 39:223–239.
- 73.Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H. Top predators, mesopredators and their prey: Interference ecosystems along bioclimatic productivity gradients. J Anim Ecol. 2010;79(4):785–794. doi: 10.1111/j.1365-2656.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 74.Ritchie EG, et al. Ecosystem restoration with teeth: What role for predators? Trends Ecol Evol. 2012;27(5):265–271. doi: 10.1016/j.tree.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Janzen DH, Martin PS. Neotropical anachronisms: The fruits the gomphotheres ate. Science. 1982;215(4528):19–27. doi: 10.1126/science.215.4528.19. [DOI] [PubMed] [Google Scholar]
- 76.Donatti CI, et al. Analysis of a hyper-diverse seed dispersal network: Modularity and underlying mechanisms. Ecol Lett. 2011;14(8):773–781. doi: 10.1111/j.1461-0248.2011.01639.x. [DOI] [PubMed] [Google Scholar]
- 77.Pires MM, et al. Reconstructing past ecological networks: The reconfiguration of seed-dispersal interactions after megafaunal extinction. Oecologia. 2014;175(4):1247–1256. doi: 10.1007/s00442-014-2971-1. [DOI] [PubMed] [Google Scholar]
- 78.Doughty CE, et al. 2015. Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests. Ecography 39:194–203.
- 79.Wigley BJ, Fritz H, Coetsee C, Bond WJ. Herbivores shape woody plant communities in the kruger national park: Lessons from three long-term exclosures. Koedoe. 2014;56(1):1–12. [Google Scholar]
- 80.Hidding B, Tremblay JP, Côté SD. A large herbivore triggers alternative successional trajectories in the boreal forest. Ecology. 2013;94(12):2852–2860. doi: 10.1890/12-2015.1. [DOI] [PubMed] [Google Scholar]
- 81.da Silva DM, Batalha MA. Defense syndromes against herbivory in a cerrado plant community. Plant Ecol. 2011;212(2):181–193. [Google Scholar]
- 82.Bond WJ, Silander JA. Springs and wire plants: Anachronistic defences against Madagascar’s extinct elephant birds. Proc Biol Sci. 2007;274(1621):1985–1992. doi: 10.1098/rspb.2007.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hobbs NT. Modification of ecosystems by ungulate. J Wildl Manage. 1996;60(4):695–713. [Google Scholar]
- 84.McNaughton SJ, Banyikwa FF, McNaughton MM. Promotion of the cycling of diet-enhancing nutrients by african grazers. Science. 1997;278(5344):1798–1800. doi: 10.1126/science.278.5344.1798. [DOI] [PubMed] [Google Scholar]
- 85.Stevenson PR, Guzmán-Caro DC. Nutrient transport within and between habitats through seed dispersal processes by woolly monkeys in north-western Amazonia. Am J Primatol. 2010;72(11):992–1003. doi: 10.1002/ajp.20852. [DOI] [PubMed] [Google Scholar]
- 86.Roman J, McCarthy JJ. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS One. 2010;5(10):e13255. doi: 10.1371/journal.pone.0013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doughty CE, et al. Interdependency of plants and animals in controlling the sodium balance of ecosystems and the impacts of global defaunation. Ecography. 2015;39:204–212. [Google Scholar]
- 88.Kaspari M, Clay NA, Donoso DA, Yanoviak SP. Sodium fertilization increases termites and enhances decomposition in an Amazonian forest. Ecology. 2014;95(4):795–800. doi: 10.1890/13-1274.1. [DOI] [PubMed] [Google Scholar]
- 89.Brault MO, Mysak LA, Matthews HD, Simmons CT. Assessing the impact of late Pleistocene megafaunal extinctions on global vegetation and climate. Clim Past. 2013;9(4):1761–1771. [Google Scholar]
- 90.Zimov S, Zimov N. Role of megafauna and frozen soil in the atmospheric CH4 dynamics. PLoS One. 2014;9(4):e93331. doi: 10.1371/journal.pone.0093331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith FA, et al. Exploring the influence of ancient and historic megaherbivore extirpations on the global methane budget. Proc Natl Acad Sci USA. 2016;113:874–879. doi: 10.1073/pnas.1502547112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320(5882):1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 93.Zimov SA. Essays on science and society. Pleistocene Park: Return of the mammoth’s ecosystem. Science. 2005;308(5723):796–798. doi: 10.1126/science.1113442. [DOI] [PubMed] [Google Scholar]
- 94.Peres CA, Emilio T, Schietti J, Desmoulière SJM, Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc Natl Acad Sci USA. 2016;113:892–897. doi: 10.1073/pnas.1516525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chapron G, et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science. 2014;346(6216):1517–1519. doi: 10.1126/science.1257553. [DOI] [PubMed] [Google Scholar]
- 96.Deinet S, et al. 2013. Wildlife Comeback in Europe: The Recovery of Selected Mammal and Bird Species (Zoological Society of London, London), Final report to Rewilding Europe by ZSL, Birdlife International, and the European Bird Census Council.
- 97.Lorimer J, et al. Rewilding: Science, practice, and politics. Annu Rev Environ Resour. 2015;40(1):39–62. [Google Scholar]
- 98.Jepson P. 2015. A rewilding agenda for Europe: creating a network of experimental reserves. Ecography 39:117–124.
- 99.Jørgensen D. Rethinking rewilding. Geoforum. 2015;65:482–488. [Google Scholar]
- 100.Reardon S. Rewilding: The next big thing? New Sci. 2014;221(2958):40–43. [Google Scholar]
- 101.Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci USA. 2008;105(34):12353–12358. doi: 10.1073/pnas.0802421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaarlejärvi E, Hoset KS, Olofsson J. Mammalian herbivores confer resilience of Arctic shrub-dominated ecosystems to changing climate. Glob Change Biol. 2015;21(9):3379–3388. doi: 10.1111/gcb.12970. [DOI] [PubMed] [Google Scholar]
- 103.Wallach AD, Ripple WJ, Carroll SP. Novel trophic cascades: Apex predators enable coexistence. Trends Ecol Evol. 2015;30(3):146–153. doi: 10.1016/j.tree.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Hunter DO, Britz T, Jones M, Letnic M. Reintroduction of Tasmanian devils to mainland Australia can restore top-down control in ecosystems where dingoes have been extirpated. Biol Conserv. 2015;191:428–435. [Google Scholar]
- 105.Harfoot MBJ, et al. Emergent global patterns of ecosystem structure and function from a mechanistic general ecosystem model. PLoS Biol. 2014;12(4):e1001841. doi: 10.1371/journal.pbio.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]