Significance
Global models indicate that the human-derived nitrogen emissions that reach the ocean through atmospheric transport and deposition directly impact biology and the oceanic carbon dioxide (CO2) sink. Here, we find that the organic nitrogen in marine aerosols derives predominantly from biological production in the surface ocean rather than from pollution on land. Our previous work has shown significant anthropogenic influence on North Atlantic nitrate deposition, whereas ammonium cycles dynamically between the upper ocean and lower atmosphere. Collectively, these findings indicate that the ocean is not a passive recipient of anthropogenic nitrogen deposition, as it has previously been considered. This implies that the contribution of atmospheric nitrogen deposition to ocean fertility, oceanic CO2 removal, and nitrous oxide emissions has been overestimated.
Keywords: organic nitrogen, marine atmosphere, rain, aerosol, atmospheric deposition
Abstract
Global models estimate that the anthropogenic component of atmospheric nitrogen (N) deposition to the ocean accounts for up to a third of the ocean’s external N supply and 10% of anthropogenic CO2 uptake. However, there are few observational constraints from the marine atmospheric environment to validate these findings. Due to the paucity of atmospheric organic N data, the largest uncertainties related to atmospheric N deposition are the sources and cycling of organic N, which is 20–80% of total N deposition. We studied the concentration and chemical composition of rainwater and aerosol organic N collected on the island of Bermuda in the western North Atlantic Ocean over 18 mo. Here, we show that the water-soluble organic N concentration ([WSON]) in marine aerosol is strongly correlated with surface ocean primary productivity and wind speed, suggesting a marine biogenic source for aerosol WSON. The chemical composition of high-[WSON] aerosols also indicates a primary marine source. We find that the WSON in marine rain is compositionally different from that in concurrently collected aerosols, suggesting that in-cloud scavenging (as opposed to below-cloud “washout”) is the main contributor to rain WSON. We conclude that anthropogenic activity is not a significant source of organic N to the marine atmosphere over the North Atlantic, despite downwind transport from large pollution sources in North America. This, in conjunction with previous work on ammonium and nitrate, leads to the conclusion that only 27% of total N deposition to the global ocean is anthropogenic, in contrast to the 80% estimated previously.
Human activities contribute substantially to the reactive nitrogen (N) load in the atmosphere over the continents (1), and modeling estimates suggest that transport of this anthropogenic N to the ocean may account for up to a third of the ocean’s external N supply (1). If so, anthropogenic N emissions on land may significantly influence open ocean biogeochemistry. However, our assessment of the role of anthropogenic N deposition to the open ocean currently relies on poorly tested assumptions regarding the origins of the “fixed” (biologically available) N deposition measured in the marine atmosphere. In particular, the complex mixture of hundreds of organic N compounds can represent 20–80% of total N deposition over the ocean (2), but there is a paucity of information on its sources, chemistry, and environmental impact. Thus, organic N represents the largest uncertainty related to total atmospheric N deposition (including nitrate, ammonium, and organic N). The water-soluble organic N (WSON) contribution is typically parameterized as averaging 30% of the total N deposition on a global basis (3), and it is generally assumed that 48–80% of WSON globally derives from anthropogenic sources (1, 4). Here, we used 18 mo of event-based rain and weekly size-segregated aerosol collections from Bermuda (32.27°N, 64.87°W), an island located downwind of the North American continent where N emissions are dominated by anthropogenic sources, to quantify the contribution of organic N to total N deposition in the subtropical North Atlantic region and to investigate anthropogenic/continental versus marine sources. All samples were analyzed for the concentration of ammonium (NH4+), nitrate (NO3−), total N, and non-sea-salt sulfate (nss-SO42−). These samples were previously analyzed for the concentration and N isotopic composition of NH4+ (δ15N–NH4+) in rain and NO3− (δ15N–NO3−) in rain and aerosol, which are discussed below but detailed in other publications (5–7). A subset of event-based rain (n = 12) and weekly aerosol (n = 20) samples were analyzed for the N isotopic composition of total N (δ15N−TN; allowing for the calculation of δ15N−WSON by mass balance) and for chemical composition by ultra-high-resolution Fourier transform-ion cyclotron resonance mass spectrometry (FT-ICR MS).
The aerosol WSON had an average concentration of 7.5 ± 13.7 nmol⋅m−3 (numerical average ± SD; n = 38 wk) and a median concentration of 1.3 nmol⋅m−3. For a given week’s collection, the fine mode (<1.0 µm) was always higher in concentration than the coarse mode (>1.0 µm) (Table S1). There were multiple instances of elevated WSON concentration ([WSON]), reaching as high as 54.7 nmol⋅m−3. These elevated concentrations are of the same order of magnitude as polluted (8) and forested (9) sites in North America and in the biomass burning season of the Amazon (10), although they are lower than concentrations reported in heavily polluted regions of China (132−180 nmol⋅m−3) (11). The high-[WSON] events at Bermuda occurred predominantly in the cool season (October to March) when air masses mainly originate from North America, compared with the warm season (April to September) when air masses are of marine origin (5, 12, 13). This might lead one to conclude that, as with NO3− deposition (5, 6, 12), cool season WSON in the atmosphere near Bermuda is largely transported from North America. However, our dataset suggests that high-[WSON] aerosols instead derive from episodes of high biological activity in the surface ocean. This has implications for existing estimates of anthropogenic organic N deposition to the subtropical North Atlantic and demonstrates the importance of N cycling between the ocean and the marine atmosphere.
Table S1.
Isotopic composition of aerosol WSON as a function of season and group (as determined from the NMS analysis), along with the concentration of aerosol WSON in the fine and coarse-mode size fractions
| Aerosol δ15N−WSON, ‰ | Aerosol [WSON], nmol⋅m−3 | Rain δ15N−WSON, ‰ | |
| Season | |||
| Warm | 10.5 ± 4.9 (10) | ||
| Cool | 15.9 ± 26.3 (10) | ||
| All data | 13.2 ± 18.6 | ||
| Group | |||
| 1 | 9.0 ± 9.4 | ||
| 2 | 7.7 ± 8.3 | ||
| Mode | |||
| Fine | 7.99 ± 13.7 | ||
| Coarse | 1.11 ± 1.02 | ||
| AMBT | |||
| Marine | −0.2 ± 4.6 (8) | ||
| Continental | 2.6 ± 9.2 (4) | ||
| All data | 0.55 ± 5.8 |
The numerical average and SD (number of samples) of the isotopic composition of rain WSON in samples with marine and continental AMBTs, as well as of all of the data, are also shown.
Results and Discussion
Aerosol WSON and Surface Ocean Productivity at Bermuda.
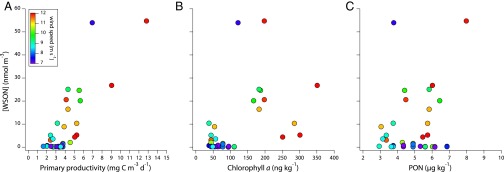
The Bermuda Atlantic Time-series Study (BATS) site, located ∼85 km southeast of Bermuda, is one of the best-studied representations of the subtropical ocean, which constitutes >50% of global ocean surface area. We observe a strong positive linear relationship of aerosol [WSON] at Bermuda with rates of surface ocean gross primary productivity (GPP) measured at BATS (bats.bios.edu) (r2 = 0.685, P value < 0.001; Fig. 1A) and a positive exponential relationship with wind speed (r2 = 0.359, P value < 0.001; indicated by the colors in Fig. 1A).
Fig. 1.
Aerosol [WSON] as a function of (A) gross primary production (GPP; r2 = 0.69), (B) chlorophyll-a concentration (r2 = 0.25), and (C) PON concentration in the surface ocean (i.e., upper 5–10 m) at BATS (r2 = 0.05). The wind speeds indicated by the color shading are the average of the daily recordings taken by the Bermuda Weather Service (www.weather.bm) for the time period during which the aerosol sampler was deployed (1 wk).
To explain these observations, we hypothesize that aerosol organic N derives primarily from active biological cycling in the surface ocean, with wind speed providing a physical mechanism for aerosol generation. During the winter and spring at BATS, low sea surface temperatures (SST) and high wind stresses drive deep mixing events that entrain subsurface nutrients into the sunlit upper ocean, supporting high rates of primary productivity by phytoplankton (14). We propose that, under these conditions, biologically derived organic N can be freely exchanged between the surface ocean and atmosphere, driving high aerosol [WSON]. By contrast, the summer is characterized by high SST, low wind stress, and lower productivity, potentially rendering the surface ocean a less important source of organic N to the atmosphere during that season.
Chlorophyll-a has long been considered the best oceanic parameter for predicting organic carbon enrichments in marine aerosols, with correlation coefficients (r2 values) ranging from 0.55 to 0.66 depending on the temporal and spatial resolution of the satellite derived chlorophyll-a (15–17). Using the chlorophyll-a data from the monthly BATS time series cruises in conjunction with weekly aerosol measurements over 18 mo, we find that surface chlorophyll-a is a weak predictor of atmospheric [WSON] at Bermuda (Fig. 1B). This is likely because the relationship between phytoplankton chlorophyll-a content and biological activity can be decoupled by the complex interactions of light, nutrients, and temperature (e.g., refs. 14 and 18). Moreover, chlorophyll-a is a proxy for phytoplankton biomass only, whereas GPP drives biological activity in general [e.g., phytoplankton growth followed by zooplankton grazing and heterotrophic bacterial activity (14)]. In these regards, the stronger correlation of [WSON] with GPP is not surprising.
It has recently been suggested that the large reservoir of mostly recalcitrant dissolved organic carbon (DOC) in the surface ocean is a better predictor of marine contributions to aerosol organic carbon than is chlorophyll-a (19). Quinn et al. (19) hypothesize that DOC is the sole source of marine aerosol organic carbon, with physical mechanisms (i.e., wind) determining the extent of this enrichment, and local biological activity not playing a significant role. At BATS, the surface concentration of dissolved organic N (DON) is high and relatively stable throughout the year [∼4 μM (20)]. Thus, DON might be hypothesized to represent an ever-present potential source of organic N to the lower marine atmosphere that is accessed only when wind speeds are adequately high to drive efflux. However, our dataset does not support a similar role for recalcitrant DON. In the presence of essentially invariant surface ocean DON concentrations ([DON]), the dataset includes aerosol collections with high [WSON] that coincide with low wind speeds but high rates of GPP (e.g., October 12, 2011; Fig. 1A and Table S2), as well as low-[WSON] samples that were collected when wind speeds were high but GPP was low (e.g., August 10, 2011; Table S2). The data thus suggest that although wind speeds must average above 8 m/s over the sampling time frame to efflux organic N out of the surface ocean, biological activity (as quantified by GPP), not the concentration of bulk DON, determines the availability of organic N for efflux.
Table S2.
Date of collection of the aerosol samples that were sector collected over 7 d, and average SST and wind speed for the 7 collection days
| Date | SST, °C | Wind speed, m s−1 | WSON, nmol⋅m−3 | Chl-a, ng⋅kg−1 | PON, μg⋅kg−1 | GPP, mg C⋅m−3⋅d−1 |
| 7/20/2011 | 27.48 | 9.07 | 10.38 | 38 | 3.75 | 3.15 |
| 7/27/2011 | 27.49 | 7.52 | 0.7 | 38 | 3.75 | 2.05 |
| 8/3/2011 | 28.35 | 8.87 | 5.16 | 44 | 3.35 | 2.41 |
| 8/10/2011 | 27.95 | 11.64 | 3.07 | 44 | 3.35 | 2.41 |
| 8/17/2011 | 28.68 | 8.87 | 0.38 | 50 | 2.94 | 2.01 |
| 9/14/2011 | 27.73 | 11.19 | 8.87 | 54 | 3.05 | 3.89 |
| 10/5/2011 | 28.1 | 8.75 | 3.58 | 57 | 3.15 | 2.67 |
| 10/12/2011 | 26.04 | 7.33 | 53.97 | 122 | 3.78 | 6.94 |
| 10/26/2011 | 26.03 | 9.97 | 24.63 | 187 | 4.41 | 5.52 |
| 11/2/2011 | 24.66 | 11.77 | 20.61 | 198 | 4.48 | 4.11 |
| 11/23/2011 | 22.49 | 9.39 | 25.08 | 183 | 5.83 | 4.31 |
| 11/30/2011 | 21.78 | 11.25 | 16.45 | 183 | 5.83 | 4.31 |
| 12/14/2011 | 21.23 | 9.77 | 20.14 | 167 | 6.44 | 5.64 |
| 1/11/2012 | 18.55 | 10.48 | 2.03 | 44 | 4.28 | 4.55 |
| 1/18/2012 | 18.83 | 11.9 | 54.74 | 198 | 7.98 | 12.82 |
| 2/15/2012 | 18.31 | 12.92 | 26.79 | 351 | 6.02 | 9.04 |
| 2/22/2012 | 18.41 | 14.42 | 5.24 | 301 | 5.74 | 5.25 |
| 2/29/2012 | 18.6 | 11.22 | 10.33 | 285 | 5.74 | 5.25 |
| 3/7/2012 | 19.73 | 12.79 | 4.39 | 251 | 5.46 | 5.04 |
| 6/6/2012 | 25.04 | 9.48 | 0.3 | 59 | 5.6 | 3.61 |
| 7/4/2012 | 26.33 | 10.29 | 0.2 | 54 | 6.02 | 2.97 |
| 7/11/2012 | 26.8 | 8.17 | 0.36 | 44 | 6.86 | 1.63 |
| 7/18/2012 | 28.53 | 7.72 | 0.2 | 44 | 6.86 | 2.63 |
| 7/25/2012 | 28.5 | 10.61 | 0.2 | 78 | 6.16 | 2.63 |
| 8/8/2012 | 29.28 | 5.72 | 0.04 | 94 | 6.16 | 3.36 |
| 8/15/2012 | 29.93 | 7.2 | 0.15 | 111 | 5.6 | 2.74 |
| 8/15/2012 | 29.93 | 7.2 | 0.16 | 111 | 5.6 | 2.74 |
| 8/22/2012 | 29.75 | 9.26 | 0.22 | 111 | 5.6 | 2.74 |
| 8/29/2012 | 29.8 | 6.82 | 0.37 | 95 | 5.32 | 2.46 |
| 9/5/2012 | 29.63 | 7.2 | 0.35 | 69 | 4.9 | 3.25 |
| 9/12/2012 | 28.83 | 10.22 | 1.62 | 78 | 4.9 | 3.75 |
| 9/19/2012 | 27.03 | 8.1 | 0.14 | 78 | 4.9 | 3.75 |
| 9/19/2012 | 27.03 | 8.1 | 1.31 | 78 | 4.9 | 3.75 |
| 9/26/2012 | 27.56 | 6.37 | 0.19 | 64 | 4.34 | 3.63 |
| 9/26/2012 | 27.56 | 6.37 | 0.82 | 64 | 4.34 | 3.63 |
| 10/3/2012 | 27.61 | 8.42 | 0.22 | 56 | 3.75 | 3.09 |
| 10/10/2012 | 27.8 | 7.2 | 0.2 | 56 | 3.64 | 3.09 |
| 10/24/2012 | 25.6 | 8.68 | 0.2 | 49 | 3.64 | 3.52 |
Dates are given as month/day/year. Oceanic parameters including chlorophyll-a (chl-a), PON, and weighted average GPP are included for BATS cruises that correspond to aerosol sampling periods, weighted as described in SI Methods.
No relationship is observed between aerosol [WSON] and surface ocean particulate organic N (PON) (Fig. 1C; r2 = 0.05). Given the small quantity of PON typical of surface waters at BATS (∼3–6 µg/kg; bats.bios.edu), its concentration would be expected to vary with aerosol [WSON] if the bulk PON standing stock were a significant source of organic N to the atmosphere. The lack of relationship with bulk DON and PON further supports the hypothesis that active biological cycling leads to newly produced, highly reactive, and potentially more volatile organic N, which is supplied to the marine atmosphere. Globally applied chlorophyll-a source functions predict marine organic aerosol of 2.8–5.8 Tg C⋅yr−1 (21), which are much lower than top-down estimates of 29–50 Tg C⋅yr−1 (22), and improving these parameterizations is a focus of global modelers. Our results suggest that the use of surface ocean GPP and wind speed data would improve the parameterization of global organic N aerosol fluxes in models.
Chemical Composition of Marine Aerosol WSON.
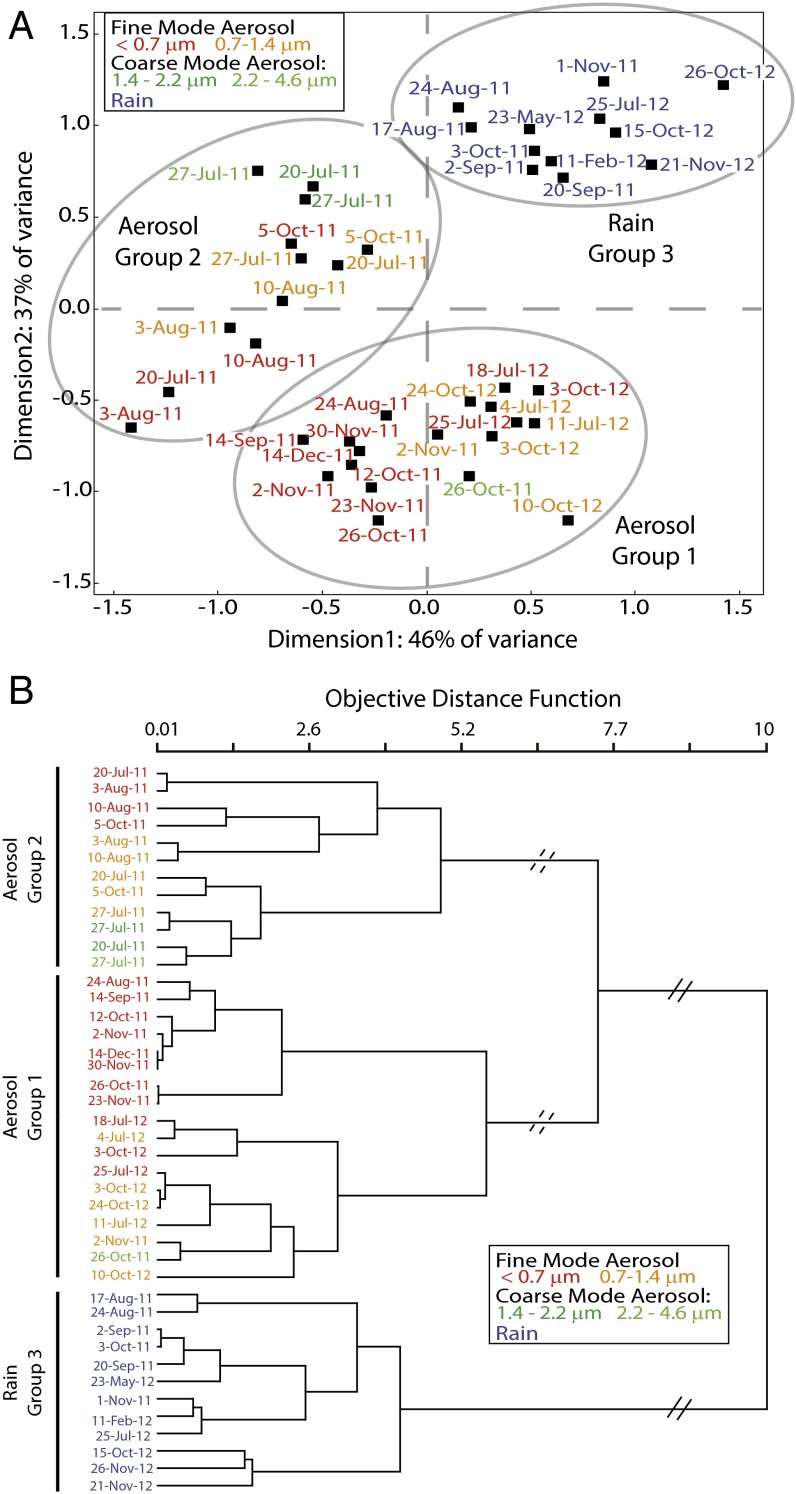
In an effort to better distinguish the sources of WSON, the compound-level chemical composition of aerosol and rainwater WSON was investigated. WSON is a complex mixture of hundreds of compounds. The large amount of information inherent in ultra-high-resolution electrospray ionization (ESI) FT-ICR MS (e.g., each sample had, on average, 750 organic N compounds identified) lends itself to multivariate statistical comparisons, including an ordination technique for nonnormally distributed data, nonmetric multidimensional scaling (NMS), hierarchical cluster analysis and indicator species analysis (ISA). Findings from both the NMS and hierarchical cluster analysis suggest that the aerosol samples fall into two distinct compositional groups (Fig. 2). Despite this distinction within the aerosols, the aerosols as a group are highly distinct in composition from the rain, even when collected on the same day (Fig. 2; discussed below).
Fig. 2.
(A) Ordination plot for NMS of the rain and aerosol samples. The two aerosol groups and one rain group are labeled Aerosol Group 1, Aerosol Group 2, and Rain Group 3, respectively. (B) Linkage diagram of rain and aerosol samples calculated from the original Bray−Curtis distance matrix and Ward’s method. The horizontal distance represents the objective distance function. The dendrogram was clipped at the highest level (solid diagonal lines) into two groups, one of rain samples and one of aerosol samples, and then a second time (dashed diagonal lines), splitting the aerosol samples into two groups.
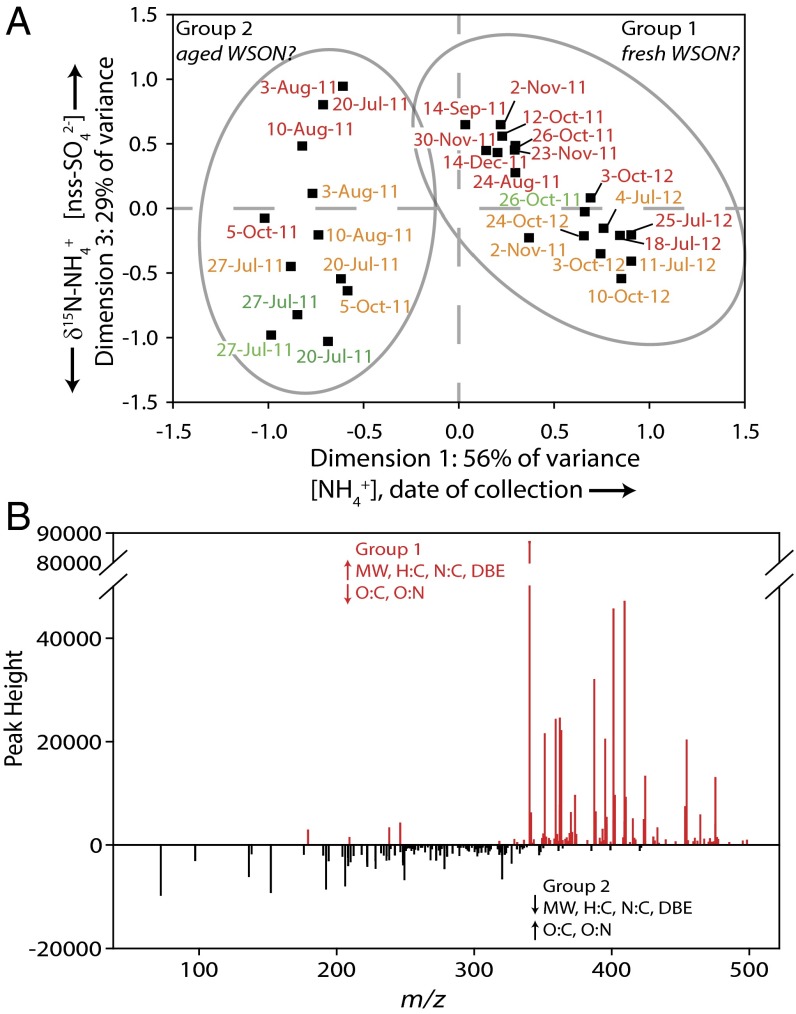
NMS analysis of the aerosol samples was used to discern the possible environmental drivers of the differences in the two groups of aerosols (Fig. 3A and Table S3). Ammonium (NH4+) concentration and date of collection are highly correlated with dimension 1 (56% of variance explained; r2 = 0.77 and 0.67, respectively). Non-sea-salt sulfate (nss-SO42−) concentration and δ15N−NH4+ (per mil vs. N2 in air) are correlated with dimension 3 (29% of variance explained; r2 = 0.48 and 0.37, respectively). The ISA analysis provides compound-specific information on what drives the compositional differences between the two groups (Fig. 3B). ISA identifies m/zs that are present in high abundance and unique to the aerosol samples in group 1 versus aerosol samples in group 2. Group 1 aerosol indicator m/zs (n = 131) are higher in molecular weight, total N content, H:C, N:C, and double bond equivalents (DBE; number of rings + number of double bonds), and lower in O:C and O:N than the group 2 indicator m/zs (n = 135). In addition, the aerosol samples in group 1 have a much higher average [WSON] than group 2 aerosols (Table 1).
Fig. 3.
(A) Ordination plot for NMS of the aerosol samples. The ordination was calculated with the N-containing elemental formulas from each sample. Dates in red and orange are fine-mode aerosols (<0.7 µm and 0.7–1.4 µm), and dates in green are coarse-mode aerosols (1.4–2.2 µm and 2.2–4.6 µm). The circles indicating groups 1 and 2 are the same designations as from the NMS ordination in Fig. 2A and the cluster dendrogram in Fig. 2B. The chemical composition data indicate that group 1 aerosols are likely fresh WSON, whereas group 2 aerosols are consistent with aged WSON. Dimension 1 is positively correlated with [NH4+] and date of collection, whereas dimension 3 is positively correlated with [nss-SO42-] and negatively correlated with δ15N−NH4+. (B) Indicator m/z values for the aerosol samples in group 1 (red lines) and group 2 (black lines) when absolute peak height was converted to presence/absence as 1/0. The group 2 aerosol indicator values are plotted as negative peak heights for comparison purposes only. Group 1 aerosols have indicator m/zs with higher molecular weight, H:C and N:C ratios, and DBE, and lower O:C and O:N ratios compared with group 2 aerosol indicator m/z’s.
Table S3.
Correlation coefficient (r2) between aerosol variables and NMS dimensions in the NMS analysis of aerosol samples alone
| Dimension 1 | Dimension 2 | Dimension 3 | |
| Date of collection | 0.67 | 0.02 | 0.02 |
| δ15N−NO3− | 0.21 | 0.04 | 0.05 |
| δ18O−NO3− | 0.00 | 0.12 | 0.14 |
| δ15N–NH4+ | 0.34 | 0.24 | 0.37 |
| δ15N−TN | 0.02 | 0.00 | 0.14 |
| δ15N−WSON | 0.03 | 0.07 | 0.08 |
| [NO3−] | 0.01 | 0.01 | 0.00 |
| [NH4+] | 0.77 | 0.01 | 0.01 |
| [nss-SO42−] | 0.00 | 0.07 | 0.48 |
| [WSON] | 0.01 | 0.08 | 0.02 |
| [TN] | 0.04 | 0.09 | 0.13 |
Dimension 1 accounts for 55.8% of variance, dimension 2 accounts for 9.8% of variance, and dimension 3 accounts for 28.7% of variance, cumulatively representing 94.3% of variance.
Table 1.
Concentration of marine rain and aerosol WSON
| Aerosol [WSON],* nmol⋅m−3 | Aerosol WSON, % of total N | Rain [WSON], µM | Rain [WSON] volume-weighted average, µM | Rain WSON,† % of total N | |
| Season | |||||
| Warm | 1.5 ± 2.6 (22) | 48.2 ± 24.5 | |||
| Cool | 14.3 ± 15.0 (16) | 52.7 ± 11.9 | |||
| All data | 7.5 ± 13.3 | 50.4 ± 18.9 | |||
| Group | |||||
| 1 | 15.6 ± 16 (18) | ||||
| 2 | 2.1 ± 2.4 (12) | ||||
| Air mass back trajectory (AMBT) | |||||
| Marine | 2.1 ± 3.1 (21) | 1.3 ± 2.5 | 18.4 ± 12.3 | ||
| Continental | 1.5 ± 0.9 (56) | 1.3 ± 1.8 | 11.6 ± 7.0 | ||
| All data | 1.9 ± 2.7 | 1.3 ± 2.4 | 16.5 ± 11.4 |
The average ±1 SD (n) concentration and percent contribution to total N (WSON/WSON + NO3− + NO2− + NH4+) in aerosols is binned by season. The average concentration of aerosol WSON is also shown for group 1 and 2, as defined in Chemical Composition of Marine Aerosol WSON and labeled on Fig. 2. The average ±1 SD (n) concentration, and percent contribution of WSON to total N in rain binned by AMBT and for the entire dataset, are also listed.
Indicates that warm vs. cool and group 1 vs. group 2 are statistically different, independent t test, P < 0.0005.
Indicates that marine vs. continental AMBT are statistically different, independent t test, P < 0.0075.
Two scenarios can explain the concentration and composition data, as well as the strong relationships among WSON composition, aerosol NH4+, and nss-SO42− concentration, and δ15N−NH4+. First, WSON may be composed of primary organic N compounds that are emitted from the ocean in an analogous manner to oceanic ammonia emissions (23) and dimethyl and diethyl amine emissions (24). The properties of group 1 indicator m/zs suggest that group 1 aerosols are composed of relatively fresh primary organic matter. We conclude that they must derive from a marine source, as there exists no primary anthropogenic source in the North Atlantic near Bermuda (25). This is supported by the higher average [WSON] of group 1 aerosols compared with group 2 (Table 1).
Second, WSON may be formed through secondary reactions of volatile organic compounds in the presence of fine-mode ammonium sulfate aerosols, the dominant form of aerosol NH4+ in the marine environment, forming N-containing organic compounds. This is analogous to known mechanisms in polluted environments such as the formation of substituted imidazoles, imines, and N-containing oligomers during reactions of glyoxal (26) and methylglyoxal (27) with ammonium sulfate. This is consistent with the properties of the group 2 aerosols. Group 2 indicator m/zs suggest greater atmospheric aging and oxidation [e.g., increase in O:C, decrease in H:C and aromatics (28)]. Group 2 aerosols occur primarily during the warm season (April to September) when air masses stagnate around the island and the longer days provide an environment conducive to oxidation and aging (i.e., photochemistry), with little anthropogenic influence. The lack of anthropogenic inorganic N precursors and the stagnant air mass back trajectories (AMBTs) are consistent with group 2 aerosols having a marine-sourced NH4+ as the inorganic N precursor. Moreover, measurements of δ15N−NH4+ point toward the open ocean as the dominant source of ammonia to the marine atmosphere at Bermuda, regardless of air mass history or season (7). Accordingly, aerosol WSON with this origin reflects open ocean/marine atmosphere N cycling, as opposed to a new/external input of N to the ocean.
The strong correlation of NH4+ concentration, as opposed to NO3−, with the organic N chemical composition is perhaps to be expected in light of work demonstrating the numerical dominance of compounds with reduced-N functionality in marine rain (29), the large contribution of amine salts and amino acids in marine aerosols (24), and the evidence that the surface ocean is the dominant source of ammonia to the marine atmosphere at Bermuda (7, 23). The prevalence of reduced-N compounds is also consistent with previous work showing that 29–75% of rain and aerosol WSON is bioavailable (30, 31). To summarize, the NMS and ISA data are consistent with high-[WSON] aerosols having a primary marine source (Fig. 3). This provides complementary evidence that high-[WSON] events derive from surface ocean biology rather than from North American pollution. By contrast, the low-[WSON] aerosols, classified primarily as group 2, have chemical compositions indicative of extensive atmospheric aging.
Sources of Organic N in Marine Rain.
Unlike aerosols, rain contains WSON with a chemical composition indicative of both anthropogenic and marine sources, consistent with previous work at Bermuda (29). At the highest level, cluster analysis resulted in two distinct groups, one composed solely of rain samples and one composed solely of aerosol samples (black diagonal lines, Fig. 2B), even for rain and aerosol samples collected on the same day. In addition, the rain and aerosol samples fall into two distinct quadrants in the NMS analysis (Fig. 2A). In general, rainwater organic matter derives from a combination of rainout, which occurs within the cloud (i.e., condensation of water vapor on aerosol particles and incorporation of gases and aerosol particles into cloud droplets, all influenced by in-cloud chemistry), and washout, which includes all below-cloud scavenging of aerosol particles and gases (ref. 32 and references therein). The relative removal of pollutants via rainout and washout is highly uncertain and compound-dependent (33), and the thousands of compounds that contribute to atmospheric organic matter render it challenging to predict the dominant wet removal process for aerosol WSON. In addition, we find that the aerosol WSON is primarily present in fine-mode particles, which is the size range associated with the “scavenging gap” that makes below-cloud scavenging inefficient (33). The separation of rainwater and aerosol organic N in the hierarchical clustering and NMS analysis and the dominance of fine-mode aerosol organic N suggest that the contribution of washout to rain WSON is small. This is consistent with a previous estimate that washout contributes only ∼30% to total aerosol mass in rainwater (34).
Implications for Estimates of Atmospheric N Deposition to the Ocean.
We present, to our knowledge, the first estimate of the flux of atmospheric WSON to the ocean based on long-term measurements at an oceanic site. Based on the [WSON] from 38 wk of aerosol samples and 77 rain events measured at Bermuda, the calculated WSON flux (wet + dry) to the North Atlantic is 0.68 Tg N⋅y−1, with WSON accounting for 19% of total N (WSON + NH4+ + NO2− + NO3−) deposition (Table 2). Scaled to the global ocean for comparison with model estimates, the calculated WSON total flux (wet + dry) to the global ocean is 18.7 Tg N⋅y−1 (Table 2). This is remarkably similar to previous model estimates of 20 Tg N⋅y−1 (1) and 15.9 Tg N⋅y−1 (4), although our observed WSON only accounts for 19% of the total N flux to the ocean at Bermuda, compared with the 30% typically used to parameterize organic N (1, 2).
Table 2.
Wet and dry depositional fluxes calculated for the North Atlantic and the global ocean using Bermuda rain (n = 77 events) and aerosol (n = 38 weeks) concentrations
| This work | Duce et al. (1) | |
| Scaled to North Atlantic | ||
| Aerosol WSON cool season, Tg N⋅y−1 | 0.32 | |
| Rain WSON continental AMBT, Tg N⋅y−1 | 0.10 | |
| Aerosol WSON warm season, Tg N⋅y−1 | 0.11 | |
| Rain WSON marine AMBT, Tg N⋅y−1 | 0.14 | |
| Total WSON (wet + dry), Tg N⋅y−1 | 0.68 | |
| % WSON of total N | 19 | |
| Scaled to global ocean | ||
| Total WSON, Tg N⋅y−1 | 18.7 | 20 (10–30) |
| % WSON of total N | 19 | 30 |
| % Anthropogenic WSON | 17 | 80 |
| % Total N that is anthropogenic | 27 | 80 |
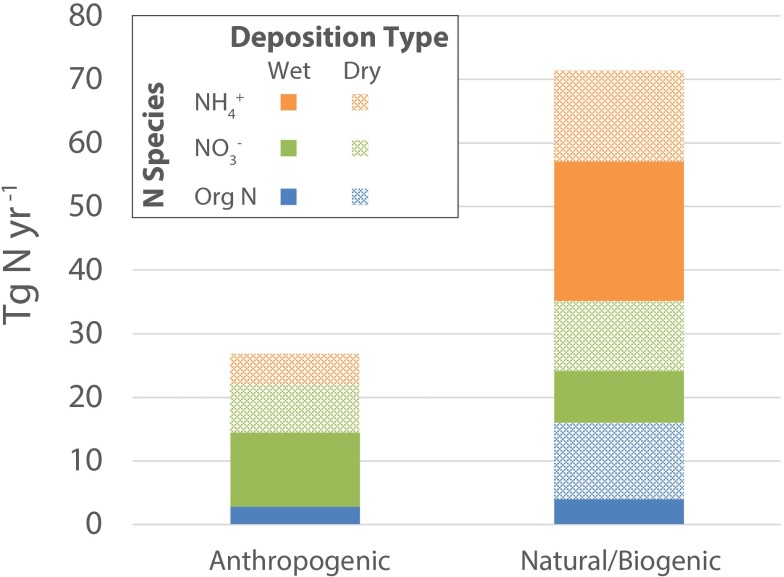
For dry deposition, the chemical composition and the relationship of aerosol [WSON] to oceanic GPP and wind speed presented here suggest that the aerosol WSON is dominated by marine biogenic sources. Rain WSON with continental AMBTs is the only anthropogenic contributor to the total WSON flux (Fig. 4 and Table S4) (29), such that 2.8 Tg N⋅y−1 of the total 18.7 Tg N⋅y−1 of WSON is anthropogenic, i.e., 17%. This is significantly lower than the two prevailing global model estimates of organic N deposition to the ocean from anthropogenic sources of 80% (1) and 48% (4) (Table 2).
Fig. 4.
Global anthropogenic and biogenic/natural N deposition scaled from concentrations at Bermuda to the global ocean and separated according to species (NH4+, NO3−, Org N/WSON; colors) and type (wet and dry deposition; solid and pattern). The anthropogenic vs. natural source apportionment is discussed in Implications for Estimates of Atmospheric N Deposition to the Ocean and presented in Table S4.
Table S4.
Eighteen months of event-based rain and weekly size-segregated samples were collected on the island of Bermuda
| Continental AMBT | Marine AMBT | Reference | |
| Org N wet deposition | anthropogenic | biogenic | (29) |
| NH4+ wet deposition | biogenic | biogenic | (7) |
| NO3− wet deposition | anthropogenic | natural | (5) |
| NO3− dry deposition | anthropogenic | natural | (6) |
| Org N dry deposition | biogenic | biogenic | this work |
| NH4+ dry deposition | anthropogenic | biogenic | this work |
The first four rows of the table present anthropogenic vs. biogenic source apportionment of the N species based on previous work on the chemical composition of rain WSON (29), the N isotopic composition of NO3− in dry (6) and wet (5) deposition, and the N isotopic composition of NH4+ in wet deposition (7). The anthropogenic vs. biogenic source apportionment of organic N and NH4+ in dry deposition is discussed further in Implications for Estimates of Atmospheric N Deposition to the Ocean. The source apportionment in this table was used to calculate the percent contribution of anthropogenic and marine N to total N deposition at Bermuda (Fig. 4).
Previous work on the concentration and N and O stable isotopic composition of NO3− in rain at Bermuda showed that rain NO3− with continental AMBTs had anthropogenic sources (5) (Table S4). WSON with continental AMBTs, in the same rain samples from Bermuda as the NO3−, also has anthropogenic sources (29) (SI Text and Table S5). By contrast, NH4+ in these samples is dominated by marine sources regardless of AMBT or season (7). The total flux of NO3− and WSON with continental AMBTs is 14.4 Tg N⋅y−1, which we take to be the anthropogenic N flux in rain at Bermuda (Fig. 4). The total wet deposition flux at Bermuda is 48.3 Tg N⋅y−1; thus, 30% of the total wet N flux can be considered anthropogenic.
Table S5.
Correlation coefficient (r2) between rain data and NMS dimensions in the NMS analysis of rain samples alone
| Dimension 1 | Dimension 2 | |
| Date of collection | 0.50 | 0.30 |
| [NO3−] | 0.08 | 0.04 |
| [NH4+] | 0.27 | 0.13 |
| [TN] | 0.39 | 0.17 |
| [WSON] | 0.27 | 0.06 |
| δ15N−NO3− | 0.00 | 0.05 |
| δ18O−NO3− | 0.12 | 0.13 |
| δ15N–NH4+ | 0.28 | 0.23 |
| Latitude of AMBT | 0.43 | 0.47 |
| Longitude of AMBT | 0.00 | 0.20 |
| δ15N–TN | 0.06 | 0.09 |
Dimension 1 accounts for 48.8% of variance, and dimension 2 accounts for 43.8% of variance, cumulatively representing 92.6% of variance.
As in the case of the rain samples described above, previous work on the concentration and N and O stable isotopic composition of aerosol NO3− at Bermuda, measured in the same samples as the organic N data presented here, has shown that NO3− with continental AMBTs derives from anthropogenic sources (6) (Table S4). Until a more detailed analysis can be conducted for the NH4+ isotopic composition of aerosol samples, the source of NH4+ aerosol is assumed to be anthropogenic during the cool season, when air masses predominantly come from North America, and biogenic during the warm season, when air masses stagnate around the island of Bermuda. Given the evidence that NH4+ in rainfall is predominantly marine in origin (7), the above assumption likely represents a bias toward overestimating the anthropogenic N input to deposition at Bermuda. By summing the aerosol N fluxes that we know or assume to derive from anthropogenic sources, we calculate that the total anthropogenic contribution to dry deposition at Bermuda is 12.5 Tg N⋅y−1 (Fig. 4). Extrapolating the 18-mo time series NO3−, NH4+, and organic N data for rain and aerosols at Bermuda to the global ocean, we find that 27% of total N deposition (26.8 Tg N⋅y−1 out of 98.3 Tg N⋅y−1; total N is the sum of NO3−, NH4+, and WSON concentrations; Fig. 4) to the global ocean is anthropogenic, compared with the 80% suggested by Duce et al. (1) (Table 2).
Conclusions
Bermuda is downwind of a major pollution zone in North America such that extrapolating the results from this region to global estimates should lead to a higher value for the anthropogenic component of global N deposition, not a significantly lower value as we report. Furthermore, recent work has shown that the surface ocean is also a source of ammonia to marine rain at Bermuda, regardless of season or air mass history (7). This is particularly interesting given the evidence presented here and elsewhere (29) suggesting that NH4+, not NO3−, is the key precursor to forming organic N compounds in the marine atmosphere. The amount of NH4+ (7) and organic N deposition at Bermuda suggested by the time series data reconciles well with the modeling estimates, but the percent contribution of anthropogenic vs. natural sources appears to be systematically overestimated in the models. Truly remote open ocean N deposition will likely have an even smaller anthropogenic component. We thus suggest that the traditional paradigm whereby the ocean is considered a passive recipient of anthropogenic N deposition is overly simplified. Instead, as we have shown, the dynamic biogeochemical cycling of the surface ocean can influence the lower atmosphere, serving as a source of aerosol organic N and NH4+ (7). The importance of anthropogenic N deposition as an external source of N to the ocean and its resulting contribution to new marine biological production, anthropogenic CO2 drawdown, and nitrous oxide emissions are thus likely also overestimated (1).
In sum, we present the most significant dataset on the chemical composition and concentration of rain and aerosol WSON in the marine environment to date. This work highlights the need for further investigation of the relationships among N species in the surface ocean and lower atmosphere, both in the subtropics and in other marine environments, to better understand the human impact on the marine atmosphere and surface ocean, with important implications for climate and marine ecosystem health.
Methods
Event-based rainwater was collected from station Prospect and the Tudor Hill Marine Atmospheric Observatory on Bermuda, filtered, and frozen at −20 °C as described in refs. 5, 7, and 29. Aerosol samples were collected weekly using a six-stage cascade impactor (Tisch 236 High Volume Cascade Impactor) fitted with precombusted glass fiber filters on stages 1–5 and a Whatman-41 filter on the backup stage. The impactor was mounted on the Tudor Hill Marine Atmospheric Observatory (32.27°N, 64.87°W) on Bermuda. The impactor’s pump is sector-controlled and turns on only when the winds are blowing from the ocean, and not from the island of Bermuda. Filters were deployed for 7 d, then removed and frozen until extraction into aqueous solution. The aqueous extracts were then analyzed in the same manner as the rain samples.
For both the rain and aerosol collections, bulk concentrations of NO3− + nitrite (NO2−) and NH4+ were determined using an automated nutrient analyzer and standard colorimetric methods as described in refs. 5 and 7. The N isotope ratios of NH4+ were determined via the combined hypobromite and azide methods with the modifications detailed in ref. 7. NO3− + NO2− N and O isotope ratios were determined using the denitrifier method as described in ref. 5. Total dissolved N (TDN) concentrations were measured using the Antek 7000 TN Analyzer, and WSON was determined as the difference between TDN and the sum of inorganic N,
| [1] |
The volume-weighted average rain concentration was converted to a flux using a precipitation rate of 1 mm⋅d−1 and the area of the global ocean as 361 million km2. Aerosol concentrations were converted to depositional fluxes using depositional velocities for coarse-mode aerosols for NO3− and fine-mode aerosols for NH4+ and WSON (35). A subset of rain and aerosol samples was analyzed for TDN−δ15N via the persulfate−denitrifier method (20). The δ15N of WSON was calculated by the mass-weighted subtraction of the δ15N of the inorganic N species from TDN−δ15N.
The same sample subset was analyzed with a 7-T electrospray ionization Fourier transform ion cyclotron resonance mass spectrometer (Thermo-Finnigan LTQ-XL, Woods Hole Oceanographic Institution Fourier Transform Mass Spectrometry Facility) operated in the positive ion mode. Scans were acquired and processed as described in ref. 29. Cluster analysis was performed on the data matrix using Bray−Curtis distance measures combined with Ward’s linkage method. NMS was used to reduce the comparisons between samples from multidimensions to fewer dimensions. The differences are presented graphically, such that samples that are closer together in space are more similar than samples located farther apart. The proportion of variation in each axis is assessed with a Mantel test that calculates the r2 between distances in the ordination space and distance in the original space. ISA is used to identify indicator values, which are the multiplication product of the relative abundance and the relative frequency of an m/z value. High indicator value m/zs must be present in most samples within the group and have high peak height values. The aerosol samples were assigned to groups based on the cluster analysis and the NMS, which both clearly showed two dominant groups of aerosols. Multivariate statistical analyses were conducted using PCOrd v 5.4.
Monthly-resolved surface (i.e., upper 5–10 m) chlorophyll-a and GPP data for the time period of our study were provided by the BATS program (bats.bios.edu). Samples are collected onboard the R/V Atlantic Explorer from the BATS station (31°40’N, 64°10’W) located in the open subtropical North Atlantic Ocean, ∼85 km southeast of Bermuda. Briefly, chlorophyll-a is analyzed by HPLC, and GPP is calculated from the incorporation of H14CO3− into biomass during dawn-to-dusk (24 h) incubations at in situ temperature and light (14). We used GPP rather than net primary production (NPP, equivalent to GPP minus phytoplankton respiration) in our analysis because our interest lies in comparing biological activity in general with aerosol [WSON]. In any case, although NPP is typically lower than GPP at BATS, NPP and GPP always show the same general trends (bats.bios.edu). Although the BATS cruises occur monthly (and sometimes even bimonthly), the aerosol and rainwater sampling intervals did not always fully coincide with a single BATS cruise. In such cases, we averaged the GPP and chlorophyll-a data from the two nearest cruises, weighting the average by the number of days after the first cruise and before the second. For example, if an aerosol sample was collected 15 d after cruise A and 5 d before cruise B, the average GPP and chlorophyll-a data were weighted by 0.25 × cruise A and 0.75 × cruise B.
SI Text
SI Sources of Organic N in Marine Rain.
The NMS analysis of the rainwater WSON results in a 2-D solution where the δ15N−WSON, date of collection, and latitude of the air mass 36 h before arriving at Bermuda are correlated with dimension 1 (49% of the variance; r2 = 0.53, 0.50, and 0.43, respectively; Table S5). The correlation with collection date and AMBT is consistent with previous work on the WSON chemical composition of rain from Bermuda suggesting that rain with continental AMBTs is similar to rain collected in North America and distinct from rain with marine AMBTs (1).
SI Methods.
Although the BATS cruises occur monthly (and sometimes even bimonthly), the aerosol and rainwater sampling intervals did not always fully coincide with a single BATS cruise. In such cases, we averaged the GPP, chlorophyll-a, and PON data from the two nearest cruises, weighting the average by the number of days after the first cruise and before the second. To account for the variability resulting from the temporal mismatch between aerosol and GPP sample collections, the weighted SE was calculated for each weighted average GPP value. The inverse of the SE was used as the weights for each average GPP value to calculate a weighted least squares regression. The linear correlation of [WSON] with GPP had an r2 value of 0.59 with a P value < 10−8. This confirms that the observed correlation between aerosol WSON concentration and GPP is robust and not driven by biases introduced by the temporal mismatch between sample collections.
Acknowledgments
We thank A. Marks, J. Rosset, J. Garcia, and A. Gobel for sample collection assistance. We acknowledge M. Soule, E. Kujawinksi, and the funding sources of the WHOI FT-MS Users’ Facility (NSF OCE-0619608 and the Gordon and Betty Moore Foundation). This work was supported by NSF ATM-1044997 (to M.G.H., A.J.P., and D.M.S.), NSF OCE-1060947 (to D.M.S.), the Grand Challenges Program at Princeton University (to D.M.S.), and the NOAA Climate and Global Change Fellowship Program (to K.E.A.). The Tudor Hill facility is supported by NSF OCE-1430741.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516847113/-/DCSupplemental.
References
- 1.Duce RA, et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science. 2008;320(5878):893–897. doi: 10.1126/science.1150369. [DOI] [PubMed] [Google Scholar]
- 2.Jickells T, Baker AR, Cape JN, Cornell SE, Nemitz E. The cycling of organic nitrogen through the atmosphere. Philos Trans R Soc Lond B Biol Sci. 2013;368(1621):20130115. doi: 10.1098/rstb.2013.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. Evidence for organic N deposition and its anthropogenic sources in China. Atmos Environ. 2008;42(6):1035–1041. [Google Scholar]
- 4.Kanakidou M, et al. Atmospheric fluxes of organic N and P to the global ocean. Global Biogeochem Cycles. 2012;26(3):GB3026. [Google Scholar]
- 5.Altieri KE, Hastings MG, Gobel AR, Peters AJ, Sigman DM. Isotopic composition of rainwater nitrate at Bermuda: The influence of air mass source and chemistry in the marine boundary layer. J Geophys Res. 2013;118(D19):11304–11316. [Google Scholar]
- 6.Gobel AR, Altieri KE, Peters AJ, Hastings MG, Sigman DM. Insights into anthropogenic nitrogen deposition to the North Atlantic investigated using the isotopic composition of aerosol and rainwater nitrate. Geophys Res Lett. 2013;40(22):5977–5982. [Google Scholar]
- 7.Altieri KE, Hastings MG, Peters AJ, Oleynik S, Sigman DM. Isotopic evidence for a marine ammonium source in rainwater at Bermuda. Global Biogeochem Cycles. 2014;28(10):1066–1080. [Google Scholar]
- 8.Zhang Q, Anastasio C, Jimemez-Cruz M. Water-soluble organic nitrogen in atmospheric fine particles (PM2.5) from northern California. J Geophys Res. 2002;107(D11) doi: 10.1029/2001JD000870. [DOI] [Google Scholar]
- 9.Lin M, Walker J, Geron C, Khlystov A. Organic nitrogen in PM2.5 aerosol at a forest site in the Southeast US. Atmos Chem Phys. 2010;10(5):2145–2157. [Google Scholar]
- 10.Mace KA. Water-soluble organic nitrogen in Amazon Basin aerosols during the dry (biomass burning) and wet seasons. J Geophys Res. 2003;108(D16):4512. [Google Scholar]
- 11.Shi J, Gao H, Qi J, Zhang J, Yao X. Sources, compositions, and distributions of water-soluble organic nitrogen in aerosols over the China Sea. J Geophys Res. 2010;115(D17):D17303. [Google Scholar]
- 12.Hastings MG. Isotopic evidence for source changes of nitrate in rain at Bermuda. J Geophys Res. 2003;108(D24):4790. [Google Scholar]
- 13.Moody JL, Galloway JN. Quantifying the relationship between atmospheric transport and the chemical composition of precipitation on Bermuda. Tellus B Chem Phys Meterol. 1988;40(5):463–479. [Google Scholar]
- 14.Lomas MWW, et al. Two decades and counting: 24-years of sustained open ocean biogeochemical measurements in the Sargasso Sea. Deep Res Part II Top Stud Oceanogr. 2013;93:16–32. [Google Scholar]
- 15.Rinaldi M, et al. Is chlorophyll-a the best surrogate for organic matter enrichment in submicron primary marine aerosol? J Geophys Res. 2013;118(10):4964–4973. [Google Scholar]
- 16.O’Dowd CD, de Leeuw G. Marine aerosol production: A review of the current knowledge. Philos Trans A Math Phys Eng Sci. 2007;365(1856):1753–1774. doi: 10.1098/rsta.2007.2043. [DOI] [PubMed] [Google Scholar]
- 17.Violaki K, et al. Atmospheric water-soluble organic nitrogen (WSON) over marine environments: A global perspective. Biogeosciences. 2015;12(10):3131–3140. [Google Scholar]
- 18.Karl DM, Church MJ. Microbial oceanography and the Hawaii Ocean Time-series programme. Nat Rev Microbiol. 2014;12(10):699–713. doi: 10.1038/nrmicro3333. [DOI] [PubMed] [Google Scholar]
- 19.Quinn PK, et al. Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol. Nat Geosci. 2014;7(3):228–232. [Google Scholar]
- 20.Knapp AN, Sigman DM, Lipschultz F. N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic Time-series study site. Global Biogeochem Cycles. 2005;19(1):GB1018. [Google Scholar]
- 21.Gantt B, Meskhidze N. The physical and chemical characteristics of marine primary organic aerosol: A review. Atmos Chem Phys. 2013;13(8):3979–3996. [Google Scholar]
- 22.Long MS, Keene WC, Kieber DJ, Erickson DJ, Maring H. A sea-state based source function for size- and composition-resolved marine aerosol production. Atmos Chem Phys. 2011;11(3):1203–1216. [Google Scholar]
- 23.Johnson MT, et al. Field observations of the ocean-atmosphere exchange of ammonia: Fundamental importance of temperature as revealed by a comparison of high and low latitudes. Global Biogeochem Cycles. 2008;22(1):GB1019. [Google Scholar]
- 24.Facchini MC, et al. Important source of marine secondary organic aerosol from biogenic amines. Environ Sci Technol. 2008;42(24):9116–9121. doi: 10.1021/es8018385. [DOI] [PubMed] [Google Scholar]
- 25.Prospero JM, et al. Atmospheric deposition of nutrients to the North Atlantic Basin. Biogeochemistry. 1996;35(1):27–73. [Google Scholar]
- 26.Yu G, et al. Glyoxal in aqueous ammonium sulfate solutions: Products, kinetics and hydration effects. Environ Sci Technol. 2011;45(15):6336–6342. doi: 10.1021/es200989n. [DOI] [PubMed] [Google Scholar]
- 27.De Haan DO, et al. Formation of nitrogen-containing oligomers by methylglyoxal and amines in simulated evaporating cloud droplets. Environ Sci Technol. 2011;45(3):984–991. doi: 10.1021/es102933x. [DOI] [PubMed] [Google Scholar]
- 28.Hall WA, 4th, Pennington MR, Johnston MV. Molecular transformations accompanying the aging of laboratory secondary organic aerosol. Environ Sci Technol. 2013;47(5):2230–2237. doi: 10.1021/es303891q. [DOI] [PubMed] [Google Scholar]
- 29.Altieri KE, Hastings MG, Peters AJ, Sigman DM. Molecular characterization of water soluble organic nitrogen in marine rainwater by ultra-high resolution electrospray ionization mass spectrometry. Atmos Chem Phys. 2012;12(7):3557–3571. [Google Scholar]
- 30.Seitzinger SP, Sanders RW. Atmospheric inputs of dissolved organic nitrogen stimulate estuarine bacteria and phytoplankton. Limnol Oceanogr. 1999;44(3):721–730. [Google Scholar]
- 31.Wedyan M, Fandi K, Al-Rousan S. Bioavailability of atmospheric dissolved organic nitrogen in the marine aerosol over the Gulf of Aqaba. Aust J Basic. 2007;1(3):208–212. [Google Scholar]
- 32.Bourcier L, et al. A new method for assessing the aerosol to rain chemical composition relationships. Atmos Res. 2012;118:295–303. [Google Scholar]
- 33.Andronache C. Estimated variability of below-cloud aerosol removal by rainfall for observed aerosol size distributions. Atmos Chem Phys. 2003;3(1):131–143. [Google Scholar]
- 34.Murakami M, Kimura T, Magono C, Kikuchi K. Observations of precipitation scavenging for water-soluble particles. J Meteorol Soc Jpn. 1983;61(3):346–358. [Google Scholar]
- 35.Spokes LJ, Yeatman SG, Cornell SE, Jickells TD. Nitrogen deposition to the eastern Atlantic Ocean. The importance of south-easterly flow. Tellus B Chem Phys Meterol. 2000;52(1):37–49. [Google Scholar]