Significance
Many CNS disorders result from dysregulation of the mesocorticolimbic dopamine projections arising from the ventral tegmental area. A mechanistic understanding of these dysregulations is critical for developing treatments for these diseases. Nicotine addiction is a global health concern, and results in part from dysregulated mesocorticolimbic dopamine function. Here we present evidence that nicotine-induced 2-arachidonoylglycerol formation in the ventral tegmental area is sensitized following chronic nicotine exposure, and that this results in the loss of GABA-mediated constraint of mesocorticolimbic dopamine activity. Potent and selective diacylglycerol lipase inhibitors are characterized and used in ex vivo electrophysiology and in vivo behavioral analyses to demonstrate that this mechanism plays a prominent role in the dysregulation of ventral tegmental dopamine neurons following nicotine exposure.
Keywords: nicotine, 2-arachidonoylglycerol, diacylglycerol lipase, GABA, ventral tegmental area
Abstract
Chronic nicotine exposure (CNE) alters synaptic transmission in the ventral tegmental area (VTA) in a manner that enhances dopaminergic signaling and promotes nicotine use. The present experiments identify a correlation between enhanced production of the endogenous cannabinoid 2-arachidonoylglycerol (2-AG) and diminished release of the inhibitory neurotransmitter GABA in the VTA following CNE. To study the functional role of on-demand 2-AG signaling in GABAergic synapses, we used 1,2,3-triazole urea compounds to selectively inhibit 2-AG biosynthesis by diacylglycerol lipase (DAGL). The potency and selectivity of these inhibitors were established in rats in vitro (rat brain proteome), ex vivo (brain slices), and in vivo (intracerebroventricular administration) using activity-based protein profiling and targeted metabolomics analyses. Inhibition of DAGL (2-AG biosynthesis) rescues nicotine-induced VTA GABA signaling following CNE. Conversely, enhancement of 2-AG signaling in naïve rats by inhibiting 2-AG degradation recapitulates the loss of nicotine-induced GABA signaling evident following CNE. DAGL inhibition reduces nicotine self-administration without disrupting operant responding for a nondrug reinforcer or motor activity. Collectively, these findings provide a detailed characterization of selective inhibitors of rat brain DAGL and demonstrate that excessive 2-AG signaling contributes to a loss of inhibitory GABAergic constraint of VTA excitability following CNE.
The mesocorticolimbic dopamine (DA) system provides a critical link between the brain regions that process cognitive information and those controlling motor behavior. Precise control of these ventral tegmental area (VTA) projections facilitates seeking rewarding stimuli, retreating from aversive stimuli, constraint of motivational state, and behavioral flexibility necessary for survival. GABAergic signaling provides robust inhibition that gates VTA DA cell excitability (1, 2), and loss of this inhibition leads to pathological dysregulation of mesocorticolimbic circuitry (3, 4).
Endocannabinoids (eCBs) regulate DAergic activity through retrograde signaling from DA cell bodies onto presynaptic cannabinoid type 1 (CB1) receptors expressed on both inhibitory and excitatory inputs. Although both 2-arachidonoylglycerol (2-AG) and anandamide (AEA) function as endogenous CB1 agonists in the brain (5–7), these lipids exhibit distinct pharmacological profiles in vivo (8, 9) and mediate differential behavioral effects (10, 11). Endocannabinoids are produced and degraded on-demand, and the primary enzymes responsible for eCB degradation have been well-characterized using selective pharmacological tools that inactivate monoacylglycerol lipase (MAGL) or fatty acid amide hydrolase (FAAH) (11–13). However, a complete evaluation of the influence of eCB signaling in the brain has been hampered by the lack of appropriate corresponding tools for selectively inactivating on-demand eCB biosynthesis.
Substantial evidence implicates eCB signaling in the etiology of nicotine addiction, and recent work demonstrates that chronic nicotine exposure (CNE) selectively enhances nicotine-induced increases in VTA 2-AG formation (14). The present study investigated the possible contribution of this effect to aberrant VTA DA cell excitation present following CNE (15). We find that sensitized nicotine-induced 2-AG release (14) strongly correlates with a loss of nicotine-induced GABA release, which may contribute to impaired inhibitory constraint of VTA DA cell excitation following CNE. To test this hypothesis, we characterized a series of selective inhibitors of 2-AG biosynthesis by diacylglycerol lipase α and β (DAGLα and DAGLβ; hereafter referred to as DAGL) (16–18) and 2-AG degradation by α/β-hydrolase domain 6 (ABHD6) and MAGL (11, 12, 19), and used these compounds to investigate the functional impact of enhanced 2-AG recruitment on GABAergic signaling at VTA synapses and nicotine self-administration.
Results
Chronic Nicotine Exposure Impairs Nicotine-Induced GABA Release in the Rat VTA.
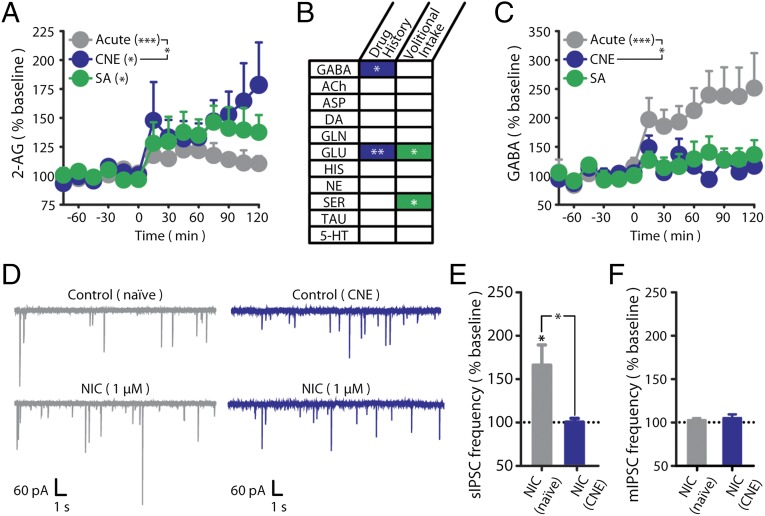
We previously demonstrated that nicotine-induced VTA 2-AG formation is sensitized following CNE in a manner independent of the volitional nature of drug exposure [e.g., similar sensitization induced by either nicotine self-administration (SA) or response-independent administration] (Fig. 1A) (14). To determine the signaling mechanisms influenced by this aberrant 2-AG response, we screened the remaining microdialysate aliquots from these same subjects for temporal changes in neurotransmitter content. Of the 11 neurotransmitters quantified, only GABA exhibited a pattern that correlated with changes in 2-AG (Fig. 1B and Fig. S1). Specifically, dialysate 2-AG and GABA levels (Fig. 1C) were inversely correlated and exhibited distinct nicotine-induced responses following chronic vs. acute nicotine exposure regardless of response contingency (14).
Fig. 1.
Chronic nicotine exposure impairs nicotine-induced GABA release in the rat VTA. (A) Dialysate 2-AG levels before (t −60 to 0 min) and during (t 0–120 min) nicotine exposure in naïve (n = 7), CNE (n = 7), and SA (n = 6) rats. Reprinted by permission from Macmillan Publishers Ltd. (14). (B) Summary of microdialysate neurotransmitter changes between groups during nicotine exposure. Corresponding microdialysate profiles are shown in Fig. S1. (C) Dialysate GABA levels before (t −60 to 0 min) and during (t 0–120 min) nicotine exposure in naïve (n = 7), CNE (n = 7), and SA (n = 6) rats. Baseline GABA levels did not significantly differ between groups. (D) Representative recordings of sIPSCs in VTA DA neurons from naïve (Left) and CNE (Right) rats during the superfusion of 1 µM nicotine (NIC). (E) Summary of sIPSC frequency during superfusion of 1 µM nicotine in VTA DA neurons relative to baseline (dashed line) from naïve (n = 7) and CNE (n = 6) rats. (F) Summary of mIPSC frequency during superfusion of 1 µM nicotine in VTA DA neurons relative to baseline (dashed line) revealed in the presence of 0.5 µM TTX [naïve (n = 6), CNE (n = 8); representative traces are in Fig. S3C]. Dashed lines reflect prenicotine baseline levels (defined as 100%). Data are presented as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
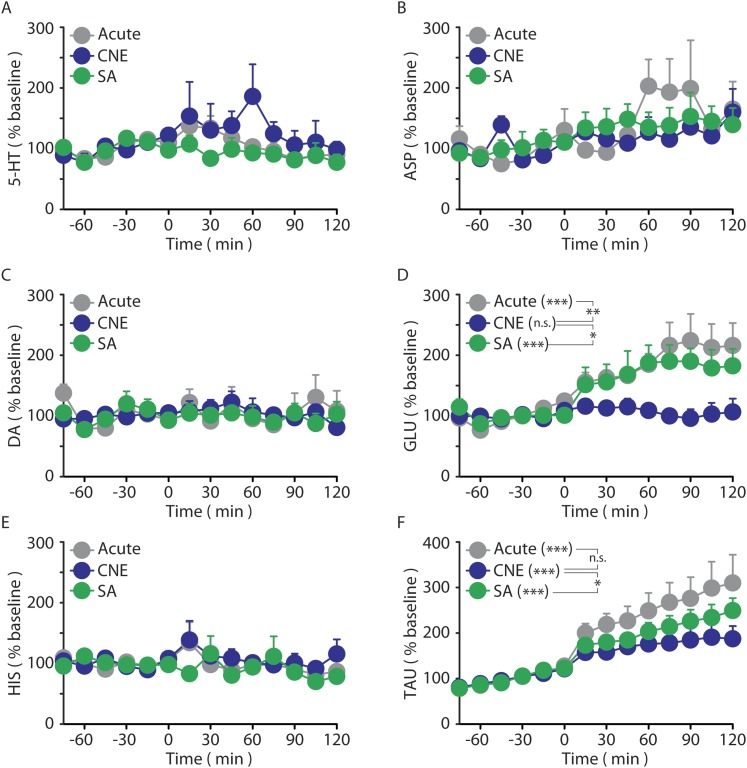
Fig. S1.
Dialysate neurotransmitter responses induced by nicotine exposure (t 0–120 min) in naïve (black), CNE (blue), and SA (green) rats. (A) Nicotine exposure did not alter dialysate 5-HT levels in naïve, CNE, or SA rats. Baseline 5-HT levels did not significantly differ between groups (naïve, 0.25 ± 0.06; CNE, 0.31 ± 0.13; SA, 0.22 ± 0.06 nM). (B) Nicotine exposure did not alter dialysate aspartate (ASP) levels in naïve, CNE, or SA rats. Baseline ASP levels did not significantly differ between groups (naïve, 276.4 ± 57.2; CNE, 364.5 ± 43.8; SA, 371.6 ± 59.2 nM). (C) Nicotine exposure did not alter dialysate DA levels in naïve, CNE, or SA rats. Baseline DA levels did not significantly differ between groups (naïve, 0.28 ± 0.13; CNE, 0.27 ± 0.04; SA, 0.53 ± 0.27 nM). (D) Nicotine exposure altered dialysate glutamate (GLU) levels in naïve and SA rats but not CNE rats. GLU was altered by both drug history (P < 0.01) as well as the volitional nature of nicotine exposure (P < 0.05). Baseline GLU levels were significantly increased by drug history (naïve, 1.5 ± 0.3; CNE, 4.4 ± 0.6 µM; P < 0.001), but were not further altered by the volitional nature of nicotine exposure (CNE, 4.4 ± 0.6; SA, 4.3 ± 0.4 µM). (E) Nicotine exposure did not alter dialysate histamine (HIS) levels in naïve, CNE, or SA rats. Baseline HIS levels did not significantly differ between groups (naïve, 84.0 ± 8.9; CNE, 70.1 ± 11.1; SA, 65.4 ± 8.7 nM). (F) Nicotine exposure increased taurine (TAU) levels in naïve, CNE, and SA rats. TAU was not affected by drug history, but was altered by the volitional nature of nicotine intake (P < 0.05). Baseline TAU levels did not significantly differ between groups (naïve, 5.6 ± 0.6; CNE, 9.3 ± 1.9; SA, 5.0 ± 0.7 µM). Data are presented as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant.
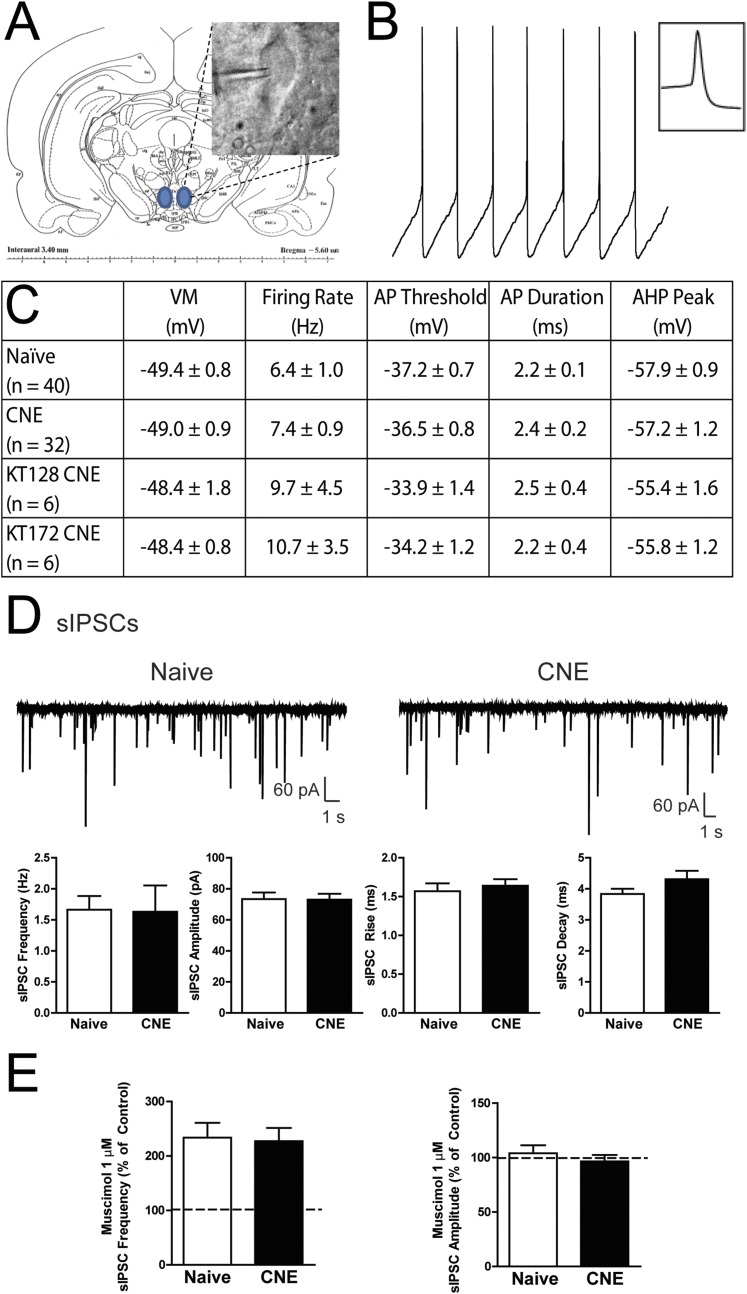
To determine the impact of nicotine exposure on GABA transmission, whole-cell voltage- and current-clamp recordings of pharmacologically isolated GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) were performed in VTA DA neurons (Fig. S2 A–C). In VTA slices from drug-naïve rats, bath application of nicotine produced a transient increase in sIPSC frequency with no effect on sIPSC amplitude (Fig. 1 D and E and Fig. S3A). However, in VTA slices from CNE rats, nicotine did not alter sIPSC frequency or amplitude (Fig. 1 D and E). There was no significant effect of prior CNE on either baseline (Fig. S2D) or muscimol-induced (Fig. S2E) sIPSCs, indicating no CNE-induced disruption in postsynaptic GABAA receptor influence. Nicotine-induced increases in sIPSC frequency were absent in the presence of tetrodotoxin (TTX), confirming a reliance on action potential firing (Fig. 1F and Fig. S3 B and C). Collectively, these data demonstrate that CNE impairs nicotine-induced increases in GABA signaling but not baseline GABA transmission in VTA DA neurons.
Fig. S2.
Cell characteristics of VTA dopamine neurons. (A) IR-DIC visualization of a VTA DA neuron during electrophysiological recording. (B) Representative current-clamp recording of a VTA DA neuron displaying spontaneous action potential firing in compressed and enlarged (Inset) time scales. (C) Summary of action potential characteristics of VTA DA neurons from naïve and CNE rats including membrane potential (VM), firing rate, action potential (AP) threshold, AP duration, and afterhyperpolarization (AHP). (D) Representative voltage-clamp recordings (Top) and summary of characteristics (Bottom) of sIPSCs in VTA DA neurons from naïve and CNE rats. (E) Summary of the change in sIPSC frequency (Left) and amplitude (Right) with 1 μM muscimol in VTA DA neurons from naïve and CNE rats relative to control (dashed line). Data are presented as mean ± SE.
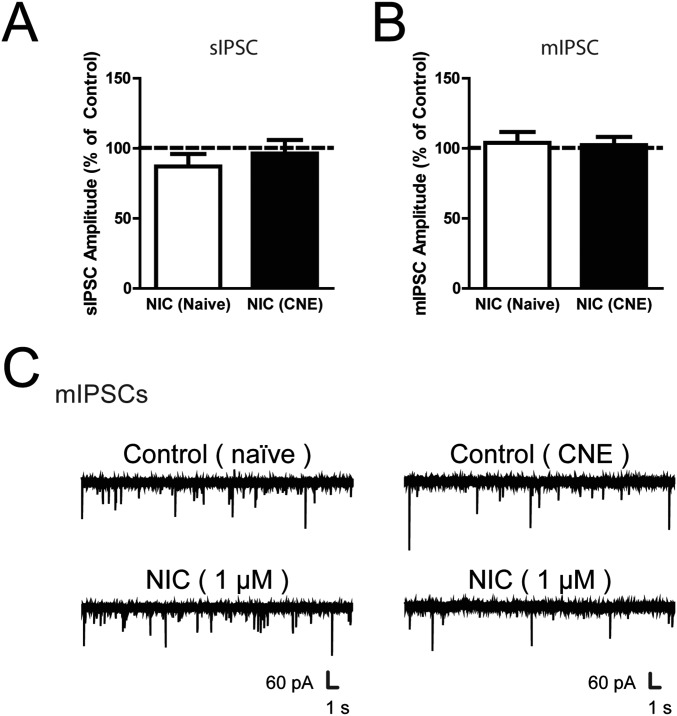
Fig. S3.
VTA dopamine neurons following nicotine exposure. (A and B) Summary of the change in (A) sIPSC and (B) mIPSC amplitude in VTA DA neurons from naïve and CNE rats following 1 μM nicotine. (C) Representative voltage-clamp recordings of mIPSCs in VTA DA neurons from naïve (Left) and CNE (Right) rats during the superfusion of 1 µM nicotine. Data are presented as mean ± SE.
CB1 Influence on VTA GABA Signaling Is Not Altered Following Chronic Nicotine Exposure.
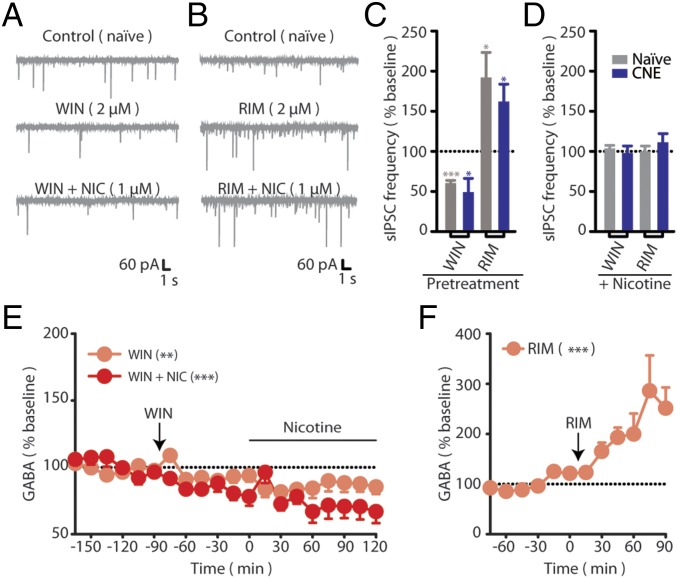
Tests with the synthetic CB1 agonist WIN 55,212-2 (WIN) and the CB1 antagonist rimonabant (RIM) were performed to probe for possible alterations in CB1 function following CNE. In nicotine-naïve slices, WIN reduced sIPSC frequency and prevented subsequent nicotine-induced increases in sIPSC frequency (Fig. 2 A, C, and D). The WIN-induced occlusion of nicotine-induced sIPSC increases likely reflects CB1-mediated suppression of presynaptic GABA release, as supported by in vivo microdialysis data demonstrating WIN-induced reductions in VTA GABA levels and prevention of subsequent nicotine-induced increases in VTA GABA release (Fig. 2E). RIM increased sIPSC frequency and also prevented subsequent nicotine-induced increases in sIPSC frequency (Fig. 2 B–D). This suggests a tonic CB1 influence on VTA GABA release, as supported by a robust increase in in vivo VTA microdialysate GABA levels following RIM administration (Fig. 2F). The robust per se effects of RIM on GABAA signaling may preclude detection of additional nicotine-induced effects. WIN and RIM induced similar effects in VTA neurons from CNE rats (Fig. 2 C and D) and, importantly, the effects of these CB1 receptor ligands on sIPSCs did not differ between nicotine-naïve and CNE VTA neurons.
Fig. 2.
CB1 influence on VTA GABA signaling is not altered following CNE. (A and B) Representative recordings of sIPSCs from nicotine-naïve rats during the superfusion of (A) 2 µM WIN or (B) 2 µM RIM, followed by 1 µM NIC. (C and D) Summary of sIPSC frequencies from naïve (gray) or CNE (blue) during (C) superfusion of RIM (n = 6 and n = 5) WIN (n = 5 and n = 5) and during (D) subsequent superfusion of nicotine compared with drug-treated baseline (n = 5 in all conditions). (E) Dialysate GABA levels in naïve rats treated with WIN (3 mg/kg i.p., t 0, n = 6) in the absence or presence of IV nicotine (matching the acute group in Fig. 1 A and C). (F) Dialysate GABA levels in CNE rats treated with RIM (3 mg/kg IP, t 0, n = 6) in the absence of nicotine. Data are presented as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Selective Inhibitors of Rat 2-AG Metabolic Enzymes.
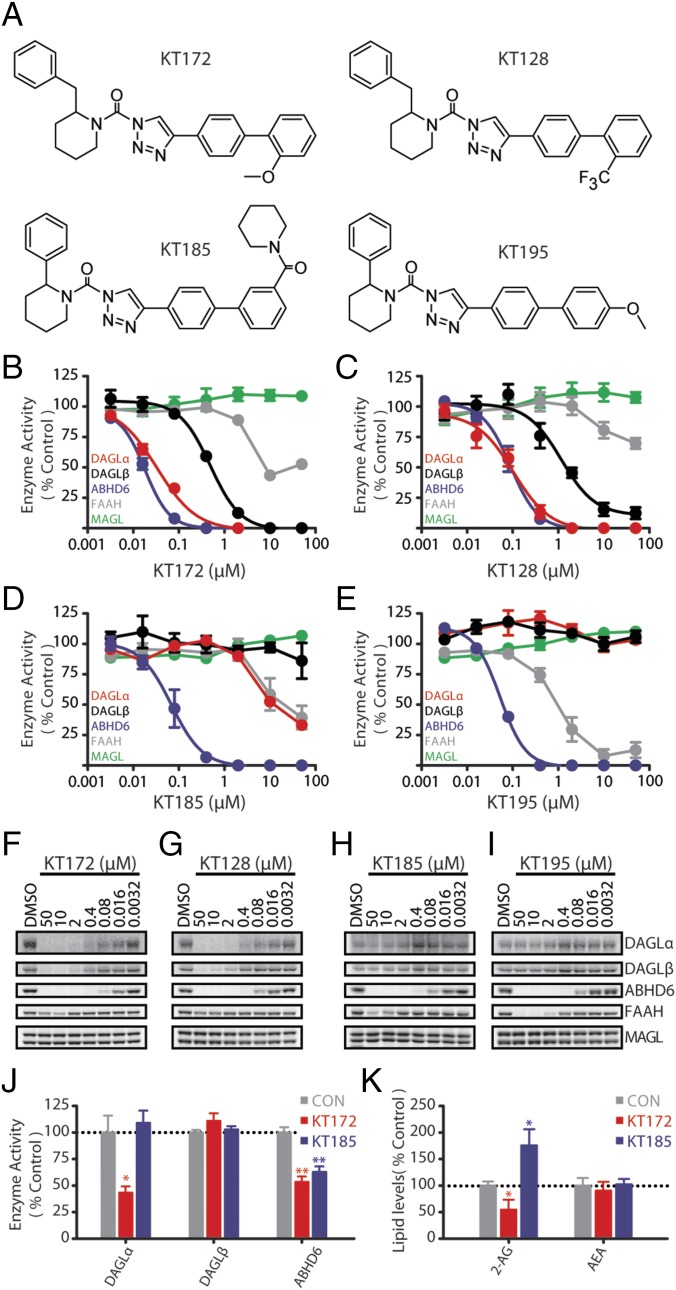
Determination of the 2-AG influence on nicotine-induced alterations in VTA GABA signaling requires manipulation of 2-AG biosynthesis. To enable this, we used an activity-based protein profiling (ABPP) assay using the broad-spectrum and DAGL-directed probes FP-Rh and HT-01 to evaluate the potency and selectivity of a series of 1,2,3-triazole ureas (1,2,3-TUs) for inhibiting the 2-AG biosynthetic enzymes DAGLα and DAGLβ (20). In the rat proteome, the 1,2,3-TU KT172 (Fig. 3 B and F) and the structurally related analog KT128 (Fig. 3 C and G) displayed greater inhibitory potency at DAGLα than DAGLβ. Both compounds exhibited good selectivity against other serine hydrolases including MAGL and FAAH but showed cross-reactivity with ABHD6, as expected (20–22). To identify appropriate negative control probes, we confirmed that the ABHD6-selective inhibitors KT185 (Fig. 3 D and H) and KT195 (Fig. 3 E and I) potently inactivated rat ABHD6 (20, 21) with minimal off-target activity against other serine hydrolases including DAGLs, with the exception of FAAH cross-reactivity by KT195 (Fig. 3, Figs. S4 and S5, and Table S1).
Fig. 3.
Selective inhibitors of rat 2-AG metabolic enzymes. (A) Chemical structures of 1,2,3-triazole urea inhibitors. (B–E) Competitive ABPP (n = 3) of (B) KT172, (C) KT128, (D) KT185, and (E) KT195 against endogenous serine hydrolases detected in rat brain proteome in vitro using either the DAGL-tailored probe (HT-01; for DAGLα, DAGLβ, and ABHD6) or broad-spectrum serine hydrolase probe (FP-Rh; for MAGL and FAAH). Proteomes were preincubated with the indicated dose of compound (30 min, 37 °C) followed by labeling with activity-based probes (1 µM FP-Rh or HT-01, 30 min, 37 °C). (F–I) Representative gels for each in vitro inhibitor treatment (full gels are in Figs. S4 and S5). (J) Activity of eCB metabolic enzymes following ex vivo incubation of striatal tissue slices with vehicle, 1 µM KT172, or 1 µM KT185 (n = 4, 10 min; full gels are in Fig. S6). (K) Levels of 2-AG and AEA following incubation of striatal tissue slices with vehicle, 1 µM KT172, or 1 µM KT185 (n = 4–6, 4 h). Data are presented as mean ± SE. *P < 0.05, **P < 0.01.
Fig. S4.
Full gel images for Fig. 3 showing in vitro activity of 1,2,3-triazole urea inhibitors in rat brain homogenates. Rat membrane proteomes were treated (37 °C, 30 min) with the indicated concentrations of (A and B) KT172 or (C and D) KT128. Proteomes were subsequently analyzed by competitive ABPP with (A and C) HT-01 or (B and D) FP-Rh (1 µM, 37 °C, 30 min) as described in Experimental Procedures.
Fig. S5.
Full gel images for Fig. 3 showing in vitro activity of 1,2,3-triazole urea inhibitors in rat brain homogenates. Rat membrane proteomes were treated (37 °C, 30 min) with the indicated concentrations of (A and B) KT185 or (C and D) KT195. Proteomes were subsequently analyzed by competitive ABPP with (A and C) HT-01 or (B and D) FP-Rh (1 µM, 37 °C, 30 min) as described in Experimental Procedures.
Table S1.
In vitro IC50 values for 1,2,3-triazole urea inhibitors measured in rat brain homogenate
| Enzyme | KT172 | KT128 | KT185 | KT195 | KML29 |
| DAGLα | 33 | 108 | >10,000 | >50,000 | N/A |
| DAGLβ | 495 | 1,130 | >50,000 | >50,000 | N/A |
| ABHD6 | 18 | 93 | 77 | 54 | >2,000 |
| MAGL | >50,000 | >50,000 | >50,000 | >50,000 | 15 |
| FAAH | >2,000 | >50,000 | >10,000 | 1,075 | >50,000 |
All IC50s were calculated from analysis of ABPP gels and are expressed as nanomolar (nM). Values for KML29 (ABHD6, MAGL, FAAH) have been previously published (1). DAGLα and DAGLβ have not been assessed for KML29 (N/A).
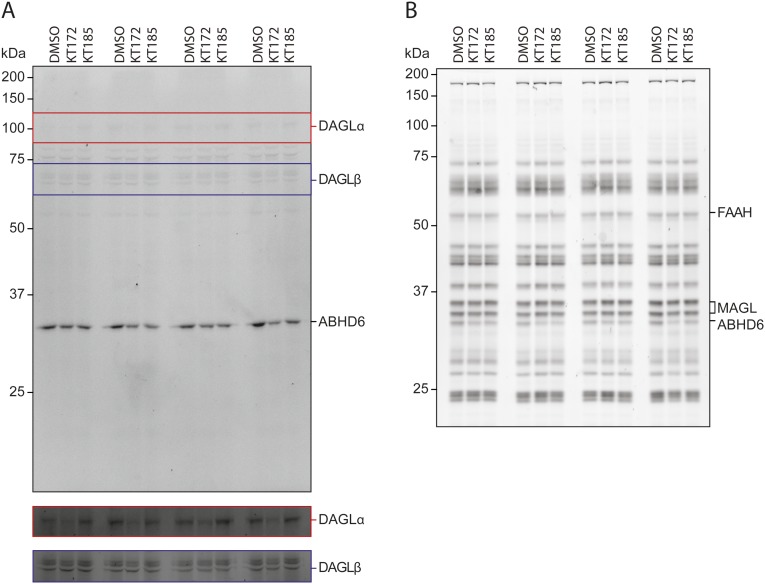
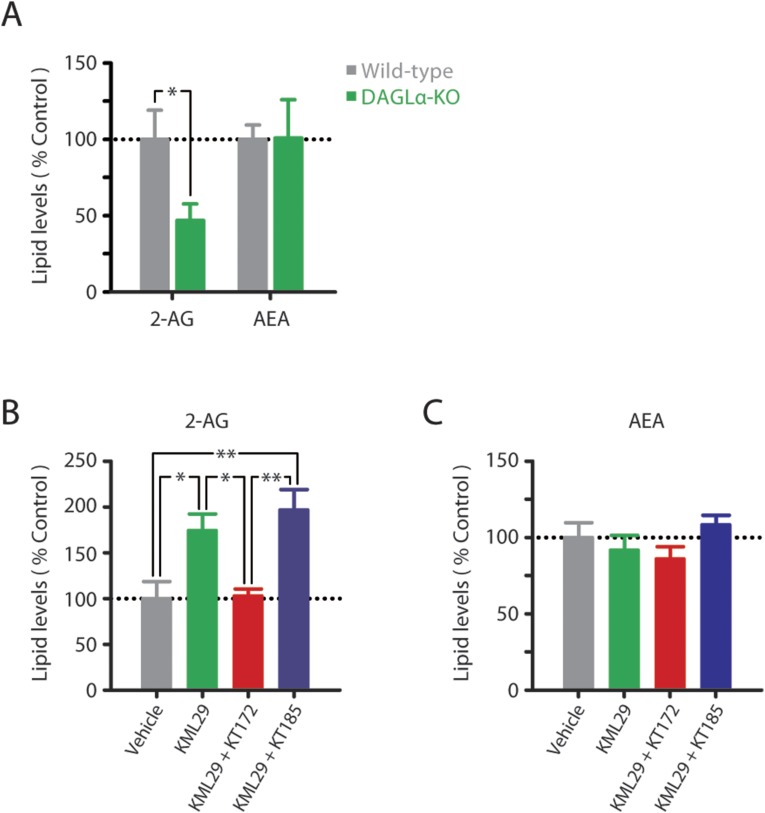
In the ex vivo brain slice conditions used for electrophysiological studies, KT172 inactivated DAGLα and ABHD6 with no significant effect on other serine hydrolases, and the negative control probe KT185 inhibited ABHD6 without disrupting DAGL activity (Fig. 3J and Fig. S6). In this same preparation, KT172 significantly and selectively reduced striatal slice 2-AG content (Fig. 3K), similar to the reduction in striatal 2-AG content evident in DAGLα-KO mice (Fig. S7A). Conversely, KT185 significantly increased striatal slice 2-AG content (Fig. 3K), consistent with a role for ABHD6 as an alternative eCB hydrolase in the rat brain (11). Although KT172 also inhibits ABHD6 activity, the upstream loss of 2-AG biosynthesis by DAGLα inhibition likely precludes 2-AG accumulation that could result from this decrement in 2-AG hydrolysis. Indeed, DAGLα inhibition by KT172 prevents the accumulation of tissue 2-AG content normally induced by the MAGL inhibitor KML29 (Fig. S7B), even though coincubation with these compounds results in the combined inhibition of ABHD6 and MAGL. Overall, KT172-induced reductions in tissue 2-AG content likely result from the combined reduction in 2-AG biosynthesis and unperturbed 2-AG hydrolysis by MAGL. No treatment altered basal AEA levels (Fig. 3K and Fig. S7C). Taken together, these results demonstrate that KT172 and KT185 serve as paired chemical probes for selectively modulating 2-AG metabolism in the rat brain.
Fig. S6.
Full gel images for Fig. 3J showing the activity of serine hydrolases in rat striatal slices. Rat striatal slices were treated for 10 min at room temperature with either vehicle (DMSO, 0.02%), KT172 (1 µM), or KT185 (1 µM). Rat membrane proteomes were immediately processed and analyzed by ABPP using either the (A) HT-01 or (B) FP probe (1 µM, 37 °C, 30 min) as described in Experimental Procedures.
Fig. S7.
Endocannabinoid levels in striatal slices. (A) Levels of 2-AG and AEA in striatal tissue slices of wild-type and DAGLα-KO mice incubated in aCSF (n = 6–8, 4 h). (B and C) Levels of (B) 2-AG and (C) AEA following incubation of rat striatal tissue slices with vehicle, 1 µM KML29, 1 µM KML29 + 1 µM KT172, or 1 µM KML29 + µM KT185 (n = 5–6, 4 h). Experiments were carried out as described in Experimental Procedures. Data are presented as mean ± SE. *P < 0.05, **P < 0.01.
DAGL Inactivation Restores Nicotine-Induced GABA Release in Rats with a History of Nicotine Exposure.
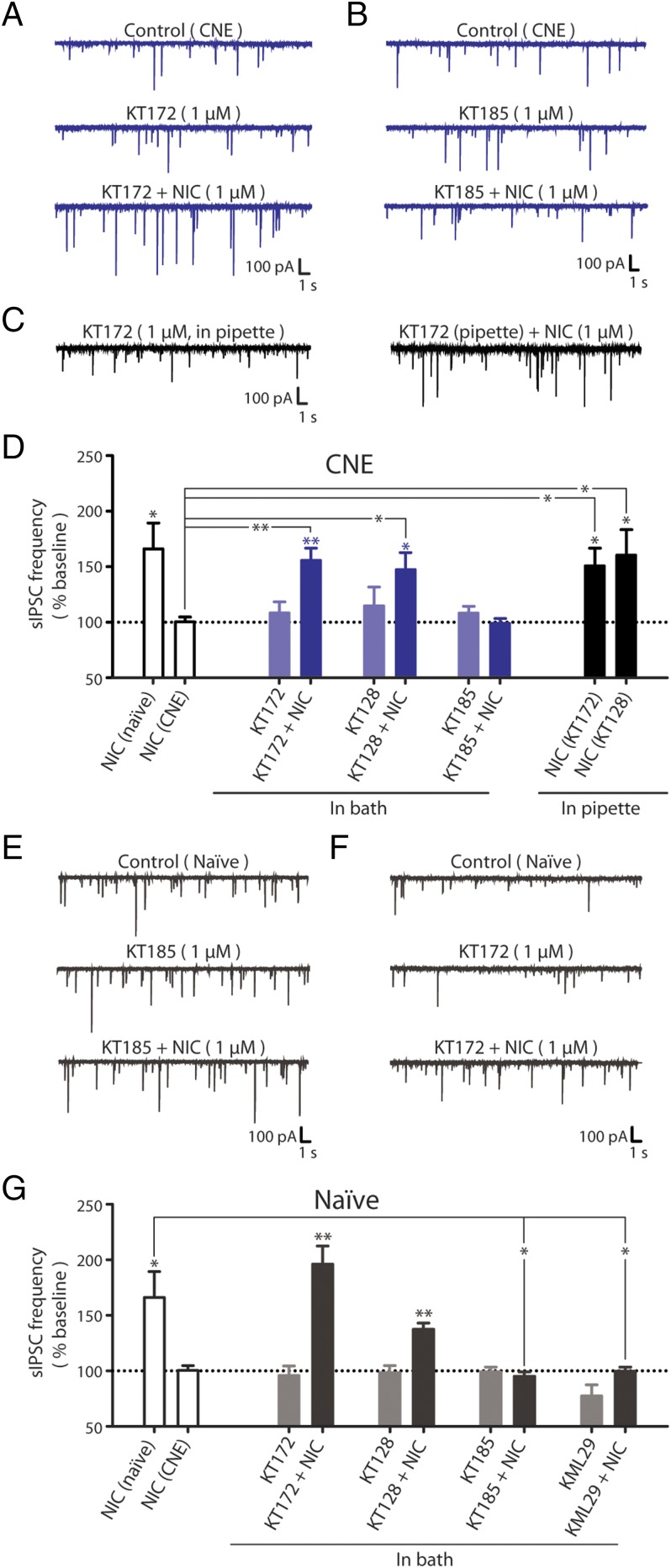
We tested whether inactivation of DAGLs could restore nicotine-elicited GABA signaling at VTA DA synapses in slices from CNE rats. Slices were superfused with KT172 or KT128, which had no per se effect on baseline sIPSC characteristics (Fig. 4 A and D). However, subsequent application of nicotine to drug-treated VTA slices significantly increased sIPSC frequency in DA neurons to levels similar to those observed in drug-naïve rats. To confirm the DAGL specificity of this effect, we treated VTA slices from CNE rats with the ABHD6-selective inhibitor KT185. KT185 had no per se effect on baseline sIPSCs (Fig. 4 B and D), and did not restore the nicotine-induced increase in GABA release. We replicated these experiments with the DAGL inhibitors included in the pipette internal solution to restrict delivery to the postsynaptic cell, and showed that either KT172 or KT128 restored nicotine-induced increases in sIPSC frequency in CNE slices to levels similar to that observed in drug-naïve slices (Fig. 4 C and D). Taken together, these data demonstrate that inhibiting postsynaptic DAGL restores nicotine-induced GABA signaling in rats with a history of nicotine exposure.
Fig. 4.
DAGL inhibition restores nicotine-induced GABA release in rats with a history of nicotine exposure. (A and B) Representative recordings of sIPSCs in VTA DA neurons from CNE rats during superfusion of (A) 1 µM KT172 followed by 1 µM NIC and (B) 1 µM KT185 followed by 1 µM NIC. (C) Representative recordings of sIPSCs in a VTA DA neuron from a CNE rat during administration of KT172 (1 µM in the pipette solution) before (Left) and during (Right) superfusion of 1 µM NIC. (D) Summary of sIPSC frequencies in VTA DA neurons from CNE rats during superfusion of either 1 µM KT172 (n = 5), 1 µM KT128 (n = 6), or 1 µM KT185 (n = 6) and during subsequent nicotine superfusion. Summary of sIPSC frequencies in VTA DA neurons before and during nicotine superfusion with either 1 µM KT172 (n = 6) or 1 µM KT128 (n = 6) in the pipette solution. Attenuation of 2-AG clearance in nicotine-naïve subjects recapitulates the effects of CNE. (E and F) Representative recordings of sIPSCs in VTA DA neurons from naïve rats during superfusion of (E) 1 µM KT185 followed by 1 µM NIC and (F) 1 µM KT172 followed by 1 µM NIC. (G) Summary of sIPSC frequencies in VTA DA neurons from naïve rats during superfusion of either 1 µM KT172 (n = 5), 1 µM KT128 (n = 5), 1 µM KT185 (n = 6), or 1 µM KML29 (n = 8) and during subsequent nicotine superfusion. The response to NIC in naïve and CNE rats is shown for comparison [open bars (Left) from Fig. 1]. Data are presented as mean ± SE. *P < 0.05, **P < 0.01.
Attenuation of 2-AG Clearance Blocks Nicotine-Induced GABA Release in Drug-Naïve Rats.
We hypothesized that enhancing 2-AG signaling in naïve rats would recapitulate the loss of nicotine-induced GABA release evident following CNE. Superfusion of naïve VTA slices with the ABHD6-selective inhibitor KT185 did not alter baseline sIPSC frequency but blocked nicotine-induced increases in sIPSC frequency (Fig. 4 E and G). Similarly, superfusion of the MAGL-selective inhibitor KML29 blocked nicotine-induced sIPSCs without significantly modifying baseline sIPSC frequency (Fig. 4G). In contrast, superfusion with the DAGL inhibitors KT172 or KT128 did not alter GABA signaling (Fig. 4 F and G). Together, these data demonstrate that the loss of nicotine-induced GABA signaling observed in the VTA of CNE rats can be reproduced in naïve rats by inhibiting 2-AG clearance with inhibitors of ABHD6 or MAGL.
Nicotine Self-Administration Is Reduced by DAGL Inhibition.
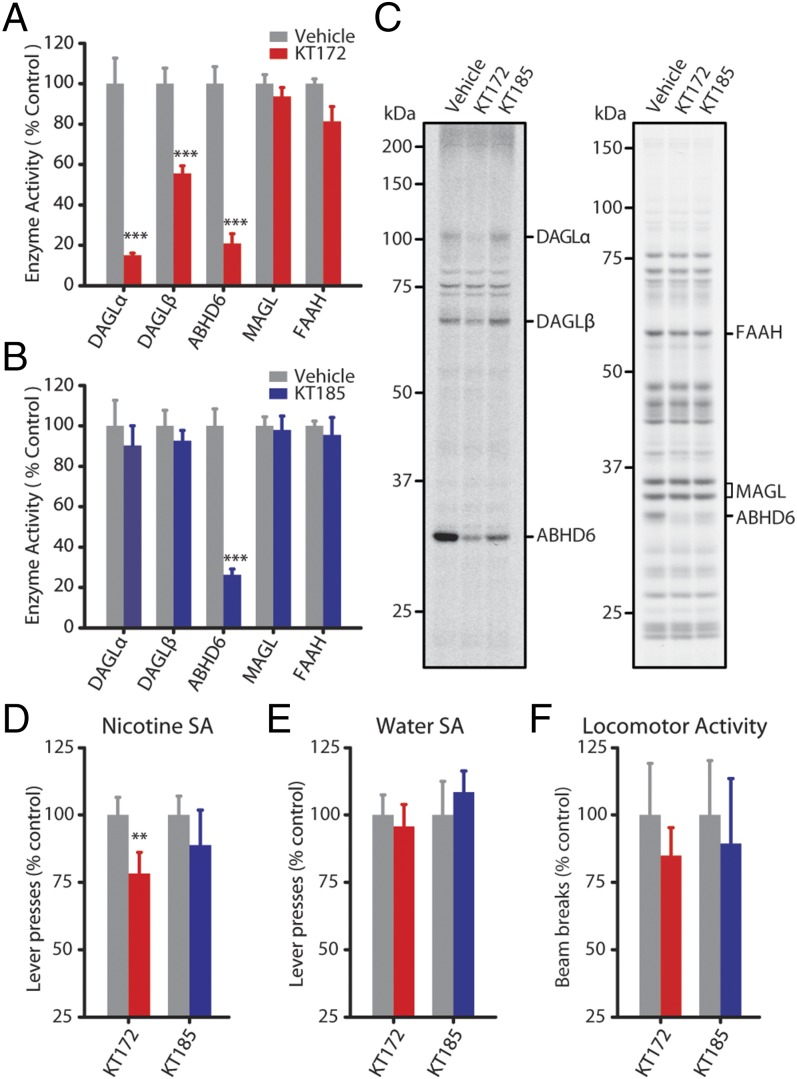
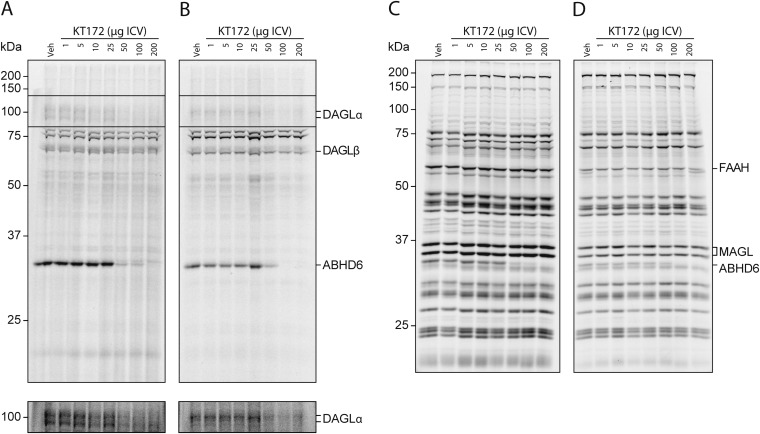
To evaluate the influence of DAGL activity on nicotine SA behavior, we established that KT172 and KT185 effectively inhibit DAGL and ABHD6 in the rat brain following intracerebroventricular (ICV) administration. Both DAGLα and ABHD6 activity were inhibited following ICV administration of KT172; DAGLβ activity was also partially inhibited in these animals (Fig. 5 A and C and Fig. S8). Treatment with KT185 resulted in comparable ABHD6 inhibition in the absence of reduced DAGL activity (Fig. 5 B and C). Neither drug exhibited significant cross-reactivity against other serine hydrolases (including MAGL and FAAH) under these conditions. Subsequent behavioral tests revealed that nicotine self-administration was significantly reduced by KT172 but not KT185 (Fig. 5D). Neither compound significantly altered operant responding for water (Fig. 5E) or spontaneous locomotor activity (Fig. 5F), thereby discounting an influence of nonspecific behavioral disruptions and highlighting a meaningful influence of DAGL activity on the motivation for nicotine vs. a nondrug reinforcer.
Fig. 5.
Nicotine self-administration is reduced by DAGL inhibition. (A and B) Gel-based ABPP using HT-01 and FP-Rh assessed the efficacy and selectivity of KT172 and KT185 in vivo by ICV injection. KT172 (A) (100 µg, 4 h, n = 4) reduces DAGLα, DAGLβ, and ABHD6 activity, whereas treatment with KT185 (B) (100 µg, 4 h, n = 4) reduces ABHD6 activity with negligible activity against DAGLs. (C) Representative HT-01 (Left) and FP-Rh (Right) gels for each inhibitor. (D) Pretreatment with KT172 (100 µg, 4 h, n = 16), but not KT185 (100 µg, 4 h, n = 10), significantly reduced nicotine self-administration (75 µg/kg per infusion; FR-1 reinforcement schedule). (E) Pretreatment with either KT172 (n = 9) or KT185 (n = 9) has no effect on oral water self-administration. (F) Pretreatment with either KT172 (n = 9) or KT185 (n = 9) has no effect on motor activity. Data are presented as mean ± SE. **P < 0.01, ***P < 0.001.
Fig. S8.
Full gel profiles of the in vivo activity in rats treated with the indicated amounts of KT172 by ICV injection. Membrane proteomes from KT172-treated rats (4 h) were analyzed by competitive ABPP: either (A) rat whole brain incubated with HT-01 (1 µM), (B) VTA homogenates incubated with HT-01 (1 µM), (C) rat whole brain incubated with FP (1 µM), or (D) VTA homogenates incubated with FP (1 µM). Experiments were carried out as described in Experimental Procedures.
Discussion
Through the use of selective inhibitors of DAGL, ABHD6, and MAGL, the present findings reveal that enhanced on-demand 2-AG formation blunts the inhibitory control of nicotine-induced VTA cell excitation following CNE (Fig. S9). Loss of this inhibitory feedback mechanism likely contributes to increased VTA sensitivity to nicotine and other stimuli (23, 24), resulting in sensitized DA release in the nucleus accumbens (15) and increased motivation for nicotine intake.
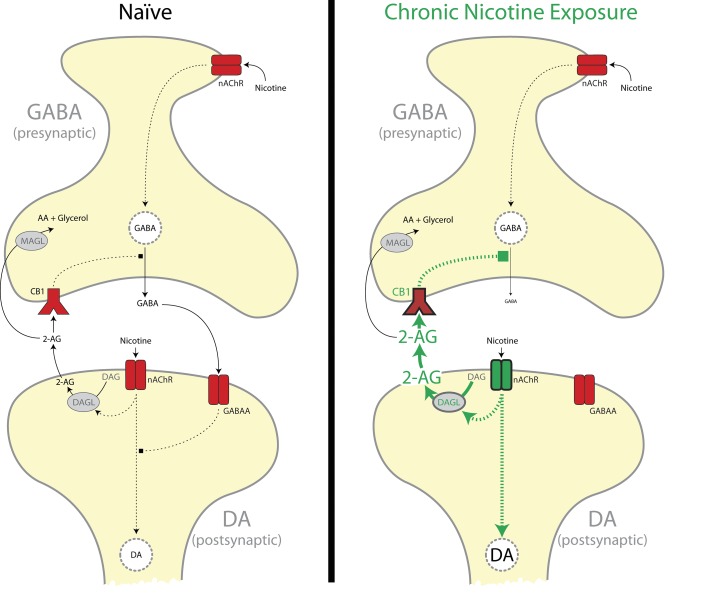
Fig. S9.
Model of on-demand 2-AG regulation of VTA GABA release. In naïve rats (Left), nicotine activates nAChRs on both DA projection neurons and GABA synapses onto DA neurons in the VTA. Activation of nicotinic acetylcholine receptors (nAChRs) on DA neurons increases DA cell activity and results in modest increases in 2-AG synthesis. Chronic nicotine exposure (Right) increases nAChR-induced postsynaptic DAGL activity, which results in enhanced 2-AG release and CB1-mediated disinhibition of GABA synapses in the VTA. AA, arachidonic acid; DAG, diacylglycerol.
Nicotine-induced increases in VTA 2-AG formation (14) likely contribute to the well-documented CB1 receptor influence on behavioral and physiological response to nicotine (25). In the VTA, CB1 receptors are thought to dynamically regulate GABA release at DAergic synapses (4), allowing the possibility that 2-AG formation dampens GABAergic constraint of DA cell excitation. A direct test of this hypothesis requires the suppression of 2-AG formation, although studies of this type have been limited by a lack of selective inhibitors of 2-AG biosynthesis (26) and suitable biochemical assays to validate inhibition of endogenous DAGL activity. To address this, we evaluated the efficacy of DAGL-selective 1,2,3-TU inhibitors for use in the rat brain using a tailored ABPP assay (20, 22). We find that KT172 selectively inhibits DAGL enzymes in the exogenous brain proteome as well as in awake animals, and that the primary off-target of this inhibitor (ABHD6) can be accounted for using the negative control probe KT185. Using targeted liquid chromatography-mass spectrometry (LC-MS) metabolomics, we show that KT172 but not KT185 significantly reduces brain 2-AG levels, supporting DAGLs as the principal 2-AG biosynthetic enzymes in the rat brain. Using this suite of chemical probes, we demonstrate that DAGL inhibition restores nicotine-induced increases in GABA signaling in the VTA of CNE rats. Conversely, attenuation of 2-AG hydrolysis by either ABHD6 or MAGL in nicotine-naïve rats recapitulates the loss of nicotine-induced GABA signaling observed following CNE. These latter effects are consistent with previous studies on hippocampal GABAergic signaling using MAGL inhibitors (27) or MAGL-knockout mice (28). Collectively, these findings support the hypothesis that nicotine-induced increases in 2-AG dampen the GABAergic constraint of DA cell excitation following CNE.
We find that the functional influence of CB1 receptors on VTA GABA release is unaltered by CNE, and demonstrate that CB1 receptor antagonism robustly increases VTA GABA levels and GABAA receptor signaling. The resultant decrease in DA cell excitability likely underlies the ability of CB1 antagonists to block nicotine-induced increases in nucleus accumbens DA release and to suppress nicotine self-administration (25, 29–34).
Nicotine and other drugs of abuse alter synaptic transmission in the VTA through additional mechanisms that may be facilitated by the loss of local inhibitory signaling. For example, nicotine enhances glutamatergic transmission in the VTA and strengthens excitatory synapses onto VTA DA neurons via the induction of long-term potentiation (35–37). A significant body of work highlights the importance of glutamatergic synaptic plasticity in long-term changes that result from a history of drug exposure (37, 38), although in the context of the present studies it is notable that the functional influence of these excitatory adaptations requires a concomitant loss of inhibitory signaling (39).
In summary, on-demand 2-AG biosynthesis represents a molecular switch that regulates inhibitory signaling in the VTA. Following CNE, enhanced 2-AG production following cholinergic receptor activation imbalances this system in a manner that facilitates DAergic cell excitability. The present results provide novel, direct evidence that pharmacological modulation of 2-AG biosynthesis is a viable approach for evaluating the influence of the eCB system on substance abuse disorders.
Experimental Procedures
Animals.
Studies involving male Wistar rats (300–450 g; Charles River Laboratories) were conducted in accordance with NIH guidelines and as approved by the Scripps Research Institute Institutional Animal Care and Use Committee. Intravenous catheterization (14), microdialysis probe implantation (14), and intracerebroventricular administration of drugs using homemade cannulae and infusers (40) were performed using published methodology.
Nicotine Administration Procedures.
For volitional nicotine exposure, SA rats were trained to operantly respond for IV nicotine (75 µg/kg per infusion; 0.1 mL per infusion over 4 s) under a continuous (fixed ratio 1; FR-1) schedule of reinforcement in daily 2-h sessions using previously published procedures (14). Rats from the SA group were individually paired with animals receiving noncontingent forced nicotine exposure (CNE) that received nicotine infusions based on the partner SA rats' patterns of intake during the training and collection sessions. Rats receiving acute noncontingent forced nicotine exposure (acute) were also individually paired with SA rats, but received saline infusions in all training sessions, and nicotine infusions during the collection session (infusion patterns based on partner SA rats' volitional intake).
Neurotransmitter Measurements.
Quantification of neurotransmitters in dialysate samples was performed by LC-MS/MS using established methodology (41, 42). Briefly, 1 µL dialysate was added to 6.5 µL borate (100 mM), derivatized with benzoyl chloride [2% (vol/vol), acetonitrile (ACN)], and supplemented with an internal standard.
Gel-Based Competitive ABPP.
Gel-based competitive ABPP experiments were performed as previously described (20, 43). For experiments using the FP-rhodamine probe, proteomes (1 mg/mL) were treated with FP-rhodamine (1 μM) in a 50-μL total reaction volume for 30 min at 37 °C. For experiments using the HT-01 probe, proteomes (2 mg/mL) were treated with HT-01 (1 μM) in a 50-μL total reaction volume for 30 min at 37 °C. Reactions were quenched with SDS/PAGE loading buffer. Proteins were separated by SDS/PAGE (10% acrylamide) and visualized using a fluorescence scanner (Hitachi; FMBIO IIe).
Electrophysiological Recording.
Electrophysiology procedures were as recently reported (44, 45) and are described briefly. Coronal sections (300 µm) through the midbrain were cut on a Leica VT1000S (Leica Microsystems) and placed in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) solution composed of 130 mM NaCl, 3.5 mM KCl, 2 mM CaCl2, 1.5 mM MgClSO4, 1.25 mM NaH2PO4, 24 mM NaHCO3, 10 mM glucose. Whole-cell recordings were made using glass pipettes (3–5 MΩ) filled with an intracellular solution containing 145 mM KCl, 5 mM EGTA, 5 mM MgCl2, 10 mM Hepes, 2 mM Na-ATP, 0.2 mM Na-GTP coupled to a MultiClamp 700B amplifier (Molecular Devices), low-pass-filtered at 2–5 kHz, digitized (Digidata 1440A; Axon Instruments), and stored on a computer using pCLAMP 10 software (Axon Instruments). To isolate inhibitory currents mediated by GABAA receptors, recordings (Vhold −60 mV) were performed in the presence of dinitroquinoxaline-2,3-dione (DNQX) (20 µM), dl-2-amino-5-phosphonovalerate (AP-5) (50 µM), and CGP55845A (1 µM). Miniature IPSCs (mIPSCs) were recorded in the additional presence of TTX (0.5 µM).
Statistics.
For microdialysis analyses, significance was determined by repeated measures ANOVA for effects of group, nicotine, and group x nicotine interaction. Significance for microdialysis baseline levels and area-under-the-curve (AUC) values was determined by ANOVA followed by Dunnett’s post hoc. For the ABPP studies, a Student’s t test (unpaired, two-tailed) was used to determine the effect of each drug. For electrophysiology experiments, sIPSC frequency was examined for a 2-min period following drug or nicotine application (36), and events were analyzed for independent significance using a one-sample t test and compared using a two-tailed t test for independent samples, a paired two-tailed t test for comparisons made within the same recording, and a one-way ANOVA with a Bonferroni post hoc analysis for comparisons made between three or more groups. All statistical analysis was performed using SPSS 18 (IBM) or Prism 5.02 (GraphPad). Data are presented as mean ± SE. In all cases, the criterion for significance was as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Supplemental Procedures.
For more details, see SI Experimental Procedures.
SI Experimental Procedures
Chemicals.
Neurotransmitters, benzoyl chloride, [13C]benzoyl chloride, borate, nicotine tartrate, and tetrodotoxin (TTX) were purchased from Sigma-Aldrich. All HPLC-grade solvents (H2O, acetonitrile, methanol), ammonium formate, and formic acid were purchased from VWR. Chemical inhibitors (KT172, KT128, KT185, KT195, and KML29) and activity probes (HT-01 and FP-rhodamine) were prepared in-house (20–22, 46, 47). 6,7-Dinitroquinoxaline-2,3-dione (DNQX), dl-2-amino-5-phosphonovalerate (AP-5), and CGP55845A were purchased from Tocris Bioscience. Rimonabant (RIM) and WIN 55,212-2 (WIN) were purchased from Cayman Chemical.
Nicotine Administration Procedures.
For volitional nicotine exposure, self-administration (SA) rats were trained to operantly respond for i.v. nicotine (75 µg/kg per infusion; 0.1 mL per infusion over 4 s) under an FR-1 schedule in daily 2-h sessions using previously published procedures (14). Drug infusions were paired with a 20-s presentation of a light stimulus above the operant lever, and a 20-s time-out period followed each drug infusion. Rats were restricted to 10–15 g chow per rat per d for the first 10 d of training, and given 25 g chow per rat per d on subsequent days. Rats receiving noncontingent forced nicotine exposure (CNE) were individually paired with animals in the SA group and received an identical pattern of nicotine infusions as obtained by the partner rat during the previous day’s self-administration session. With the exception of response contingency, all experimental parameters (stimulus light presentation, food restriction, handling, etc.) were identical between the SA and CNE groups. Rats receiving acute noncontingent forced nicotine exposure were individually paired with animals in the SA group, and received an identical pattern of nicotine infusions only during the final session.
Water Oral Self-Administration Procedures.
For volitional nicotine exposure, SA rats were trained to operantly respond for oral water access (120-µL delivery over 4 s) under an FR-1 schedule in daily 30-min sessions using previously published procedures (34). Water access was paired with a 20-s presentation of a light stimulus above the operant lever. There was no time-out period in this paradigm. Following each session, rats were given access to unlimited water for 3 h.
Locomotor Activity.
Locomotor activity was assessed using dual photobeam chamber methodology as previously published (48). Rats were placed in individual mesh cages (36-cm length, 25-cm width, 20-cm height) that were equipped with two photobeams placed 16 cm apart along the length of the cage. Rats were acclimated to the locomotor cages for 30 min and subsequently tested for locomotor activity for 30 min. Locomotor activity was assessed as the number of breaks in the photobeam by the rat, which indicates movement by the animal into and out of the beam path.
Nicotine Withdrawal Assessments.
To probe for nicotine dependence, rats were isolated in a Plexiglas field and videotaped for somatic signs of withdrawal across two separate, 10-min observation periods. The first of these periods was conducted 2 h following the penultimate session of nicotine self-administration, and the second was conducted 24 h following the last self-administration session. The scored behaviors included blinks, writhes, body/head shakes, teeth chatters, gasps, backing, scratching, paw tremors, and ptosis. These measures of withdrawal have been used as a reliable index of the physical signs of withdrawal in nicotine-dependent rats (49, 50). The animals were continuously observed for 10 min, during which time the number of times the animals exhibited any of the above signs was recorded. Multiple successive observations of any sign were counted as a single sign until a distinct pause was observed. If present continuously, ptosis was only counted once. The total number of somatic signs was defined as the sum of individual occurrences of the aforementioned withdrawal signs during the entire 10-min observation period.
Neurotransmitter Measurements.
Quantification of neurotransmitter levels in microdialysate samples was performed using LC-MS/MS [using previously published methodology (41, 42)]. Samples were prepared for analysis by off-line derivatization with benzoyl chloride. Briefly, 1 µL dialysate was added to 6.5 µL borate (100 mM), derivatized with benzoyl chloride (2%, ACN), and supplemented with 2.5 µL internal standard. The internal standard contained [13C]benzoyl chloride-derivatized amino acids and monoamines and d4-acetylcholine in H2O [1% (vol/vol)] supplemented with DMSO:formic acid [98 and 1% (vol/vol), respectively]. Neurotransmitters were separated by reversed-phase LC on an Agilent ZORBAX Rapid Resolution High Definition C18 column (2.1 mm × 50 mm, 1.8 µm) at a flow rate of 300 µL/min at 50 °C. The column was equilibrated in solvent A (H2O, 0.15% formic acid, 10 mM ammonium formate). Sample (10 of 12.5 µL) was injected using a 40-µL injection loop, and solvent A was held until 1 min. Solvent B (acetonitrile) was increased in a linear gradient to 65% solvent B until 7.0 min, increased to 75% until 8.5 min, dropped to 0% at 8.51 min, and held until 14 min. Neurotransmitters were analyzed using a tandem quadrupole mass spectrometer (Agilent; 6460 QQQ) via multiple-reaction monitoring in positive-ion mode. The capillary voltage was 4,000 V, the ion spray source temperature was 350 °C, the drying gas flow rate was 10 L/min, and the nebulizer pressure was 55 psi. Collisional activation of neurotransmitter precursor ions used nitrogen as a collision gas. The following neurotransmitters were quantified using the standard isotope dilution method (precursor→product): serotonin (5-HT, 385→264), acetylcholine (ACh, 146→87), aspartate (Asp, 238→105), dopamine (DA, 466→105), gamma-aminobutyric acid (GABA, 208→105), glutamate (Glu, 252→105), glutamine (Gln, 251→105), histamine (His, 216→105), norepinephrine (NE, 482→105), serine (Ser, 210→105), and taurine (Tau, 230→105). Although adenosine (Ado, 372→136) and glycine (Gly, 180→105) were included in the analytical method, it was not possible to detect them from test samples containing beta-cyclodextrin and thus they were excluded from the analysis. Neurotransmitters were identified in samples by matching their multiple-reaction monitoring signal and LC retention time with those of a pure standard. Quantitative analysis was performed by the stable isotope dilution method. Internal standard solution was mixed with an appropriate amount of natural (nondeuterated) primary standard. The final baseline values were normalized to the original sample volume and expressed as nanomolar or micromolar, as appropriate. For each group, outliers were removed using the following criteria. First, if the resting baseline for a given neurotransmitter was not stable (two or more samples were more than twofold different from the average), it was removed from analysis. For the remaining samples, if one or more animals had a baseline neurotransmitter concentration that was greater than 1.8σ, the greatest outlier was removed. The temporal in-session data for each animal were normalized to the baseline and expressed as a percent control. In addition, the in-session data were expressed using area-under-the-curve (AUC) analysis, calculated as the sum total of the difference between the percent control and 100% of each time point.
Endocannabinoid Tissue Measurements.
Endocannabinoids were analyzed from slice tissue samples using previously published methodology (14). Briefly, flash-frozen tissue was homogenized in ice-cold ethyl acetate containing internal standard (50 pmol d5-2-AG, 1 pmol d4-AEA) using a dounce homogenizer and transferred to a 1.5-mL Eppendorf tube. Samples were centrifuged (16,000 × g, 4 °C) for 30 min to precipitate protein and other insoluble material. The precipitated pellet was reconstituted in PBS and analyzed for total protein content using the BCA assay. The supernatant was transferred to a 75-mL silica tube and evaporated to dryness via a SpeedVac. Samples were reconstituted in 50 µL solution containing 50% LC solvent A [water/methanol/acetic acid (90:10:0.1; vol/vol/vol), 10 mM ammonium acetate] and 50% LC solvent B [isopropyl alcohol/methanol/water/acetic acid (60:30:10:0.1; vol/vol/vol/vol), 10 mM ammonium acetate] for analysis. Endocannabinoids were analyzed using a tandem quadrupole using the following mass transitions: 2-AG (379→287), d5-2-AG (384→287), AEA (348→62), d4-AEA (352→66).
Brain Proteomes.
Brains, slices, and regional punches were obtained from rats killed under anesthesia and immediately homogenized in lysis buffer (1 M Hepes, 2.5 M sucrose, 1 M DTT, 1 M MgCl2) using a dounce homogenizer (51). Unhomogenized debris was removed by low-speed centrifugation (2,300 × g, 5 min), and the membrane proteins were pelleted by high-speed centrifugation (100,000 × g, 45 min) and resuspended in assay buffer (1 M Hepes).
Brain Slice Preparation.
Rats (n = 33, 2–6 mo, 300–450 g) were subjected to brief anesthesia (3–5% isoflurane) followed by rapid decapitation and removal of the brain to an ice-cold high-sucrose solution (pH 7.3–7.4) that contained 206.0 mM sucrose, 2.5 mM KCl, 0.5 mM CaCl2, 7.0 mM MgCl2, 1.2 mM NaH2PO4, 26.0 mM NaHCO3, 5.0 mM glucose, 5.0 mM Hepes. Brains cut into transverse sections (300 µm) as previously described (44) through the midbrain from −4.5 to −5.7 mm from bregma (52) were cut on a vibrating microtome (Leica VT1000S; Leica Microsystems) and placed in an oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) solution composed of 130 mM NaCl, 3.5 mM KCl, 2.0 mM CaCl2, 1.5 mM MgClSO4∙7H2O, 1.25 mM NaH2PO4, 24 mM NaHCO3, 10 mM glucose. Slices were incubated in this solution for 30 min at 35–37 °C, followed by a 30-min equilibration at room temperature (21–22 °C). Following equilibration, a single slice was transferred to a recording chamber mounted on the stage of an upright microscope (Olympus; BX50WI).
Electrophysiological Recording.
We visualized neurons using infrared differential interference contrast (IR-DIC) optics and an EXi Aqua camera (QImaging). A 60× magnification water immersion objective (Olympus) was used for identifying and approaching neurons, and images were captured using QCapture software (QImaging). Whole-cell (voltage-clamp and current-clamp) recordings were made as previously described (45) using glass pipettes (3–5 MΩ; Warner Instruments) coupled to a MultiClamp 700B amplifier (Molecular Devices), low-pass-filtered at 2–5 kHz, digitized (Digidata 1440A; Axon Instruments), and stored on a computer using pCLAMP 10 software (Axon Instruments). Series resistance was typically <10 MΩ, and was continuously monitored with a hyperpolarizing 10-mV pulse. Electrophysiological properties of cells were determined by pCLAMP 10 Clampex software online during voltage-clamp recording using a 5-mV pulse delivered after breaking into the cell. The resting membrane potential was determined online after breaking into the cell using the zero-current (I 0) recording configuration, and the liquid junction potential was included in the determination.
The intracellular solution used for voltage- and current-clamp recordings was composed of 145 mM KCl, 5 mM EGTA, 5 mM MgCl2, 10 mM Hepes, 2 mM Na-ATP, 0.2 mM Na-GTP. Drugs were either dissolved in aCSF and included in the bath perfusion or included in the internal pipette solution. Pretreatment drugs (WIN, RIM, KT172, KT128, KT189, KT195, KML29) were bath-applied for a period of 10 min to allow full wash-in and maximal effects.
Nicotine has been shown to produce a transient-effect increase in IPSC frequency followed by a prolonged depression (36). To isolate the early phase of nicotine effects, nicotine was bath-applied for 5–7 min, but the period of analysis was restricted to 2 min after full wash-in. To isolate only the inhibitory currents mediated by GABAA receptors, recordings (Vhold −60 mV) were performed in the presence of the glutamate receptor blockers DNQX (20 µM) and AP-5 (50 µM) and the GABAB receptor antagonist CGP55845A (1 µM). Miniature IPSCs (mIPSCs) were recorded in the presence of DNQX (20 µM), AP-5 (50 µM), CGP55845A (1 µM), and TTX (0.5 µM). All voltage- and current-clamp recordings were performed in a gap-free acquisition mode with a sampling rate per signal of 10,000 Hz or a total data throughput equal to 20 kHz (2.29 MB/min) as defined by pCLAMP 10 Clampex software.
Statistics.
Statistical analyses were performed using PASW Statistics (version 18). For microdialysis lipid data, statistical significance was determined by repeated measures ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001) for effects of group, nicotine, and group x nicotine interaction. Here, groups were defined as drug history (acute vs. CNE) and contingency (CNE vs. SA). Effect of nicotine on each individual condition was determined by ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001). Statistical significance for microdialysis baseline levels and AUC values was determined by ANOVA followed by Dunnett’s post hoc (*P < 0.05, **P < 0.01, ***P < 0.001). For the ABPP studies, a Student’s t test (unpaired, two-tailed) was used to determine differences between two groups. A P value of <0.05 was considered significant. Action potential characteristics were evaluated using threshold-based event detection analysis in Clampfit 10.2 (Molecular Devices).
For electrophysiology analysis, the frequency, amplitude, and decay of IPSCs were analyzed and visually confirmed using semiautomated threshold-based mini detection software (Mini Analysis; Synaptosoft). We determined averages of IPSC characteristics from baseline and experimental drug conditions containing a minimum of 60 events (time period of analysis varied as a product of individual event frequency) and determined decay kinetics using exponential curve fittings and reported as decay time (ms). All detected events were used for event frequency analysis, but superimposed events were eliminated for amplitude and decay kinetic analysis. Action potential characteristics were evaluated using threshold-based event detection analysis in Clampfit 10.2 (Molecular Devices). Events were analyzed for independent significance using a one-sample t test and compared using a two-tailed t test for independent samples, a paired two-tailed t test for comparisons made within the same recording, and a one-way ANOVA with a Bonferroni post hoc analysis for comparisons made between three or more groups. All statistical analysis was performed using Prism 5.02 (GraphPad). Data are presented as mean ± SE. In all cases, P <0.05 was the criterion for statistical significance.
Acknowledgments
This work was supported by NIH Grants R01 AA020404 (to L.H.P.), P60 AA006420 (to L.H.P. and M.R.), R01 AA013498 (to M.R.), R01 DA009789 (to B.F.C.), R01 DA033760 (to B.F.C.), R01 MH084512 (to B.F.C.), K99 DA035864 (to K.-L.H.), F32 AA020430 (to M.A.H.), F32 DA029994 (to M.W.B.), and K99 DA035865 (to M.W.B.). This is manuscript no. 28034 from The Scripps Research Institute.
Footnotes
Conflict of interest statement: B.F.C. is a cofounder and advisor for a biotechnology company interested in developing inhibitors of serine hydrolases as therapeutic targets.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522672113/-/DCSupplemental.
References
- 1.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: A dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16(8):2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jentsch JD, Pennington ZT. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76(Pt B):479–486. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melis M, Pistis M. Hub and switches: Endocannabinoid signalling in midbrain dopamine neurons. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3276–3285. doi: 10.1098/rstb.2011.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura T, et al. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 7.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 8.Goodfellow CE, Glass M. Anandamide receptor signal transduction. Vitam Horm. 2009;81:79–110. doi: 10.1016/S0083-6729(09)81004-2. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45(5):405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Long JZ, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA. 2009;106(48):20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrs WR, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13(8):951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 14.Buczynski MW, Polis IY, Parsons LH. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology. 2013;38(4):574–584. doi: 10.1038/npp.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry. 2012;71(3):184–191. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisogno T, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30(6):2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanimura A, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65(3):320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Long JZ, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5(1):37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu KL, et al. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol. 2012;8(12):999–1007. doi: 10.1038/nchembio.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu KL, et al. Discovery and optimization of piperidyl-1,2,3-triazole ureas as potent, selective, and in vivo-active inhibitors of alpha/beta-hydrolase domain containing 6 (ABHD6) J Med Chem. 2013;56(21):8270–8279. doi: 10.1021/jm400899c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu KL, et al. Development and optimization of piperidyl-1,2,3-triazole ureas as selective chemical probes of endocannabinoid biosynthesis. J Med Chem. 2013;56(21):8257–8269. doi: 10.1021/jm400898x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vihavainen T, et al. Chronic nicotine modifies the effects of morphine on extracellular striatal dopamine and ventral tegmental GABA. J Neurochem. 2008;107(3):844–854. doi: 10.1111/j.1471-4159.2008.05676.x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DH, Blomqvist O, Engel JA, Söderpalm B. Subchronic intermittent nicotine treatment enhances ethanol-induced locomotor stimulation and dopamine turnover in mice. Behav Pharmacol. 1995;6(2):203–207. [PubMed] [Google Scholar]
- 25.Le Foll B, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: Insights from pre-clinical and clinical studies. Addict Biol. 2008;13(2):239–252. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoover HS, Blankman JL, Niessen S, Cravatt BF. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg Med Chem Lett. 2008;18(22):5838–5841. doi: 10.1016/j.bmcl.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan B, et al. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31(38):13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrié P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13(5-6):451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Eich HT, et al. Biophysical analysis of the acute toxicity of radiotherapy in Hodgkin’s lymphoma—A comparison between extended field and involved field radiotherapy based on the data of the German Hodgkin Study Group. Int J Radiat Oncol Biol Phys. 2005;63(3):860–865. doi: 10.1016/j.ijrobp.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 31.Cheer JF, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27(4):791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;54(2):438–444. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration—Comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205(4):613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- 34.Simonnet A, Cador M, Caille S. Nicotine reinforcement is reduced by cannabinoid CB1 receptor blockade in the ventral tegmental area. Addict Biol. 2013;18(6):930–936. doi: 10.1111/j.1369-1600.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 35.Gao M, et al. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30(41):13814–13825. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 37.Mao D, McGehee DS. Nicotine and behavioral sensitization. J Mol Neurosci. 2010;40(1-2):154–163. doi: 10.1007/s12031-009-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology. 2011;61(7):1070–1087. doi: 10.1016/j.neuropharm.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437(7061):1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parsons LH, Weiss F, Koob GF. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18(23):10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natividad LA, Buczynski MW, Parsons LH, Torres OV, O’Dell LE. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. J Neurochem. 2012;123(4):578–588. doi: 10.1111/j.1471-4159.2012.07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song P, Mabrouk OS, Hershey ND, Kennedy RT. In vivo neurochemical monitoring using benzoyl chloride derivatization and liquid chromatography-mass spectrometry. Anal Chem. 2012;84(1):412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachovchin DA, et al. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc Natl Acad Sci USA. 2010;107(49):20941–20946. doi: 10.1073/pnas.1011663107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nimitvilai S, et al. Dopamine D2 receptor desensitization by dopamine or corticotropin releasing factor in ventral tegmental area neurons is associated with increased glutamate release. Neuropharmacology. 2014;82:28–40. doi: 10.1016/j.neuropharm.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 2013;33(8):3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang JW, et al. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19(5):579–588. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Direct visualization of serine hydrolase activities in complex proteomes using fluorescent active site-directed probes. Proteomics. 2001;1(9):1067–1071. doi: 10.1002/1615-9861(200109)1:9<1067::AID-PROT1067>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 48.Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP. Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology. 2014;39(7):1722–1731. doi: 10.1038/npp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malin DH, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43(3):779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- 50.Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry. 2013;4:38. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baggelaar MP, et al. Development of an activity-based probe and in silico design reveal highly selective inhibitors for diacylglycerol lipase-α in brain. Angew Chem Int Ed Engl. 2013;52(46):12081–12085. doi: 10.1002/anie.201306295. [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic; San Diego: 1998. [DOI] [PubMed] [Google Scholar]