Significance
Human-caused extinctions are normally thought to result from overexploitation or habitat alteration. A third possible cause of extinction is the loss of interactions associated with keystone species. Using recent and historical information on sea otters and kelp forests, we show that the extinction of Steller's sea cow from the Commander Islands in the mid-1700s would have been a nearly inevitable consequence of the overhunting of sea otters, which occurred a decade earlier.
Keywords: Steller’s sea cow, extinction, sea otter, Commander Islands, kelp
Abstract
The late Pleistocene extinction of so many large-bodied vertebrates has been variously attributed to two general causes: rapid climate change and the effects of humans as they spread from the Old World to previously uninhabited continents and islands. Many large-bodied vertebrates, especially large apex predators, maintain their associated ecosystems through top-down forcing processes, especially trophic cascades, and megaherbivores also exert an array of strong indirect effects on their communities. Thus, a third possibility for at least some of the Pleistocene extinctions is that they occurred through habitat changes resulting from the loss of these other keystone species. Here we explore the plausibility of this mechanism, using information on sea otters, kelp forests, and the recent extinction of Steller's sea cows from the Commander Islands. Large numbers of sea cows occurred in the Commander Islands at the time of their discovery by Europeans in 1741. Although extinction of these last remaining sea cows during early years of the Pacific maritime fur trade is widely thought to be a consequence of direct human overkill, we show that it is also a probable consequence of the loss of sea otters and the co-occurring loss of kelp, even if not a single sea cow had been killed directly by humans. This example supports the hypothesis that the directly caused extinctions of a few large vertebrates in the late Pleistocene may have resulted in the coextinction of numerous other species.
Explanations for the sudden extinction of more than half of the New World’s megafauna at the Pleistocene/Holocene transition is a topic of long-standing interest and debate. Argument has focused on the relative importance of two would-be causes: rapid environmental change associated with late Pleistocene glacial recession, and the effect of early humans crossing Beringia and spreading into the New World. The absence of extinctions in the New World during earlier interglacial periods, together with discovery of similar large-animal losses after the peopling of various other continents and islands, but at widely differing times, has cast doubt on climate change as the extinctions’ principal cause (1, 2). This reasoning led to the now widely held belief that human impacts figured prominently in megafaunal extinctions worldwide (3, 4).
The intriguing question is just how aboriginal peoples did this. Was the sudden disappearance of so many large animals entirely a consequence of hunting and overexploitation (5, 6), a pandemic from diseases introduced by early humans or domesticated animals they may have brought with them (7), human-induced environmental changes (e.g., as might have accompanied deforestation or burning) (8), or some combination of these processes?
Another possibility, less discussed in the literature on megafaunal extinctions, is that reductions and complete extinctions of directly hunted species may have led to the loss of other species through cascading indirect interactions. Most speculation about such effects has focused on the loss or decline of predators or scavengers that relied on human-depleted prey [e.g., California condors (9) and Haast’s eagle (10)]. Although that is one possible pathway to coextinction, there are others. Ecosystems are organized around complex interaction webs in which certain species, variously referred to as keystones (11, 12), foundation species (13), and ecosystem engineers (14), can have disproportionately strong influences on population and ecosystem dynamics. Included among these strong interactors are many large-bodied vertebrates (15). Given the abilities of such species to shape the ecosystems in which they occur, it is possible that loss of one or more species by direct human exploitation led to ecosystem changes that in turn caused the demise of multiple other species. Although there has been conjecture that such losses could have significantly contributed to the Pleistocene extinctions (16, 17), detailed understanding of the ecological roles of now-extinct species makes direct examination of this idea difficult. Nonetheless, reliance of some species on the direct or indirect effects of others in complex natural communities mean that a loss of one species may have led to losses of others (18, 19).
Here we explore an example (that of Steller’s sea cow, Hydrodamalis gigas) that can help inform this hypothesis for Pleistocene extinctions. This particular case is intriguing and potentially informative because various aspects of the rise and fall of sea cows are reasonably well known; the final step in the sea cows’ demise occurred just several hundred years ago, in the presence of modern human observers; and dynamics of the coastal kelp forest interaction web, to which the sea cow was intimately linked, have been extensively studied and are well understood. Although aboriginal people (and early Russian fur traders in the Commander Islands) exploited sea cows and may have reduced or even exterminated local populations through that process, humans also hunted and reduced sea otter populations to such low levels that the otter’s keystone role in maintaining the kelp forest ecosystem (20) was lost (21–23). Sea cows lived in kelp forests and fed on kelp, thus raising the question of the relative importance in driving the sea cow’s demise of the direct effects of human exploitation vs. the indirect effects of reduced food or altered habitat caused by the ecological extinction of sea otters.
In this article, we use historical records of sea otter harvest from the early maritime fur trade, the functional relationship between sea otter density and kelp abundance, and data on the demographic and behavioral responses to food shortages of dugongs (the sea cow’s closest living relative) to argue that the sea cow’s extinction in the Commander Islands would have almost inevitably occurred without a single direct human take of the species. We discuss the broader ramifications of this finding to the extinction of sea cows elsewhere in the North Pacific and to extinctions of other large vertebrates after the peopling of the world.
Results and Discussion
Steller’s Sea Cow.
Hydrodamaline sirenians, which included Steller’s sea cow as the last surviving species, radiated from the tropics to coastal waters of the North Pacific Ocean with the onset of late Cenozoic polar cooling (24). On the basis of their cranial morphology (24) and Steller’s direct observations (25), sea cows are thought to have been obligate algivores, feeding largely on the diverse and highly productive kelps (order Laminariales) that appear also to have originated or at least diversified in the North Pacific with late Cenozoic polar cooling (26). Steller’s sea cow ranged across the Pacific Rim from the northern Japanese archipelago to the Pacific coast of Baja California, Mexico (24), a distribution that was roughly coincident with those of both sea otters and kelps. Sea cows, however, disappeared from most of this region sometime between the Pleistocene and the arrival of the first European explorers to the North Pacific region in the 18th century. The Commander Islands, where an abundance of sea cows survived in 1741 when the Bering Expedition shipwrecked and overwintered there (25), is a notable exception to the sea cow’s earlier extinction across the remainder of its range.
Aboriginal humans colonized the Aleutian archipelago by dispersing westward from the New World and inhabiting the more westerly islands at increasingly later times (27). Numerous midden sites attest to a sustained presence of these early peoples on nearly every island of sufficient size. Because of their great distance (>400 km) from Attu Island, westernmost of the Aleutians, the Commander Islands may never have been discovered by New World aboriginals. While artifacts of aboriginal people have been found in association with the Bering expedition's camp site (28), the absence of both living human populations at the time of Bering’s shipwreck in 1741 and the lack of midden sites in the Commander Islands suggest these islands were neither permanently occupied nor regularly visited.
The fact that sea cows disappeared from those segments of their historical range that were peopled with aboriginals but survived in abundance at the one location that was not (the Commander Islands) argues for human impacts and against climate change as the principal cause of the sea cow’s contraction to a tiny fraction of its former range. With ensuing growth of the North Pacific maritime fur trade, the last sea cow had disappeared from the Commander Islands by 1768 (29).
Exactly how did humans exterminate these last sea cows? The simplest and most straightforward explanation is overkill: rampant exploitation of a behaviorally naive prey by a novel and highly effective human predator. Although sea cow flesh was reportedly delicious (25), and sea cows seemingly would have been vulnerable to human hunters, Anderson (30) proposed that the sea cow’s decline and extinction was either caused or helped along by the ecological extinction of sea otters and the resulting collapse of the sea otter–sea urchin–kelp trophic cascade (20). Supporting this possibility is the commonness of starvation in driving periodic rapid declines of many large mammal populations (31) and Steller’s observations that over the winter months of low kelp growth, sea cows frequently appeared emaciated (29).
Because it occurred so recently, the extinction of sea cows in their final stronghold, the Commander Islands, provides a possible “Rosetta Stone” (32) for the broader question of how likely extinctions of past megafauna as a result of indirect community effects really is. Turvey and Risely (33) concluded from a population viability analysis that the extinction of Steller’s sea cows in the Commander Islands was an inevitable consequence of human exploitation; the pre-exploitation size of this remnant population was larger than Stejneger’s (29) often-cited estimate of 1,500 individuals, and contrary to proposals by Anderson (30) and others, “environmental changes caused by sea otter declines are unlikely to have contributed to this extinction event.” We agree that direct human hunting of sea cows occurred and may well have figured prominently in the sea cow’s extinction. However, it is not possible to establish a link between sea cow mortality and human hunting. The mere presence of overwintering fur hunters in the Commanders does not demonstrate that these people were substantially exploiting sea cows [although they surely did some of this (29)], much less the number of sea cows they may have killed and eaten. Direct human effects are thus likely, but not inevitable. Conversely, Anderson’s (30) proposal is backed up by compelling evidence: kelp forest collapse after the loss of sea otters is a virtual certainty. The timing of sea otter exploitation and the sea cow’s loss on the Commander Islands has led us to believe that the sea cow’s extinction from these islands would have occurred as an indirect effect of removing sea otters, even if no direct hunting of sea cows had occurred or it was of minimal importance. In the following sections, we provide data, analyses, and arguments to support our hypothesis.
Kelp and Sea Cows.
Kelps and other fleshy macroalgae (hereafter collectively referred to as “kelps”) provide the majority of production to coastal marine ecosystems in the North Pacific Ocean (34). Various and sundry evidence suggests that kelp was also the sea cow’s primary food source. First, all extant sirenians are obligate herbivores, and extinct species probably were as well (24, 35–37). Second, macroalgae are the only autotrophs that would have been accessible to sea cows in sufficient amounts to support viable populations. Sea cows could not possibly have subsisted on terrestrial plants or phytoplankton. Seagrasses (Phyllospadix and Zostera), the only remaining group of autotrophs that might have provided food for sea cows, do occur in some areas of the North Pacific but are rare or absent throughout much of the sea cow’s northernmost range, including the Aleutian and Commander islands. Third, loss of dentition and development of a keratinous palatine plate suggest a transition in diet from siliceous sea grasses by primitive tropical dugongids to nonsiliceous macroalgae by hydrodamalines as they radiated into the North Pacific (24). Finally, while shipwrecked on Bering Island during winter and spring of 1741–1742, Steller (25) observed sea cows feeding on kelp.
Sea Otters and Kelp in the Western Aleutian Islands.
A trophic cascade in which predation by sea otters limits herbivorous sea urchins is essential for the maintenance of kelp forests across much of the North Pacific Ocean’s rocky reefs at higher latitudes (20, 21, 38, 39). Kelp density and standing biomass (a reasonable surrogate for sea cow carrying capacity) is >10-fold lower at islands where sea otter populations have been reduced or lost compared with otherwise similar islands where sea otters abound (21, 40). Moreover, transitions between kelp-dominated and deforested ecosystem states with varying sea otter densities occur rapidly, as sharply punctuated phase shifts (41, 42).
The equilibrium population density for sea otters in the central and western Aleutian archipelago is about 12–15 individuals⋅km−1 of shoreline, and the breakpoint density of sea otters for the transition from a kelp-dominated to deforested state is about 6 individuals⋅km−1 of shoreline (43). Hence, a reduction in sea otter abundance below this ecologically effective population size (sensu ref. 44) would have rapidly and profoundly reduced the environmental carrying capacity for sea cows (K). The important question is whether or not sea otter population reductions in the Commander Islands occurred in such a manner as to have influenced kelp abundance during times that would have mattered to the sea cows. We suspect they did, and in the following section, we explain why.
Sea Otters and Kelp Forests in the Commander Islands.
Sea otters and sea cows abounded in the Commander Islands when these formerly uninhabited islands were discovered by the Bering expedition in 1741. We have no direct knowledge of kelp abundance or the status of the reef systems surrounding the islands at that time. However, we do know that shallow reef systems at Bering Island were extensively deforested in the mid-1970s, before sea otters recovered from the fur trade in the Commander Islands; that these systems have since become kelp-dominated with the sea otter’s recovery to carrying capacity; and that reef species composition is similar from the Commander through the central Aleutian Islands. From this knowledge and the broad occurrence of a sea otter–urchin–kelp trophic cascade elsewhere in the North Pacific Ocean (21, 38, 39), we surmise that reef habitats in the Commander Islands were kelp-dominated in 1741.
Sea otter population declines after the onset of the Pacific maritime fur trade, which began in earnest in 1743, were rapid and precipitous. Only 25 of the 8,226 sea otters reportedly harvested from the Commander Islands were taken after 1753 (45, 46). Direct information on any associated changes in sea urchin and kelp abundance are, of course, lacking. However, the rate and magnitude of this decline in sea otter abundance in the Commander Islands are roughly similar to those purportedly caused by killer whale predation (47) in the nearby western Aleutian Islands during the 1990s (48, 49), for which records of change in sea urchin and kelp abundance do exist (47) (Fig. 1). These data provide a surrogate for the approximate rate, magnitude, and timing of kelp forest decline that must have followed the loss of sea otters from the Commander Islands in the 1740s and 1750s.
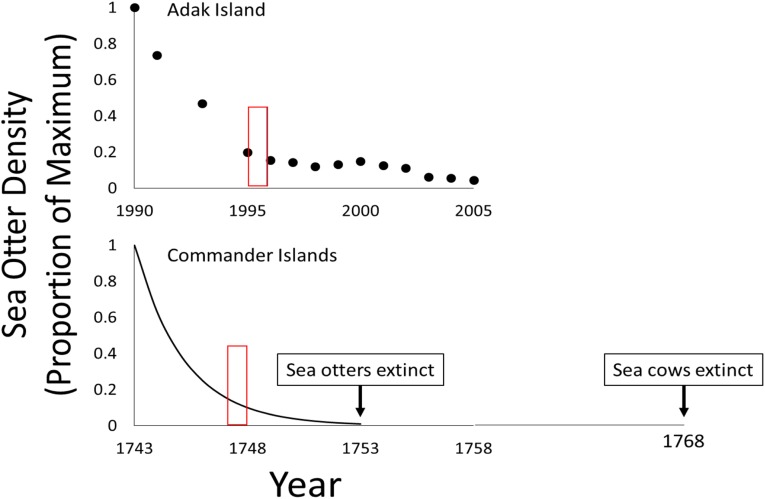
Fig. 1.
Trajectories of sea otter population declines in the Aleutian Islands during the 1990s and early 2000s (Upper) and the Commander Islands after the onset of the Pacific maritime fur trade in 1743 (Lower). (Upper) Data points are from skiff surveys of Adak Island. (Lower) Line assumes that sea otters were at maximum density in 1743 and extinct by 1753, and that the decline was exponential. Open red boxes indicate time window of kelp forest phase shift at Adak Island and the corresponding estimated time of kelp forest phase shift in the Commander Islands.
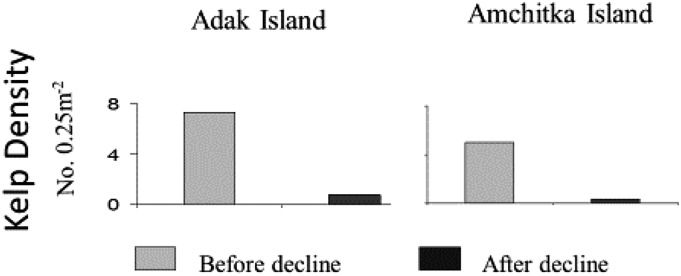
Sea otter populations at Adak and Amchitka islands, which existed at or near carrying capacity at the decline’s onset in the late 1980s or early 1990s, had fallen about 90% by the late 1990s, and about 95% by the early to mid-2000s (Fig. 1A). In response to the sea otter decline, sea urchin density had increased by about 50% by 1994 (50), but with no measurable change in the associated kelp assemblage. By 1997, urchin densities had increased by another 50%, maximum sea urchin test diameter had increased by about 15%, and kelp density had declined 10-fold (47). By 1999, the shallow reef communities at Amchitka and Adak islands were indistinguishable from those that had lacked sea otters for decades (47) (Fig. 2). The phase shift from a kelp- to urchin-dominated state thus occurred some 5–8 y after the onset of the sea otter decline (Fig. 1, Upper).
Fig. 2.
Declines in kelp density after sea otter population collapse at Adak and Amchitka Islands. Predecline data were obtained in 1987. Postdecline data from Adak and Amchitka were obtained in 1997 and 1999, respectively. Error bars (SEs) are too small to show on the graphs.
Although the exact timing and even the existence of a kelp forest collapse in the Commander Islands can only be surmised, the phase shift probably occurred soon after the onset of the fur trade in 1743. Sea otters in the Commander Islands had been hunted to virtual extinction by 1753. Although the precise timing of the associated kelp forest to urchin barrens phase shift depends on the exact trajectory of decline in sea otter density, those details are of little consequence to our argument. Sea otters were ecologically extinct by 1753, and the kelp forest collapse therefore preceded that date if our data from the western Aleutians are a reasonable proxy for what happened 250 y earlier in the Commander Islands. If the time course of the sea otter decline in the Commander islands was roughly exponential, then the kelp forest collapse probably occurred around 1750, just 7 y after the onset of the fur trade and 16 y before the last record of a living sea cow (Fig. 1, Lower).
Modeling the Sea Cow’s Response.
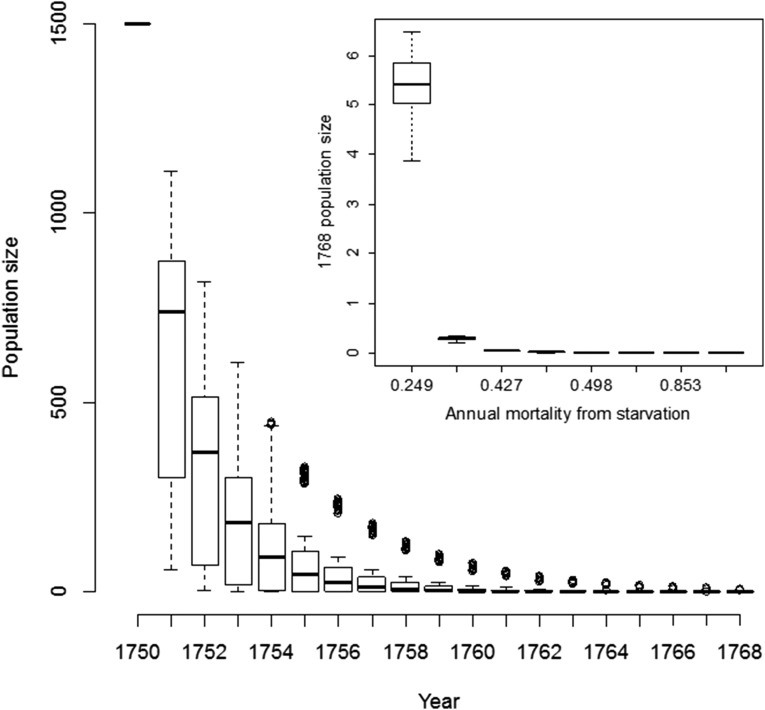
Even though we are reasonably sure that the kelp forest collapse preceded the sea cow’s final extinction in the Commander Islands by at least a decade, and probably closer to 2 decades, reconstructing the sea cow’s response to the greatly reduced food availability that would have followed the sea otter’s loss is more difficult. We have done this by applying the demographic and behavioral responses of dugongs (Dugong dugong), the sea cow’s closest living relative, to catastrophic food loss (51), using simple age-structured population models and general sirenian demographic rates (33, 52). In making projections, we accounted for uncertainty in both life history and rates of starvation-induced mortality. Starvation-induced increases in mortality and cessation in reproduction are predicted to have resulted in rapid declines in a sea cow population (Fig. 3). Starting with 1,500 sea cows at stable stage distribution in 1750 and averaging across all life history and starvation-caused mortality rates, the models predict a mean of one surviving animal by 1768, the year of the recorded extinction; the median number of survivors is zero, and the maximum is six.
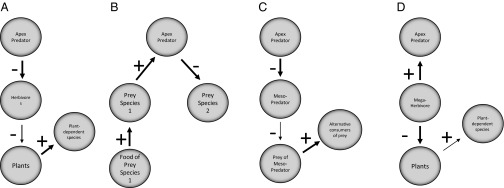
Fig. 3.
Some known food web pathways by which changes in abundance of predators and other large vertebrates influence the abundance of other species through indirect interactions involving top-down forcing. (A) Classic trophic cascades. (B) Apparent competition. (C) Mesopredator release. (D) Trophic cascade beginning with megaherbivore and with megaherbivores sustaining predators via scavenging. Arrow line weights correspond with interaction strengths. Extinctions might occur as a result of increased interaction strength at any of the negative linkages. See text for examples.
We also ran simulations to ask what annual starvation mortality rate would be needed to predict a median of one survivor in 1768; this would be a mortality of 31%, which is considerably lower than any of the direct estimates for mortality in the face of starvation we made from dugong data (50). Furthermore, these effects do not include sea cow emigration. As Preen and Marsh (51) show, dugongs readily move when faced with starvation, and this emigration can account for the majority of immediate local dugong declines. Oral tradition of Aleuts on Attu island (425 km east of Bering Island) states that sea cows were present and being hunted after their extinction on Bering Island (archived notes by L.M. Turner, recounted in ref. 53), thus supporting the possibility that some movement of animals away from Bering Island, their supposed last population, may have occurred. Sea cow bone fragments from the western Aleutian Islands and dated to the past several millennia also may be from Commander Island’s emigrants (53).
Conclusions
Using our knowledge of sea otter–kelp forest interactions in the nearby western Aleutians and data on the demographic and behavioral responses of dugongs to food reduction (51), we show that sea cows around Bering Island would have reached near or complete extinction by or very close to 1768, the year of the last reported sighting of a living sea cow (29). Human hunting clearly occurred and may have been largely responsible for the sea cow decline at Bering Island. However, our analyses suggest that the sea cow’s extinction from this last stronghold also was a nearly inevitable consequence of the loss of sea otters and kelp forests and would have occurred without any loss of sea cows to human hunting.
Whether similar ecological processes led to the extinction of Steller’s sea cows elsewhere in the North Pacific is more speculative. Aboriginal peoples inhabited all such areas for long periods of time, and the exact timing of the sea cow’s disappearance from these areas is unknown. There is evidence that aboriginal humans exploited sea otters, thus driving coastal reefs to the urchin-dominated phase state (23), and few sea cow bones have been recovered from midden sites anywhere (53). However, sea otters were sufficiently abundant throughout this range to support a lucrative fur trade, thus implying that kelp forests were abundant as well. All we can infer with reasonable certainty is that aboriginal peoples and sea cows did not coexist when these peoples were first encountered by western civilization.
Other megafauna, especially large predators and megaherbivores, have comparably important ecological influences to those of sea otters on their associated ecosystems (15, 54, 55). As some of these keystone species were depleted or lost to early peoples, the loss of their community effects may have dragged other species down as well, just as we have proposed for the coextinction of sea otters and sea cows. If some of these other species were also keystones, the process of megafaunal extinction might have progressed rapidly as an ecological chain reaction, thus obviating the need to attribute all or even most of these massive losses directly to humans or a changing climate. The possibility is at least worth further consideration.
Whereas the mechanisms of prehistoric extinctions are difficult to disentangle from paleontological and archaeological records, coextinctions associated with the loss of large vertebrates are known or suspected in modern ecological studies, and can be generated from a variety of ecological effects (Fig. 4). For example, local extinctions of birds and other small vertebrates followed the loss of coyotes from chaparral habitat fragments in southern California (56), a result of increased mortality from mesopredators that grew into coyote-free environments (Fig. 4C). The small vertebrate losses that accompanied mesopredator release in turn may have driven declines of other predators that depended on these small vertebrates for food. Ripple et al. (57) hypothesized one such chain of events, with extirpation of wolves in the northern United States causing increased coyote numbers, thus purportedly forcing snowshoe hare populations downward and forcing the hare’s obligate predator, lynx, to extinction in local areas as well. Elk and moose population increases in the Canadian Rockies (caused in some cases by forest management practices) have elevated wolf densities, in turn driving caribou numbers downward (58) (Fig. 4B). The loss of wolves, pumas, and grizzly bears caused reductions or losses of various plant, invertebrate, and small vertebrate species from certain riparian habitats in the North American intermountain west (59, 60), a direct consequence of overgrazing by the elevated numbers of large ungulates (in the case of plants) or the knock-on effects of plant reductions (in the case of the various animal species). Other indirect interaction web pathways of obligate relationships between large predators and co-occurring species have been proposed, and still others that may well exist in nature have not yet even been imagined. As our analyses demonstrate, these chains of interactions can be powerful enough to cause significant changes in the abundance of other species, including extinctions. Although similarly detailed knowledge of community interactions are lacking for Pleistocene communities and their many now-extinct species, consideration of such chains of effects should be given when judging the likelihood of overkill as a mechanism that could have generated the wide-ranging extinctions seen wherever human hunters entered novel habitats as they spread around the globe.
Fig. 4.
Modeled sea cow population trajectories in response to kelp forest phase shift after the ecological extinction of sea otters in the Commander Islands. Main figure shows boxplots for the number of living sea cows each year after the onset of starvation, including variation across both estimated starvation-caused mortality rates and life history parameters. (Inset) Boxplot for the number of living animals predicted to remain in 1768, the year of the historically recorded extinction, segregated by the assumed starvation-caused annual mortality rate. Only simulations using the lowest of the eight mortality rates predict more than one survivor by 1768, and even this most optimistic scenario always predicts fewer than seven survivors by that year. Note that these predictions do not account for emigration, which would likely reduce local numbers far below those caused just by elevated mortality.
Materials and Methods
Much of this article is based on a reanalysis and synthesis of published data from our studies of sea otters and kelp forests in the western Aleutian Islands. We have documented the influences of sea otters on coastal ecosystems by measuring changes that occurred with the recovery of local populations from the Pacific maritime fur trade (21), and then with the more recent collapse of recovered sea otter populations because of killer whale predation (47).
Sea otter populations were surveyed by counting animals from a skiff run parallel to shore. Methodological details are reported by Doroff et al. (48) and Estes et al. (43). Reported trends in sea otter abundance are based on these survey data, except for the mid-1700s population trend at Bering Island, which is taken from ref. 45.
Sea urchin and kelp abundance was measured in 0.25-m2 plots, randomly placed on the seafloor at randomly selected locations along the perimeter of islands with concurrently measured sea otter densities. Methodological details are reported by Estes and Duggins (21) and Watson and Estes (39).
Existence of distinct phase states (kelp forests vs. deforested sea urchin barrens) in the Aleutian archipelago was inferred from cluster and discriminate function analyses of the joint sea urchin/kelp abundance measurements at islands and times where sea otter densities ranged from zero to about 16 animals⋅km−1 shoreline (43). The functional relationship between sea otter density and reef phase state was determined by logistic regression (see ref. 43 for details).
To project sea cow population responses to kelp reductions that followed the loss of sea otters, we first used the demographic values applied to sea cows by Turvey and Risley (33) and second, we used values for a general sirenian life cycle proposed by Heinsohn et al. (52) in their dugong population viability analysis. We also created 100 random versions of the Turvey and Risley life history, following them in drawing life history values from normal distributions. We also generated all 36 alternative sets of vital rates for the Heinsohn et al. model, based on their maximum and minimum values for the three vital rates (other than interbirth interval) they varied. For each model, we then adjusted interbirth interval to yield a stable population and used the stable age distribution of the resulting model, applied to a total population of 1,500 animals, to initialize simulations of starvation effects. We assume sex-independent survival.
To simulate the effects on sea cow numbers of the loss of kelp forests after sea otter harvest, we require some estimate of starvation-caused mortality. Although we have no data on sea cows and their reactions to rapid declines in their food (kelp), we use documented effects of rapid food (seagrass) losses on dugongs (51), the sea cow’s closest living relative. After catastrophic loss of a formerly abundant food source, Preen and Marsh (51) document three responses: movement out of the affected area, near-cessation of reproduction, and dramatically increased mortality. To estimate survival over this starvation event, we used the estimated starting numbers in the most intensively influenced area (1,753 animals) and in a wider area (2,206 animals), in combination with estimates of dugong numbers both immediately (71 and 1,106) and more than a year after the event (257 and 604, respectively, for the smaller and larger areas). The resulting survival rates are 0.041, 0.147, 0.501, and 0.274. The last two estimates of survivor numbers are likely to include most survivors, who moved back to the affected area (51), but we also used another set of estimates, assuming that a substantial number of survivors did not return, and thus those mortality rates were only half the base estimates (survival equal to 0.520, 0.573, 0.751, and 0.637). We multiplied these eight probabilities of surviving starvation with the natural age-dependent survival rates in each of the 236 alternate demographic descriptions and also stopped all reproduction (as reported by ref. 51 for dugongs and conforming to reactions of most large mammals to severe food shortage) to estimate the rate at which a sea cow population would decline in the face of severe food shortages. We assumed that increased mortality began in 1750 and continued unchanged into the future. Although continuing lack of adequate food would likely result in rapidly increasing mortality, we conservatively kept the risk of starvation-caused death constant each year.
Acknowledgments
We thank Michael Graham, Douglas Rasher, Robert Steneck, and two anonymous referees for discussion and comments on earlier versions of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.M. is a guest editor invited by the Editorial Board.
References
- 1.Flannery TF. Debating extinction. Science. 1999;283:182–183. [Google Scholar]
- 2.Burney DA, Flannery TF. Fifty millennia of catastrophic extinctions after human contact. Trends Ecol Evol. 2005;20(7):395–401. doi: 10.1016/j.tree.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306(5693):70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 4.Koch PL, Barnosky D. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 5.Martin PS. Prehistoric overkill. In: Martin PS, Wright HE, editors. Pleistocene Extinctions: The Search for a Cause. Yale Univ. Press; New Haven, CT: 1967. [Google Scholar]
- 6.Zuo W, Smith FA, Charnov EL. A life-history approach to the late Pleistocene megafaunal extinction. Am Nat. 2013;182(4):524–531. doi: 10.1086/671995. [DOI] [PubMed] [Google Scholar]
- 7.MacFee RDE, Marx PA. The 40,000-year plunge. In: Goodman S, Patterson BD, editors. Humans, Hyperdisease and First-Contact Extinctions. Natural Change and Human Impact in Madagascar. Smithsonian Press; Washington, DC: 1997. pp. 169–217. [Google Scholar]
- 8.Flannery TF. The Future Eaters: An Ecological History of the Australasian Lands and People. Grove/Atlantic, Inc.; New York: 2002. [Google Scholar]
- 9.Emslie SD. Age and diet of fossil California condors in grand canyon, Arizona. Science. 1987;237(4816):768–770. doi: 10.1126/science.237.4816.768. [DOI] [PubMed] [Google Scholar]
- 10.Worthy TH, Holdaway RN. The Lost World of the Moa: Prehistoric Life of New Zealand. Indiana Univ. Press; Bloomington, IN: 2002. [Google Scholar]
- 11.Paine RT. A note on trophic complexity and community stability. Am Nat. 1969;103(929):91–93. [Google Scholar]
- 12.Power ME, et al. Challenges in the quest for keystones. Bioscience. 1996;46:609–620. [Google Scholar]
- 13.Dayton PK. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In: Parker BC, editor. Proceedings of the Colloquium on Conservation Problems in Antarctica. Allen Press; Lawrence, KS: 1972. pp. 81–95. [Google Scholar]
- 14.Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. [Google Scholar]
- 15.Terborgh J, Estes JA. In: Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature. Terborgh J, Estes JA, editors. Island Press; Washington, DC: 2010. [Google Scholar]
- 16.Alroy J. A multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction. Science. 2001;292(5523):1893–1896. doi: 10.1126/science.1059342. [DOI] [PubMed] [Google Scholar]
- 17.Zimov S, et al. Steppe-Tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am Nat. 1995;146(5):765–794. [Google Scholar]
- 18.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 19.Koh LP, et al. Species coextinctions and the biodiversity crisis. Science. 2004;305(5690):1632–1634. doi: 10.1126/science.1101101. [DOI] [PubMed] [Google Scholar]
- 20.Estes JA, Palmisano JF. Sea otters: Their role in structuring nearshore communities. Science. 1974;185(4156):1058–1060. doi: 10.1126/science.185.4156.1058. [DOI] [PubMed] [Google Scholar]
- 21.Estes JA, Duggins DO. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol Monogr. 1995;65:75–100. [Google Scholar]
- 22.Kenyon KW. Sea otters in the eastern North Pacific Ocean. North Am Fauna. 1969;68:1–352. [Google Scholar]
- 23.Simenstad CA, Estes JA, Kenyon KW. Aleuts, sea otters, and alternate stable-state communities. Science. 1978;200(4340):403–411. doi: 10.1126/science.200.4340.403. [DOI] [PubMed] [Google Scholar]
- 24.Domning DP. Sirenian evolution in the North Pacific Ocean. Univ Calif Publ Geol Sci. 1978;118:176. [Google Scholar]
- 25.Steller GW. De Bestiis Marinus. Novi Comm Acad Sci Petropolitanae. 1751;2:289–398. [Google Scholar]
- 26.Estes JA, Steinberg PD. Predation, herbivory, and kelp evolution. Paleobiology. 1988;14:19–36. [Google Scholar]
- 27.Balter M. Archaeology. The peopling of the Aleutians. Science. 2012;335(6065):158–161. doi: 10.1126/science.335.6065.158. [DOI] [PubMed] [Google Scholar]
- 28.Stanyukovich AK, Chernosvttov PYu, Stanyukovich YL. Investigation of the earliest settlement with stone tools on the Commander Islands. Arctic Anthropology. 1994;31:45–56. [Google Scholar]
- 29.Stejneger L. How the great northern sea cow (Rytina) became exterminated. Am Nat. 1887;21:1047–1054. [Google Scholar]
- 30.Anderson P. Competition, predation, and the evolution and extinction of Steller’s sea cow, Hydrodamalis gigas. Mar Mamm Sci. 1995;11:391–394. [Google Scholar]
- 31.Young TP. Natural die offs of large mammals: Implications for conservation. Conserv Biol. 1994;8(2):410–418. [Google Scholar]
- 32.Diamond JM. Historic extinctions: A Rosetta Stone for understanding prehistoric extinctions. In: Martin PS, Klein RG, editors. Quaternary Extinctions. Univ. of Arizona Press; Tucson: 1984. pp. 824–862. [Google Scholar]
- 33.Turvey ST, Risley CL. Modelling the extinction of Steller’s sea cow. Biol Lett. 2006;2(1):94–97. doi: 10.1098/rsbl.2005.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duggins DO, Simenstad CA, Estes JA. Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science. 1989;245(4914):170–173. doi: 10.1126/science.245.4914.170. [DOI] [PubMed] [Google Scholar]
- 35.Clementz MT, Koch PL. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia. 2001;129:461–472. doi: 10.1007/s004420100745. [DOI] [PubMed] [Google Scholar]
- 36.Clementz MT, Hoppe KA, Koch PL. A paleoecological paradox: The habitat and dietary preferences of the extinct tethythere Desmostylus, inferred from stable isotope analysis. Paleobiology. 2003;29(4):506–519. [Google Scholar]
- 37.Clementz MT, Goswami A, Gingerich PD, Koch PL. Isotopic records from early whales and sea cows: Contrasting patterns of ecological transition. J Vertebr Paleontol. 2006;26:355–370. [Google Scholar]
- 38.Kvitek RG, Iampietro PJ, Bowlby CE. Sea otters and benthic prey communities: A direct test of the sea otter as keystone predator in Washington State. Mar Mamm Sci. 1998;14(4):895–902. [Google Scholar]
- 39.Watson J, Estes JA. Stability, resilience, and phase shifts in rocky subtidal communities along the west coast of Vancouver Island, Canada. Ecol Monogr. 2011;81:215–239. [Google Scholar]
- 40.Wilmers CC, Estes JA, Edwards M, Laidre KL, Konar B. Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Front Ecol Environ. 2012;10:409–415. [Google Scholar]
- 41.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29(4):436–459. [Google Scholar]
- 42.Konar B, Estes JA. The stability of boundary regions between kelp beds and deforested areas. Ecology. 2003;84(1):174–185. [Google Scholar]
- 43.Estes JA, Tinker MT, Bodkin JL. Using ecological function to develop recovery criteria for depleted species: Sea otters and kelp forests in the Aleutian archipelago. Conserv Biol. 2010;24(3):852–860. doi: 10.1111/j.1523-1739.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 44.Soulé ME, Estes JA, Berger J, Martinez Del Rio C. Ecological effectiveness: Conservation goals for interactive species. Conserv Biol. 2003;17(5):1238–1250. [Google Scholar]
- 45.Berkh VN. The Chronological History of Discovery of Aleutian Islands, or Feats of Russian Merchantmans. N. Grecha; St. Petersburg: 1823. [Google Scholar]
- 46.Geptner VG, Naumov NP. Sea Cows and Carnivores. Mammals of the Soviet Union. Vshaya Shkola; Moscow: 1967. [Google Scholar]
- 47.Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282(5388):473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 48.Doroff AM, Estes JA, Tinker MT, Burn DM, Evans TJ. Sea otter population declines in the Aleutian Archipelago. J Mammal. 2003;84:55–64. [Google Scholar]
- 49.Estes JA, Tinker M, Doroff AM, Burn DM. Continuing decline of sea otter populations in the Aleutian archipelago. Mar Mamm Sci. 2005;21:169–172. [Google Scholar]
- 50.Watt J, Siniff DB, Estes JA. Inter-decadal patterns of population and dietary change in sea otters at Amchitka Island, Alaska. Oecologia. 2000;124:289–298. doi: 10.1007/s004420000373. [DOI] [PubMed] [Google Scholar]
- 51.Preen A, Marsh H. Response of dugongs to large-scale loss of seagrass from Hervey Bay, Queensland Australia. Wildl Res. 1995;22:507–519. [Google Scholar]
- 52.Heinsohn R, et al. Unsustainable harvest of dugongs in Torres Strait and Cape York (Australia) waters: Two case studies using population viability analysis. Anim Conserv. 2004;7(4):417–425. [Google Scholar]
- 53.Domning DP, Thomason J, Corbett DG. Steller’s sea cow in the Aleutian Islands. Mar Mamm Sci. 2007;23(4):976–983. [Google Scholar]
- 54.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 55.Ripple WJ, et al. Status and ecological effects of the world’s largest carnivores. Science. 2014;343(6167):1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 56.Crooks K, Soulé M. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 1999;400:563–566. [Google Scholar]
- 57.Ripple WJ, Wirsing AJ, Beschta RL, Buskirk SW. Can restoring wolves aid in lynx recovery? Wildl Soc Bull. 2011;35:514–518. [Google Scholar]
- 58.Hebblewhite M, et al. Conditions for caribou persistence in the wolf-elk-caribou systems of the Canadian Rockies. Rangifer. 2007;17:79–91. [Google Scholar]
- 59.Berger J, Stacey PB, Bellis L, Johnson MP. A mammalian predator-prey imbalance: Grizzly bear and wolf extinction affect avian neotropical migrants. Ecol Appl. 2001;11:947–960. [Google Scholar]
- 60.Ripple WJ, Beschta RL. Linking a cougar decline, trophic cascade, and catastrophic regime shift in Zion National Park. Biol Conserv. 2006;133(4):397–408. [Google Scholar]