Significance
Brain endothelial cells, pericytes, and astrocytes participate in maintenance of the blood–brain barrier (BBB). Juxtavascular microglial cells are also an integral part of the neurovascular unit. We show here that, in response to capillary injury, microglial processes rapidly form a dense plexiform aggregate at the site of injury. Photoablation of microglial cells abolished closure of BBB leakage, whereas inhibition of P2RY12 (purinergic receptor P2Y, G-protein coupled, 12) receptors suppressed microglial process motility and prolonged BBB closure. Thus, microglial cells mediate rapid resealing of injury-induced leaks in BBB. These observations may have clinical importance as P2RY12 receptor antagonists are widely used as platelet inhibitors in patients with coronary artery and cerebrovascular disease at risk for stroke and its attendant disruption of the injured BBB.
Keywords: purinergic receptors, microglia, blood–brain barrier, stroke, clopidogrel
Abstract
Microglia are integral functional elements of the central nervous system, but the contribution of these cells to the structural integrity of the neurovascular unit has not hitherto been assessed. We show here that following blood–brain barrier (BBB) breakdown, P2RY12 (purinergic receptor P2Y, G-protein coupled, 12)-mediated chemotaxis of microglia processes is required for the rapid closure of the BBB. Mice treated with the P2RY12 inhibitor clopidogrel, as well as those in which P2RY12 was genetically ablated, exhibited significantly diminished movement of juxtavascular microglial processes and failed to close laser-induced openings of the BBB. Thus, microglial cells play a previously unrecognized protective role in the maintenance of BBB integrity following cerebrovascular damage. Because clopidogrel antagonizes the platelet P2Y12 receptor, it is widely prescribed for patients with coronary artery and cerebrovascular disease. As such, these observations suggest the need for caution in the postincident continuation of P2RY12-targeted platelet inhibition.
The resident immune cells of the central nervous system (CNS) play a variety of roles in both CNS development and homeostatic maintenance (1). During development, microglia engulf apoptotic immature neurons and prune synapses to eliminate redundant or inappropriate synaptic connections (2–5). In addition, microglia release a variety of both neurotrophic and gliotrophic factors (6), whereas antiinflammatory M2-stage microglia have been shown to accelerate oligodendrocytic differentiation during remyelination (7). In addition, a number of studies have highlighted microglial activation as a hallmark of neurodegenerative disease (8, 9), as well as in the tissue response to acute ischemic and traumatic brain injury (10, 11). However, despite this multiplicity of roles, microglia have not hitherto been viewed as significant contributors to maintenance of the blood–brain barrier (BBB), despite the fact that a large fraction of juxtavascular microglial cells are localized in the perivascular space (12).
Recent observations show that a coordinated microglial response to cortical compression injury can reinforce the glial limitans. In response to meningeal cell death, microglia extended processes through the compromised glial limitans into the meninges, forming a stable contiguous network resembling a “honeycomb” structure concurrently with their other retracted processes. The honeycomb network formed quickly, within an hour of injury. It was dependent upon purinergic signaling, because transcranial application of P2RY12 (purinergic receptor P2Y, G-protein coupled, 12) or P2RX4 (purinergic receptor P2X, ligand gated ion channel, 4) inhibitors before compression injury prevented formation of the honeycomb barrier (13). Microglial cells express high levels of P2RY12 (14, 15), which serve as chemotactic receptors, directing movement of microglial cell processes toward local sites of CNS injury (16–19). We here assessed whether microglial cells via activation of P2RY12 receptors contribute to closures of the small opening of the BBB. Microscopic opening of the BBB may occur during the normal replacement of brain endothelial cells as part of their life cycle or as a consequence of localized ischemic events.
The purinergic receptor P2RY12 is a clinical target in both cardiovascular and cerebrovascular disease in that inhibition of platelet P2RY12 prevents ADP-induced platelet aggregation and thereby reduces the risk of thrombosis (14, 20). Since its approval in 1997 by the Food and Drug Administration, clopidogrel has been prescribed to over 52 million patients worldwide (21). Clopidogrel is a thienopyridine prodrug, whose active metabolite acts as an irreversible inhibitor of P2RY12 (22). Systemic expression of P2RY12 is limited to platelets, so that clopidogrel exhibits few side effects in addition to the prolongation of bleeding time and hemorrhagic risk ascribable to its anti-platelet actions. Other organ systems are essentially devoid of P2RY12, with the exception of CNS microglial cells.
Although clopidogrel reduces the risk of stroke, a large number of treated patients nonetheless experience cerebral ischemic events; in one study, 9% of patients taking clopidogrel suffered an ischemic event on clopidogrel over a 2.4-year observation period (23). Because BBB compromise is a hallmark of stroke, clopidogrel and its active metabolite may thereby gain entry into the affected CNS, resulting in the local suppression of P2RY12-mediated microglial activation within ischemic tissue.
Results
Systemic Clopidogrel Suppresses Juxtavascular Microglial Cell Activation After BBB Breakdown.
The active metabolite of clopidogrel [molecular weight (MW) 353 Da] has low-BBB permeability (24). Clopidogrel would thus not be expected to interfere with the movement of microglia or their ramified processes under control conditions. To test this supposition, we evoked small focal lesions within the cerebral parenchyma using two-photon focused laser excitation. We observed a rapid chemotaxic response of nearby microglial cell processes in CX3CR1+/EGFP mice (Fig. 1 A and B and Movie S1). Earlier studies have shown that P2RY12 drives microglial cell process movement toward focal lesions (18). We confirmed that mice with deletion of P2RY12 (P2RY12−/−) exhibited significantly less process accumulation around focal lesions (Fig. 1 A and B and Movie S2). In contrast, pretreatment of P2RY12+/+ mice with 20 mg/kg clopidogrel for 3 d before the experiment did not suppress microglia process motility, suggesting that clopidogrel do not inhibit microglial P2RY12 in the normal mouse brain in the absence of vascular injury (Fig. 1 A and B and Movie S3). We next asked whether clopidogrel could inhibit microglial process motility in the setting of vascular injury. The focal laser injury was targeted to induce injury in single capillaries, located 80–150 µm below the pial surface. The capillary injury was calibrated to cause minimal, nonhemorrhagic damage, evaluated by the lack of an extravascular leakage of 70 kDa of Texas Red-dextran (Fig. 1C). Similarly to brain parenchyma, juxtavascular microglial processes in control mice were immediately attracted to the site of the capillary lesion; within 20–30 min, they formed a dense sheet of EGFP+ processes plastered around the vessel wall (Fig. 1 C and D and Movie S4), which was significantly reduced in CX3CR1/P2RY12−/− mice (P < 0.05, Tukey–Kramer test) (Fig. 1 C and D and Movie S5). Moreover, mice pretreated with clopidogrel exhibited a significant suppression of movement of EGFP+ juxtavascular microglial processes toward laser-injured capillaries (P < 0.01, Tukey–Kramer test) (Fig. 1 C and D and Movie S6). Of note, we chose a dose of 20 mg/kg clopidogrel, which increased the bleeding time by 84.8% and reduced platelet aggregation by 35.5% (Fig. 1E); patients receiving 75 mg of clopidogrel daily experienced a mean increase in bleeding time of ∼140% (25) and an increase in platelet aggregation time of 35% (26). Clopidogrel’s only targets in the adult CNS were confirmed to be microglial cells (14, 15, 18), because the immunohistochemical labeling of P2RY12 colocalized with Cx3CR1-EGFP (Fig. 1F); blood-borne platelets also expressed high levels of P2RY12 (27, 28). However, suppression of platelet activity in blood by clopidogrel is unlikely to be the cause of juxtavascular microglial motility reduction, because non-P2RY12–dependent platelet antagonists—acetylsalicylic acid (10 mg/kg per day for 3 d) and heparin (200 IU/kg i.v.)—did not reduce the motility of juxtavascular microglial processes (P > 0.05, Tukey–Kramer test) (Fig. 1D), even though both agents completely suppressed hemostasis of tail bleeding for over 20 min (n = 3–7). In addition, the same laser injury failed to initiate platelet accumulation inside the capillary at the injured site (P > 0.05 with vs. without injury, Tukey–Kramer), whereas collagen injection induced the accumulation of platelets in random positions in capillaries (Fig. 1 G and H). Taken together, whereas P2RY12 deletion reduced juxtavascular microglial chemotaxis in response to both vascular and nonvascular injury, clopidogrel suppressed only microglial motility when the injury was targeted to the local vascular bed, the presumed entry site of clopidogrel and its metabolites.
Fig. 1.
Movement of juxtavascular microglia processes toward injured vessels requires P2RY12. (A) Representative time-lapse imaging of laser injury targeted outside the vasculature in CX3CR1+/EGFP/P2RY12+/+ mice (Upper), CX3CR1+/EGFP/P2RY12−/− mice (Middle), and CX3CR1+/EGFP/P2RY12+/+ mice receiving clopidogrel (Lower). (Scale bars, 25 µm.) (B, Upper) Juxtavascular microglial cell process accumulation in response to laser injury outside the vasculature as shown in A. Fluorescence signal of EGFP around the capillaries was normalized to fluorescence signal in the whole field. (Lower) Comparisons of the kinetics of process accumulation. n = 4–11 injuries from four animals; ns, P > 0.05; **P < 0.01, Kruskal–Wallis test. (C) Time-lapse images of juxtavascular microglial cell activation in response to laser injury in a CX3CR1+/EGFP/P2RY12+/+ mouse. The laser was targeted to the center of a capillary located 120 µm below the cortical surface (yellow star). Laser injury in a CX3CR1+/EGFP/P2RY12−/− mouse and a CX3CR1+/EGFP/P2RY12+/+ mouse treated with clopidogrel (20 mg/kg). (Scale bars, 20 µm.) (D, Upper) Kinetics of juxtavascular microglial process accumulation around the injured capillaries shown in C. Fluorescence signal of EGFP around the capillaries was normalized to fluorescence signal in the whole field. (Lower) Comparison of juxtavascular microglial cell processes around the injured capillary in CX3CR1+/EGFP/P2RY12+/+, CX3CR1+/EGFP/P2RY12−/−, and CX3CR1+/EGFP/P2RY12+/+ mice treated with clopidogrel; in CX3CR1+/EGFP/P2RY12+/+ mice treated with acetylsalicylic acid (10 mg/kg i.p.); and in CX3CR1+/EGFP/P2RY12+/+ mice treated with heparin (200 IU/kg i.v.). n = 5–9 capillaries from four to eight animals; ns, P > 0.05; *P < 0.05, **P < 0.01, one-way ANOVA with Tukey–Kramer test. (E, Upper) Tail bleeding time in vehicle control (n = 7), clopidogrel (5, 20, 30, 40, and 100 mg/kg i.p. daily for 3 d; n = 7–9), and acetylsalicylic acid (10 mg/kg, i.p. daily for 3 d, n = 5). (Lower) Platelet aggregation in whole blood from animals treated with vehicle control (n = 9–15), clopidogrel (5, 20, 30, 40, and 100 mg/kg i.p. daily for 3 d; n = 8–18), and acetylsalicylic acid (10 mg/kg, i.p. daily for 3 d; n = 11). (F) P2RY12 is predominantly, if not exclusively, expressed by juxtavascular microglial cells. DAPI (blue), CX3CR1-EGFP (green), P2RY12 (red), Laminin (white). Orthogonal views of XYZ stacked images are shown with planes of sections shown by white dotted lines. (Scale bar, 25 µm.) (G) Laser injury to a capillary did not induce accumulation of platelets at the injury site. Platelets were labeled with Calcein AM (green) inside capillaries labeled with Texas Red-dextran (red). Systemic administration of collagen (1 mg/mL) caused aggregations of platelets in random positions. (Scale bars, 20 µm.) (H, Upper) Kinetics of platelet accumulation inside the injured capillaries shown in G. (Lower) Comparison of platelet accumulation inside the capillary at 10 min after with or without the laser injury or collagen administration. n = 11 capillaries from four animals; ns, P > 0.05; **P < 0.01; one-way ANOVA with Tukey–Kramer test.
Motility of Juxtavascular Microglial Cells Contributes to the Rapid Closure of the BBB.
Our data suggest that at sites of vascular injury opening of the BBB may lead to influx of low-molecular-weight compounds, including clopidogrel (MW 353 Da), which in turn suppress the P2RY12-dependent movement of juxtavascular microglial processes to sites of vascular injury (Fig. 2 A–D). To establish whether the laser injury indeed triggered opening of the BBB, we developed a technique by which BBB permeability could be serially assessed (Fig. 2A). Alexa Fluor 488 (MW 640 Da, 10 µL) was injected into the internal carotid artery every 10 min after laser injury. Leakage across the BBB was calculated as the peak fluorescence signal outside the capillary, divided by the fluorescence signal inside the vessel lumen within the same frame (Fig. 2B and Movies S7 and S8). Using this approach, we noted that the efflux of Alexa Fluor 488 gradually decreased after laser injury and that the BBB defect was resealed at 39.6 ± 8.6 min in P2RY12+/+ mice. Similarly, neither acetylsalicylic acid nor heparin significantly slowed the closure of BBB leakage after injury (P > 0.05, Tukey–Kramer test) (Fig. 2 C and D). In contrast, both P2RY12−/− and clopidogrel-treated mice exhibited much slower rates of BBB resealing (P < 0.01, Tukey–Kramer test) (Fig. 2 C and D). Because microglia are the only cells of the neurovascular unit that express detectable levels of P2RY12 (14, 15, 18) (Fig. 1F), these observations suggest that juxtavascular microglia are critical to the rapid closure of BBB defects. Ultrastructural analysis based on electron microscopy (EM) of the laser injury revealed, as expected, aggregation of densely packed processes, which completely ensheathed the site of injury. Adjacent processes exhibited closely apposed membrane (Fig. 3A). Immunolabeling revealed that the juxtavascular microglial cell processes extending toward the site of laser injury exhibited very high P2RY12 expression (Fig. 3B), as well as polarized expression of the adherens junction molecule E-cadherin. In contrast, a tight junction protein occludin was not detected (Fig. 3B). These observations showed that microglial cell processes were in direct contact with each other after they aggregated around the site of injury. It is possible that the E-cadherin–positive membrane appositions will restrict diffusion between the lesion and surrounding tissue and thus reseal acute BBB openings. An alternative possibility—that juxtavascular microglial process-mediated restriction of the injured capillary wall reduced capillary perfusion—was not supported because capillary diameter, erythrocyte flow velocity, and flux did not differ among P2RY12+/+, P2RY12−/−, and P2RY12+/+ mice treated with clopidogrel (P > 0.05, ANOVA) (Fig. 3 C–F).
Fig. 2.
P2RY12 is required for rapid closure of the BBB, and juxtavascular microglia processes may temporarily seal BBB openings. (A) Experimental setup. The large MW weight tracer, Texas Red-dextran, was injected i.v. to outline the vasculature, and the small molecular Alexa Fluor 488 (10 µL, 80 μM) was repeatedly delivered by a catheter inserted into the internal carotid artery every 10 min to map the duration of closure of BBB openings induced by laser injury of single capillaries. (B, Upper panels) Time lapse of Alexa Fluor 488 (green) passage through a control, noninjured capillary. (Lower panels) Similar time lapse of Alexa Fluor 488 (green) passage through a capillary exposed to laser injury. The capillary is outlined by Texas Red-dextran (red). The dotted white square indicates the region used for quantification of Alexa Fluor 488 leakage. Alexa Fluor 488 leakage was defined as “peak fluorescence signal intensity outside the vessel divided by fluorescence signal intensity inside the vessel.” (Scale bar, 10 µm.) (C) Scatter histograms of Alexa Fluor 488 leakage in P2RY12+/+ mice (black), P2RY12−/−mice (orange), P2RY12+/+ mice receiving clopidogrel (20 mg/kg, red), acetylsalicylic acid (10 mg/kg, blue), or heparin (200 IU/kg, turquoise). Different color gradients indicate an individual set of capillaries. The lines indicate the average of linear regression curves, obtained by averaging the slopes and Y-intercept of each regression line from a single capillary. The average regression lines were used to obtain BBB closure time (X-intercept). (D) Summary histogram of BBB closure time. n = 4–7 capillaries from four to seven animals; ns, P > 0.05; **P < 0.01; one-way ANOVA with Tukey–Kramer test.
Fig. 3.
Laser injury induces accumulatation of juxtavascular microglia processes and does not affect capillary perfusion. (A) Electron microscopic image of laser injury in cerebral cortex. Yellow dotted line with a yellow star indicates the site of the focal injury. Green dotted line indicates the accumulated juxtavascular microglia processes, with arrows indicating the close apposition of adjacent microglia processes extended toward the injury site. (B) Immunohistochemical analysis of focal laser injury site in cerebral cortex of CX3CR1+/EGFP animals. P2RY12 and E-cadherin (red) colocalized with EGFP (green) and are highly expressed in microglial cell processes encircling the injury site. In contrast, occludin (red) was detected only in vascular endothelial cells, but not in microglial cells. (Scale bars, 20 µm.) (C) Time lapse of a laser-injured capillary (red) with microglia (gray) in CX3CR1+/EGFP/P2RY12+/+, CX3CR1+/EGFP/P2RY12−/−, and CX3CR1+/EGFP/P2RY12+/+ mice treated with 20 mg/kg clopidogrel. (Scale bars, 10 µm.) (D) Plots of capillary diameter at the site of laser injury plotted as a function of time in CX3CR1+/EGFP/P2RY12+/+, CX3CR1+/EGFP/P2RY12−/−, and CX3CR1+/EGFP/P2RY12+/+ mice treated with 20 mg/kg clopidogrel. n = 3–5 capillaries from three to five animals. (E, Left) Strategy for collecting time series of XT line-scan images in capillaries filled with Texas Red-dextran (red). (Right) Line scans were collected at 0–64 min after laser injury. (F) Plots of RBC velocity and flux of capillary exposed to laser injury in P2RY12+/+, P2RY12−/−, and P2RY12+/+ mice treated with 20 mg/kg clopidogrel. n = 5–12 capillaries from three animals.
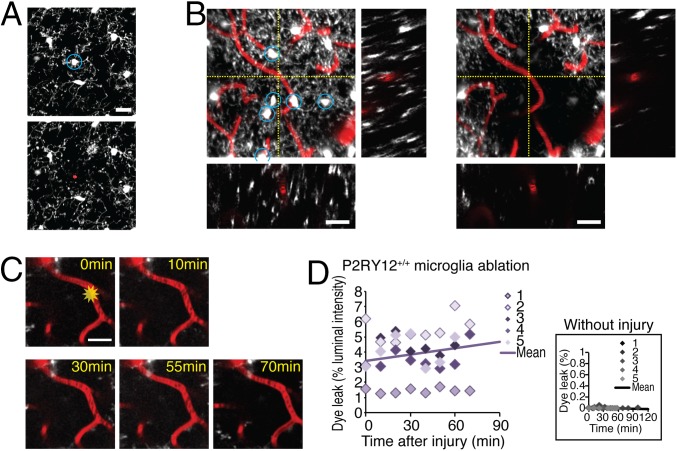
To assess the role of juxtavascular microglial cells in BBB resealing using an alternative approach, we next used laser injury to ablate juxtavascular microglial cells. Pulsed two-photon laser ablation of EGFP+ cells yields a higher degree of localized injury than continuous lasers, and has been successfully used to ablate organelles in single cells (29), as well as to sever individual dendrites of sensory neurons (30), and to functionally inactivate individual interneurons (31). The femtosecond pulsed laser was tuned for high absorbance by EGFP (910 nm) and focused on the center of juxtavascular microglial soma. Constant laser exposure (60–120 s) resulted in the irreversible loss of fluorescence signal in the targeted microglial cells (Fig. 4 A and B). Repeated collection of 3D Z-stacks confirmed that EGFP did not recover after laser ablation, and that ablated juxtavascular microglial cells exhibited nuclear staining with propidium iodide, a marker of irreversible membrane damage (32). Remarkably, BBB leaks failed to reseal during a 70-min observation period when all juxtavascular microglia located within a radius of 40 µm from the capillary (six to nine cells) were ablated (Fig. 4 C and D). Of note, no dye leak was observed after ablation of juxtavascular microglial cells in the absence of laser injury to the capillary (Fig. 4D). Thus, the congregation of activated juxtavascular microglial processes at sites of capillary injury appears to contribute significantly to the closure of BBB defects following capillary injury. Clopidogrel inhibits juxtavascular microglial process movement, thereby impairs resealing of the BBB, and by so doing prolongs injury-associated transudation.
Fig. 4.
Juxtavascular microglia ablation attenuates vascular closure. (A) Ablation of juxtavascular microglial cell. Propidium iodide (30 µM) was applied after a juxtavascular microglia was ablated with focused laser radiation. Only the ablated microglial cell (blue circle) that lost EGFP fluorescence was stained with a cell death marker propidium iodide (red). (Scale bar, 20 µm.) (B) Projection images (55 µm in z direction) and orthogonal views (XZ and YZ planes at yellow dotted lines) of a field before (Left) and after (Right) laser ablation of six juxtavascular microglial cells (white with blue circles) located within a radius of 40 µm around the target capillary (red, at the crosshair) in a CX3CR1+/EGFP/P2RY12+/+ mouse. (Scale bars, 20 µm.) (C) Time series of experiment with ablation of juxtavascular microglial cells shows that the region around the injured capillary remained free of microglial cell processes for the duration of the experiment. No juxtavascular microglial cell processes were in contact with the injured capillary at 70 min. (Scale bar, 20 µm.) (D) Scatter histogram of Alexa Fluor 488 leakage in microglia-ablated CX3CR1+/EGFP/P2RY12+/+ mice. Different color gradients indicate an individual set of capillaries. The line indicates the average of linear regression curves (y = 0.0144x–1125.84), obtained by averaging slopes and Y-intercept of each regression line from each capillary. Rate of BBB closure was 1.44 ± 0.87%/min (n = 5 capillaries), indicating that the leak worsened over time rather than gradually closing. (Inset) Scatter histogram of Alexa Fluor 488 leakage following juxtavascular microglial cell ablation but without laser injury to the capillary. The line indicates the average of linear regression curves (y = 0.00021x–0.01092; n = 5 capillaries).
Discussion
The experiments in this study show that P2RY12-mediated activation of juxtavascular microglial cells contributes to the rapid closure of small openings in the BBB, and that the movement of microglial cell processes toward the site of vascular injury is a key determinant of how rapidly those leaks are closed (Figs. 1 and 2). Our in vivo analysis suggested that juxtavascular microglial cell processes aggregate to form a physical barrier, consisting of E-cadherin–expressing membrane appositions, and that the microglial cuff around the vessel wall temporally assumes the functions of the BBB lost in the setting of acute vascular injury (Fig. 3). In contrast, laser-targeted elimination of juxtavascular microglial cells (Fig. 4), as well as both pharmacological inhibition and genetic deletion of P2RY12 receptors, delayed resealing of small openings in the BBB. Although our EM analysis suggests that juxtavascular microglial processes formed a physical barrier that temporarily sealed the BBB, our data also permit the possibility that these microglial processes released trophic factors that accelerated endothelial cell closure of the BBB opening. As such, this study, to our knowledge, is the first to identify a key role for microglial cells as integral to the structural integrity of the neurovascular unit, and paramount in the acute closure of injury-associated BBB leaks. Juxtavascular microglia thus join pericytes and astrocytes as critical contributors to the unique barrier functions of brain endothelial cells (33, 34).
Although most relevant studies have highlighted the relative specificity of brain P2RY12 expression to microglial cells, some controversy on the point exists, in that studies have variably reported that P2RY12 might also be expressed in brain endothelial cells (35), human smooth muscle vascular cells (36), and astrocytes (37). However, these reports have without exception been based on analysis of cultured cells or ex vivo preparations. In contrast, a recent transcriptome analysis showed that microglial cells are the only cell type in brain that express P2RY12 (38), confirming earlier studies (15, 18) as well as our own analysis (Fig. 1F). Another question is how selective clopidogrel inhibits P2RY12 receptors. Our analysis suggested that clopidogrel is a highly specific P2RY12 inhibitor in accordance with the literature (39, 40) because clopidogrel had no effect on juxtavascular microglia process motility or the duration of closure of small BBB openings in mice with deletion of P2RY12 (P2RY12−/− KO) (Figs. 1 B and D and 2C).
Clopidogrel is widely prescribed to patients with cardio- and cerebrovascular disease on the basis of its platelet-targeted anti-thrombotic actions, which are affected through inhibition of the platelet P2RY12. Large prospective studies have supported clopidogrel’s use for the prevention of both heart attack and stroke, principally as an adjunct to acetylsalicylic acid, and recently as prophylaxis for stent restenosis following angioplasty, in a patient population similarly at risk for coronary, carotid, and intracerebral thrombotic events; these studies have included clopidogrel in unstable angina to prevent recurrent events (CURE) (41), clopidogrel for the reduction of events during observation (CREDO) (42), atrial fibrillation clopidogrel trail with irbesartan for prevention of vascular events (ACTIVE) (43), and clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) (44–46), among others. However, a significant number of patients proceed to develop stroke despite optimal medical management and prophylactic clopidogrel (23). Because a hallmark of ischemic brain injury is BBB breakdown (47, 48), which in turn can allow the influx of both clopidogrel and its active metabolite into the ischemic tissue, we asked whether clopidogrel might the affect microglial P2RY12 receptors, essentially as unintended targets. Specifically, we asked whether clopidogrel—and its active metabolite, already present in the bloodstream at the time of an acute stroke—might reduce local P2RY12-dependent microglial activation, and thereby worsen stroke outcome. Our analysis showed that clopidogrel indeed suppressed the movement of juxtavascular microglial processes toward photolytic lesions in the vessel wall, and by so doing suppressed the closure of BBB leaks. If microglial P2RY12 plays a similar role in humans, then these observations might have significant implications for current platelet-directed strategies for the prevention of thrombosis, which call for P2RY12 inhibition. However, two prior studies have shown that P2RY12 antagonist administration after ischemia is beneficial: The first study induced global stroke in mice and reported that clopidogrel administered 5 min to 4 h after stroke reduced delayed neuronal loss after transient occlusion of the common carotid arteries (49). The second study exposed rats to occlusion of the middle cerebral artery followed by injection of ticagrelor (a reversible P2RY12 antagonist) 10 min to 36 h later (50). Both studies ascribed the apparent neuroprotective effect of P2RY12 inhibition to a suppression of the inflammatory response to ischemic cellular injury.
How then can these observations be reconciled with our findings that clopidogrel potently inhibited the repair of vascular injury, thus potentially aggravating edema and ischemic injury? Clinically, clopidogrel must be administered for several consecutive days to achieve a therapeutic effect, as the active metabolite of clopidogrel is produced in the liver (51). The typical patient takes clopidogrel for months or years before an ischemic event. By administering clopidogrel after the ischemic event, the two aforementioned studies (49, 50) would not have been expected to achieve functional P2RY12 inhibition until several days after injury, long after the acute role of microglia in reannealing the BBB would have been accomplished. As such, the designs of these studies would have effectively eliminated any deleterious effects of clopidogrel at the time of acute injury. In contrast, our study was designed to model a more clinically relevant scenario, by administering clopidogrel over 3 consecutive days before the ischemic event, so that functional P2RY12 inhibition would be in effect at the time of vascular occlusion, as might be expected of a patient on clopidogrel prophylaxis who proceeds to nonetheless have a cerebral ischemic event. Also, we selected a dose of clopidogrel that induced a clinically relevant prolongation of bleeding time and platelet aggregation, which was verified to be so at the time of the ischemic event. Using this design, we found that clopigogrel indeed prolongs the opening of the BBB at the time of experimental vaso-injury and thereby may aggravate ischemic injury in those patients who proceed to have an ischemic event while on clopidogrel as a prophylactic. Together, these data suggest the hitherto unappreciated importance of juxtavascular microglial cells in the structural integrity and functional maintenance of the gliovascular unit and BBB, while highlighting the need for further studies modeling the potential risks of inadvertent microglial inhibition when targeting P2RY12 for purposes of platelet inhibition.
Materials and Methods
Mouse Strains.
CX3CR1+/EGFP mice were purchased from Jackson Labs (strain name B6.129P-CX3CR1tm1Litt/J, stock no. 005582), P2RY12−/− mice were obtained from the European Mutant Mouse Association (stock no. EM:02301). Both strains are in the C57BL/6J background, and CX3CR1+/EGFP/P2RY12+/+ and CX3CR1+/EGFP/P2RY12−/− littermates were generated by crossing the two lines. All experiments were performed in accordance with protocols approved by Animal Use Committees at the University of Rochester.
Animal Preparation for in Vivo Imaging.
The 8- to 12-wk-old male mice were anesthetized with a mixture of ketamine (70 mg/kg) and xylazine (10 mg/kg) i.p. and artificially ventilated (SAR-830, CWE). A custom-made metal plate was glued to the skull with dental acrylic cement, and a 1.5-mm cranial window was prepared over the parietal cortex (2 mm posterior and 3 mm lateral from bregma). The dura was left intact and artificial cerebrospinal fluid containing 1% agarose was placed between the window and a glass coverslip (52). Silastic catheters (PE10) were inserted into the left femoral artery and vein. During two-photon imaging the anesthesia was changed to isoflurane (1.2%), and body temperature was maintained at 37 °C by a circulating warm-water blanket (T/Pump, Stryker). Blood samples (40 µL) from the femoral artery were analyzed by a blood-gas analyzer (Rapidlab 248, Bayer), and experiments were completed only if physiological variables remained within normal limits. The normal limits for pCO2 were set at 35–45 mm Hg; for pO2, 80–115 mm Hg; and for arterial blood pH, 7.35–7.45 (52).
In Vivo Two-Photon Laser Scanning Microscopy.
A custom-built microscope attached to a Ti:Sapphire laser (Mai Tai, SpectraPhysics), a scanning box (FV300, Olympus) operated by FluoView software (Olympus), and a 20× water-immersion objective lens (0.95 N.A., Olympus) was used for imaging. Excitation wavelength was set to 910 nm, whereas two-channel detection of emission wavelength was achieved by a 565-nm dichroic mirror (Chroma Technology) and two external photomultiplier tubes. A 515/50 bandpass filter (Chroma Technology) detected EGFP and Alexa Fluor 488 (Invitrogen) emission wavelength, and a 620/60 bandpass filter (Chroma Technology) detected Texas Red-dextran. Texas Red-dextran (MW 70 kDa, 0.1 mL of 1% in saline; Invitrogen) was administered through a femoral-vein catheter before imaging. Time-lapse images of CX3CR1+/EGFP microglial cells were recorded every 10 s at a depth of 80–150 µm from the cortical surface. The two-photon laser power was adjusted daily to 40 mW below the objective lens. Individual capillaries with an average diameter of 4–6 µm were injured by repeated line scanning (1 µm) targeted to the center of the capillary lumen for 20 s. Tissue injury was carried out the same way except by focusing the laser to a site devoid of blood vessels. Microglial cell ablation was accomplished by focusing the imaging two-photon laser on the center of the soma of EGFP+ cells. The duration of laser exposure (typically 60–120 s) was adjusted to irreversibly ablate the EGFP signal in soma and processes. Propidium iodide (30 µM; Sigma) was locally applied with a glass micropipette (tip: 2–3 µm) after ablation. Accumulation of microglial cell processes in CX3CR1+/EGFP mice was quantified as the increase in EGFP fluorescence signal surrounding the site of laser injury in a field of ∼10 × 4 µm and normalized to the EGFP fluorescence signal of the whole field (180 × 180 µm) (18). For quantification of dye leakage, Alexa Fluor 488 cadaverine (10 µL of 80 μM dissolved in saline) was injected through a catheter (PE10) inserted through the external carotid artery into the right internal carotid artery while imaging the injured capillary at high speed (1–1.2 Hz) for 30 s. An image frame with highest fluorescence signal intensity outside the capillary was chosen, and Alexa Fluor 488 leakage was quantified as peak fluorescence signal intensity in a field (10 × 4 µm) positioned immediately outside the injured capillary and normalized to fluorescence signal intensity in the capillary in the same frame. In each experiment, this image sequence of Alexa Fluor 488 diffusion was collected at 10-min intervals, the percentage of peak dye leak was plotted over time, and then a linear curve was fitted by the least-square method. The slope (closure rate) and Y-intercept of each regression line were pooled together to obtain the average slope and Y-intercept. BBB closure time was obtained using average slope and Y-intercept fitted to the linear line. Capillary flow velocity and flux were measured using two-photon imaging in line-scan mode (2–3 ms/line, 10 µm length, 2,000 lines per image). Average velocity and flux per image were calculated by Matlab software (MathWorks) with custom software by detecting the movement of red blood cells as black lines devoid of Texas Red-dextran (53). Platelet aggregation in vivo was imaged with Texas Red-dextran and Calcein AM (100 µL of 5 mM; Invitrogen) administered through a femoral vein before the imaging (54, 55). Collagen (10–50 µL of 1 mg/mL dissolved in saline; Gibco) was administered through a catheter inserted into the external carotid artery to induce platelet aggregation.
Assessments of Bleeding Time and Platelet Aggregation.
Mice were fasted overnight before experiments. Clopidogrel (Tocris Bioscience) was prepared as a 100-mM stock solution in DMSO and administered i.p. at a dose of 5, 20, 30, 40, and 100 mg/kg animal in 0.2 mL saline for 3 d before the experiment. Acetylsalicylic acid was prepared as 10 mg/mL in saline and administered i.p. (10 mg/kg) for 3 d. For bleeding tests and platelet aggregation assays, the mice were anesthetized with ketamine (70 mg/kg) and xylazine (10 mg/kg) i.p. Their tails were transected 2 mm from the tip with a no. 10 scalpel blade and immersed in a 20-mL scintillation vial filled with normal saline at 37 °C. Bleeding time was determined as time to cessation of bleeding within a 20-min observation period (27). Bleeding was considered stopped if no bleeding was observed for 30 s and was done only once in each mouse. Whole-blood platelet aggregation was measured by an impedance aggregometer (Chronolog). Blood samples were collected with a 19-gauge needle by direct venipuncture into a syringe containing 1:10 volume of 3.2% sodium citrate and left at room temperature for 15 min. The sample was mixed with saline (1:2 dilution) to make 1 mL in a test vial and allowed to warm to 37 °C for 5 min. The electrode was placed and the sample was continuously stirred at 1,200 × g. The sample was stimulated with either 0.2 µg/mL collagen (Chronolog) or 0.1 mM arachidonic acid (Chronolog) with 0.2 mM CaCl2, and the reaction during the 15-min recording time was recorded.
Immunohistochemistry and Electron Microscopy for Analysis of Microglial Processes.
Forty-five minutes before the tissue harvest, animals received focal laser injuries as described above. Deeply anesthetized mice were then transcardially perfused with PBS and 4% (wt/vol; dissolved in PBS solution, pH 7.4) paraformaldehyde (PFA) followed by brain isolation and postfixation in 4% PFA for 3 h at room temperature. Vibratome coronal sections (50 μm) were prepared (Vibratome Series 3000) and blocked in PBS containing 0.2% Triton X and 7% normal donkey serum (Vector Labs). Specific antibodies for P2RY12 (1:2,000, kindly provided by David Julius, University of California, San Francisco) and Laminin (1:400, Abcam 14055) were applied with 0.1% Triton X and 1% normal donkey serum overnight at 4 °C. Antibodies against occludin (1:50, Life Technologies 33–1500) or E-cadherin (1:100, Abcam 76055 and Life Technologies 33–4000) were applied after treating the sections with proteases (0.2 mg/mL, 10 min at 37 °C; Sigma P5147). The antibodies were visualized following a 2-h incubation period (at room temperature) with appropriated secondary antibodies conjugated to fluorophore (1:250, Jackson ImmunoResearch). DAPI was used for nuclear counterstaining, and slides were mounted with ProLong Antifade (Life Technologies). Immunolabeled brain sections were imaged and analyzed using the confocal microscope with a 60× oil-immersion objective lens (1.25 N.A.; Olympus).
For electron microscopy of focal injury, the brains from Cx3CR1+/EGFP mice were perfusion-fixed with 4% PFA, and transverse sections (50 µm) were cut. The sections were examined with the confocal microscope to locate the positions of the focal injuries before being postfixed with 2.5% glutaraldehyde/4% PFA overnight at 4 °C. The brain sections were then postfixed in 1.0% osmium tetroxide for 30 min and dehydrated in a graded series of ethanol up to 100%, transitioned into proplyene oxide, and infiltrated with EPON/Arialdite epoxy resin. The brain slices were then polymerized for 24 h between two glass slides (pretreated with Sialane to prevent adhesion). Thin sections were cut at 70 nm, and the images were captured with a transmission electron microscope (Hitachi 7650) and a Gatan 11 megapixel digital camera system.
Statistical Analysis.
All histograms are expressed as mean ± SE. Normality of the data was examined with the Shapiro–Wilk test. One-way ANOVA, one-way repeated measure ANOVA, two-way ANOVA with the Tukey–Kramer post hoc test, and t test were used where appropriate. The Mann–Whitney nonparametric test with Bonferroni-corrected alpha level was used where normality was not assumed.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01DE022743, R01NS075177, and R01AT007945.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520398113/-/DCSupplemental.
References
- 1.Aguzzi A, Barres BA, Bennett ML. Microglia: Scapegoat, saboteur, or something else? Science. 2013;339(6116):156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marín-Teva JL, et al. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41(4):535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 3.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 4.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueno M, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 7.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingwell K. Neurodegenerative disease: Microglia in early disease stages. Nat Rev Neurol. 2012;8(9):475. doi: 10.1038/nrneurol.2012.172. [DOI] [PubMed] [Google Scholar]
- 9.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 10.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 11.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 12.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol Appl Neurobiol. 2005;31(3):325–338. doi: 10.1111/j.1365-2990.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 13.Roth TL, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollopeter G, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki Y, et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44(3):242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- 16.Catalin B, Cupido A, Iancau M, Albu CV, Kirchhoff F. Microglia: First responders in the central nervous system. Rom J Morphol Embryol. 2013;54(3):467–472. [PubMed] [Google Scholar]
- 17.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 18.Haynes SE, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 19.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 20.Foster CJ, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107(12):1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju NC, Eikelboom JW, Hirsh J. Platelet ADP-receptor antagonists for cardiovascular disease: Past, present and future. Nat Clin Pract Cardiovasc Med. 2008;5(12):766–780. doi: 10.1038/ncpcardio1372. [DOI] [PubMed] [Google Scholar]
- 22.Savi P, et al. P2y(12), a new platelet ADP receptor, target of clopidogrel. Biochem Biophys Res Commun. 2001;283(2):379–383. doi: 10.1006/bbrc.2001.4816. [DOI] [PubMed] [Google Scholar]
- 23.Diener HC, et al. Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study group Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: A double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7(10):875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereillo JM, et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos. 2002;30(11):1288–1295. doi: 10.1124/dmd.30.11.1288. [DOI] [PubMed] [Google Scholar]
- 25.Wilhite DB, et al. Managing PAD with multiple platelet inhibitors: The effect of combination therapy on bleeding time. J Vasc Surg. 2003;38(4):710–713. doi: 10.1016/s0741-5214(03)01029-2. [DOI] [PubMed] [Google Scholar]
- 26.Boneu B, Destelle G. Platelet anti-aggregating activity and tolerance of clopidogrel in atherosclerotic patients. Thromb Haemost. 1996;76(6):939–943. [PubMed] [Google Scholar]
- 27.Andre P, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112(3):398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113(3):340–345. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe W, Matsunaga S, Higashi T, Fukui K, Itoh K. In vivo manipulation of fluorescently labeled organelles in living cells by multiphoton excitation. J Biomed Opt. 2008;13(3):031213. doi: 10.1117/1.2939401. [DOI] [PubMed] [Google Scholar]
- 30.Chung SH, Clark DA, Gabel CV, Mazur E, Samuel AD. The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci. 2006;7:30. doi: 10.1186/1471-2202-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, et al. A cortical circuit for gain control by behavioral state. Cell. 2014;156(6):1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 33.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Simon J, et al. Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J Biol Chem. 2002;277(35):31390–31400. doi: 10.1074/jbc.M110714200. [DOI] [PubMed] [Google Scholar]
- 36.Wihlborg AK, et al. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol. 2004;24(10):1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- 37.Krzeminski P, Misiewicz I, Pomorski P, Kasprzycka-Guttman T, Barańska J. Mitochondrial localization of P2Y1, P2Y2 and P2Y12 receptors in rat astrocytes and glioma C6 cells. Brain Res Bull. 2007;71(6):587–592. doi: 10.1016/j.brainresbull.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34(36):11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marteau F, et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64(1):104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 40.Savi P, et al. The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA. 2006;103(29):11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusuf S, et al. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 42.Steinhubl SR, et al. CREDO Investigators. Clopidogrel for the Reduction of Events During Observation Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA. 2002;288(19):2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 43.Connolly SJ, et al. ACTIVE Investigators Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360(20):2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 44.Hankey GJ, et al. CHARISMA Trial Investigators Effect of clopidogrel on the rate and functional severity of stroke among high vascular risk patients: A prespecified substudy of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) trial. Stroke. 2010;41(8):1679–1683. doi: 10.1161/STROKEAHA.110.586727. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt DL, et al. CHARISMA Investigators Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt DL, et al. CHARISMA Investigators Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 47.del Zoppo GJ, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38(2, Suppl):646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 48.Lo EH, Pan Y, Matsumoto K, Kowall NW. Blood-brain barrier disruption in experimental focal ischemia: Comparison between in vivo MRI and immunocytochemistry. Magn Reson Imaging. 1994;12(3):403–411. doi: 10.1016/0730-725x(94)92533-x. [DOI] [PubMed] [Google Scholar]
- 49.Webster CM, et al. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS One. 2013;8(8):e70927. doi: 10.1371/journal.pone.0070927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelosa P, et al. Microglia is a key player in the reduction of stroke damage promoted by the new antithrombotic agent ticagrelor. J Cereb Blood Flow Metab. 2014;34(6):979–988. doi: 10.1038/jcbfm.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taubert D, et al. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb Haemost. 2004;92(2):311–316. doi: 10.1160/TH04-02-0105. [DOI] [PubMed] [Google Scholar]
- 52.Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura N, et al. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: Three models of stroke. Nat Methods. 2006;3(2):99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- 54.Bonnefoy A, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107(3):955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez P, Alarcón M, Fuentes E, Palomo I. Thrombus formation induced by laser in a mouse model. Exp Ther Med. 2014;8(1):64–68. doi: 10.3892/etm.2014.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.