Abstract
Until recently in Earth history, very large herbivores (mammoths, ground sloths, diprotodons, and many others) occurred in most of the World’s terrestrial ecosystems, but the majority have gone extinct as part of the late-Quaternary extinctions. How has this large-scale removal of large herbivores affected landscape structure and ecosystem functioning? In this review, we combine paleo-data with information from modern exclosure experiments to assess the impact of large herbivores (and their disappearance) on woody species, landscape structure, and ecosystem functions. In modern landscapes characterized by intense herbivory, woody plants can persist by defending themselves or by association with defended species, can persist by growing in places that are physically inaccessible to herbivores, or can persist where high predator activity limits foraging by herbivores. At the landscape scale, different herbivore densities and assemblages may result in dynamic gradients in woody cover. The late-Quaternary extinctions were natural experiments in large-herbivore removal; the paleoecological record shows evidence of widespread changes in community composition and ecosystem structure and function, consistent with modern exclosure experiments. We propose a conceptual framework that describes the impact of large herbivores on woody plant abundance mediated by herbivore diversity and density, predicting that herbivore suppression of woody plants is strongest where herbivore diversity is high. We conclude that the decline of large herbivores induces major alterations in landscape structure and ecosystem functions.
Keywords: browsers, ecosystem functions, herbivore diversity, landscape structure, megaherbivore
During the late Quaternary, megafaunas were drastically reduced in most regions (1, 2), representing the start of an ongoing trophic downgrading that has resulted in the loss of entire functional guilds and relaxation of top-down control in today’s ecosystems (3). A high proportion of the large herbivores that have survived into the Anthropocene (4) are now drastically reduced in range and abundance, rendering them functionally extinct, or have been replaced by livestock in much of their historic ranges (5–8). How has this loss of wild-living large herbivores affected landscape structure and ecosystem functioning?
Contemporary large herbivores have strong effects on the abundance of woody species, plant diversity, nutrient cycling, and other biota (9). Most likely, the ecological effects of preextinction herbivores were as large, possibly much more so given the great size and diversity of the lost large herbivore assemblages (10, 11). We hypothesize that Pleistocene herbivore assemblages, including large and megaherbivore browsers, would have greatly reduced woody plant abundance and altered species composition and landscape structure, if present at sufficient densities. We review the impact of large herbivores (≥45 kg in body weight) on woody vegetation, with a focus on megaherbivores (≥1,000 kg), and combine information from modern exclosure experiments with paleoecological records to estimate herbivore impacts and the consequences of their late-Quaternary declines.
Impact of Large-Herbivore Assemblages on Woody Plant Abundance
Much of our understanding of the impact of large herbivores on woody vegetation comes from Africa, where the Pleistocene herbivore assemblage has remained fairly intact, albeit at a much reduced distribution and abundance (8). Exclosure studies in African savannas reveal that species-rich herbivore assemblages may reduce woody species cover by 15–95% (Fig. 1A) (12–17). This broad range reflects factors such as body size and feeding mode (18) and soil fertility, topography, and hydrology (12, 19, 20).
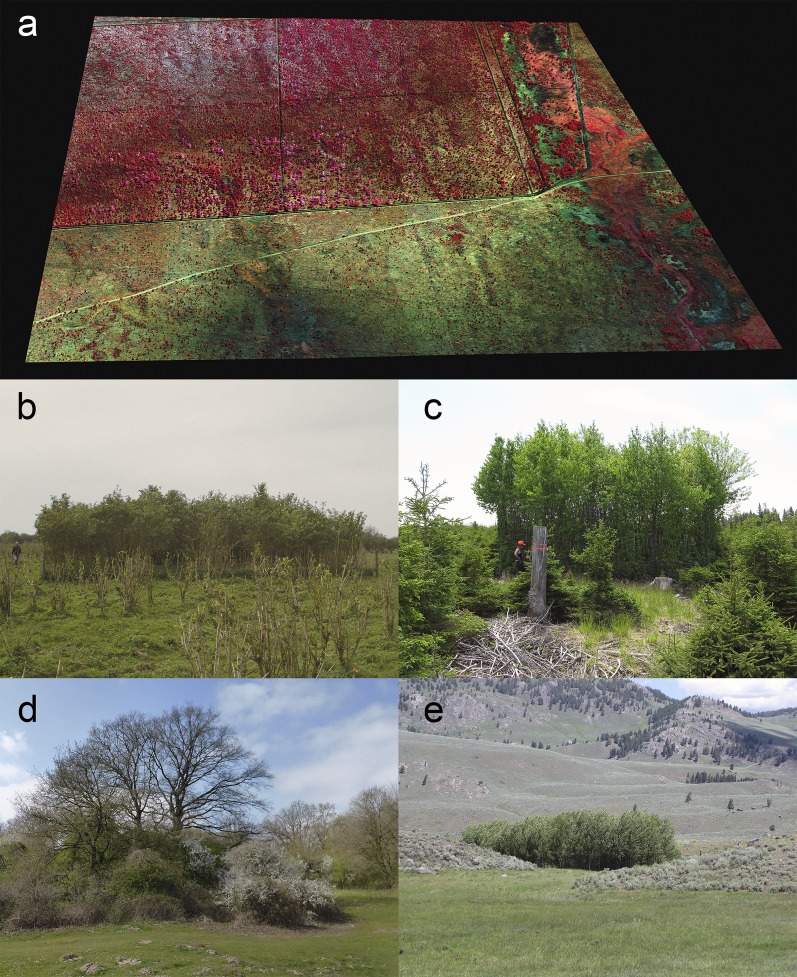
Fig. 1.
Modern exclosure experiments demonstrate strong impacts of large herbivore assemblages on woody plants. (A) In subtropical savanna, a diverse large herbivore assemblage (>5 kg) greatly reduces the abundance of woody plants outside the 302-ha exclosure (upper part of the picture) (12). The 3D infrared color indicates woody vegetation (in red, more intense red revealing more gross primary productivity) and herbaceous vegetation (in green–blue). Fire is controlled both inside and outside of the exclosure [Carnegie Airborne Observatory image (122), Kruger National Park, South Africa (12)]. (B) In temperate wetland grasslands, Heck cattle, Konik horses, and red deer (Cervus elaphus) break down the established elderberry woodland (Sambucus nigra) where it is not protected by fencing (123) (Oostvaardersplassen, The Netherlands). (C) In the boreal forest, after logging, white-tailed deer strongly influence the recruitment of woody species, with the exclosure dominated by palatable deciduous species whereas a spruce parkland developed under intense browsing (38) (Anticosti Island, Quebec, Canada). (D) Thorny shrubs (Prunus spinosa) function as natural exclosures, where they protect establishing palatable oak (Quercus sp.) from herbivory (35, 47) (Borkener Paradies, Germany). (E) In temperate sagebrush and grassland vegetation, American bison (Bison bison) and elk (Cervus elaphus) strongly suppress establishment of palatable trees (Populus sp.), which abundantly regenerate inside the exclosure (center of the picture) (Yellowstone National Park, United States). C courtesy of Bert Hidding.
African elephants (Loxodonta africana) have strong effects on woody plants due to their physical strength and height (21), causing disproportionate mortality of adult shrubs, by pulling them out (16), and trees, by pushing them over (22). Experiments with size-selective exclosures on savannas showed that elephants accounted for more than 80% of all woody plant loss across all plant height classes (22) whereas exclusion of elephants resulted in 42% more trees (23). Furthermore, elephants and all large herbivores debark trees and also feed on saplings and adult shrubs and trees without killing them, but with the effect of limiting woody plant growth and abundance (Fig. 1B) (17, 24). The removal of large browsers is thought to generally lead to a net increase in abundance of woody plants (20), but this effect depends in part on the compensatory response of smaller herbivores, which can have strong impacts, particularly on the recruitment of woody species (14, 16, 25–28).
Ground-dwelling browsers control woody plants mainly by increasing mortality in early life stages or suppressing growth to maturity by injuring plants or removing photosynthetic tissue. These impacts depend on plant height and the reach of the herbivore assemblage (29): Plants can be subject to a “browser trap” where they experience high impact while within the reach of browsers, but escape this trap by growing beyond the browse height. In species-rich herbivore communities, containing large as well as small browsers and a variety of feeding strategies, browsing impacts extend to a wider range of plant growth stages. Demographic bottlenecks imposed by browsing are therefore more difficult to escape (13, 14, 16). Temporary reductions in herbivore numbers allow trees to regenerate and grow into taller height classes, escaping herbivory by the time herbivore populations have recovered (17). Fruit and seed consumption might offset the demographic effects of browsing injury by seed dispersal, but the net effect of large herbivore assemblages on seed predation versus dispersal remains unclear (16).
The feeding mode of herbivores dictates their impact on woody plants. Browsers generally have direct inhibitory effects on growth and survival of woody plants. Grazers can suppress woody plants through trampling or occasional feeding, but can also promote recruitment and survival by reducing competition with herbaceous vegetation, thereby reducing rodent densities and reducing fire frequency by preventing fuel accumulation (15, 30, 31). Nevertheless, feeding mode impacts are complex and poorly understood because large herbivores are often mixed feeders (32).
Impact of Large Herbivores on Woody Plant Species Composition
By selecting palatable species, large herbivores affect woody species composition and promote the abundance of defended browsing-tolerant shrubs and trees (33, 34); furthermore, by creating a certain degree of vegetation openness, they promote the abundance of light-demanding woody species (35). Exclosure studies have documented these effects across a broad range of biomes. For example, in the North American boreal forest, moose (Alces americanus) and white-tailed deer (Odocoileus virginianus) selectively feed on hardwoods and the soft-needled balsam fir (Abies balsamea), but avoid the hard-needled white spruce (Picea glauca) (36), creating a spruce parkland, whereas hardwood species dominate in exclosures (Fig. 1C) (37, 38). In deciduous forest, deer browsing likewise reduces regeneration of palatable hardwood species, resulting in a more open habitat resembling oak savanna, with many light-demanding plant species (39). Browsers shift the species composition of African savanna from dominance by palatable shrubs to dominance by thorny acacias (14) and chemically defended or browsing-tolerant species (40).
Similar dynamics are seen in response to forest management. In European forest reserves, removal of domestic cattle and horses and culling of wild ungulates, such as deer and European bison (Bison bonasus), have resulted in the expansion of shade-tolerant tree species such as lime (Tilia spp.), hornbeam (Carpinus betulus) and beech (Fagus sylvatica), creating closed canopy forest and out-competing the shade-intolerant oak (Quercus spp.) (41–44).
Spatially Structured Landscapes
In landscapes characterized by intense herbivory, woody plants can persist by defending themselves, by associating with defended species, or by growing in areas that are physically inaccessible or that are risky for herbivores because of high activity of predators (34, 45). The resulting variation in the local intensity of herbivory can create spatial mosaics of herbaceous plants, shrubs, and trees.
Palatable woody species can regenerate in the vicinity of thorny or poisonous forbs and shrubs that protect them from browsing (Fig. 1D) (46–48). Cyclic succession may occur as grasslands are colonized by thorny shrubs, from which palatable trees can grow, which outcompete the shrubs; with death of the trees, herbivory suppresses woody plant regeneration, returning the system to a grassland state. Because, spatially, patches are out-of-phase over time, this cyclic succession may result in a mosaic of grasslands, shrubs, single trees, and clumps of trees at the landscape scale (35).
Alternatively, palatable species may grow in areas that are physically inaccessible, such as steep slopes or between rocks and logs (49, 50). Spatial heterogeneity in landscape structure can also be induced by the presence of predators imposing a landscape of fear (51). As a response to perceived predation risk, often heterogeneously distributed across the landscape (52), herbivores may select less risky areas, creating spatial variability in herbivore pressure and thus varying impacts on vegetation (34, 53). Therefore, the presence of predators can allow local increases in the abundance of woody species, such as observed after the introduction of wolves in temperate woodlands followed by reduced browsing pressure from deer and locally enhanced recruitment of palatable shrubs and trees (45, 54–56), resembling that observed in exclosures (Fig. 1E).
Because extremely large size confers a high degree of invulnerability to predation (21), adult megaherbivores may prefer areas with a higher density of trees, because of greater forage availability, whereas smaller herbivores often prefer open grassland due to higher risk of ambush by predators in the woodland (53). As a result, assemblages of different-sized herbivores will exert spatially heterogeneous grazing and browsing pressure across the landscape, which affects woody plant abundance and species composition (34).
How Did Extinct Late Pleistocene Megaherbivores Affect Woody Plants?
The interpretation of extinct megaherbivore impact relies on the comparison with the ecology of modern megaherbivores. There are 8 extant megaherbivores, from three orders (Cetartiodactyla, Perissodactyla, Proboscidea), and 35 extinct megaherbivores from the Late Pleistocene, from seven orders (Cetartiodactyla, Cingulata, Diprotodontia, Notoungulata, Perissodactyla, Pilosa, and Proboscidea) (1). An open question is whether extinct megaherbivores would have had similar effects on woody plants as their contemporary closest relatives. The nine extinct Late Pleistocene proboscideans had divergent feeding strategies, from the predominantly grazing woolly mammoth (Mammuthus primigenius) to the browsing American mastodon (Mammut americanum) (57–59), the latter being supposedly most similar in feeding ecology to present-day browsing black rhinoceros (Diceros bicornis) or moose (A. americanus) (60, 61). Whereas some niche separation was evident (57, 61, 62), recent multiproxy data on megaherbivore paleodiets suggests that many were mixed feeders that adapted their diets to local plant availability (62–64). Similarly, extant megaherbivores are mostly mixed feeders with a few grazing specialists like the hippopotamus (Hippopotamus amphibius) and white rhinoceros (Ceratotherium simum) (65). Further work comparing the guild structures of megaherbivores both in the present and the Late Pleistocene would provide better understanding of the potential impact of these species on vegetation structure.
Extinct megaherbivores would have impacted woody plants through consumption, but also by other physical impacts. Wear patterns on the teeth and tusks of mastodons have been interpreted as indication of their bark stripping behavior (60, 66), which would undoubtedly have killed many trees and shrubs as observed with contemporary African elephants (59). Selective feeding of mastodons on spruce may have contributed to the spruce–pine transition in the US Great Lakes region in the Late Pleistocene (57). Furthermore, many extinct and extant megaherbivores are avid fruit consumers and thus contributed strongly to the abundance of woody plants through dispersal of fruits, in particular those that bear the megafaunal dispersal syndrome (67, 68).
The paleoecological record provides evidence of geomorphological engineering by mammoths, presumably digging for water and mineral-rich sediments, trail formation, and trampling, similar to what elephants do today (69). Combined geo-engineering and enhanced nutrient cycling by extinct megaherbivores would have significantly contributed to the maintenance of open habitats, dominated by fast growing palatable herbaceous vegetation over slower growing woody species (59, 70). The impact of Pleistocene herbivore assemblages may have been amplified by lower atmospheric CO2 concentrations during glacial episodes. Low CO2 probably limited woody plant growth, impeding recovery from herbivory and increasing total impact of herbivores (71, 72).
Evidence of Large-Herbivore Impact from the Paleoecological Record
The extinction and density reductions of Late Pleistocene large herbivores represent a grand removal experiment (1). We assess this experiment by comparing landscape structure and vegetation composition in the presence and absence of Late Pleistocene diverse large-herbivore faunas over time, under more or less similar climatic conditions. Similarities between patterns of woody plant response to large herbivore removal in the paleoecological record and modern exclosure experiments are indicated in Table 1.
Table 1.
Examples of the impact of large herbivores on woody plants, species composition, and landscape structure as found in contemporary (exclosure) studies and from the paleoecological record
| Process | Contemporary pattern | Paleoecological record |
| Large herbivores reduce the abundance of woody plants. | Higher woody plant cover in exclosures and after removal of large herbivores (12–16) | Landscapes of previous interglacials seem to have been more open than after Pleistocene extinctions in the early Holocene (81, 82). |
| Moas may have maintained mosaics of open canopied woodland and scrub (95). | ||
| Large herbivores induce shifts in woody species composition. | Under intense browsing, unpalatable and thorny species thrive and palatable species are suppressed (14, 37, 40). | Increase in palatable and shade-tolerant hardwoods immediately after the Pleistocene extinction in North America (75, 78). |
| Browsing may also promote browsing-tolerant species (102). | Increase in unpalatable trees during historically high herbivore densities in European forest (111). | |
| Under intense herbivory, light-demanding trees and shrubs are promoted (35, 39). | ||
| Large herbivore impact is mediated by soil fertility. | More thorny shrub species in fertile habitats may indicate higher browsing pressure (35). | Vegetation openness was greater in fertile lowland areas, compared with less fertile upland areas (82). |
| Higher elephant impact on treefall at fertile soils (22) | ||
| Herbivores modify vegetation responses of woody plants to climate and soils. | In tundras, herbivores can inhibit shrub encroachment with climate warming (87), but this effect is site-dependent (88). | Mosaic forest tundra in northeastern Siberia during the Last Interglacial, with browsing tolerant trees frequent—likely (at least partly) due to large herbivores (86). |
| In savannas, woody species cover does frequently not reach its abiotic potential due to fire and herbivory (115, 116). | Large herbivore presence maintained the mammoth steppe in northeastern Siberia, which disappeared after Late Pleistocene extinctions (70, 85). | |
| Higher openness of vegetation in last interglacial than expected based on climate and soil may be mediated by large herbivores (81). | ||
| Herbivores reduce fuel load for fires. | Herbivores reduce herbaceous biomass and fire frequency, which benefits woody species, unless these woody plants are also browsed (30, 116, 117). | Increased fire activity immediately after the Pleistocene extinctions (73–76, 78) |
The initial ecological adjustment of plant communities to release of browsing and grazing after megafauna extinctions should be completed within relatively short periods of decades or centuries and can thus seem rapid in paleoecological records spanning thousands of years. After that, long-term changes, such as through reduced seed dispersal, would continue to influence plant species distributions up until present times (67). The ecological consequences of megafaunal extinctions have received little study, which is in part due to limitations inherent to comparing a discontinuous vertebrate bone record with a vegetation record constructed primarily from lake sediment records that are typically not associated with megafaunal fossils. Recently, the use of Sporormiella and other coprophilous fungi has shown promise for determining the abundance of large herbivores and testing their impact on vegetation in the paleoecological record. Sporormiella spores are preserved in lakes and mires along with pollen and so can be used to provide the ecological context of functional large herbivore collapse (73–77).
Several pollen records from eastern North America show an increase in hardwood deciduous taxa immediately after the Sporormiella-indicated megafaunal decline, including increases in palatable and shade-tolerant woody species (74, 75, 78), and a more closed vegetation, consistent with release from browsing pressure. The continued postextinction presence of light-demanding oak (Quercus alba) indicates that the surviving large herbivores could have maintained a certain degree of openness of the landscape (42), which has also been ascribed to the effect of dry climate and anthropogenic fires (79). Similarly, the now-endangered grass balds of the southern Appalachian mountains are hypothesized to be remnants of past herbivory (later maintained by Native American burning) (80).
Pollen and Sporormiella records from northeastern Australia (76) during the last glaciation record a decline of large herbivores around 40,000 years ago, followed by a shift from a mixed and relatively open vegetation, consisting of elements of angiosperm and gymnosperm rainforest along with sclerophyll species, to pure sclerophyllous vegetation, in apparent absence of major climate change. This vegetation shift was evidently due to a combination of relaxed herbivory pressure and increased fire that closely followed the onset of herbivore decline (76).
Evidence from fossil beetles indicates that regions of European vegetation were more open in the Last Interglacial and supported more dung beetles, than after the extinctions, in the preagricultural Holocene. Some wood-pasture and moderate open vegetation remained in the early Holocene, indicating a role for the remaining wild herbivores (81–84).
In northeastern Siberia, the productive pastures of the mammoth steppe disappeared after the removal of the high densities of large herbivores that may have maintained this ecosystem and was replaced by mossy forests and tundras (85, 86). The modern climate of this area is inside the mammoth steppe climatic envelope, suggesting that the removal of high densities of large herbivores determined the biome transition from pasture to tundra with woody plants (70). Modern experiments show that herbivores can inhibit shrub encroachment on tundra with climate warming, but this effect is site-dependent (87, 88).
The presence and loss of megafauna might also have long-term impacts on biotic communities that are still ongoing and witnessed by current plant traits that coevolved with megafauna, which may be less adaptive in modern landscapes (e.g., ecological anachronisms) (89). For example, woody species that are adapted to megafauna dispersal (67) may still be experiencing slow declines (90), depending on whether megafauna have been substituted by smaller wild animals, domestic livestock, or humans. Similarly, coevolution with the recently exterminated moa and elephant birds, flightless ratite birds 20–500 kg (91), can explain some remarkable idiosyncrasies of New Zealand and Madagascar vegetation, especially the high representation of “wire plants,” a growth form likely to have reduced the foraging efficiency of moa, thus providing protection from browsing (92–94). Browsing by moa might also have created canopy gaps that sustained high diversity of light-loving herbs and regeneration of light-demanded conifer seedlings (95).
In summary, modern studies and paleo-studies indicate that removal of large herbivores is followed by increased abundance of woody plants and altered vegetation composition and structure toward less open landscapes, with more shade-tolerant and palatable species (Table 1).
Under What Conditions Would Pleistocene Large Herbivore Assemblages Have Had Most Impact?
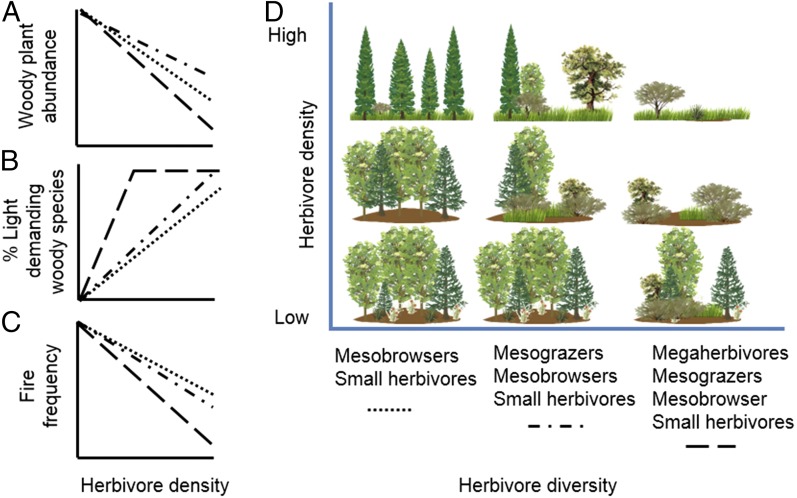
Based on the evidence from modern exclosure studies and the paleoecological record, we expect that the Late Pleistocene large herbivore assemblages would have had at least equal, but probably stronger impacts on woody plant abundance than most contemporary assemblages, due to their broader range of body sizes and higher species-richness imposing more complete inhibition of woody plant life stages (Fig. 2). However, a central question that needs to be answered is under what conditions were herbivore densities high enough to have strong impacts on landscape structure.
Fig. 2.
Hypothesized impact of large herbivore removal on landscape structure, proportion of light-demanding woody species, and fire frequency. All of these landscapes represent sites where the climate and soil allow trees to dominate. The dotted and dashed lines in A–C correspond to the three herbivore assemblages indicated on the x axis of D. The three herbivore combinations represent a series of herbivore diversity indicating simplification from the full Pleistocene fauna to the common late Holocene condition. We predict that removal of megaherbivores would result in (A) increased woody plant abundance, (B) reduced percentage of light-demanding species, and (C) increased fire frequency, depending on the densities of the remaining wild herbivores. (D) The resulting landscape structure. In essence, over time, the landscape developed in many areas from open in the Late Pleistocene, with high densities of diverse herbivore assemblages (D, Top Right), to defaunated wild herbivore communities controlled at low densities in the Holocene, resulting in a wooded landscape (D, Bottom Left), unless livestock is introduced, which could take over the role of native extinct grazers, resulting in a wood pasture (D, Middle). In the wood pasture, palatable light-demanding trees can regenerate within the protection of light-demanding thorny shrubs. When browsers are not managed, they can reach high densities, resulting in an open landscape with unpalatable, light-demanding trees (D, Top Left).
Landscape geomorphology plays an important role in sustaining particularly megaherbivore densities because these animals often tend to avoid steep slopes. Therefore, high concentrations of large herbivores are more often found in a plains habitat than in steep terrain, both in modern times and in the Pleistocene, resulting in higher impacts on woody plants and greater openness on plains (96, 97). The effect of terrain may be amplified by hydrology because both modern and Pleistocene large and megaherbivores frequently visit water bodies, resulting in enhanced local landscape openness around fresh water (98). Modern large herbivore communities reach their highest densities and diversities at sites of high soil fertility, due to high food quantity and quality (99, 100). Plant traits confirm this pattern because woody species in fertile areas defend themselves heavily with thorns or tolerate herbivory through rapid growth, indicating adaptations to high browsing pressure (35, 47, 101, 102). Similarly, during the Last Interglacial, open vegetation was found in European lowlands whereas less fertile uplands were more wooded, suggesting a larger herbivore impact in fertile habitats (Table 1) (82).
Although the places in the landscape attracting the highest densities of large herbivores can be identified, the absolute densities and the fluctuations therein of Pleistocene large herbivore assemblages remain difficult to determine. In the Late Pleistocene, communities of large predators were also more diverse than today, and they probably limited the densities or habitat use of large herbivores (103). Despite this predator diversity, Late Pleistocene densities of large herbivores at the mammoth steppe in Alaska and northeastern Siberia have been estimated at 88 and 105 kg⋅ha−1, respectively, during the Last Glacial (61, 85). In the Last Interglacial in Great Britain, densities were estimated at ≥2.5 large ungulates per hectare in over half of the studied sites (81), which amounts to ≥125 kg⋅ha−1 (at a mean ungulate weight of 50 kg). These densities of Pleistocene large herbivores are in the range of the African game reserves (9–191 kg⋅ha−1 [in Pachzelt et al. (104)] and imply strong impacts on woody plants. They also suggest that at least some landscapes were kept open by herbivory, given that to allow regeneration of modern temperate closed-canopy forests herbivore densities (deer) have to be very low (<3.5 kg⋅ha−1) and that at densities of >25 kg⋅ha−1 the forest is transformed toward oak savanna or wood-pasture (39). The temperate wood–pastures, in turn, respond fundamentally differently to herbivory than closed-canopy forests because here trees can persist by regeneration within light-demanding thorny shrubs, also in the presence of high densities of large ungulates at fertile soils (110–187 kg⋅ha−1) (35, 47, 105, 106). More estimates of densities of Pleistocene herbivores would greatly advance our understanding of their ecological impacts.
Perspectives for Future Research
The paleoecological record provides several examples that support the hypothesis that the Quaternary extinctions of megaherbivores rapidly changed vegetation composition and structure in different regions, but further tests are needed to confirm the generality of these findings. We propose the following approaches to provide such evidence.
Geographically Spread Paleoecological Records.
More detailed, chronological records of herbivore abundance and vegetation change from the Late Pleistocene-to-Holocene transition are needed to determine whether herbivore decline preceded vegetation change or not. Sampling more lake and peatland cores for both pollen and Sporormiella would yield such data to allow generalization of the few currently available studies (75, 76), taking into account improvements in the calibration of Sporormiella (77). Furthermore, comparisons of landscape openness and herbivore abundance before and after megafaunal extinctions under similar climatic conditions yields evidence of the impact of large herbivores [e.g., Sandom et al. (81)]. In both cases, the use of multiple proxies for herbivore density and abundance of woody plants would be valuable (Table 2) because each proxy will have its own limitations. Proxies need to be calibrated with modern data. Geographical spread of the sample locations across continents would allow for further generalization of herbivore impact across taxonomically very different faunas. These samples should be distributed over gradients of climate, fertility, and topography to assess the abiotic factors governing the impact of large herbivores on vegetation.
Table 2.
Characteristics of a herbivore-influenced landscape that can be found in the paleoecological record
| Component | Large herbivore-influenced landscape has | Proxies in the paleoecological record |
| Herbivores | Presence of large herbivores | Fungi: dung fungi spores (77, 118) |
| Fossil beetles: dung beetles (81, 119) | ||
| Herbivores: fossil remains of herbivores or dung (61, 66, 85) | ||
| Fossilized herbivore tracks (69, 98) | ||
| Vegetation composition | Light-demanding plant species | Plants: pollen, macrofossils, ancient DNA, stable isotopes (63, 75, 76, 120) |
| Thorny, unpalatable or herbivory-tolerant plant species | ||
| Higher ratio of C4 grasses over C3 forbs | ||
| Woody plant abundance and landscape structure | Landscape openness where climatic conditions predict more closed woody plant cover | Fossil beetles: proportion of wood beetles from terrestrial beetles (83, 84) |
| Plants: pollen, macrofossils, ancient DNA, stable isotopes to determine woody plant abundance (delta 13C) (121) | ||
| Other fauna: bat species associated with open or more wooded landscapes (120) | ||
| Plant biomass | Reduced plant biomass and low fire frequency in fire-prone landscapes | Charcoal (10, 75) |
Single characteristics may also be caused by other factors: in particular, fire and climatic conditions. The combination of properties should provide clues to the likelihood of large herbivores or other factors driving the observed patterns, which could further be tested by large-scale modeling or experiments, combining modern ecology and paleoecology.
Large-Scale Modeling.
Modeling climate envelopes in which biomes occur, when regulated by climate conditions alone, allows identification under what conditions large herbivore impacts would potentially make a strong difference. Especially where alternative vegetation states are climatically possible, this approach generates testable hypotheses about the impact of herbivores, such as in the case of the mammoth steppe and savannas (85, 107). These hypotheses could subsequently be addressed either by adding herbivores in the model, using literature data or performing experiments. Similarly, the present-day landscape structure and woody species composition can be linked to abundance and species richness of large herbivore assemblages over large biogeographical areas [see, for instance, Greve et al. (108)]. This relationship will yield baseline data that can be applied to Late Pleistocene conditions to predict what the landscape structure was, given estimates of large herbivore abundances. Furthermore, mechanistic models can be used to predict the interaction between herbivore communities and vegetation. Recently, large-scale coupling of physiologically based vegetation and herbivore population models has been applied to predict herbivore population dynamics at continental scales (104). This approach could also be used to predict herbivore impact on the vegetation.
Experiments.
Because woody species have long generation times and spatial heterogeneity in landscape structure is a key feature of wooded habitats under herbivory, small-scale and short-term exclosures may capture only part of the resulting woody plant dynamics at the landscape scale. Therefore, long-term and large-scale experiments—including unintentional experiments—are extremely valuable to determine large herbivore impact on woody plants (12). These conditions can be found, for instance, at the Finnish–Russian border [which had markedly different reindeer densities across the border (109)], in ongoing and future rewilding projects (110), and in forest reserves where large herbivores were removed to protect tree regeneration (42). In this respect, the forestry literature may offer valuable information about the regeneration ecology, competitive ability, and herbivore tolerance of woody species (42, 44). Such long-term fencing studies are also very useful to test proxies for herbivore and woody species abundance in an experimental setting (111).
Study of Contemporary Large Herbivore-to-Megaherbivore Impacts.
Studies on current megaherbivore impacts are extremely valuable because these animals are the only proxies that we have for extinct megafauna. Better insight into the behavior, habitat preferences, and whole ecosystem functions of large herbivores is required (112) to predict their impact on landscape structure. Because most are experiencing alarming declines, some may already be too rare to study whereas, for several species, the wider impacts of their ecosystem engineering effects only very recently have started to become clear (8, 113, 114).
Conclusions
Given the ecological importance of modern large herbivores, we see the end-Pleistocene reduction in diversity and biomass of such animals as being a significant event in global ecology. Growing evidence supports the hypothesis that the loss of large herbivores strongly affected woody plants and triggered regime shifts across the World’s biomes. Linking large herbivores and their impact on vegetation at Quaternary timescales is an enormous task, but an interdisciplinary approach that combines proxy records and modeling grounded by modern studies will help to link pattern and process in the paleoecological record (Table 2). Modern large herbivores are now among some of the most threatened species, facing the combined threats of anthropogenic land use and climate change (8). The ecological consequences of the end-Pleistocene extinctions are therefore relevant not only to understanding the vegetation changes of the early Holocene, but also to the management of ecosystems in the Anthropocene. In this respect, modern and paleoecological analyses have much to contribute to one another. Testing hypotheses to explain the variation in effects of large herbivores and their decline on woody plants and landscape structure should be a priority for future work. This hypothesis testing should integrate the effects of fire and herbivory across large abiotic and geographical gradients to obtain a better understanding of herbivore regulation of woody plants and the importance of herbivore body size, density, and diversity.
Acknowledgments
We thank the people who set up and maintain the exclosure in Yellowstone National Park that we photographed as illustration in Fig. 1E. J.-C.S. was supported by European Research Council Grant ERC-2012-StG-310886-HISTFUNC. We additionally consider this article a contribution to the Danish National Research Foundation Niels Bohr Professorship Project Aarhus University Research on the Anthropocene. This manuscript is number 5926 from the Netherlands Institute of Ecology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.E.D. is a guest editor invited by the Editorial Board.
References
- 1.Sandom C, Faurby S, Sandel B, Svenning JC. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc R Soc Lond B Biol Sci. 2014;281(1787):20133254. doi: 10.1098/rspb.2013.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch PL, Barnosky AD. Late quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 3.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 4.Zalasiewicz J, et al. When did the Anthropocene begin? A mid-twentieth century boundary level is stratigraphically optimal. Quat Int. 2015 doi: 10.1016/j.quaint.2014.11.045. in press. [DOI] [Google Scholar]
- 5.McCauley DJ, et al. Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 6.Young HS, et al. Effects of mammalian herbivore declines on plant communities: Observations and experiments in an African savanna. J Ecol. 2013;101(4):1030–1041. doi: 10.1111/1365-2745.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 8.Ripple WJ, et al. Collapse of the world’s largest herbivores. Sci Adv. 2015;1(4):e1400103:1–12. doi: 10.1126/sciadv.1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danell K, Bergström R, Duncan P, Pastor J, editors. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge Univ Press; Cambridge, UK: 2006. [Google Scholar]
- 10.Johnson CN. Ecological consequences of Late Quaternary extinctions of megafauna. Proc R Soc Lond B Biol Sci. 2009;276(1667):2509–2519. doi: 10.1098/rspb.2008.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill JL. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 2014;201(4):1163–1169. doi: 10.1111/nph.12576. [DOI] [PubMed] [Google Scholar]
- 12.Asner GP, et al. Large-scale impacts of herbivores on the structural diversity of African savannas. Proc Natl Acad Sci USA. 2009;106(12):4947–4952. doi: 10.1073/pnas.0810637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sankaran M, Augustine DJ, Ratnam J. Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna. J Ecol. 2013;101(6):1389–1399. [Google Scholar]
- 14.Augustine DJ, McNaughton SJ. Regulation of shrub dynamics by native browsing ungulates on East African rangeland. J Appl Ecol. 2004;41(1):45–58. [Google Scholar]
- 15.Keesing F, Young TP. Cascading consequences of the loss of large mammals in an African savanna. Bioscience. 2014;64(6):487–495. [Google Scholar]
- 16.Pringle RM, et al. Low functional redundancy among mammalian browsers in regulating an encroaching shrub (Solanum campylacanthum) in African savannah. Proc R Soc Lond B Biol Sci. 2014;281(1785):20140390. doi: 10.1098/rspb.2014.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staver AC, Bond WJ. Is there a ‘browse trap’? Dynamics of herbivore impacts on trees and grasses in an African savanna. J Ecol. 2014;102(3):595–602. [Google Scholar]
- 18.Owen-Smith N. Megafaunal extinctions: The conservation message from 11,000 years B.p. Conserv Biol. 1989;3(4):405–412. doi: 10.1111/j.1523-1739.1989.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 19.Guldemond R, Van Aarde R. A meta-analysis of the impact of African elephants on savanna vegetation. J Wildl Manage. 2008;72(4):892–899. [Google Scholar]
- 20.Maron JL, Crone E. Herbivory: Effects on plant abundance, distribution and population growth. Proc Biol Sci. 2006;273(1601):2575–2584. doi: 10.1098/rspb.2006.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen-Smith RN. Megaherbivores: The Influence of Very Large Body Size on Ecology. Cambridge Univ Press; Cambridge, UK: 1988. [Google Scholar]
- 22.Asner GP, Levick SR. Landscape-scale effects of herbivores on treefall in African savannas. Ecol Lett. 2012;15(11):1211–1217. doi: 10.1111/j.1461-0248.2012.01842.x. [DOI] [PubMed] [Google Scholar]
- 23.Kimuyu DM, Sensenig RL, Riginos C, Veblen KE, Young TP. Native and domestic browsers and grazers reduce fuels, fire temperatures, and acacia ant mortality in an African savanna. Ecol Appl. 2014;24(4):741–749. doi: 10.1890/13-1135.1. [DOI] [PubMed] [Google Scholar]
- 24.Kuiters AT, van der Sluijs LAM, Wytema GA. Selective bark-stripping of beech, Fagus sylvatica, by free-ranging horses. For Ecol Manage. 2006;222(1–3):1–8. [Google Scholar]
- 25.Prins HHT, Van der Jeugd HP. Herbivore population crashes and woodland structure in East Africa. J Ecol. 1993;81(2):305–314. [Google Scholar]
- 26.O’Kane CAJ, Duffy KJ, Page BR, Macdonald DW. Are the long-term effects of mesobrowsers on woodland dynamics substitutive or additive to those of elephants? Acta Oecol. 2011;37(5):393–398. [Google Scholar]
- 27.Moe SR, Rutina LP, Hytteborn H, du Toit JT. What controls woodland regeneration after elephants have killed the big trees? J Appl Ecol. 2009;46(1):223–230. [Google Scholar]
- 28.Keesing F. Cryptic consumers and the ecology of an African Savanna. Bioscience. 2000;50(6):205–215. [Google Scholar]
- 29.du Toit JT. Feeding height stratification among African browsing ruminants. Afr Ecol. 1990;28(1):55–61. [Google Scholar]
- 30.Goheen JR, Palmer TM, Keesing F, Riginos C, Young TP. Large herbivores facilitate savanna tree establishment via diverse and indirect pathways. J Anim Ecol. 2010;79(2):372–382. doi: 10.1111/j.1365-2656.2009.01644.x. [DOI] [PubMed] [Google Scholar]
- 31.Maclean JE, Goheen JR, Doak DF, Palmer TM, Young TP. Cryptic herbivores mediate the strength and form of ungulate impacts on a long-lived savanna tree. Ecology. 2011;92(8):1626–1636. doi: 10.1890/10-2097.1. [DOI] [PubMed] [Google Scholar]
- 32.Codron D, et al. Diets of savanna ungulates from stable carbon isotope composition of faeces. J Zool (Lond) 2007;273(1):21–29. [Google Scholar]
- 33.Gill R. The influence of large herbivores on tree recruitment and forest dynamics. In: Danell K, Bergström R, Duncan P, Pastor J, editors. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge Univ Press; Cambridge, UK: 2006. pp. 170–202. [Google Scholar]
- 34.Ford AT, et al. Large carnivores make savanna tree communities less thorny. Science. 2014;346(6207):346–349. doi: 10.1126/science.1252753. [DOI] [PubMed] [Google Scholar]
- 35.Vera FWM, Bakker ES, Olff H. Large herbivores: Missing partners of western European light-demanding tree and shrub species? In: Danell K, Bergström R, Duncan P, Pastor J, editors. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. Cambridge Univ Press; Cambridge, UK: 2006. pp. 203–231. [Google Scholar]
- 36.Pastor J, Dewey B, Naiman RJ, McInnes PF, Cohen Y. Moose browsing and soil fertility in the boreal forest of Isle Royale National Park. Ecology. 1993;74(2):467–480. [Google Scholar]
- 37.McInnes PF, Naiman RJ, Pastor J, Cohen Y. Effects of moose browsing on vegetation and litter of the boreal forest, Isle Royale, Michigan, USA. Ecology. 1992;73(6):2059–2075. [Google Scholar]
- 38.Hidding B, Tremblay JP, Côté SD. A large herbivore triggers alternative successional trajectories in the boreal forest. Ecology. 2013;94(12):2852–2860. doi: 10.1890/12-2015.1. [DOI] [PubMed] [Google Scholar]
- 39.Tanentzap AJ, et al. Seeing the forest for the deer: Do reductions in deer-disturbance lead to forest recovery? Biol Conserv. 2011;144(1):376–382. [Google Scholar]
- 40.Wigley BJ, Fritz H, Coetsee C, Bond WJ. Herbivores shape woody plant communities in the Kruger National Park: Lessons from three long-term exclosures. Koedoe. 2014;56(1):1–12. [Google Scholar]
- 41.Bernadzki E, Bolibok L, Brzeziecki B, Zajaczkowski J, Zybura H. Compositional dynamics of natural forests in the Bialowieza National Park, northeastern Poland. J Veg Sci. 1998;9(2):229–238. [Google Scholar]
- 42.Vera FWM. Grazing Ecology and Forest History. CAB International; Wallingford, UK: 2000. [Google Scholar]
- 43.Jedrzejewska B, Jedrzejewski W, Bunevich AN, Milkowski L, Krasinski ZA. Factors shaping population densities and increase rates of ungulates in Bialowieza Primeval Forest (Poland and Belarus) in the 19th and 20th centuries. Acta Theriol (Warsz) 1997;42(4):399–451. [Google Scholar]
- 44.Pigott D. Lime-Trees and Basswoods: A Biological Monograph of the Genus Tilia. Cambridge Univ Press; Cambridge, UK: 2012. [Google Scholar]
- 45.Kuijper DPJ, et al. Landscape of fear in Europe: Wolves affect spatial patterns of ungulate browsing in Bialowieza Primeval Forest, Poland. Ecography. 2013;36(12):1263–1275. [Google Scholar]
- 46.Olff H, et al. Shifting mosaics in grazed woodlands driven by the alternation of plant facilitation and competition. Plant Biol. 1999;1(2):127–137. [Google Scholar]
- 47.Bakker ES, et al. Ecological anachronisms in the recruitment of temperate light-demanding tree species in wooded pastures. J Appl Ecol. 2004;41(3):571–582. [Google Scholar]
- 48.Smit C, Vandenberghe C, den Ouden J, Müller-Schärer H. Nurse plants, tree saplings and grazing pressure: Changes in facilitation along a biotic environmental gradient. Oecologia. 2007;152(2):265–273. doi: 10.1007/s00442-006-0650-6. [DOI] [PubMed] [Google Scholar]
- 49.Milchunas DG, Noy-Meir I. Grazing refuges, external avoidance of herbivory and plant diversity. Oikos. 2002;99(1):113–130. [Google Scholar]
- 50.Smit C, Kuijper DPJ, Prentice D, Wassen MJ, Cromsigt J. Coarse woody debris facilitates oak recruitment in Bialowieza Primeval Forest, Poland. For Ecol Manage. 2012;284:133–141. [Google Scholar]
- 51.Lima SL, Dill L. Behavioral decisions made under the risk of predation: A review and prospectus. Can Zool. 1990;68(4):619–640. [Google Scholar]
- 52.Kauffman MJ, et al. Landscape heterogeneity shapes predation in a newly restored predator-prey system. Ecol Lett. 2007;10(8):690–700. doi: 10.1111/j.1461-0248.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 53.Riginos C, Grace JB. Savanna tree density, herbivores, and the herbaceous community: Bottom-up vs. top-down effects. Ecology. 2008;89(8):2228–2238. doi: 10.1890/07-1250.1. [DOI] [PubMed] [Google Scholar]
- 54.Laundre JW, Hernandez L, Altendorf KB. Wolves, elk, and bison: Re-establishing the “landscape of fear” in Yellowstone National Park, USA. Can Zool. 2001;79(8):1401–1409. [Google Scholar]
- 55.Painter LE, Beschta RL, Larsen EJ, Ripple WJ. Recovering aspen follow changing elk dynamics in Yellowstone: Evidence of a trophic cascade? Ecology. 2015;96(1):252–263. doi: 10.1890/14-0712.1. [DOI] [PubMed] [Google Scholar]
- 56.Callan R, Nibbelink NP, Rooney TP, Wiedenhoeft JE, Wydeven AP. Recolonizing wolves trigger a trophic cascade in Wisconsin (USA) J Ecol. 2013;101(4):837–845. [Google Scholar]
- 57.Metcalfe JZ, Longstaffe FJ, Hodgins G. Proboscideans and paleoenvironments of the Pleistocene Great Lakes: Landscape, vegetation, and stable isotopes. Quat Sci Rev. 2013;76:102–113. [Google Scholar]
- 58.Teale CL, Miller NG. Mastodon herbivory in mid-latitude late-Pleistocene boreal forests of eastern North America. Quat Res. 2012;78(1):72–81. [Google Scholar]
- 59.Owen-Smith N. Pleistocene extinctions: The pivotal role of megaherbivores. Paleobiology. 1987;13(3):351–362. [Google Scholar]
- 60.Green JL, Semprebon GM, Solounias N. Reconstructing the palaeodiet of Florida Mammut americanum via low-magnification stereomicroscopy. Palaeogeogr Palaeocl. 2005;223(1-2):34–48. [Google Scholar]
- 61.Mann DH, Groves P, Kunz ML, Reanier RE, Gaglioti BV. Ice-age megafauna in Arctic Alaska: Extinction, invasion, survival. Quat Sci Rev. 2013;70:91–108. [Google Scholar]
- 62.Franca LD, et al. Review of feeding ecology data of Late Pleistocene mammalian herbivores from South America and discussions on niche differentiation. Earth Sci Rev. 2015;140:158–165. [Google Scholar]
- 63.Willerslev E, et al. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature. 2014;506(7486):47–51. doi: 10.1038/nature12921. [DOI] [PubMed] [Google Scholar]
- 64.van Asperen EN, Kahlke RD. Dietary variation and overlap in Central and Northwest European Stephanorhinus kirchbergensis and S. hemitoechus (Rhinocerotidae, Mammalia) influenced by habitat diversity. Quat Sci Rev. 2015;107:47–61. [Google Scholar]
- 65.Kissling WD, et al. Establishing macroecological trait datasets: Digitalization, extrapolation, and validation of diet preferences in terrestrial mammals worldwide. Ecol Evol. 2014;4(14):2913–2930. doi: 10.1002/ece3.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lister A, Bahn P. Mammoths: Giants of the Ice Age. Univ of California Press; Oakland, CA: 2009. [Google Scholar]
- 67.Janzen DH, Martin PS. Neotropical anachronisms: The fruits the gomphotheres ate. Science. 1982;215(4528):19–27. doi: 10.1126/science.215.4528.19. [DOI] [PubMed] [Google Scholar]
- 68.Campos-Arceiz A, Blake S. Megagardeners of the forest: The role of elephants in seed dispersal. Acta Oecol. 2011;37(6):542–553. [Google Scholar]
- 69.Haynes G. Elephants (and extinct relatives) as earth-movers and ecosystem engineers. Geomorphology. 2012;157:99–107. [Google Scholar]
- 70.Zimov SA, et al. Steppe-tundra transition: A herbivore-driven biome shift at the end of the pleistocene. Am Nat. 1995;146(5):765–794. [Google Scholar]
- 71.Kgope BS, Bond WJ, Midgley GF. Growth responses of African savanna trees implicate atmospheric CO2 as a driver of past and current changes in savanna tree cover. Austral Ecol. 2010;35(4):451–463. [Google Scholar]
- 72.Ward JK, et al. Carbon starvation in glacial trees recovered from the La Brea tar pits, southern California. Proc Natl Acad Sci USA. 2005;102(3):690–694. doi: 10.1073/pnas.0408315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burney DA, Robinson GS, Burney LP. Sporormiella and the late Holocene extinctions in Madagascar. Proc Natl Acad Sci USA. 2003;100(19):10800–10805. doi: 10.1073/pnas.1534700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson GS, Burney LP, Burney DA. Landscape paleoecology and megafaunal extinction in southeastern New York state. Ecol Monogr. 2005;75(3):295–315. [Google Scholar]
- 75.Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–1103. doi: 10.1126/science.1179504. [DOI] [PubMed] [Google Scholar]
- 76.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 77.Johnson CN, et al. Using dung fungi to interpret decline and extinction of megaherbivores: problems and solutions. Quat Sci Rev. 2015;110:107–113. [Google Scholar]
- 78.Gill JL, Williams JW, Jackson ST, Donnelly JP, Schellinger GC. Climatic and megaherbivory controls on late-glacial vegetation dynamics: A new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat Sci Rev. 2012;34:66–80. [Google Scholar]
- 79.Faison EK, Foster DR, Oswald WW, Hansen BCS, Doughty E. Early holocene openlands in southern New England. Ecology. 2006;87(10):2537–2547. doi: 10.1890/0012-9658(2006)87[2537:ehoisn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 80.Weigl PD, Knowles TW. Temperate mountain grasslands: A climate-herbivore hypothesis for origins and persistence. Biol Rev Camb Philos Soc. 2014;89(2):466–476. doi: 10.1111/brv.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sandom CJ, Ejrnæs R, Hansen MDD, Svenning JC. High herbivore density associated with vegetation diversity in interglacial ecosystems. Proc Natl Acad Sci USA. 2014;111(11):4162–4167. doi: 10.1073/pnas.1311014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svenning J-C. A review of natural vegetation openness in north-western Europe. Biol Conserv. 2002;104(2):133–148. [Google Scholar]
- 83.Alexander K. Wood decay, insects, palaeoecology, and woodland conservation policy and practice: Breaking the halter. Antenna. 2005;29:171–178. [Google Scholar]
- 84.Whitehouse NJ, Smith D. How fragmented was the British Holocene wildwood? Perspectives on the “Vera” grazing debate from the fossil beetle record. Quat Sci Rev. 2010;29(3-4):539–553. [Google Scholar]
- 85.Zimov SA, Zimov NS, Tikhonov AN, Chapin FS. Mammoth steppe: A high-productivity phenomenon. Quat Sci Rev. 2012;57:26–45. [Google Scholar]
- 86.Kienast F, et al. Paleontological records indicate the occurrence of open woodlands in a dry inland climate at the present-day Arctic coast in western Beringia during the Last Interglacial. Quat Sci Rev. 2011;30(17-18):2134–2159. [Google Scholar]
- 87.Olofsson J, et al. Herbivores inhibit climate-driven shrub expansion on the tundra. Glob Change Biol. 2009;15(11):2681–2693. [Google Scholar]
- 88.Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci USA. 2008;105(34):12353–12358. doi: 10.1073/pnas.0802421105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barlow C. The Ghosts of Evolution: Nonsensical Fruit, Missing Partners, and Other Ecological Anachronisms. Basic Books; New York: 2002. [Google Scholar]
- 90.Zaya DN, Howe HF. The anomalous Kentucky coffeetree: Megafaunal fruit sinking to extinction? Oecologia. 2009;161(2):221–226. doi: 10.1007/s00442-009-1372-3. [DOI] [PubMed] [Google Scholar]
- 91.Perry GLW, Wheeler AB, Wood JR, Wilmshurst JM. A high-precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes) Quat Sci Rev. 2014;105:126–135. [Google Scholar]
- 92.Bond WJ, Lee WG, Craine JM. Plant structural defences against browsing birds: A legacy of New Zealand’s extinct moas. Oikos. 2004;104(3):500–508. [Google Scholar]
- 93.Wilson JB, Lee WG. Is New Zealand vegetation really ‘problematic’? Dansereau’s puzzles revisited. Biol Rev Camb Philos Soc. 2012;87(2):367–389. doi: 10.1111/j.1469-185X.2011.00202.x. [DOI] [PubMed] [Google Scholar]
- 94.Bond WJ, Silander JA. Springs and wire plants: Anachronistic defences against Madagascar’s extinct elephant birds. Proc Biol Sci. 2007;274(1621):1985–1992. doi: 10.1098/rspb.2007.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee WG, Wood JR, Rogers GM. Legacy of avian-dominated plant–herbivore systems in New Zealand. New Zeal Ecol. 2010;34(1):28–47. [Google Scholar]
- 96.Colgan MS, Asner GP, Levick SR, Martin RE, Chadwick OA. Topo-edaphic controls over woody plant biomass in South African savannas. Biogeosciences. 2012;9(5):1809–1821. [Google Scholar]
- 97.Diedrich CG. Cave bear killers and scavengers from the last ice age of central Europe: Feeding specializations in response to the absence of mammoth steppe fauna from mountainous regions. Quat Int. 2012;255:59–78. [Google Scholar]
- 98.Pop E, Bakels C. Semi-open environmental conditions during phases of hominin occupation at the Eemian Interglacial basin site Neumark-Nord 2 and its wider environment. Quat Sci Rev. 2015;117:72–81. [Google Scholar]
- 99.Fritz H, Duncan P. On the carrying capacity for large ungulates of African savanna ecosystems. Proc Biol Sci. 1994;256(1345):77–82. doi: 10.1098/rspb.1994.0052. [DOI] [PubMed] [Google Scholar]
- 100.Olff H, Ritchie ME, Prins HHT. Global environmental controls of diversity in large herbivores. Nature. 2002;415(6874):901–904. doi: 10.1038/415901a. [DOI] [PubMed] [Google Scholar]
- 101.Cromsigt J, Kuijper DPJ. Revisiting the browsing lawn concept: Evolutionary interactions or pruning herbivores? Perspect Plant Ecol Evol Syst. 2011;13(3):207–215. [Google Scholar]
- 102.Kuijper DPJ, et al. Bottom-up versus top-down control of tree regeneration in the Bialowieza Primeval Forest, Poland. J Ecol. 2010;98(4):888–899. [Google Scholar]
- 103.Van Valkenburgh B, Hayward MW, Ripple WJ, Meloro C, Roth VL. The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc Natl Acad Sci USA. 2016;113:862–867. doi: 10.1073/pnas.1502554112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pachzelt A, Rammig A, Higgins S, Hickler T. Coupling a physiological grazer population model with a generalized model for vegetation dynamics. Ecol Modell. 2013;263:92–102. [Google Scholar]
- 105.Rackham O. Ancient Woodland: Its History, Vegetation and Uses in England. Edward Arnold; London: 1980. [Google Scholar]
- 106.Putman RJ. Grazing in Temperate Ecosystems: Large Herbivores and the Ecology of the New Forest. Croom Helm; London, UK: 1986. [Google Scholar]
- 107.Staver AC, Archibald S, Levin SA. The global extent and determinants of savanna and forest as alternative biome states. Science. 2011;334(6053):230–232. doi: 10.1126/science.1210465. [DOI] [PubMed] [Google Scholar]
- 108.Greve M, et al. Continental-scale variability in browser diversity is a major driver of diversity patterns in acacias across Africa. J Ecol. 2012;100(5):1093–1104. [Google Scholar]
- 109.Vare H, Ohtonen R, Mikkola K. The effect and extent of heavy grazing by reindeer in oligotrophic pine heaths in northeastern Fennoscandia. Ecography. 1996;19(3):245–253. [Google Scholar]
- 110.Svenning J-C, et al. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc Natl Acad Sci USA. 2015;113:898–906. doi: 10.1073/pnas.1502556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitchell FJG, Cole E. Reconstruction of long-term successional dynamics of temperate woodland in Bialowieza Forest, Poland. J Ecol. 1998;86(6):1042–1059. [Google Scholar]
- 112.Kerley GIH, Kowalczyk R, Cromsigt J. Conservation implications of the refugee species concept and the European bison: King of the forest or refugee in a marginal habitat? Ecography. 2012;35(6):519–529. [Google Scholar]
- 113.Cromsigt J, te Beest M. Restoration of a megaherbivore: Landscape-level impacts of white rhinoceros in Kruger National Park, South Africa. J Ecol. 2014;102(3):566–575. [Google Scholar]
- 114.Pennisi E. The river masters. Science. 2014;346(6211):802–805. doi: 10.1126/science.346.6211.802. [DOI] [PubMed] [Google Scholar]
- 115.Bond WJ. What limits trees in C4 grasslands and savannas? Annu Rev Ecol Evol Syst. 2008;39:641–659. [Google Scholar]
- 116.Sankaran M, Ratnam J, Hanan N. Woody cover in African savannas: The role of resources, fire and herbivory. Glob Ecol Biogeogr. 2008;17(2):236–245. [Google Scholar]
- 117.Bond WJ, Keeley JE. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol Evol. 2005;20(7):387–394. doi: 10.1016/j.tree.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 118.Gill JL, et al. Linking abundances of the dung fungus Sporormiella to the density of bison: Implications for assessing grazing by megaherbivores in palaeorecords. J Ecol. 2013;101(5):1125–1136. [Google Scholar]
- 119.Smith D, Nayyar K, Schreve D, Thomas R, Whitehouse N. Can dung beetles from the palaeoecological and archaeological record indicate herd concentration and the identity of herbivores? Quat Int. 2014;341:119–130. [Google Scholar]
- 120.Widga C, Colburn M. Paleontology and paleoecology of guano deposits in Mammoth Cave, Kentucky, USA. Quat Res. 2015;83(3):427–436. [Google Scholar]
- 121.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 122.Asner GP, et al. Carnegie Airborne Observatory: In-flight fusion of hyperspectral imaging and waveform light detection and ranging (wLiDAR) for three-dimensional studies of ecosystems. J Appl Remote Sens. 2007;1:013536. [Google Scholar]
- 123.Cornelissen P, Gresnigt MC, Vermeulen RA, Bokdam J, Smit R. Transition of a Sambucus nigra L. dominated woody vegetation into grassland by a multi-species herbivore assemblage. J Nat Conserv. 2014;22(1):84–92. [Google Scholar]