Significance
Hendra virus (HeV) is a deadly member of the Henipavirus genus of paramyxoviruses, which causes high mortality in humans and horses. We determined the crystal structure of the HeV fusion protein, F, in its metastable, prefusion conformation. The structure is highly conserved compared with parainfluenza virus 5 (PIV5) F, but divergent from respiratory syncytial virus (RSV) F. The structural similarities suggest a common mode of activation for PIV5 and HeV F despite low sequence homology. Structural differences in the HeV F cleavage/activation loop are observed that may be explained by a requirement for cleavage by cathepsins. The HeV F structure was used to predict disulfide bonds that stabilize its prefusion conformation, providing a construct for vaccine and functional studies.
Keywords: paramyxovirus F protein, F-protein atomic structure, metastable F-protein stabilization, Hendra virus, membrane fusion
Abstract
Hendra virus (HeV) is one of the two prototypical members of the Henipavirus genus of paramyxoviruses, which are designated biosafety level 4 (BSL-4) organisms due to the high mortality rate of Nipah virus (NiV) and HeV in humans. Paramyxovirus cell entry is mediated by the fusion protein, F, in response to binding of a host receptor by the attachment protein. During posttranslational processing, the fusion peptide of F is released and, upon receptor-induced triggering, inserts into the host cell membrane. As F undergoes a dramatic refolding from its prefusion to postfusion conformation, the fusion peptide brings the host and viral membranes together, allowing entry of the viral RNA. Here, we present the crystal structure of the prefusion form of the HeV F ectodomain. The structure shows very high similarity to the structure of prefusion parainfluenza virus 5 (PIV5) F, with the main structural differences in the membrane distal apical loops and the fusion peptide cleavage loop. Functional assays of mutants show that the apical loop can tolerate perturbation in length and surface residues without loss of function, except for residues involved in the stability and conservation of the F protein fold. Structure-based disulfide mutants were designed to anchor the fusion peptide to conformationally invariant residues of the F head. Two mutants were identified that inhibit F-mediated fusion by stabilizing F in its prefusion conformation.
The Paramyxoviridae family of viruses includes many species known to cause human and animal disease, including Nipah virus (NiV) and Hendra virus (HeV) of the genus Henipavirus (1). This emergent genus was first described in 1994 with a disease outbreak of HeV, followed by a disease outbreak of NiV in 1999 (2), and was recently expanded by the discovery of the Cedar virus (3) and evidence for 19 new species of African henipaviruses (4). Both NiV and HeV have caused outbreaks of encephalitic and respiratory illness in humans in Malaysia, Bangladesh, Australia, and several neighboring countries, with high morbidity and mortality, and are designated biosafety level 4 (BSL-4) organisms (5, 6). The animal reservoir for NiV and HeV is Pteropus spp. fruit bats. These viruses are transmitted to humans from an intermediate animal vector, pigs in the case of NiV and horses in the case of HeV. Serological and genetic evidence for henipaviruses has been discovered in Pteropus far from known locations of disease incidence (6).
Like other paramyxoviruses, the henipaviruses are enveloped viruses that are densely studded on their outer surfaces with the two transmembrane-anchored glycoproteins involved in entry of the virion into host cells via membrane fusion (7). These glycoproteins are the fusion glycoprotein, F, which mediates fusion of the viral lipid envelope with the host cell plasma membrane, and the attachment glycoprotein, G, in henipaviruses, which acts as a trigger for fusion upon specific recognition of the host cell receptors ephrinB2 and ephrinB3 (8, 9). Triggering is thought to occur via sequential conformational changes in G that are communicated to F while they associate in F–G complexes (10, 11). Exposure of stalk residues in G, which are thought to be occluded by its receptor-binding head domains before triggering, appear key to initiating F refolding and membrane fusion.
The trimeric F protein in its prefusion form has a globular conformation consisting of three domains (DI, DII, and DIII), followed by a C-terminal stalk, transmembrane domain, and cytoplasmic tail (12). DI and the Ig-like fold DII are implicated in interactions with the attachment protein (13, 14). F also contains two heptad repeats, HRA in DIII and HRB in the stalk. During posttranslational processing, the F0 precursor is cleaved by the cellular protease cathepsin L at a defined site following a basic residue, K109 in HeV F, resulting in release of the fusion peptide segment located C-terminal to the cleavage site. Cleavage results in two disulfide-linked fragments, F1 and F2 (15, 16). Upon activation, the F protein undergoes large-scale refolding, mostly in DIII. HRA is extended into a long α-helix, which forms a six-helix bundle with the HRB region of the stalk. This refolded F forms a golf tee-shaped postfusion conformation (17), which is modeled to bring the host and virus membranes together as the fusion peptides inserted into the host-membrane oligomerize with the virion-embedded portion of the HRB stalk (12).
The atomic resolution structures of the prefusion forms of the F protein have been solved for two paramyxoviruses, parainfluenza virus 5 (PIV5) and respiratory syncytial virus (RSV) (12, 18). These two proteins exhibit fairly low sequence identity and significant differences in their structures, although several key domain features are conserved (18). HeV F shares low sequence similarity with PIV5 and lower sequence similarity with RSV (27.9% and 21.3% identity, respectively, calculated with ClustalX). The fusion (F)-attachment (HN) protein pair of PIV5, along with the fusion-attachment protein pair of Newcastle disease virus and human parainfluenza virus type 3, behave according to the “association” model of fusion activation. Although interaction is required for fusion, the F-HN pairs of these proteins have relatively low affinity for each other and host receptor binding is thought to bring the proteins into greater association at initiation of fusion. The F protein of RSV does not require its cognate attachment protein for virus fusion and replication. In contrast, the fusion-attachment protein pairs of the Morbillivirus genus (measles and canine distemper virus) and henipaviruses, NiV and HeV, behave more consistently with the “dissociation” model of fusion activation. Biochemical evidence suggests a relatively higher affinity for the fusion-attachment proteins and preassociation in complexes where dissociation only occurs upon receptor binding (8, 9, 19). To date, no high-resolution structural information has been available for fusion proteins in the dissociation class.
Here, we present the crystal structure of the HeV F ectodomain. Its structure has a high degree of structural similarity to the structure of PIV5 F despite low sequence similarity. The areas of greatest structural deviation are at the pair of loops at the apical region of the trimer and at the fusion peptide cleavage site. Site-directed mutagenesis within the apical loops showed that residues that have a role in maintaining tertiary structural integrity of the region have effects on fusion and cell surface expression. Based on the crystal structure, amino acid pairs with a likelihood of forming disulfide bonds were predicted computationally. Double-Cys mutants of HeV F were generated with the intent of preventing refolding of the HRA region that is crucial for fusion. Two of the mutants show loss of fusion activity, while being expressed at the cell surface and recognized by a mAb (5B3) specific for the prefusion conformational state.
Results and Discussion
Overall Structure of HeV F Is Similar to PIV5 F.
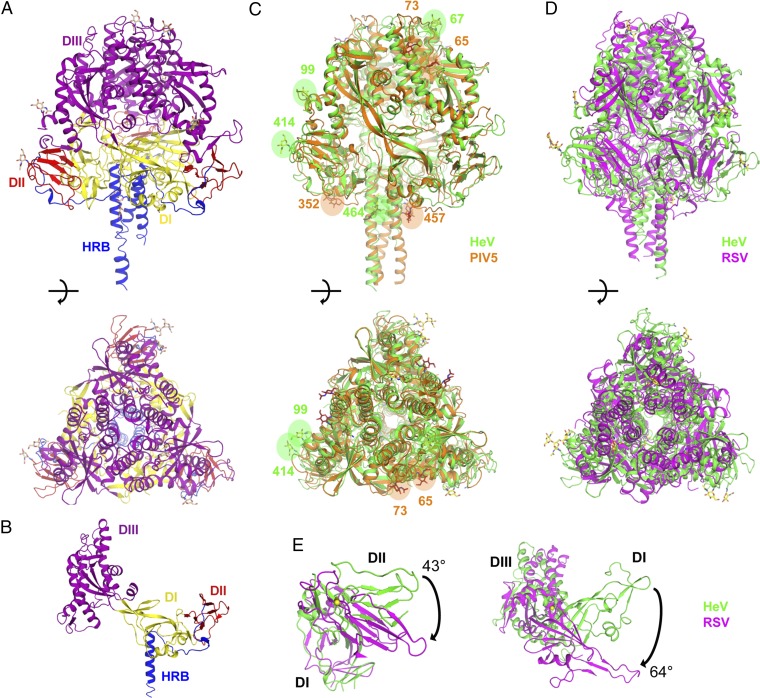
The HeV F ectodomain consisting of residues 26–482 fused to the GCN4 trimerization domain (12) was expressed in 293F cells and crystallized. X-ray diffraction data were collected to 3.2 Å, and a molecular replacement solution was found with the PIV5 prefusion F ectodomain (12) as a search model (Fig. S1 A–C and Table S1). The crystal structure of the HeV F ectodomain (Fig. 1 A–C) shows a high degree of structural conservation with the crystal structure of PIV5 F. In addition to high similarity between the folds of DI, DII, and DIII (Fig. 1B and Fig. S1), relative domain orientations are similar, resulting in an rmsd by secondary structure matching (SSM) of 2.27 Å between the ectodomains of HeV F and PIV5 F (Fig. 1C). Although conserved fold elements exist between the individual domains of RSV F (18) and HeV F (Fig. 1D), the overall correspondence of the structures is much poorer. In contrast to PIV5 F, significant differences in relative domain orientations exist between all three domains of the globular head between HeV F and RSV F (Fig. S1C). These differences are expressed as rotation of a domain about an axis following SSM superposition of a fixed domain, calculated with DynDom (20). The positions of DII in HeV F and RSV F relative to DI differ by 43°, and the positions of DIII relative to DI differ by 64° (Fig. 1E), resulting in an rmsd of 5.11 Å between the RSV and HeV F structures.
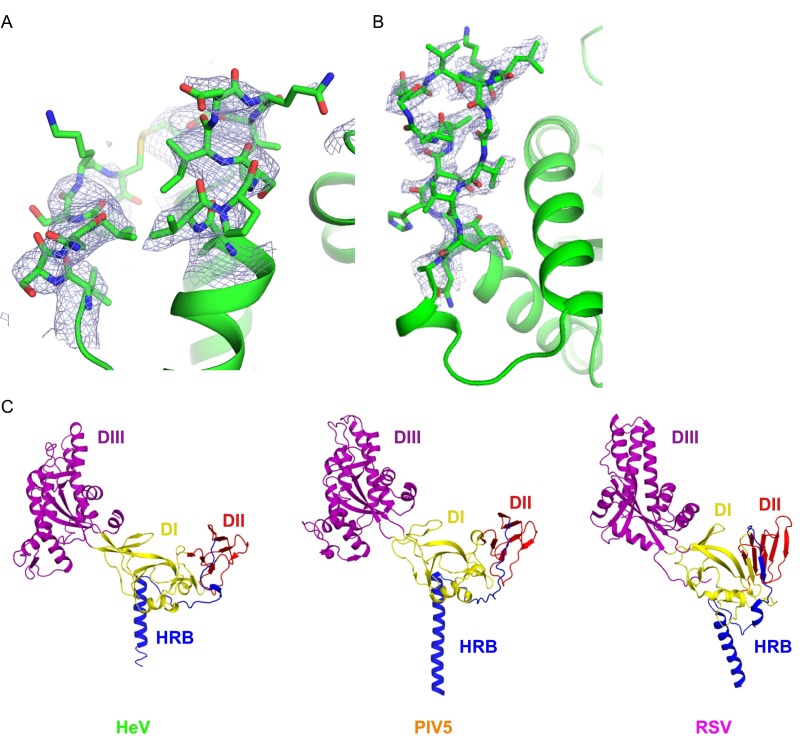
Fig. S1.
Iterative build omit electron density maps of HeV F and paramyxovirus prefusion F structure comparison. (A) Apical loops, map contoured at 0.8 σ in PyMOL. The map was generated with PHENIX. (B) Fusion peptide region, map contoured at 1.6 σ in PyMOL. The map was generated with PHENIX. (C) Crystal structures of monomeric chains of prefusion HeV F, PIV5 (PDB ID code 2B9B), and RSV F (PDB ID code 4JHW).
Table S1.
Crystallographic data collection and refinement of the Hendra F ectodomain
| Data collection | |
| Space group | P21 |
| Cell dimensions | |
| a, b, c; Å | 108.6, 163.5, 147.9 |
| α, β, γ; ° | 90, 94.1, 90 |
| Wavelength, Å | 0.99969 |
| Resolution, Å | 50.0–3.20 |
| Reflections (unique) | 338,511 (84,644) |
| Rsym* | 0.012 (0.667)† |
| I/σI | 8.3 (1.9)† |
| Redundancy | 4.0 (4.1)† |
| Completeness, % | 99.6 (99.9)† |
| Refinement | |
| Resolution, Å | 49.62–3.20 |
| No. of unique reflections (free) | 80,411 (4,233) |
| Rwork‡/Rfree§, % | 25.27/28.33 |
| No. of residues (atoms) in asymmetrical unit | 2,699 (20,443) |
| B-factor (overall) | 95.0 |
| Bond angle rmsd, ° | 1.541 |
| Bond length rmsd, Å | 0.0109 |
| Ramachandran | Favored: 91.7% |
| Allowed: 7.6% | |
| Disallowed: 0.7% |
Rsym = ΣhklΣi| Ii(hkl) − <I(hkl)>|/ ΣhklΣi Ii(hkl), where Ii(hkl) is the intensity for an observation of a reflection and <I(hkl)> is the average intensity of all symmetry-related observations of a reflection.
Data of the highest resolution shell (3.28–3.20 Å) are shown in parentheses.
Rwork = Σhkl||Fobs|−|Fcalc||/ Σhkl|Fobs|.
Rfree = Rwork calculated for 5% of reflections excluded from refinement.
Fig. 1.
Crystal structure of prefusion HeV F ectodomain and comparison with prefusion F structures from other paramyxoviruses. (A) HeV F fusion glycoprotein ectodomain in prefusion conformation, orthogonal views. (B) HeV F prefusion monomer. (C) Superposition of prefusion HeV F and PIV5 F (PDB ID code 2B9B), orthogonal views. HeV F is shown in green, and PIV5 F is shown in orange. Crystallographically refined N-linked carbohydrates are circled and indicated by Asn residue number. (D) Superposition of prefusion HeV F and RSV F (PDB ID code 4JHW), orthogonal views. HeV F is shown in green, and RSV F is shown in purple. (E) Rotation between domains of HeV F and RSV F. The axes of rotation are indicated by yellow rods.
N-linked carbohydrate residues were observed for all of the previously reported glycosylation sites of HeV F, N67, N99, N414, and N464 (21) (Fig. 1C). N67 is on the N-terminal apical loop, and N99 is at the base of the fusion peptide cleavage site loop. The only N-glycan site location conserved between HeV F and PIV5 is HeV N464, which coincides with PIV5 457 on the HRB stalk. Notably, there is no functional conservation between the sites, because HeV F N464 is dispensable for fusion and processing (21), whereas the stalk glycan of PIV5 is essential for stability, cell surface expression, and proteolytic processing (22). The only HeV F glycan necessary for F expression and function, N414 (21), is in DII on the lower extremity of the globular head.
Investigation of Apical Loop Differences in HeV F.
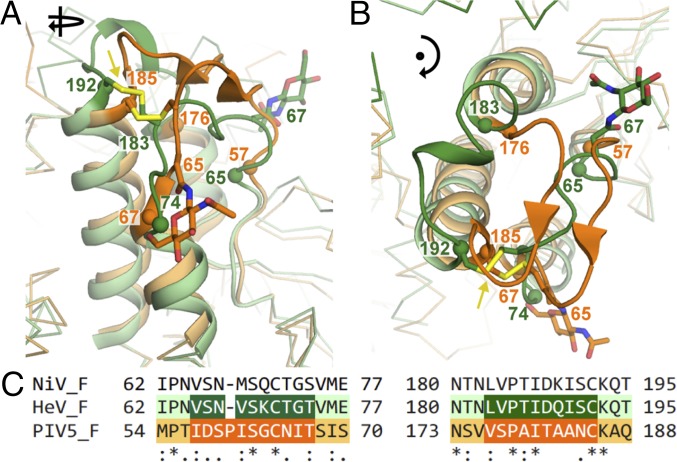
A notable difference between the HeV F and PIV5 F structures occurs in the pair of loops at the topmost surface of the trimer. Compared with the PIV5 loops, the HeV F loops are more oriented toward the central axis and have more of an α-helical than β-sheet secondary structure (Fig. 2 A and B) and the N-terminal loop is shorter by one amino acid (Fig. 2C). These differences result in a slight overall compaction in structure in the apical region. V68 has a role in structural stabilization of the double loops due to insertion of its side chain in a hydrophobic pocket that includes I179, L183, V184, and I187 from the second apical loop (Fig. 3A). The disulfide bond between C71 and C185 joining the first and second apical loops is conserved with PIV 5. Both HeV and PIV5 F have N-linked glycans on the N-terminal apical loop, but the N-linked glycan of HeV at N67 is N-terminal, whereas the N-linked glycan of PIV5 at N65 is C-terminal to the loop (Fig. 3A).
Fig. 2.
Apical loops of HeV F in comparison to PIV5 F. (A) Apical portion of the HeV F trimer with PIV5 F superposed. HeV F is shown in green, and PIV5 F is shown in orange, with the apical loops lightened to pale green in HeV F and to orange in PIV5 F. The threefold axis of rotational symmetry is indicated. The disulfide bond between the two apical loops is indicated with sticks, and the sulfur atoms are colored olive-yellow. Carbohydrates of HeV F are shown as green sticks, and carbohydrates of PIV5 F are shown as orange sticks. (B) View down the rotational axis of the HeV F and PIV5 F trimers. (C) Sequence alignment of the apical loops of HeV F, PIV5 F, and NiV F generated with ClustalW. Loop structures confirmed by available crystal structures are lightened. Degree of conservation is indicated as follows: *, perfect; :, strong; ., weak.
Fig. 3.
Fusion activity of HeV F apical loop mutants. (A) Site-directed mutants of the apical region of HeV F. Residues mutated to Ala are indicated in purple, and Ala insertion mutants are indicated by B. (B) Fusion levels of site-directed mutants measured by luciferase reporter gene assay. R.L.U., relative light units.
Site-directed mutants were generated in the apical loop region of HeV F to assess the contribution of individual residues to fusion function (Fig. 3A), because these residues could represent structural differences associated with G interaction and/or activation. Single Ala insertions were made following residues 65, 68, and 70 (mutants 65B, 68B, and 70B) to mimic the longer PIV5 loop and to test if loop length has an impact on HeV F function. Ala mutants were made of the polar surface residues K70 and D185 and the aliphatic buried residue V68. Residue T164 was mutated to Leu because it is highly exposed at the end of a protruding β-strand hairpin, where it could potentially interact with G.
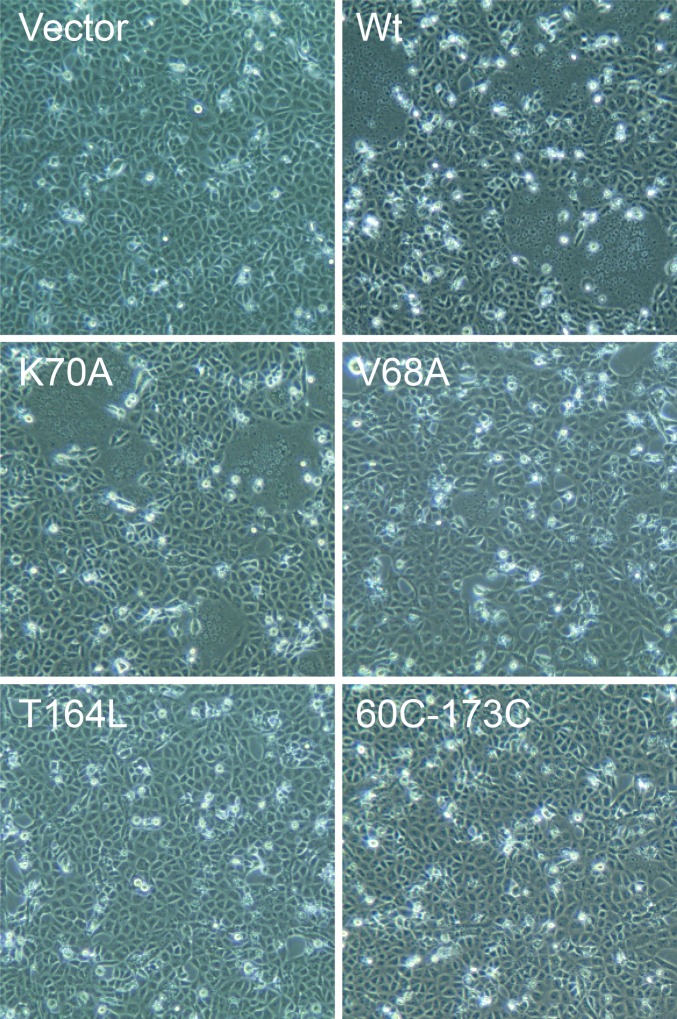
Of the site-directed mutants, only V68A, T164L, and 65B (an insertion mutant), listed in order of increasing defect, show significant effects on fusion (Fig. 3B and Fig. S2). Mutant V68A shows reduced cell surface protein expression, which would contribute, at least partially, to its decreased fusogenicity (Fig. S3 and Table S2), but the mutation may also affect the stability of the prefusion conformation. Mutant T164L has robust total protein expression but undergoes abnormal proteolysis, resulting in a smaller F1 fragment, which is a likely contributor to its decreased cell surface expression and fusogenicity (Fig. S3). Mutant 65B has normal proteolytic processing, a mild decrease in total expression level, and a more significant decrease in cell surface expression to ∼25% of WT, but it shows negligible fusion activity (Fig. S3 and Table S2). Therefore, Ala insertion after residue 65 affects fusion activity more severely than cell surface expression.
Fig. S2.
Syncytia formation in HeV F- and G-expressing cells. Representative samples of HeV F apical loop mutants, double-Cys mutants, WT, and empty vector controls are shown at 160× magnification. Cells at 90% confluency were transfected with HeV F and HeV G in pCAGGS, and syncytia were observed 16–20 h later.
Fig. S3.
Total and cell surface expression of HeV F mutants. (A) Total immunoprecipitated HeV F fraction. Vero cells were transfected with HeV F and HeV G in pCAGGS, and then grown in Cys−Met− medium with Easy-Tag EXPRESS35S Protein Labeling Mix for radiolabeling. Cells were biotinylated with Sulfo-NHS-Biotin and lysed in radioimmunoprecipitation assay buffer. HeV F was immunoprecipitated from the cell lysate with polyclonal anti-HeV F 527–540 peptide serum and protein A agarose. (B) Cell surface HeV F fraction. The biotinylated cell surface fraction was separated with streptavidin agarose from the total immunoprecipitated HeV F fraction.
Table S2.
Hendra F protein expression and fusion activity
| Construct | Total protein expression* (normalized to WT) | Cell surface protein† expression (normalized to WT) | Fusion activity‡ (normalized to WT) |
| 65B | 0.49 | 0.25 | 0.04 |
| 68B | 0.64 | 0.61 | 1.00 |
| 70B | 0.70 | 0.74 | 1.01 |
| K70A | 1.00 | 0.74 | 0.91 |
| D188A | 0.54 | 0.44 | 0.95 |
| V68A | 0.49 | 0.37 | 0.44 |
| T164L | 0.42 | 0.25 | 0.18 |
| WT | 1.00 | 1.00 | 1.00 |
| Vector | 0.09 | 0.12 | 0.02 |
| K60C-T173C | 0.35 | 0.22 | 0.02 |
| P63C-V175C | 0.28 | 0.12 | 0.03 |
| Y97C-G131C | 0.81 | 0.87 | 0.02 |
| N100C-A116C | 0.33 | 0.15 | 0.02 |
| N100C-A119C | 1.34 | 1.03 | 0.03 |
| A137C-V267C | 0.28 | 0.16 | 0.03 |
Total protein expression was assessed by radioimmunoprecipitation of cell lysates with anti-HeVF 527–540 antiserum. In brief, Vero cells were transfected with HeV F expression plasmid metabolically protein-labeled with S35 and cell surface biotinylated. Cells were lysed with radioimmunoprecipitation assay (RIPA) buffer, and anti-HeVF 527–540 antiserum was incubated with the lysate, followed by Ab pull-down with protein A beads. Ten percent of the protein A bead eluate was saved for total protein fraction analysis.
Cell surface protein expression was assessed by streptavidin pull-down of cell surface biotinylated HeV F from the total HeV pulled down by anti-HeV F 527–540 antiserum. In brief, 90% of the total fraction was incubated with streptavidin-Sepharose beads, and all of the eluate was saved for cell surface protein fraction analysis.
Fusion activity was assessed by luciferase reporter gene assay. In brief, Vero cells transfected with HeV F, HeV G, and luciferase expression plasmids were overlaid with BSR-T7 cells expressing T7 polymerase. Because luciferase expression is under control of the T7 promoter, cell fusion results in luciferase protein production, which is then measured by a luminescent substrate activity assay. Reported values are the means of four measurements for single-site mutants, and the means of three measurements for double-Cys mutants.
Based on the fusion and protein expression assays, we conclude that the protein surface residues K70 and D188 alone are unlikely to be crucial residues involved in HeV F interaction with potential binding partners. The one-residue-length decrease of the first apical loop in HeV F is not crucial for function, because only 65B of the three loop insertion mutants resulted in a fusion level or protein processing defect (Fig. 3B and Fig. S3). The 68B insertion has the additional effect of disrupting the N-X-S glycosylation motif beginning at residue N67, resulting in an F0 fragment with decreased molecular weight relative to WT (Fig. S3), showing that this region is tolerant of various types of perturbations. The common feature of V68A and 65B, the two mutants that disrupt total fusion activity, and the amount of cell surface expression is that they could disrupt the secondary structure of the apical loop region. V65 of HeV F is in a strand that is structurally conserved with PIV5 just before it begins to diverge in the apical loop region, and 65B may disrupt a conserved feature that is important for the function of F. The hydrophobic packing of the two apical loops by V68 may substitute for the β-sheet interactions between the loops in PIV5. Maintaining these contacts may be necessary to avoid trafficking defects or the early protein degradation that may lead to the decreased amount of V68 and 65B mutant proteins on the cell surface.
HeV F Fusion Peptide Cleavage Site Region.
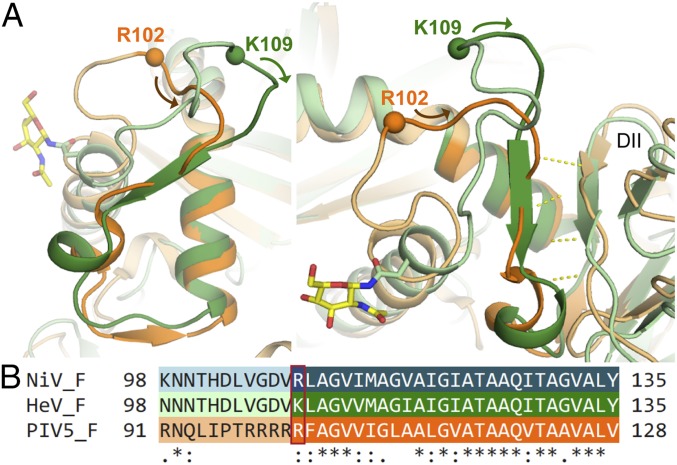
Residues 100–116 of HeV F, which surround the F1/F2 cleavage site following residue K109, form an elongated, antiparallel β-sheet–like structure with the cleavage site at the turn. This structure is in contrast to residues 94–109 of PIV5, which form a circular, open loop (Fig. 4). The residues C-terminal to the cleavage site in HeV F form a more defined β-strand structure. This strand makes β-sheet backbone interactions with a neighboring β-strand consisting of residues 425–428 from DII that is conserved between HeV F and PIV5 F (Fig. 4).
Fig. 4.
Fusion peptide region of HeV F in comparison to PIV5 F. (A) HeV and PIV5 F fusion peptides and preceding regions superimposed. HeV F is shown in light green, and PIV5 F is shown in light orange, with the fusion peptides darkened in HeV F and PIV5 F. Basic protease cleavage site residues are indicated by spheres, and the N- to C-terminal fusion peptide direction is indicated by arrows. (B) Sequence alignment of the fusion peptide regions of HeV, PIV5, and NiV F generated with ClustalW. The basic residue upstream of the N terminus of the fusion peptide is boxed in red, and the remainder of the fusion peptide is darkened. The degree of conservation is indicated as in Fig. 2.
These differences in fusion peptide conformation may reflect their recognition by different proteases during posttranslational processing. Unlike HeV F, which is cleaved by cathepsin L, PIV5 is cleaved into its fusion-active form by furin (23). Crystal structures exist for furin in complex with peptide-derived synthetic inhibitors (24) and for cathepsin L in complex with a natural peptide substrate (25), peptide-derived inhibitors (26), and full-length protein inhibitors (27, 28). The furin substrate-binding pocket is well-suited to accommodate an open-loop conformation (Fig. S4A). Its wide and rounded shape would force a linear peptide substrate to curve around to exit. The entirety of the binding pocket surface is electronegative, which is suitable for complementary electrostatic interactions with the poly-Arg preceding the cleavage site. In comparison to furin, the substrate-binding pocket of cathepsin L forms a longer and narrower groove (Fig. S4B). The crystal structures of cathepsin L in complex with the MHC class II invariant chain p41 fragment and chagasin show that the turn of an elongated loop binds in the positions of the substrate-binding groove immediately preceding the cleavage site, whereas additional binding from the rest of the protein inhibitor is provided by insertion of two additional turns at separate points in the binding groove (Fig. S4C). The elongated loop structure surrounding the cleavage site in HeV F suggests that it may insert into the cathepsin L active site in a similar fashion.
Fig. S4.
Fusion peptide cleavage site of PIV5 and HeV F. (A) Catalytic site of furin in complex with the inhibitor m-guanidinomethyl-phenylacetyl-Arg-Val-Arg-(amidomethyl)-benzamidine (m-guanidinomethyl-Phac-RVR-Amba) (PDB ID code 4OMC). (B) Structural alignment of cathepsin L in complex with a natural peptide substrate, histone H3 (PDB ID code 3K24) and the inhibitor AZ12878478 (PDB ID code 3HHA). (C) Structural alignment of cathepsin L in complex with the natural polypeptide inhibitors chagasin (PDB ID code 2NQD) and MHC class II Ii p41 variant (PDB ID code 1ICF).
Prefusion HeV F Disulfide Bond Mutants.
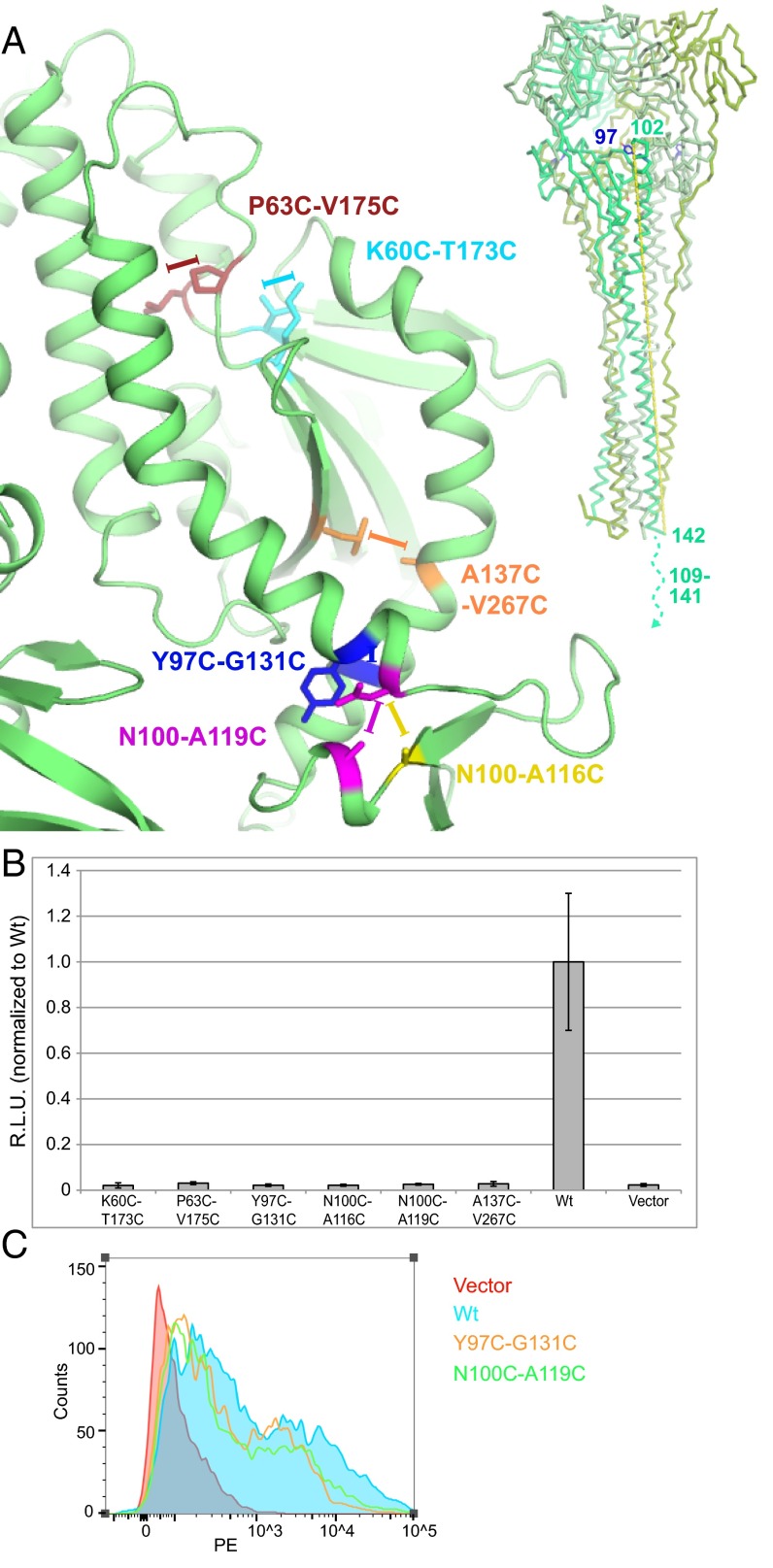
The crystal structure of the HeV F protein provides the opportunity to predict F variants that could be stabilized in the prefusion conformation, which would be useful for functional studies, antibody (Ab) epitope mapping, and vaccine antigen design. To stabilize prefusion F, double-Cys mutants were generated throughout the HRA region of HeV F with the intent of locking it in its prefusion conformation via disulfide bonds. Potential pairs of disulfide bond-forming residues within the HeV F crystal structure were found with Disulfide by Design (29), and six were chosen for further analysis (Fig. 5A). All six mutants expressed to appreciable levels, but only two, Y97C-G131C and N100C-A119C, had normal proteolytic processing and were expressed at WT levels on the cell surface (Fig. S3 and Table S2). The other four mutants had some combination of decreased or abnormal proteolytic processing and greatly decreased cell surface expression. All six mutants exhibited negligible fusion (Fig. 5B). This result suggests that the Y97C-G131C and N100C-A119C mutants are locked in the prefusion conformation while on the cell surface and are unable to undergo the prefusion-to-postfusion conformational transition triggered by G. Although both residues are adjacent in the prefusion conformation, they end up on essentially opposite sides of the protein after fusion due to Y97 and N100 being on one side of the fusion peptide cleavage site and F131 and A119 on the other (Fig. 5A). Residues N-terminal to the cleavage site are adjacent to the head in the postfusion conformation, whereas residues C-terminal to the cleavage site are embedded in the membrane at the base of the stalk (Fig. 5A).
Fig. 5.
Fusion activity of predicted disulfide bond-forming mutants in HeV F. (A) Disulfide bond double mutants of HeV F HRA. Prefusion conformation of HeV F with residue pairs chosen for Cys mutations (Left) and a model of postfusion HeV F based on postfusion hPIV3 F (PDB ID code 1ZTM) (Right) are shown. Y97, the only ordered aligned residue in the crystal structure, is colored purple. The distance between the last visible residue preceding the fusion peptide and first visible residue of HRB is shown as a yellow dashed line. (B) Fusion levels of disulfide mutants measured by luciferase reporter gene assay. (C) Binding of prefusion-specific mAb 5B3 to HeV F disulfide mutants measured by flow cytometry. PE, phycoerythrin.
To confirm if the HeV F protein on the cell surface is in the prefusion conformation, HEK293T cells transfected with HeV F-expressing vector were analyzed by flow cytometry with an anti-HeV F mAb (5B3) specific for its prefusion conformation (30). The median fluorescence intensities (MFIs) (Fig. 5C, Fig. S5, and Table S3) show that the Y97C-G131C and N100C-A119C mutants are present at the surface in the prefusion conformation at ∼60% of the WT level, consistent with the predicted disulfide bond stabilization. The radioimmunoprecipitation assay indicates that the total amount of HeV F on the cell surface may be similar to the total amount of HeV F of WT (Table S2), potentially suggesting that a lower percentage of prefusion F accumulates for the disulfide bond mutants. However, variability in the radioimmunoprecipitation assay and in transfection efficiency, which could have an impact on the MFI, is too significant to determine the efficiency of folding of the disulfide bond mutants to the prefusion state. Nonetheless, the observed fusion defects in Y97C-G131C and N100C-A119C are much greater than the reduction in the amount of prefusion F at the cell surface detected by mAb 5B3 compared with WT. Therefore, we conclude that the majority of the decrease in fusion is most consistent with the F protein being locked in the prefusion conformation by disulfide bonds.
Fig. S5.
Assay for prefusion conformation of cell surface HeV F by flow cytometry. HeV F-pCAGGS–transfected HEK293T cells were incubated with prefusion conformation-specific mAb 5B3, followed by phycoerythrin (PE)-conjugated 2° Ab. Representative histograms from each sample are shown.
Table S3.
Cell surface expression of prefusion Hendra F measured by flow cytometry
| Construct | MFI | SD |
| Mock transfection, no Ab | 0.03 | N/A |
| Vector, 2° Ab only | 0.03 | 0.01 |
| WT, 2° Ab only | 0.03 | 0.01 |
| 97–131, 2° Ab only | 0.03 | 0.01 |
| 100–119, 2° Ab only | 0.03 | 0.01 |
| 65B | 0.12 | 0.07 |
| 68B | 0.63 | 0.06 |
| 70B | 0.41 | 0.03 |
| K70A | 0.80 | 0.23 |
| D188A | 0.60 | 0.15 |
| V68A | 0.25 | 0.08 |
| T164L | 0.24 | 0.08 |
| K60C-T173C | 0.12 | 0.09 |
| P63C-V175C | 0.08 | 0.04 |
| Y97C-G131C | 0.57 | 0.08 |
| N100C-A116C | 0.08 | 0.04 |
| N100C-A119C | 0.61 | 0.08 |
| A137C-V267C | 0.21 | 0.01 |
| WT | 1.00 | 0.06 |
| Vector | 0.17 | 0.06 |
Prefusion HeV F on the cell surface was detected by incubating 293T cells with mAb 5B3, followed by phycoerythrin-conjugated secondary Ab. The amount of mAb 5B3 binding was measured by flow cytometry detection of phycoerythrin fluorescence. Median intensities were calculated for all samples with n = 2, except for WT, Y97C-G131C, and N100C-A119C, which had n = 4. MFIs did not take into account the transfection efficiency of each replicate. N/A, not applicable.
Engineering of vaccine antigens with introduced disulfide bonds has been explored as a means of generating more stable and/or more immunogenic variants (31, 32). The metastability of viral fusion glycoproteins can be an obstacle for their expression, purification, and antigenicity due to their tendency to transition spontaneously from the prefusion to postfusion conformation (18, 33). The successful engineering of disulfide bonds for maintaining fusion proteins in the prefusion conformation has been reported for measles, RSV, PIV5 F, influenza HA, and HIV gp120-gp41 trimers (32, 34–37). An additional benefit of prefusion-stabilizing disulfide bonds may be increased thermal stability during storage, because the WT prefusion-to-postfusion transition can be triggered by heat (38). Disulfide bond-enhanced thermal stability and shelf life have been demonstrated in subunit vaccines for Lyme borreliosis (39) and ricin A toxin (40). Our results with the HeV F Y97C-G131C and N100C-A119C mutants provide tools for further studies of HeV F fusion and antigenicity, as well as an additional example of how the engineering of disulfide bonds into metastable fusion proteins can be used to control their conformational and functional properties.
Conclusions
The HeV F ectodomain has a structure very similar to the structure of PIV5 F, suggesting that the mechanism for receptor-dependent triggering of fusion is conserved. However, HeV F preassociates with its attachment protein, G, before receptor binding, whereas PIV5 F does not. Significant structural differences are observed in their fusion peptide regions and the double apical loops. The more narrow and elongated loop of HeV F surrounding the K109 cleavage site likely reflects differences in the recognition site of its specific activating protease, cathepsin L, as opposed to furin, which is used by PIV5. The functional role of the double apical loops appears limited to its structural integrity based on our functional studies. Residues in these loops involved in structure stabilization and conservation are important for maintaining protein expression and function. Other residues within the F trimer must promote the preferential assembly of HeV into prefusion complexes with G, in contrast to PIV5 F, and these residues have yet to be identified. However, the overall structural conservation is consistent with a similar mode of F activation for Henipavirus and parainfluenza viruses. We have generated two HeV F disulfide mutants that fold to the prefusion form, while blocking F fusion activity. Both disulfide mutants generate covalent links between residues immediately preceding and following the fusion peptide cleavage site, so that they are unable to move apart in the conformational transition to the postfusion state. Fusion peptide immobilization via the structure-based introduction of such disulfide bonds provides a promising route for design of other stabilized viral fusion protein variants.
Materials and Methods
Protein Expression and Purification.
The HeV F ectodomain (Fecto) was expressed in HEK293F cells transiently transfected with the HeV Fecto-pCAGGS3 plasmid according to the high-density method of Backliwal et al. (41). Cell culture medium was collected 5 d after transfection and dialyzed against 25 mM sodium phosphate (pH 7.6), 200 mM NaCl, and 10 mM imidazole. HeV F was purified from the medium by nickel-nitrilotriacetic acid (Ni-NTA) chromatography and eluted with a stepwise imidazole gradient. The peak fractions were further purified by size exclusion on a Superdex S200 (GE Healthcare Life Sciences) column in 25 mM Tris (pH 8.0), 200 mM NaCl, and 100 mM imidazole buffer.
Crystallization and Structure Solution of HeV Fecto.
The His 8-tag was removed from HeV Fecto before crystallization by digestion with Factor XA (New England Biolabs) in 20 mM Tris (pH 7.4), 100 mM NaCl, and 2 mM CaCl2 at a ratio of 1 μg of protease to 20 μg of F overnight at 4 °C. Cleaved His-tags and uncleaved protein were removed by incubation with Ni-NTA beads, followed by removal of protease by size exclusion chromatography. HeV Fecto peak fractions were buffer-exchanged into 20 mM Tris (pH 8.0), 100 mM NaCl, and 1 mM EDTA, and then concentrated to 5 mg/mL for crystallization setup. Crystals were grown by vapor diffusion in a 1:1 protein/reservoir solution ratio at room temperature. The reservoir composition was 100 mM sodium acetate (pH 5.0), 1.75 M lithium sulfate, 100 mM magnesium sulfate, and 3.4% (vol/vol) isopropanol. Crystals were washed in reservoir solution before flash-freezing in liquid nitrogen. Diffraction data were collected at Advanced Light Source Beamline 8.2.2, and data were scaled and indexed with XDS (42). A molecular replacement solution was found using MOLREP (43) and the PIV5 prefusion F structure [Protein Data Bank (PDB) ID code 2B9B] as a search model. The model was refined with Crystallography & NMR System (CNS) (44)-simulated annealing and REFMAC5 (45). Model building was done with Coot (46) and the lego_ca function of O (47). Coordinates have been deposited in the PDB with ID code 5EJB.
Flow Cytometry Assay of Prefusion HeV F Conformation.
HEK293T cells in six-well plates were transfected with 1 μg of full-length HeV F pCAGGS, 5 μL of lipofectamine, and 4 μL of Plus Reagent (Life Technologies). After overnight incubation, cells were detached with 500 μL of enzyme-free cell dissociation buffer, PBS (Life Technologies). A total of 25,000 cells were washed by dilution in 1 mL of FACS buffer (1× PBS, Corning Cellgro; 1% BSA) and pelleting at 400 × g for 5 min. Cells were resuspended in 100 μL of FACS buffer for staining with 1 μg of mAb 5B3 for 1 h, and then were washed by dilution and pelleting with 2 mL of FACS buffer. Cells were resuspended in 100 μL of FACS buffer for staining with 1 μg of goat anti-mouse F(ab′)2 fragment-phycoerythrin (eBioscience) and 0.5 μL of LIVE/DEAD Fixable Aqua stain (Life Technologies) for 1 h. Cells were washed by dilution and pelleting with 2 mL of FACS buffer twice and were then resuspended in 100 μL of FACS buffer and fixed in 1% paraformaldehyde. Data were collected on ∼7,000 cells per sample on a Becton Dickinson FACScan flow cytometer (Becton Dickinson) with Cytek DxP multicolor upgrades. MFIs were calculated on live gated cells with FlowJo (TreeStar). Variation in transfection efficiency was not taken into account in calculations of MFI.
SI Materials and Methods
Cloning of HeV Fecto and Full-Length HeV F Mutants.
Codon-optimized DNA encoding for the gp64 signal peptide, HeV F residues 26–482, GCN4 trimerization domain (12), factor XA cleavage site, and His 8-tag was obtained by gene synthesis and cloned into the XmaI and NheI sites of pCAGGS3 to make HeV Fecto. For the mutant proteins, HeV F bases 138–1,079 containing mutated codons were obtained by GeneArt Strings synthesis (Life Technologies), and bases 1,054–1,642 with the 3′ flanking regions of the vector were PCR-amplified from the WT HeV F-pCAGGS plasmid. Gene fragments 138–1,079 and 1,054–1,642 were cloned into the BclI-BglII fragment of the HeV F-pCAGGS plasmid containing HeV F bases 1–173 by Gibson Assembly to make each full-length HeV F mutant, except for 60C–173C. The 5′ flanking region of the vector to HeV F bases 1–555 was obtained by GeneArt Strings synthesis, and bases 520–1,642 with the 3′ flanking vector regions were PCR-amplified from the WT HeV F-pCAGGS plasmid. Gene fragments 138–555 and 520–1,642 were cloned into the EcoRI-BglII fragment of the HeV F-pCAGGS plasmid by Gibson Assembly to make HeV F K60C-V173C.
Luciferase Fusion Activity Assay.
Vero cells in six-well plates were transfected with 1 μg each of full-length HeV F-pCAGGS, HeV G-pCAGGS, and pT7-luciferase plasmid using 5 μL of lipofectamine and 4 μL of Plus Reagent (Life Technologies). After overnight incubation, BSR-T7 cells (which stably express the T7 RNA polymerase) were overlaid on the Vero cells and incubated for 5 h. Cells were lysed in Glo-Lysis buffer (Promega), and luciferase activity was measured using Bright-Glo Reagent (Promega) with a Synergy 4 plate reader (Biotek).
Radioimmunoprecipitation of Total and Cell Surface-Expressed Hendra F.
Vero cells in 60-mm plates were transfected with 3 μg of full-length HeV F pCAGGS, and with 5 μL of lipofectamine and 4 μL of Plus Reagent. After overnight incubation, cells were washed with Dulbecco’s PBS (DPBS) with calcium and starved with Cys−Met− DMEM for 45 min. Starve medium was aspirated, and 2 mL of S35 labeling medium [50 μCi/mL Easy-Tag EXPRESS35S Protein Labeling Mix (PerkinElmer) in Cys−Met− DMEM and 2% (vol/vol) FBS] was added, followed by 5 h of incubation. At the end of labeling, cells were washed twice with ice-cold DPBS (pH 8.0), and then biotinylated in 1 mL of 1 mg/mL EZ-Link Sulfo-NHS-Biotin (Thermo Scientific) for 40 min at 4 °C. Cells were washed twice in ice-cold DPBS (pH 8.0) and once in ice-cold DPBS (pH 8.0) + 20 mM Tris.
Cells were detached from the plate with 500 μL of Enzyme-free cell dissociation buffer, PBS (Life Technologies) and cell lifters, and were centrifuged for 1 min at 1,000 × g to pellet them. The supernatant was aspirated, and the cells were lysed with 50 μL of radioimmunoprecipitation assay buffer [100 mM Tris (pH 7.4), 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholic acid] and protease inhibitors [1 mM PMSF, 2 KIU/mL aprotinin (Calbiochem), 1× Complete protease inhibitor tablet (Roche) stock solution]. A total of 450 μL of NET-2 buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 0.1% Igepal CA 630] was added to the lysate, and 8 μL of anti-HeVF 427–440 rabbit polyclonal antiserum was added for overnight incubation at 4 °C. Ab was pulled down by incubation with 30 μL of protein A agarose beads (Pierce, ThermoFisher) for 1 h. The beads were washed five times with 1 mL of NET-2 buffer and boiled for 10 min in 60 μL of 10% (wt/vol) SDS and then 40 μL of 10% SDS for a total of 100 μL of eluate. Ten microliters was saved for total protein evaluation, and 500 μL of NET-2 buffer was added to the remainder for cell surface expression analysis. Thirty microliters of streptavidin beads (Pierce, ThermoFisher) was added to the cell surface fraction and incubated for 1 h at 4 °C on a nutator. The beads were washed five times with 1 mL of NET-2 buffer, and the bound fraction was collected by boiling the beads in 30 μL of 2× SDS/PAGE loading buffer. Band intensities were quantified by ImageJ (NIH).
SI Text
Only residues with visible electron density where the model could be refined were included in the final model. Unbuilt residues are loops 248–251 in all chains, fusion peptide loop residues, chain A 106–112, chain C 103–112, chain E 103–113, chain F 103–113, and stalk residues past 477 in all chains except chains C and D. The entire stalk up to the last membrane proximal stalk residue 484 was visible for chains C and D and then the first five and nine amino acids of the GCNt trimerization domain, respectively. Side chains with no visible density were also not built.
Acknowledgments
We thank the staff at Advanced Light Source Beamline 8.2.2 and Stanford Synchrotron Radiation Lightsource for their assistance in crystallographic data collection; Rebecca Dutch and members of the Dutch laboratory for providing the HeV F pCAGGS, HeV G pCAGGS, polyclonal anti-HeV F 527-540 Ab, and HeV F radioimmunoprecipitation assay protocols; Gabriele Fuchs and the Peter Sarnow laboratory for advice and facilities for performing radioimmunoprecipitation assays; Christopher Broder for providing the mAb 5B3; and Luke Pennington and the Kari Nadeau laboratory for assistance and instrumentation for performing flow cytometry. This work was supported, in part, by NIH Research Grants AI-23173 (to R.A.L.) and GM-61050 (to T.S.J.). R.A.L. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 5EJB).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523303113/-/DCSupplemental.
References
- 1.Rota PA, Lo MK. Molecular virology of the henipaviruses. Curr Top Microbiol Immunol. 2012;359:41–58. doi: 10.1007/82_2012_211. [DOI] [PubMed] [Google Scholar]
- 2.Field H, et al. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3(4):307–314. doi: 10.1016/s1286-4579(01)01384-3. [DOI] [PubMed] [Google Scholar]
- 3.Marsh GA, et al. Cedar virus: A novel Henipavirus isolated from Australian bats. PLoS Pathog. 2012;8(8):e1002836. doi: 10.1371/journal.ppat.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drexler JF, et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahalingam S, et al. Hendra virus: An emerging paramyxovirus in Australia. Lancet Infect Dis. 2012;12(10):799–807. doi: 10.1016/S1473-3099(12)70158-5. [DOI] [PubMed] [Google Scholar]
- 6.Clayton BA, Wang LF, Marsh GA. Henipaviruses: An updated review focusing on the pteropid reservoir and features of transmission. Zoonoses Public Health. 2013;60(1):69–83. doi: 10.1111/j.1863-2378.2012.01501.x. [DOI] [PubMed] [Google Scholar]
- 7.Hyatt AD, Zaki SR, Goldsmith CS, Wise TG, Hengstberger SG. Ultrastructure of Hendra virus and Nipah virus within cultured cells and host animals. Microbes Infect. 2001;3(4):297–306. doi: 10.1016/s1286-4579(01)01383-1. [DOI] [PubMed] [Google Scholar]
- 8.Bossart KN, Fusco DL, Broder CC. Paramyxovirus entry. Adv Exp Med Biol. 2013;790:95–127. doi: 10.1007/978-1-4614-7651-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jardetzky TS, Lamb RA. Activation of paramyxovirus membrane fusion and virus entry. Curr Opin Virol. 2014;5:24–33. doi: 10.1016/j.coviro.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, et al. Unraveling a three-step spatiotemporal mechanism of triggering of receptor-induced Nipah virus fusion and cell entry. PLoS Pathog. 2013;9(11):e1003770. doi: 10.1371/journal.ppat.1003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, et al. Nipah virus attachment glycoprotein stalk C-terminal region links receptor binding to fusion triggering. J Virol. 2015;89(3):1838–1850. doi: 10.1128/JVI.02277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin HS, Wen X, Paterson RG, Lamb RA, Jardetzky TS. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439(7072):38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apte-Sengupta S, et al. Base of the measles virus fusion trimer head receives the signal that triggers membrane fusion. J Biol Chem. 2012;287(39):33026–33035. doi: 10.1074/jbc.M112.373308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bose S, et al. Mutations in the parainfluenza virus 5 fusion protein reveal domains important for fusion triggering and metastability. J Virol. 2013;87(24):13520–13531. doi: 10.1128/JVI.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pager CT, Dutch RE. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J Virol. 2005;79(20):12714–12720. doi: 10.1128/JVI.79.20.12714-12720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moll M, Diederich S, Klenk HD, Czub M, Maisner A. Ubiquitous activation of the Nipah virus fusion protein does not require a basic amino acid at the cleavage site. J Virol. 2004;78(18):9705–9712. doi: 10.1128/JVI.78.18.9705-9712.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin HS, Paterson RG, Wen X, Lamb RA, Jardetzky TS. Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc Natl Acad Sci USA. 2005;102(26):9288–9293. doi: 10.1073/pnas.0503989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLellan JS, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee B, Ataman ZA. Modes of paramyxovirus fusion: A Henipavirus perspective. Trends Microbiol. 2011;19(8):389–399. doi: 10.1016/j.tim.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayward S, Kitao A, Berendsen HJ. Model-free methods of analyzing domain motions in proteins from simulation: A comparison of normal mode analysis and molecular dynamics simulation of lysozyme. Proteins. 1997;27(3):425–437. doi: 10.1002/(sici)1097-0134(199703)27:3<425::aid-prot10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Carter JR, Pager CT, Fowler SD, Dutch RE. Role of N-linked glycosylation of the Hendra virus fusion protein. J Virol. 2005;79(12):7922–7925. doi: 10.1128/JVI.79.12.7922-7925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagai S, Lamb RA. Individual roles of N-linked oligosaccharide chains in intracellular transport of the paramyxovirus SV5 fusion protein. Virology. 1995;209(1):250–256. doi: 10.1006/viro.1995.1251. [DOI] [PubMed] [Google Scholar]
- 23.Garten W, et al. Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie. 1994;76(3-4):217–225. doi: 10.1016/0300-9084(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 24.Dahms SO, et al. X-ray structures of human furin in complex with competitive inhibitors. ACS Chem Biol. 2014;9(5):1113–1118. doi: 10.1021/cb500087x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams-Cioaba MA, Krupa JC, Xu C, Mort JS, Min J. Structural basis for the recognition and cleavage of histone H3 by cathepsin L. Nat Commun. 2011;2:197. doi: 10.1038/ncomms1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asaad N, et al. Dipeptidyl nitrile inhibitors of Cathepsin L. Bioorg Med Chem Lett. 2009;19(15):4280–4283. doi: 10.1016/j.bmcl.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 27.Guncar G, Pungercic G, Klemencic I, Turk V, Turk D. Crystal structure of MHC class II-associated p41 Ii fragment bound to cathepsin L reveals the structural basis for differentiation between cathepsins L and S. EMBO J. 1999;18(4):793–803. doi: 10.1093/emboj/18.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljunggren A, et al. Crystal structure of the parasite protease inhibitor chagasin in complex with a host target cysteine protease. J Mol Biol. 2007;371(1):137–153. doi: 10.1016/j.jmb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Dombkowski AA. Disulfide by Design: A computational method for the rational design of disulfide bonds in proteins. Bioinformatics. 2003;19(14):1852–1853. doi: 10.1093/bioinformatics/btg231. [DOI] [PubMed] [Google Scholar]
- 30.Chan YP, et al. Biochemical, conformational, and immunogenic analysis of soluble trimeric forms of henipavirus fusion glycoproteins. J Virol. 2012;86(21):11457–11471. doi: 10.1128/JVI.01318-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dormitzer PR, Ulmer JB, Rappuoli R. Structure-based antigen design: A strategy for next generation vaccines. Trends Biotechnol. 2008;26(12):659–667. doi: 10.1016/j.tibtech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cozzi R, Scarselli M, Ferlenghi I. Structural vaccinology: A three-dimensional view for vaccine development. Curr Top Med Chem. 2013;13(20):2629–2637. doi: 10.2174/15680266113136660187. [DOI] [PubMed] [Google Scholar]
- 33.Magro M, et al. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci USA. 2012;109(8):3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JK, Prussia A, Snyder JP, Plemper RK. Reversible inhibition of the fusion activity of measles virus F protein by an engineered intersubunit disulfide bridge. J Virol. 2007;81(16):8821–8826. doi: 10.1128/JVI.00754-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zokarkar A, Connolly SA, Jardetzky TS, Lamb RA. Reversible inhibition of fusion activity of a paramyxovirus fusion protein by an engineered disulfide bond in the membrane-proximal external region. J Virol. 2012;86(22):12397–12401. doi: 10.1128/JVI.02006-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLellan JS, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemble GW, Bodian DL, Rosé J, Wilson IA, White JM. Intermonomer disulfide bonds impair the fusion activity of influenza virus hemagglutinin. J Virol. 1992;66(8):4940–4950. doi: 10.1128/jvi.66.8.4940-4950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connolly SA, Leser GP, Yin HS, Jardetzky TS, Lamb RA. Refolding of a paramyxovirus F protein from prefusion to postfusion conformations observed by liposome binding and electron microscopy. Proc Natl Acad Sci USA. 2006;103(47):17903–17908. doi: 10.1073/pnas.0608678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comstedt P, Hanner M, Schüler W, Meinke A, Lundberg U. Design and development of a novel vaccine for protection against Lyme borreliosis. PLoS One. 2014;9(11):e113294. doi: 10.1371/journal.pone.0113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Compton JR, Legler PM, Clingan BV, Olson MA, Millard CB. Introduction of a disulfide bond leads to stabilization and crystallization of a ricin immunogen. Proteins. 2011;79(4):1048–1060. doi: 10.1002/prot.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Backliwal G, Hildinger M, Hasija V, Wurm FM. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol Bioeng. 2008;99(3):721–727. doi: 10.1002/bit.21596. [DOI] [PubMed] [Google Scholar]
- 42.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vagin A, Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 1):22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- 44.Brünger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 45.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]