Significance
Muscle contraction begins with release of neurotransmitter molecules from motor-nerve terminals onto muscle fibers. Strength of contraction depends on high-frequency stimulation of the nerve, which induces short-term increases in neurotransmitter release through presynaptic facilitation. Neurotransmitter release is initiated via calcium influx through voltage-gated calcium channels, which are regulated by calcium sensor (CaS) proteins. We found that genetically modified mice with a mutation in the binding site for CaS proteins on presynaptic calcium channels have reduced synaptic facilitation during high-frequency stimulation, resulting in reduced muscle strength, endurance, and coordination. Our results demonstrate critical roles for the regulation of calcium channels by CaS proteins in short-term presynaptic facilitation, with important consequences for motor control and muscle function in vivo.
Keywords: calcium channels, synaptic facilitation, calcium sensor proteins, calmodulin, synaptic plasticity
Abstract
Facilitation and inactivation of P/Q-type calcium (Ca2+) currents through the regulation of voltage-gated Ca2+ (CaV) 2.1 channels by Ca2+ sensor (CaS) proteins contributes to the facilitation and rapid depression of synaptic transmission in cultured neurons that transiently express CaV2.1 channels. To examine the modulation of endogenous CaV2.1 channels by CaS proteins in native synapses, we introduced a mutation (IM-AA) into the CaS protein-binding site in the C-terminal domain of CaV2.1 channels in mice, and tested synaptic facilitation and depression in neuromuscular junction synapses that use exclusively CaV2.1 channels for Ca2+ entry that triggers synaptic transmission. Even though basal synaptic transmission was unaltered in the neuromuscular synapses in IM-AA mice, we found reduced short-term facilitation in response to paired stimuli at short interstimulus intervals in IM-AA synapses. In response to trains of action potentials, we found increased facilitation at lower frequencies (10–30 Hz) in IM-AA synapses accompanied by slowed synaptic depression, whereas synaptic facilitation was reduced at high stimulus frequencies (50–100 Hz) that would induce strong muscle contraction. As a consequence of altered regulation of CaV2.1 channels, the hindlimb tibialis anterior muscle in IM-AA mice exhibited reduced peak force in response to 50 Hz stimulation and increased muscle fatigue. The IM-AA mice also had impaired motor control, exercise capacity, and grip strength. Taken together, our results indicate that regulation of CaV2.1 channels by CaS proteins is essential for normal synaptic plasticity at the neuromuscular junction and for muscle strength, endurance, and motor coordination in mice in vivo.
Classic work on the frog neuromuscular junction (NMJ) first described facilitation and depression of synaptic transmission during trains of action potentials (1). These forms of short-term plasticity are widespread among different types of synapses, and they transmit information encoded in the frequency and pattern of action potential generation to postsynaptic cells (2). Calcium (Ca2+)-dependent synaptic transmission is mediated by multiple types of voltage-gated Ca2+ (CaV) channels. Mature mammalian NMJ synapses use exclusively CaV2.1 channels to initiate synaptic transmission (3), in contrast to central nervous system synapses that use combinations of CaV2.1, CaV2.2, and CaV2.3 channels (4–6). Disruption of CaV2.1 channels by elimination of their pore-forming α1 subunit greatly reduces facilitation at the calyx of Held synapse (7, 8) and the NMJ (9) in mice, suggesting a key role for CaV2.1 channels in short-term synaptic plasticity.
CaV2.1 channels in transfected nonneuronal cells are regulated in a biphasic manner by calmodulin and other related Ca2+ sensor (CaS) proteins through interaction with a bipartite regulatory site in their C-terminal domain composed of an IQ-like motif (IM) and a calmodulin-binding domain (CBD) (10–13). CaS proteins interact with the IM motif to initiate Ca2+-dependent facilitation in response to local increases in Ca2+, and then interact with the CBD to induce Ca2+-dependent inactivation in response to longer, more global increases in Ca2+ (10–13).
The facilitation and inactivation of CaV2.1 channels during trains of repetitive stimuli induces synaptic facilitation, followed by a rapid phase of synaptic depression in cultured superior cervical ganglion neurons transiently expressing CaV2.1 channels (14). The Ile-Met→Ala-Ala (IM-AA) mutation prevents this synaptic plasticity by altering the interaction of CaV2.1 channels with CaS proteins (14). Other CaS proteins can displace calmodulin from their common regulatory site, enhance either facilitation or inactivation of the Ca2+ current, and thereby control the direction and amplitude of synaptic plasticity in cultured superior cervical ganglion neurons (15–18). Although previous studies revealed that regulation of CaV2.1 channels by CaS proteins can induce and regulate short-term synaptic plasticity in transfected neurons in cell culture, whether this mechanism makes an important contribution to short-term synaptic plasticity in native synapses has remained unknown.
To define the functional role of regulation of endogenous CaV2.1 channels by CaS proteins in short-term synaptic plasticity in vivo, we introduced the IM-AA mutation into the CaS protein-binding site in the C-terminal domain of CaV2.1 channels in mice, and investigated the effects of this mutation on synaptic transmission and short-term synaptic plasticity of NMJ synapses. The IM-AA mutation did not affect basal neuromuscular transmission; however, this mutation blocked short-term synaptic facilitation in response to paired stimuli with short interstimulus intervals (ISIs). Similarly, during high-frequency trains in which the intervals between stimuli are short, the IM-AA mutation reduced synaptic facilitation. In contrast, during trains of stimuli at low frequency with long ISIs, the IM-AA mutation slowed synaptic depression and thereby allowed increased synaptic facilitation. Hindlimb tibialis anterior (TA) muscles of IM-AA mice exhibited reduced peak specific force at 50-Hz stimulation, along with increased muscle fatigue. These defects in muscle function were accompanied by impaired motor control, reduced exercise capacity, and loss of grip strength in IM-AA mice in vivo.
Forceful muscle contractions require high-frequency stimulation by the presynaptic motor nerve. Therefore, our results with IM-AA mice link reduced paired-pulse facilitation (PPF) at short ISIs and reduced facilitation during high-frequency trains of stimuli at the cellular level with impaired strength, coordination, and exercise capacity in vivo. These findings demonstrate a critical role for the modulation of CaV2.1 channels by CaS proteins in regulating short-term synaptic plasticity, with important consequences for muscle strength and motor control.
Results
Spontaneous and Nerve-Evoked Neuromuscular Transmission in IM-AA Mice.
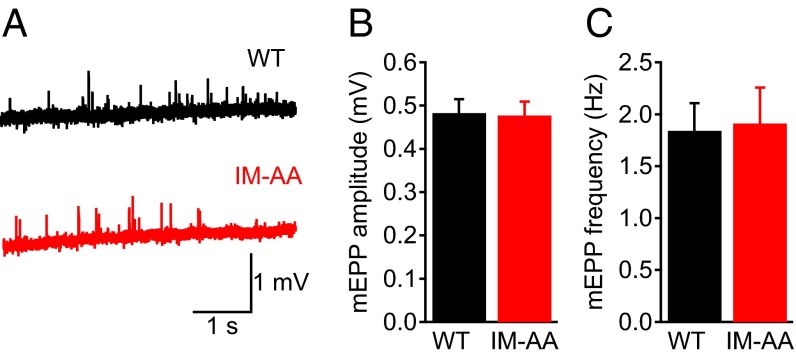
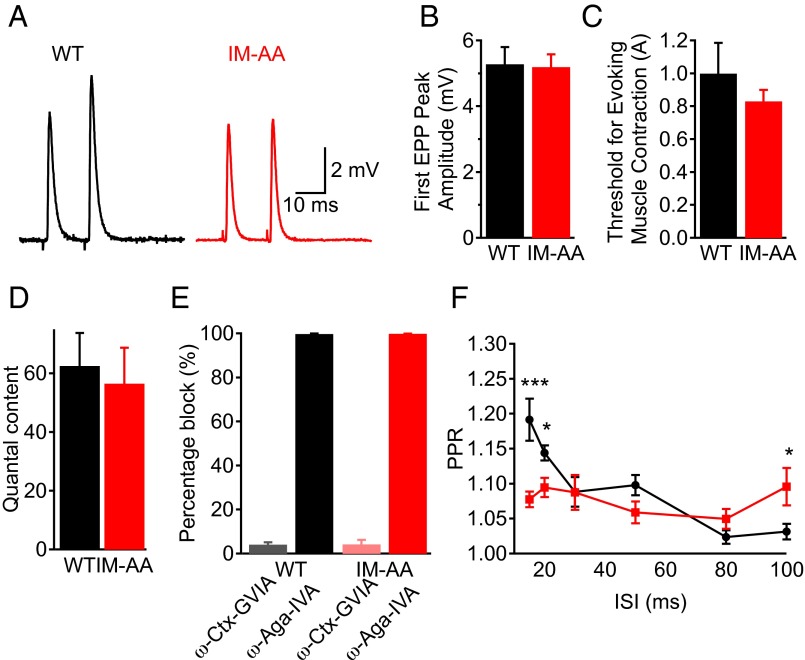
To examine the effect of the IM-AA mutation on synaptic function, we first evaluated spontaneous neurotransmitter release by analyzing miniature endplate potentials (mEPPs) from WT and IM-AA synapses at NMJs in the forelimb epitrochleoanconeus (ETA) muscle, which is ideal for electrophysiological recordings (19, 20). We quantified the amplitude (Fig. 1 A and B) and frequency (Fig. 1 A and C) of mEPPs and found no difference in spontaneous synaptic transmission between WT and IM-AA NMJ synapses. We then evaluated the effect of the IM-AA mutation on nerve-evoked synaptic transmission (Fig. 2 A–C). We found that the amplitude of the first EPP elicited by stimulation of the nerve (Fig. 2B), the threshold for evoking muscle contraction (Fig. 2C), and the quantal content of the EPPs (Fig. 2D) were indistinguishable in WT and IM-AA mice.
Fig. 1.
Mutation of the IM motif of CaV2.1 channels does not affect basal synaptic transmission in NMJ synapses. (A) Example of mEPPs in WT (black) and IM-AA (red) mice. (B) Average mEPP amplitude in WT (black; n = 10) and IM-AA (red; n = 12) mice. (C) Average mEPP frequency in WT (black; n = 10) and IM-AA (red; n = 12) mice. All recordings were made in the presence of 2 mM Ca2+.
Fig. 2.
Mutation of the IM motif of CaV2.1 channels impairs synaptic facilitation in paired-pulse stimuli. (A) Example paired EPP from NMJ synapses at a 15-ms ISI in WT (black) and IM-AA (red) preparations. (B) Mean amplitudes of EPPs in response to the first stimulus in WT (black; n = 11) and IM-AA (red; n = 20). (C) Threshold stimuli for evoking muscle contraction in WT (black; n = 9) and IM-AA (red; n = 13) mice. (D) Average quantal content in WT (black, n = 10) and IM-AA (red; n = 12) mice. (E) Percentage block of EPP amplitude by ω-Aga-IVA in WT (black; n = 3) and IM-AA (red; n = 3) mice, and by ω-conotoxin GVIA in WT (gray; n = 3) and IM-AA (pink; n = 3) mice. (F) PPR plotted as a function of ISI in WT (black; n = 16) and IM-AA (red; n = 23) mice. All recordings were made in the presence of 0.75 mM Ca2+, except for measurements of quantal content, which were done in the presence of 2 mM Ca2+. *P < 0.05; **P < 0.01; ***P < 0.001.
Neuromuscular transmission in neonatal skeletal muscle is initiated by both CaV2.1 and CaV2.2 channels, whereas CaV2.1 channels exclusively initiate neuromuscular transmission in adult muscle (21, 22). We examined whether the IM-AA mutation up-regulates CaV2.2 channels by recording evoked synaptic transmission from WT and IM-AA NMJs after blocking CaV2.2 channels by application of ω-conotoxin GVIA. We found that IM-AA synaptic transmission remained unaffected by the CaV2.2 channel block, whereas it was completely abolished when CaV2.1 channels were blocked by application of ω-agatoxin IVA (Fig. 2E). Taken together, these results show that inserting the IM-AA mutation in endogenous CaV2.1 channels does not alter either spontaneous neurotransmitter release or basal nerve-evoked synaptic transmission at NMJs.
Short-Term Synaptic Plasticity in IM-AA Mice.
Because the IM-AA mutation impaired short-term plasticity in cultured superior ganglion neurons expressing exogenous CaV2.1 channels (14), we investigated whether short-term facilitation is impaired by the IM-AA mutation in NMJs of ETA muscle fibers. We evaluated short-term plasticity at ISIs ranging from 15 to 100 ms (Fig. 2 A and F). WT NMJ synapses exhibited significant PPF at ISIs of 15 ms and 20 ms, which was abolished in IM-AA NMJ synapses (Fig. 2 A and F); however, IM-AA synapses retained more facilitation at long ISIs (e.g., 100 ms).
To examine whether the reduction in paired-pulse ratio (PPR) at short ISIs is caused by a change in initial neurotransmitter release probability, we quantified the amplitude of the first EPP for WT and IM-AA synapses, and found no difference between them (Fig. 2B). Taken together, our results show that the IM-AA mutation in CaV2.1 channels impairs short-term facilitation in NMJ synapses without altering basal synaptic transmission.
Short-Term Synaptic Plasticity in Trains of Stimuli in IM-AA Mice.
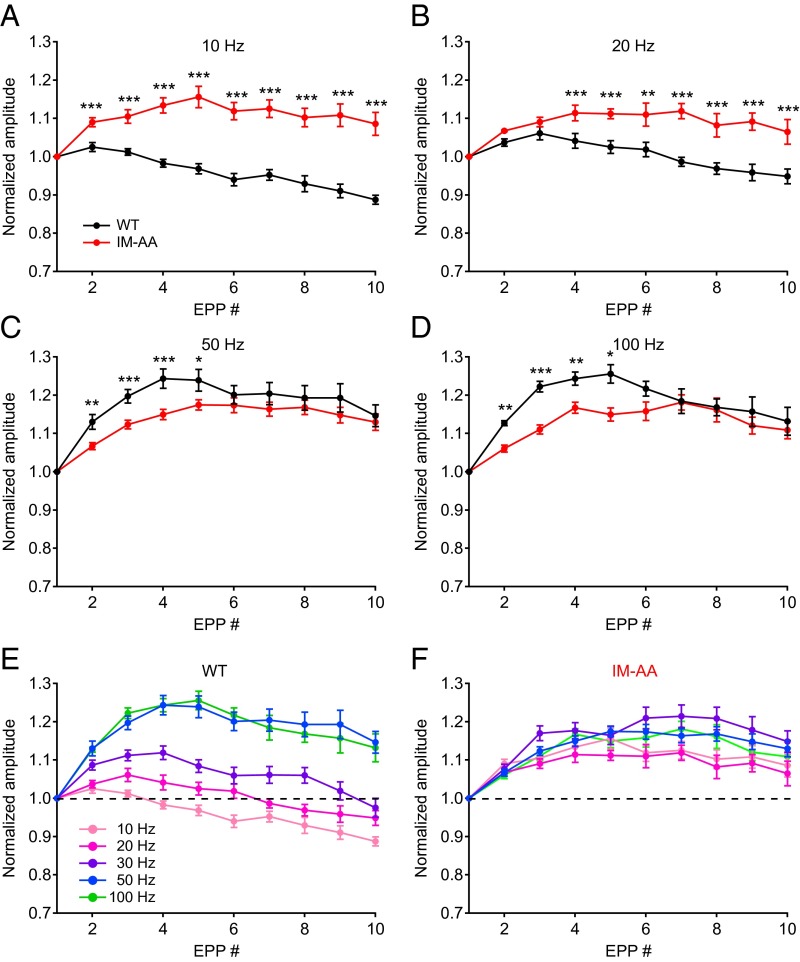
To investigate whether impaired short-term facilitation is also observed for EPPs evoked by trains of action potentials, we evaluated short-term synaptic plasticity during trains of stimuli presented at different frequencies. Repetitive stimulation at 10 Hz produced only depression of EPPs in WT NMJ synapses, whereas stimulation at 20–100 Hz resulted in facilitation followed by depression (Fig. 3 A–D, black). Stimulation at 50 Hz and 100 Hz (Fig. 3 C and D, black) substantially increased the level of facilitation compared with stimulation at 10 Hz or 20 Hz in WT synapses (Fig. 3 A and B, black). In contrast, NMJ synapses from IM-AA mice exhibited more facilitation, followed by very slow depression at 10–20 Hz, as if impaired depression allowed the accumulation of weak facilitation in repetitive stimuli (Fig. 3 A and B, red). The increased facilitation observed for WT synapses at stimulus frequencies of 50 Hz and 100 Hz was lost in IM-AA synapses (Fig. 3 C and D, red), suggesting that this component of synaptic facilitation observed at high frequencies of stimulation requires facilitation of CaV2.1 channels. Thus, the IM-AA mutation reduced short-term facilitation and slowed short-term depression, altering the frequency-dependent information processing in response to trains of action potentials. The mutation also blocked a specific component of synaptic facilitation observed at stimulus frequencies of 50 Hz and 100 Hz, which are required to generate peak force of muscle contraction (23, 24).
Fig. 3.
Mutation of the IM motif of CaV2.1 channels slows both facilitation and depression in trains of stimuli. (A–D) Average normalized peak amplitudes of evoked EPPs during trains in WT (black) and IM-AA (red) mice. (A) 10 Hz: WT, n = 11; IM-AA, n = 7. (B) 20 Hz: WT, n = 11; IM-AA, n = 9. (C) 50 Hz: WT, n = 15; IM-AA, n = 21. (D) 100 Hz: WT, n = 12; IM-AA, n = 24. (E) Average normalized peak amplitude of evoked EPPs during trains at frequencies from 10 Hz to 100 Hz in WT mice. (F) Average normalized peak amplitude of evoked EPPs during trains at frequencies from 10 Hz to 100 Hz in IM-AA mice. All recordings were made in the presence of 0.75 mM Ca2+. The dotted line in E and F indicates 1.0, no facilitation or depression. *P < 0.05; **P < 0.01; ***P < 0.001.
In classical studies of the frog NMJ, synaptic facilitation was found to be both frequency- and Ca2+-dependent (1). Facilitation at the NMJ of ETA muscles is also frequency-dependent (Fig. 3E). Surprisingly, the frequency dependence of facilitation is almost completely lost in NMJs of IM-AA mice (Fig. 3F). Therefore, the component of synaptic facilitation that requires regulation of CaV2.1 channels by CaS proteins may be essential for frequency-dependent facilitation at mammalian NMJs. This specific component of facilitation that confers frequency dependence may be related to the F1 component of facilitation described at frog NMJs, which decays with a time constant of ∼60 ms (25).
Force of Contraction in IM-AA Mice.
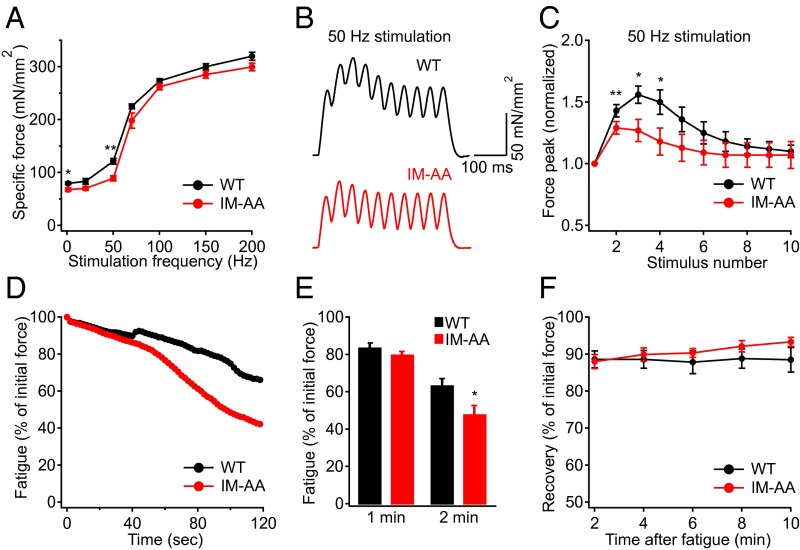
To investigate the physiological role of the IM-AA mutation in vivo, we evaluated hindlimb muscle function in response to nerve stimulation at different frequencies (Fig. 4A). We found that the peak specific force of the IM-AA muscle was decreased by 27% compared with WT at 50 Hz (Fig. 4 A and B), the frequency at which the greatest loss of synaptic facilitation occurs (Fig. 3C). At that frequency, normalized peak specific force during each stimulus was significantly smaller compared with that in WT during the first four pulses (Fig. 4 B and C), in correlation with the reduction of synaptic facilitation in the first four stimuli of trains at 50 Hz (Fig. 3C). In addition to the reduction in specific force, we found that compared with WT, IM-AA muscles fatigued to a greater extent after 2 min (Fig. 4 D and E). In contrast, recovery from fatigue was similar in the IM-AA and WT mice (Fig. 4F). Taken together, these results show that the IM-AA mutation impairs in vivo muscle function in response to direct nerve stimulation at the same frequency and in the same pattern as its impairment of synaptic facilitation.
Fig. 4.
IM-AA mice exhibit impaired muscle function during direct nerve stimulation. (A) Specific force of TA muscle measured at various stimulation frequencies in WT (black; n = 4) and IM-AA (red; n = 4) mice. (B) Representative traces showing the force peaks during each stimulus in WT (black) and IM-AA (red) muscles stimulated at 50 Hz. (C) Pooled data showing the force peaks, normalized to the first peak, for each stimulus of a 50-Hz contraction in WT (black; n = 4) and IM-AA (red; n = 4) mice. (D) Representative traces showing the force decline during a muscle fatigue protocol over 2 min in WT (black) and IM-AA (red) mice. (E) Pooled data showing the force after 1 min or 2 min of fatigue in WT (black; n = 5) and IM-AA (red; n = 5) mice. (F) Pooled data showing the force recovery for 10 min after muscle fatigue in WT (black; n = 5) and IM-AA (red; n = 5) mice. *P < 0.05; **P < 0.01.
Coordination, Strength, and Exercise Capacity of IM-AA Mice.
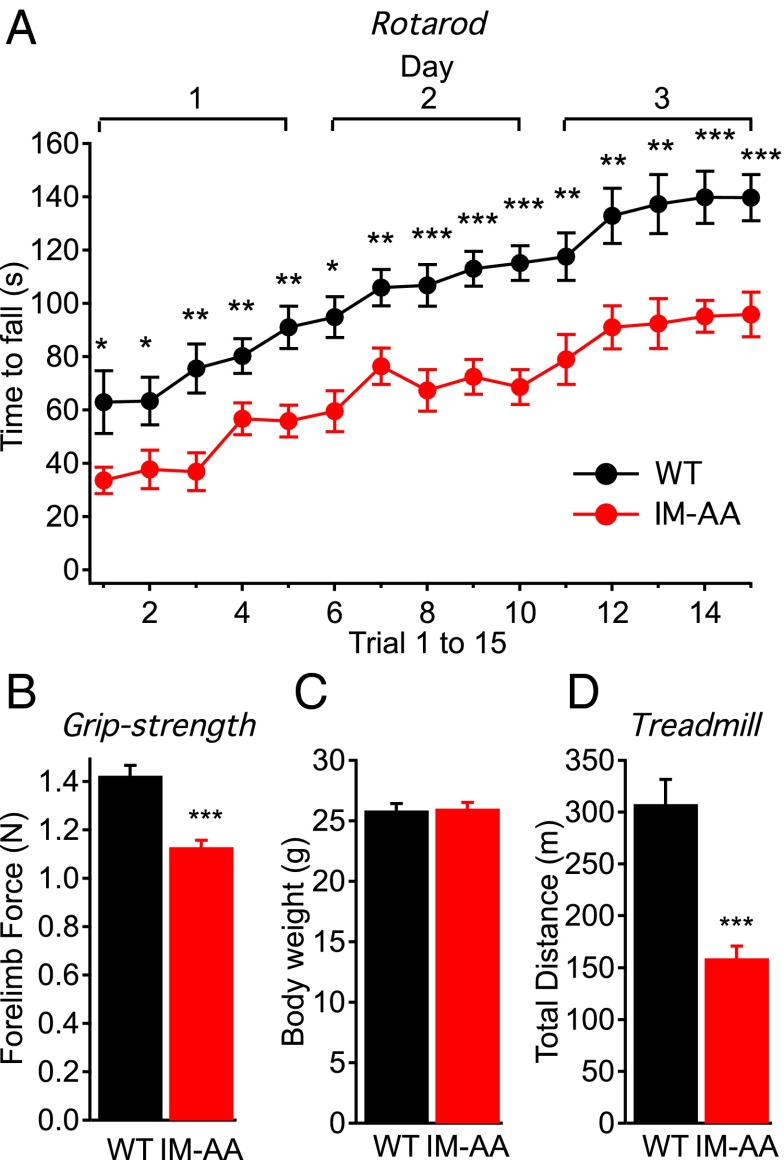
Because the reduced specific force and increased fatigue in IM-AA muscles suggested that muscle function would be compromised in vivo, we tested the IM-AA mice for motor control, exercise capacity, and grip strength. In the rotarod test of muscle strength and coordination, IM-AA mice fell from the rotarod significantly faster than WT mice in all trials (Fig. 5A), indicating impaired motor control in the IM-AA mice. Even in the first trial, IM-AA mice remained on the rotarod for a significantly shorter time. Although the performance of IM-AA mice improved, indicating significant motor learning capacity, their performance remained lower than that of WT mice throughout the series of trials (Fig. 5A).
Fig. 5.
IM-AA mice have impaired motor control, muscle strength, and exercise capacity. (A) Average time to fall from the rotating rod plotted against trials in WT (black; n = 14) and IM-AA (red; n = 17) mice. (B) Average forelimb force in WT (black; n = 18) and IM-AA (red; n = 20) mice. (C) Average body weight in WT (black; n = 18) and IM-AA (red; n = 20) mice. (D) Average distance traveled on the treadmill escaping an electrical shock in WT (black; n = 9) and IM-AA (red; n = 12) mice. *P < 0.05; **P < 0.01; ***P < 0.001.
To test for muscle strength in vivo, we measured grip strength in WT and IM-AA mice. We found significantly reduced peak forelimb force in the IM-AA mice (Fig. 5B), suggesting that these mice were weaker than the WT mice, even though their average body weight was similar (Fig. 5C). To directly test for muscle endurance, we evaluated the exercise capacity of IM-AA mice on an inclined treadmill with an electrical grid to provide a shock at the bottom. Compared with the WT mice, the IM-AA mice ran a significantly shorter distance before exhaustion (Fig. 5D), indicating decreased maximal exercise capacity. Taken together, these data reveal that the IM-AA mutation in presynaptic CaV2.1 channels greatly impairs muscle performance in vivo.
Discussion
Our results show that regulation of CaV2.1 channels by CaS proteins in native NMJ synapses contributes substantially to short-term plasticity. Both short-term facilitation and short-term depression were impaired and their temporal pattern was altered by the IM-AA mutation in the CaS-binding site in the C termini of CaV2.1 channels. These impairments in short-term plasticity in IM-AA synapses were coupled to decreased peak specific force of muscle contraction and increased muscle fatigue. IM-AA mice also had impaired motor function, with decreased muscle strength and coordination, and decreased exercise capacity. Overall, our results forge a causal link between regulation of CaV2.1 channels by CaS proteins and short-term synaptic plasticity, and provide direct functional evidence for a key role of short-term synaptic plasticity in muscle strength and motor control.
The IM-AA Mutation Impairs a Specific High-Frequency Component of Short-Term Synaptic Plasticity at the NMJ.
Consistent with previous work on transiently transfected superior cervical neurons (14), our results show that native NMJ synapses exhibit decreased facilitation in response to pairs or trains of high-frequency stimuli in IM-AA mice. The specific effect of the IM-AA mutation to reduce facilitation during trains of stimuli presented at 50 Hz and 100 Hz exactly correlates with the frequency of stimulation that supports forceful muscle contractions (23, 24); therefore, it is likely that the facilitation of CaV2.1 channel activity and the resulting facilitation of synaptic transmission contribute in an important way to the development of peak force of contraction in skeletal muscle.
In contrast, when paired pulses or trains of stimuli were presented at low frequency, the IM-AA NMJs exhibited increased facilitation and slowed depression compared with WT NMJs. Slowed inactivation of CaV2.1/IM-AA channels could account for decreased depression at lower frequencies, given that the synaptic depression caused by depletion of the readily releasable pool of vesicles is minor at such frequencies (26). Evidently, the IM-AA mutation alters the balance of synaptic facilitation and synaptic depression at these lower stimulus frequencies. Alteration of the balance of facilitation and depression in trains of stimuli at different frequencies would alter the encoding of physiological information in neuromuscular transmission and contribute to the loss of motor coordination in IM-AA mice.
Although the amplitude of facilitation is frequency-dependent in WT synapses, synaptic facilitation in IM-AA synapses is small (∼10%), nearly independent of frequency, and very stable, showing no noticeable decline in trains of 10 stimuli at 10–100 Hz. The loss of frequency-dependent facilitation and the remaining stable, frequency-independent facilitation also could contribute to the abnormal encoding of physiological information and loss of coordination of muscle function in IM-AA mice.
The IM-AA Mutation Reduces Muscle Strength.
Impaired synaptic plasticity at the NMJ during high-frequency stimulation would be expected to have profound consequences for forceful contractions in response to high-frequency stimulation of the motor nerve (Fig. 4). Consistent with this expectation, we found significantly decreased peak specific force and increased muscle fatigue in IM-AA TA muscles in response to high-frequency stimulation. Such reduced muscle strength would have profound effects at high exercise intensities in vivo.
Impaired Motor Control, Exercise Capacity, and Muscle Strength in IM-AA Mice.
Decreased strength and enhanced fatigue of muscles in IM-AA mice would be expected to cause significant impairment of strength, coordination, and endurance in vivo. We found decreased grip strength, impaired motor coordination on the rotarod test, and loss of endurance in forced exercise on the treadmill test. Overall, these results reveal a substantial functional role for regulation of CaV2.1 channels by CaS proteins in maintaining strong and precise motor function.
Regulation of CaV2.1 Channels and Short-Term Synaptic Plasticity.
This study adds to a growing body of evidence supporting an important role for regulation of CaV2.1 channels in short-term synaptic plasticity. In the calyx of Held synapse, CaV2.1 channels are required for synaptic facilitation, and both synaptic facilitation and depression are correlated with the facilitation and inactivation of P/Q-type Ca2+ currents (26–29). In transfected superior cervical ganglion neurons, facilitation requires the expression of CaV2.1 channels and is blocked by the IM-AA mutation (14). Moreover, expression of CaS proteins can change the mode of synaptic plasticity from facilitation to depression and vice versa (17, 18). The results presented here and in the accompanying report on hippocampal synapses provide, to our knowledge, the first reported evidence that regulation of CaV2.1 channels by CaS proteins is required for normal facilitation and depression in native synapses, and that failure of regulation of CaV2.1 channels has crucial consequences for in vivo physiology of nerve and muscle.
Materials and Methods
Animals.
IM-AA mice with a mutation in the IM motif of CaV2.1, in which Ile1913-Met1914 were changed to Ala1913-Ala1914 via conversion of the nucleotide sequence ATCATG to GCCGCT, were generated by Ingenious Targeting Laboratory. The mutation (within exon 40) was generated by PCR mutagenesis and confirmed by sequencing. Traditional blastocyst injection of ES cells expressing the targeting vector resulted in chimeric mice. These chimeric mice were mated first to generate heterozygotes, which were then backcrossed for 10 generations with C57BL/6J to generate homozygous IM-AA mutant mice in a pure genetic background. The experimenter was blind to genotype during all experiments with intact animals. All experiments were performed according to the guidelines for the care and use of animals approved by the Institutional Animal Care and Use Committee at the University of Washington.
Intracellular Recordings at Mouse NMJs.
Sharp electrode intracellular recordings were made from an ex vivo nerve–ETA muscle preparation (19, 20) isolated from WT and IM-AA mice (8–12 wk old). The ETA muscle, located on the medial surface of the upper forelimb, contains ∼380 muscle fibers (∼35 μm diameter, 9 mm long). It is a thin layer of muscle, ∼5–10 fibers thick, making it ideal for intracellular recording (30). This ex vivo nerve–muscle preparation was placed in a bath containing 118 mM NaCl, 3.45 mM KCl, 11 mM glucose, 26.2 mM NaHCO3, 1.7 mM NaH2PO4, 0.7 mM MgCl2, and 0.75 mM CaCl2, pH 7.4. The nerve was stimulated with a suction electrode to evoke EPPs, and muscle contraction was blocked by exposure to 1 μM μ-conotoxin GIIIB (Peptides International). Borosilicate electrodes (∼40–60 MΩ) were filled with 3 M potassium chloride. EPP amplitudes were averaged and normalized to the first EPP of each trial and plotted against the EPP number. mEPPs were recorded for 2 min in each muscle fiber, followed by single nerve-evoked synaptic activity (20 EPPs) collected with an ISI of 5 s. Recordings of mEPPs were made in the presence of 2 mM CaCl2. Quantal content was calculated by dividing the evoked EPP amplitude recorded in 2 mM Ca2+ by the mEPP amplitude in 2 mM Ca2+. All data are presented as mean ± SEM. Statistical significance was calculated using the Student's t test.
Skeletal Muscle Contraction.
TA muscle function of WT and IM-AA mice (8–11 wk old) was measured in situ as described previously (31). In brief, mice were anesthetized with Avertin (625 mg/kg i.p.) and placed on a heated metal platform (∼37 °C). The distal TA tendon was dissected and sutured to the lever arm of the force/length control system (305C-LR; Aurora Scientific). Muscle contraction was provided by sciatic nerve stimulation. A length–force curve was produced by stimulation at 70 Hz (200-ms duration) every 90 s from short to long muscle lengths, to establish the optimum length for peak force production. At the optimum length, a force–frequency curve was then measured over a range of stimulation frequencies from single twitch to 200 Hz. At each frequency, the stimulation time was kept constant at 200 ms. Specific force was calculated by dividing force values by the muscle cross-sectional area (mN/mm2). A protocol to assess muscle fatigue was then carried out. The muscle was stimulated at 120 Hz (200-ms duration) every 2 s, for a total of 2 min. Fatigue recovery was measured every 2 min up to 10 min postfatigue. All data are presented as mean ± SEM. Statistical significance was calculated using the Student's t test.
Rotarod Test.
Motor control was examined using an automated five-lane rotarod unit (Rotarod; IITC Life Science). On the first day, mice (8-12 wk old) were habituated to the rotarod for 5 min and then became familiar with the rotarod by being subjected to one trial. Then the animals were tested for 3 d with five trials each day. The rotarod was set to accelerate from 4 to 40 rpm over a maximum of 5 min. Mice were given 30-min rest in between trials to prevent stress and fatigue. The duration that each animal was able to stay on the rotating rod was recorded as time period to fall. All data are presented as mean ± SEM. Statistical significance was calculated using the Student's t test.
Grip Strength Test.
The grip strength test was used to measure neuromuscular function as the maximal muscle strength of forelimbs in 8- to 12-wk-old mice. Maximal muscle strength was assessed by the grasping force (in Newtons) applied by the mouse on a grid that was connected to a sensor. Three trials were carried out in succession to measure forelimb strength. All data are presented as mean ± SEM. Statistical significance was calculated using the Student's t test.
Exercise Tolerance Test.
The 8- to 12-wk-old mice were trained for 1 d on the treadmill before the test day. On the training day, the mice were first acclimated to the treadmill chamber (Columbus Instruments) for 10 min. After this short acclimation period, the belt, at a 5% incline, was started at a rate of 5 m/min. The speed was increased by 2.5 m/min every 3 min, with a maximum speed of 20 m/min. The mice were motivated to run by a mild electric shocking grid located at the back of the treadmill belt. On the test day, the mice ran until exhaustion was reached, as defined by >5 s of consecutive contact with the shock grid without an attempt to return to the treadmill. Total distance was calculated based on speed and time. All data are presented as mean ± SEM. Statistical significance was calculated using the Student's t test.
Acknowledgments
This work was supported by National Institutes of Health Research Grants R01 NS022625 (to W.A.C.) and R01 NS033145 (to S.C.F.). E.N. was supported by a postdoctoral fellowship from the Swedish Society for Medical Research. M.J.K. was supported by National Heart, Lung, and Blood Institute Cardiovascular Training Grant T32HL007828. S.C.F. received funding from the Raymond and Beverly Sackler Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 3.Rosato Siri MD, Uchitel OD. Calcium channels coupled to neurotransmitter release at neonatal rat neuromuscular junctions. J Physiol. 1999;514(Pt 2):533–540. doi: 10.1111/j.1469-7793.1999.533ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18(2):89–98. [PubMed] [Google Scholar]
- 5.Poncer JC, McKinney RA, Gähwiler BH, Thompson SM. Either N- or P-type calcium channels mediate GABA release at distinct hippocampal inhibitory synapses. Neuron. 1997;18(3):463–472. doi: 10.1016/s0896-6273(00)81246-5. [DOI] [PubMed] [Google Scholar]
- 6.Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21(22):8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inchauspe CG, Martini FJ, Forsythe ID, Uchitel OD. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal. J Neurosci. 2004;24(46):10379–10383. doi: 10.1523/JNEUROSCI.2104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maejima T, et al. Postnatal loss of P/Q-type channels confined to rhombic lip-derived neurons alters synaptic transmission at the parallel fiber to purkinje cell synapse and replicates genomic Cacna1a mutation phenotype of ataxia and seizures in mice. J Neurosci. 2013;33(12):5162–5174. doi: 10.1523/JNEUROSCI.5442-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbano FJ, et al. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci USA. 2003;100(6):3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee A, et al. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399(6732):155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 11.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20(18):6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411(6836):484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Zhou H, Scheuer T, Catterall WA. Molecular determinants of Ca2+/calmodulin-dependent regulation of CaV2.1 channels. Proc Natl Acad Sci USA. 2003;100(26):16059–16064. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic CaV2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57(2):210–216. doi: 10.1016/j.neuron.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Lee A, et al. Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci. 2002;5(3):210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci. 2005;25(30):7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leal K, Mochida S, Scheuer T, Catterall W. Fine-tuning synaptic plasticity by modulation of CaV2.1 channels with Ca2+ sensor proteins. Proc Natl Acad Sci USA. 2012;109(42):17069–17074. doi: 10.1073/pnas.1215172109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, et al. Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation. Mol Cell Neurosci. 2014;63:124–131. doi: 10.1016/j.mcn.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogozhin AA, Pang KK, Bukharaeva E, Young C, Slater CR. Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. J Physiol. 2008;586(13):3163–3182. doi: 10.1113/jphysiol.2008.153569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarr TB, et al. Evaluation of a novel calcium channel agonist for therapeutic potential in Lambert-Eaton myasthenic syndrome. J Neurosci. 2013;33(25):10559–10567. doi: 10.1523/JNEUROSCI.4629-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz E, Protti DA, Ferro PA, Rosato Siri MD, Uchitel OD. Effects of Ca2+ channel blocker neurotoxins on transmitter release and presynaptic currents at the mouse neuromuscular junction. Br J Pharmacol. 1997;121(8):1531–1540. doi: 10.1038/sj.bjp.0701290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosato-Siri MD, Piriz J, Tropper BA, Uchitel OD. Differential Ca2+-dependence of transmitter release mediated by P/Q- and N-type calcium channels at neonatal rat neuromuscular junctions. Eur J Neurosci. 2002;15(12):1874–1880. doi: 10.1046/j.1460-9568.2002.02015.x. [DOI] [PubMed] [Google Scholar]
- 23.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks SV, Faulkner JA. Contraction-induced injury: Recovery of skeletal muscles in young and old mice. Am J Physiol. 1990;258(3 Pt 1):C436–C442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- 25.Zengel JE, Magleby KL. Augmentation and facilitation of transmitter release: A quantitative description at the frog neuromuscular junction. J Gen Physiol. 1982;80(4):583–611. doi: 10.1085/jgp.80.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46(4):633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Borst JG, Sakmann B. Facilitation of presynaptic calcium currents in the rat brainstem. J Physiol. 1998;513(Pt 1):149–155. doi: 10.1111/j.1469-7793.1998.149by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol. 1998;512(Pt 3):723–729. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20(4):797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 30.Bradley SA, Lyons PR, Slater CR. 1989. The epitrochleoanconeus muscle (ETA) of the mouse: A useful muscle for the study of motor innervation in vivo. J Physiol 415:3P.
- 31.Whitehead NP, Kim MJ, Bible KL, Adams ME, Froehner SC. A new therapeutic effect of simvastatin revealed by functional improvement in muscular dystrophy. Proc Natl Acad Sci USA. 2015;112(41):12864–12869. doi: 10.1073/pnas.1509536112. [DOI] [PMC free article] [PubMed] [Google Scholar]