Abstract
Syntaxin (Syn)-1A mediates exocytosis of predocked insulin-containing secretory granules (SGs) during first-phase glucose-stimulated insulin secretion (GSIS) in part via its interaction with plasma membrane (PM)-bound L-type voltage-gated calcium channels (Cav). In contrast, Syn-3 mediates exocytosis of newcomer SGs that accounts for second-phase GSIS. We now hypothesize that the newcomer SG Syn-3 preferentially binds and modulates R-type Cav opening, which was postulated to mediate second-phase GSIS. Indeed, glucose-stimulation of pancreatic islet β-cell line INS-1 induced a predominant increase in interaction between Syn-3 and Cavα1 pore-forming subunits of R-type Cav2.3 and to lesser extent L-type Cavs, while confirming the preferential interactions between Syn-1A with L-type (Cav1.2, Cav1.3) Cavs. Consistently, direct binding studies employing heterologous HEK cells confirmed that Syn-3 preferentially binds Cav2.3, whereas Syn-1A prefers L-type Cavs. We then used siRNA knockdown (KD) of Syn-3 in INS-1 to study the endogenous modulatory actions of Syn-3 on Cav channels. Syn-3 KD enhanced Ca2+ currents by 46% attributed mostly to R- and L-type Cavs. Interestingly, while the transmembrane domain of Syn-1A is the putative functional domain modulating Cav activity, it is the cytoplasmic domain of Syn-3 that appears to modulate Cav activity. We conclude that Syn-3 may mimic Syn-1A in the ability to bind and modulate Cavs, but preferring Cav2.3 to perhaps participate in triggering fusion of newcomer insulin SGs during second-phase GSIS.
Introduction
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, including target- (t-) membrane SNAREs (Syntaxins [Syn]) and synaptosomal-associated proteins of 25 kDa (SNAP25) and vesicle-associated membrane proteins (VAMPs), are the fundamental components of the exocytotic machinery required for the docking and fusion of secretory granules (SGs) with the plasma membrane (PM), which have been well studied in neurons [1, 2] and neuroendocrine cells, particularly pancreatic islet β-cells [3–5]. t-SNAREs Syn-1A and SNAP25 through their interactions with PM-bound voltage-gated calcium channels (Cav), L-type in β-cells and N-type in neurons, position the predocked SGs to the site of maximum Ca2+ influx for efficient exocytosis [6–12].
Cavs regulate secretion in neurons and β-cells [13, 14]. Cavα1 pore-forming subunits, Cav1 and Cav2, exist as heteromeric complexes by association with auxiliary subunits, β and α2δ subunits, which mediate trafficking of Cavs to the PM and fine-tune their biophysical properties [13, 14]. In β-cells, L-type Cav1s (Cav1.2 is abundant in rodents; Cav1.3 is abundant in human) [15, 16], are believed to effect first-phase GSIS by acting on the readily releasable pool (RRP) of predocked SGs [17–20]. Genetic deletion of R-type/Cav2.3 suppressed only the second-phase GSIS from the mouse islets; and did not affect the early component of depolarization-induced exocytosis (corresponding to the RRP) in the β-cells [21, 22], leaving intact the late component, referred to as SG mobilization from the reserve pool, which corresponds to the newcomer SGs. This led us and others to hypothesize that predocked SGs mediating a major portion of first-phase GSIS and newcomer SGs accounting for all of second-phase GSIS are respectively mediated by L- and R-type Cavs.
Of the four Syns that mediate exocytosis, Syn-1A, Syn-2 and Syn-4 are present and localized predominantly to the β-cell PM, whereas Syn-3 is more abundant in SGs [5, 23–25]. Genetic deletion of Syn-1A in mice blunted first-phase GSIS, which was attributed to loss of ability of predocked insulin SGs to undergo exocytosis, without perturbation of recruitment and fusion of newcomer SGs [5]. Syn-1A binding and inhibition of L-type Cav [9, 10] was demonstrated to be via the two highly conserved cysteines, Cys271 and Cys272 at its transmembrane domain [26–28]. In contrast, depletion of endogenous Syn-3 by RNA interference (RNAi) in INS-1 cells inhibited GSIS by impairing the recruitment and fusion of newcomer SGs affecting predominantly the second-phase GSIS, without affecting predocked SGs [23]. Overexpression of Syn-3 was reported to also inhibit β-cell L-type Cav [9]. However, it remains unclear whether endogenous Syn-3 modulates Cav channels in β-cells. Furthermore, the putative Cav-interacting transmembrane cysteine residues in Syn-1A are not conserved in Syn-3. Therefore, more work is required to clarify which β-cell Cavs Syn-3 acts on and the putative Cav-binding domain within Syn-3.
In this work, we assessed the endogenous function of Syn-3 on β-cell Cav activity by siRNA depletion and provide detailed biochemical and functional evidence for the interactions between endogenous Syn-3 and R-type (Cav2.3) and to lesser degree also L-type (Cav1.2 and Cav1.3) Cavs.
Methods
Cell Culture
INS-1 832/13 cells and HEK293 cells lines were cultured as previously reported [29, 30]. INS-1 832/13 cell line (herein called INS-1) was a gift from Christopher Newgard (Duke University, Durham, North Carolina) [31]. Syn-3 siRNA/mCherry plasmid (Dharmacon, Chicago, IL, USA) used here we previously reported to efficiently knockdown (KD) Syn-3 expression in INS-1 cells [23]. A mCherry plasmid was used as control [23]. After the cells were transfected with these plasmids for 48 h, cellular entry of the plasmids was confirmed by the mCherry expression observed by epifluorescence imaging. These mCherry-expressing cells were subjected to electrophysiology and TIRF imaging studies.
Immunoprecipitation
This was performed on INS-1 cells as previously reported [30, 32]. INS-1 cells at 80%–85% confluence were washed with PBS (37°C) and incubated for 30 min at 37°C in Krebs–Ringer HEPES buffer (KRB, in mM: 125 NaCl, 5.6 KCl, 1.28 CaCl2, 5 Na2CO3, 25 HEPES, pH 7.4. with 0.1% BSA) containing basal 0.8 mM glucose concentration. Cells intended for stimulation were preincubated for 30 min with 0.8 mM glucose, and then stimulated with 16.7 mM glucose plus 10 nM glucagon-like peptide (GLP)-1 for 30 min. 1 mg protein extract of cell lysates were used for each condition. Immunoprecipitation (IP) was with 2 μg Syn-1A or Syn-3 antibodies or pre-immune IgG (as control). Precipitated proteins were immunodecorated and identified using the indicated primary antibodies, which include anti-Cav1.2, -Cav1.3, -Cav2.3, -Cav2.2, -Cavα2δ-1, -Cavβ3, -SNAP25, -Syn-1A and–Syn-3. All Cav subunits antibodies are from Alomone Labs (Jerusalem, Israel), and SNAP25, Syn-1A and Syn-3 are from SySy (Goettingen, Germany); the specificity of these antibodies was well characterized by these companies, which been used broadly. IP experiments on HEK293 cells transfected with Syn-1A, Syn-3, Cav1.3 or Cav2.3, were similar to those performed on INS-1 cells.
In Vitro Binding Assay and Western Blotting
In vitro binding assays were performed according to the method we previously described [30, 32, 33], which also showed the specificity of the SNARE protein antibodies. Briefly, GST-Syn-1A (cytoplasmic domain a.a. 1–265), GST-Syn-3 (cytoplasmic domain a.a. 1–263) and GST (as control, 300 pM protein each, all bound to glutathione agarose beads) were incubated with HEK293 cell lysate at 4°C for 2 h with constant agitation. The beads were then washed three times with washing buffer (in mM: 20 HEPES (pH 7.4), 150 KOAC, 1 EDTA, 1 MgCl2; with 5% glycerol and 0.1% Triton X-100). Precipitated proteins were separated on 10% SDS-PAGE and identified with anti-Cav1.3 or -Cav2.3 antibody (1:200).
All of the Western blot bands were quantified using image J software (http://rsb.info.nih.gov/ij). We employed two approaches to quantify the ‘input control’ and ‘co-immunoprecipitated (co-IPed)’ blots. For quantification of ‘input control’ bands, we considered maximum intense band for each protein from each experiment as 100 and expressed other bands for that particular protein as % of maximum. For quantification of ‘co-IPed’ blots, we measured intensities of both ‘co-IPed’ and ‘input control’ bands that are processed in parallel. The intensity of ‘co-IPed’ band was then calculated as a ratio to the corresponding ‘input control’ band intensity and multiplied by 5 (as ‘input control’ is 5% of total protein used for IP) and expressed as ‘percentage of recovery’.
Electrophysiology
Recording pipettes were pulled from 1.5-mm borosilicate glass capillary tubes using a programmable micropipette puller. Pipettes were heat polished to a tip resistance ranging from 2 to 3 MOhm when filled with the intracellular solution. For measurement of Cav currents, barium was used as charge carrier. Pipettes were filled with the buffer containing (in mM): 120 CsCl, 20 tetraethylammonium chloride, 5 EGTA, 5 MgATP and 5 HEPES (pH 7.2 with CsOH). The external solution contained (in mM): 100 NaCl, 20 BaCl2, 20 tetraethylammonium chloride, 4 CsCl, 1 MgCl2, 10 glucose and 5 HEPES (pH 7.4 with NaOH). L-type Cav inhibitor nifedipine (10 μM), R-type Cav inhibitor SNX482 (400 nM) and N-type Cav inhibitor ω-Conotoxin GVIA (100 nM) are all from Alomone labs (Jerusalem, Israel). Cells were held at −70 mV for 2 min after formation of whole-cell mode, and currents elicited by stepped 300 or 500 milliseconds depolarizations in 10 mV increments. Recordings were conducted using an EPC10 patch clamp amplifier equipped with Pulse and X-Chart software programs (HEKA Electronik, Lambrecht, Germany).
Statistical Analysis
Data are presented as mean±SEM. Statistical significance was evaluated by Student’s t test or Mann-Whitney rank sum test (SigmaStat 3.11. Systat Software Inc., Chicago, IL, USA) and considered significant when P<0.05.
Results
Syn-3 Binds to Distinct β-cell Cavs than Syn-1A
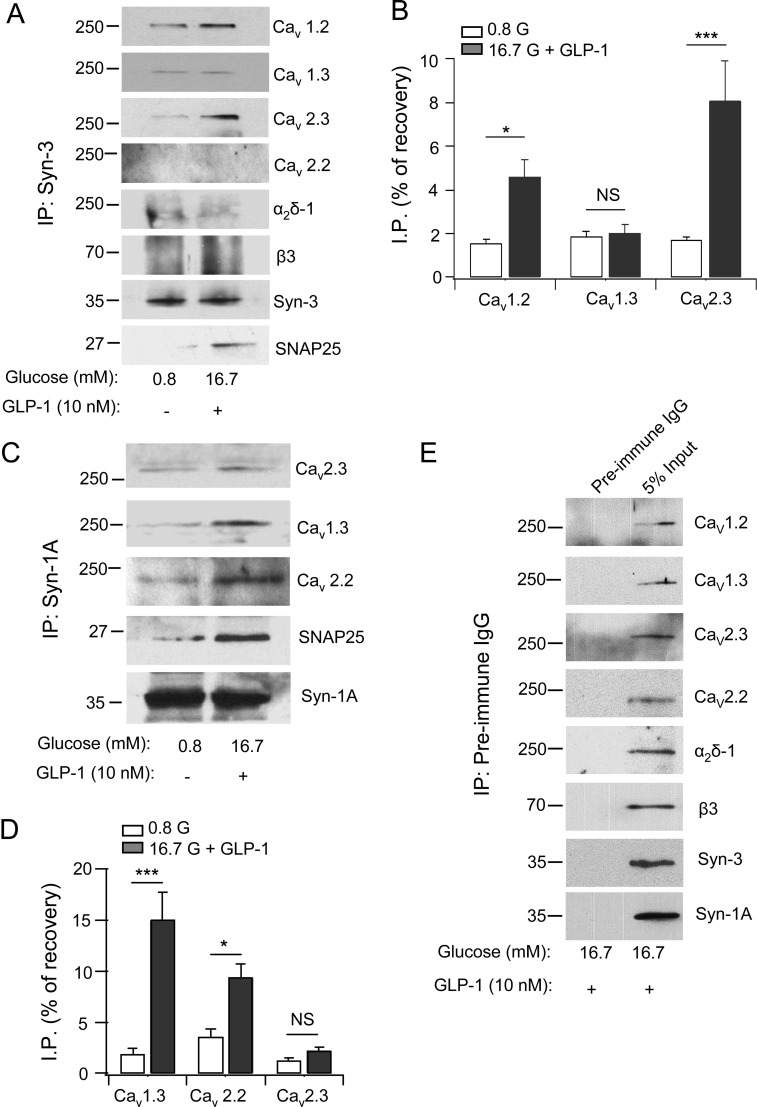
We postulated that Syn-3’s actions on Cav channels [9] may be by direct physical binding to Cav subunits as had been demonstrated for Syn-1A [11, 12]. This was assessed with Syn-3 antibody co-immunoprecipitation of INS-1 at basal (0.8 mM glucose) and maximal stimulated conditions (16.7 mM glucose plus 10 nM GLP-1). Fig 1A and 1B show that Syn-3 co-precipitated Cavα1 pore-forming subunits including Cav1.2, Cav1.3 and Cav2.3, but not Cav2.2. Syn-3 also brought down small amounts of auxiliary subunits α2δ-1 and β3. Remarkably, GLP-1/high glucose stimulation caused a large increase in the amount of Cav2.3 co-precipitated (from 1.7% to 8.1%, a 4.7 fold increase; p<0.001), a more moderate increase in Cav1.2 co-precipitated (from 1.6% to 4.6%, a 2.9 fold increase; p<0.05), and no significant increase in Cav1.3 co-precipitated (1.9% to 2.0%). There was no increase in the levels of co-precipitated auxiliary subunits α2δ-1 and β3; and there was no detectable Cav2.2 brought down. There was also an expected large increase in the amount of SNAP25 co-precipitated (from 0.9% to 7.5%, p<0.001). These results indicate a preferential formation of the Syn-3/Cav2.3 α1 complex, and the more moderate formation of the Syn-3/Cav1.2 complex could explain our previous report on why Syn-3 overexpression also affected L-type Cav current [9].
Fig 1. Syn-3 co-immunoprecipitates (IP) distinct Cavs than Syn-1A in INS-1 cells.
Syn-3 (A) and Syn-1A (C) interactions with the indicated Cavα1 subunits (Cav1.2, Cav1.3, Cav2.3 and Cav2.2) and auxiliary subunits (α2δ-1 and β3) and SNAP25 in INS-1 cells. Densitometric analysis of Syn-3 co-IP (B) and Syn-1A co-IP (D), expressed as percent recovery of total lysate inputs (which showed equal protein loading in S1 Fig), shows that high glucose (16.7 mM) plus GLP-1 (10 nM) increased the association of these syntaxins with the respective Cavs. Values are means±SEM, n = 3. *p<0.05, ***p<0.001, NS: not significant. As control (E) shows representative blots from five separate co-IP experiments with pre-immune IgG, which did not bring down syntaxins or Cavs (left lanes). Right lanes show the input lysates. All five experiments probed for the Cav α subunits and α2δ-1, whereas β3, Syn-3 and Syn-1A were probed on two blots from separate experiments.
Syn-1A antibody co-precipitated Cav1.3, Cav2.2 and Cav2.3 α1 subunits (Fig 1C). However, only the amounts of Cav1.3 (from 1.9% at basal to 15% when stimulated, a 7.8 fold increase; p<0.001; N = 3; Fig 1C and 1D) and Cav2.2 (from 3.6% to 9.4%; p<0.05; N = 3; Fig 1C and 1D) to lesser degree increased with stimulation, whereas there was no further increase in the amount of Cav2.3 co-precipitated. SNAP25 co-precipitated increased from 1.25% to 6.3% (p<0.05; N = 3) after stimulation. The latter results confirm the previous hypothesis that Syn-1A preferentially associates with L-type Cav to form a complex exictosomes, which is functionally important in mediating exocytosis of predocked insulin SG [6, 7]. While our results support that Syn-1A could also bind R-type Cav [34], this complex is perhaps less important in β-cell or at least subordinate to the more abundant Syn-3-R-type Cav complex that formed. Syn-1A is known to also bind N-type Cav in neurons [35] that is also found in INS-1 [34], albeit less abundant, thus presumably less important in β-cell. As control, at maximal stimulation with high glucose plus GLP-1, preimmune IgG did not pull down the syntaxins or any of the Cav subunits (Fig 1E). S1 Fig shows the corresponding input proteins with the Syn-3 or Syn-1A IP experiments in Fig 1 in both control and stimulated INS-1 cells.
Syn-3 Preferentially Binds Cav2.3, whereas Syn-1A Preferentially Binds Cav1.2 and Cav1.3
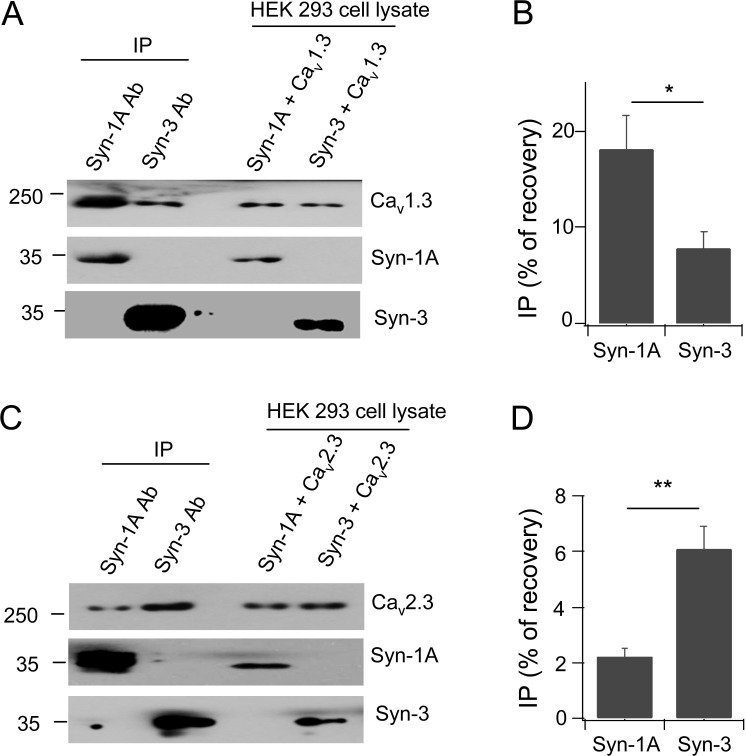
The co-IP studies of endogenously interacting proteins (Fig 1) showed a preference of Syn-3 for R-type/Cav2.3 over L-type Cavs. We therefore next critically assessed whether Syn-3 vs Syn-1A do indeed preferentially bind R- and L-type Cavs, respectively, by employing the HEK cell model. HEK cells, devoid of endogenous Cavs and SNARE proteins, allow these proteins to be individually exogenously expressed presumably in their native conformations, unperturbed by any other proteins that might affect them or their interactions as may be the case using native β-cells (Fig 1).
Immunoprecipitation experiments were conducted in HEK cells expressing only Syn-3 or Syn-1A with Cav1.3 or Cav2.3 α1 subunits. When calculated as the percentage of protein recovery from total lysate input, Syn-1A co-precipitated Cav1.3 with an average (N = 3) of 18.1±3.5% versus Syn-3 of 7.8±1.8%, which is 2.3 times higher (Fig 2A and 2B). In contrast, Syn-3 antibody co-precipitated more Cav2.3 (6.1±0.8%) than Syn-1A antibody (2.2±0.3%), which is 2.8 times higher (Fig 2C and 2D). Therefore, while there is some promiscuity in the binding of Syn-1A and Syn-3 for these Cavs, Syn-1A preferentially binds Cav1.3 and Syn-3 preferentially binds Cav2.3. This is consistent with the current thinking that while the Syn-1A-Cav1.3 complex targets the PM sites of maximal Ca2+ influx to where predocked insulin SGs exocytose [6, 7], we further postulate that the Syn-3-Cav2.3 complex likely targets the PM sites of Ca2+ influx where exocytosis of newcomer insulin SGs [23] would likely occur. This thinking is also consistent with the role of Cav2.3 in second-phase GSIS [21].
Fig 2. Syn-3 preferentially binds Cav2.3 while Syn-1A preferentially binds Cav1.3.
Representative blots show HEK293 cells co-transfected with Cav1.3 (A) or Cav2.3 (C) with either Syn-1A or Syn-3, then subjected to co-IP with anti-Syn-1A or Syn-3 antibody. Co-precipitated proteins were identified with the indicated antibodies. Densitometric analysis of the co-precipitated Cav1.3 (B) or Cav2.3 (D), expressed as percent recovery of total lysate inputs. Values are means±SEM, n = 3; *p<0.05, **p<0.01.
Depletion of Endogenous Syn-3 Selectively Enhances Cav Channels Activity
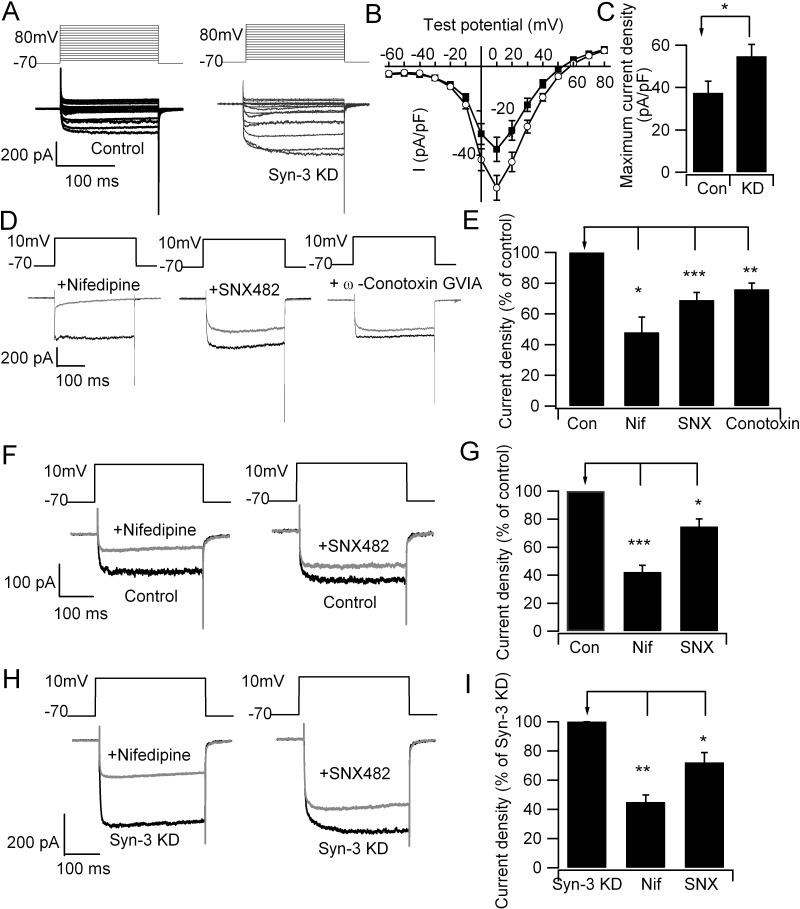
We then assessed the functional consequence of Syn-3 interactions with these Cavs by depletion of endogenous Syn-3. Syn-3 siRNA plasmid co-expressing mCherry was used to transfect INS-1 832/13 cells, which effectively reduced Syn-3 protein expression by >70%, as shown in our previous report [23]. INS-1 cells expressing mCherry would be expected to exhibit near-total depletion of Syn-3, thus ideal for single cell analysis by electrophysiology. Whole cell Cav current recording of Syn-3-depleted INS-1 cells showed the Cav current amplitudes were increased by 46% (54.8±5.6 pA/pF; n = 11; Fig 3A–3C) compared to control (mCherry transfected) cells (37.4±5.5 pA/pF; p<0.05; n = 16). L- and R-type Cavs have been postulated to be the major Cavs in rodents to affect first- and second-phase GSIS, respectively [17–22]. N-type Cav was reported to also contribute to first-phase GSIS [36]. We thus used selective antagonists to determine the contribution of each of these Cavs to the overall Cav current density in INS-1 cells (Fig 3D). Fig 3E is the summary analysis of their Cav current densities normalized to control values, showing that amounts of Cav current blocked was 52% by nifedipine (L-type antagonist) (n = 8; p<0.05), 31% (n = 10; p<0.001) by SNX482 (R-type antagonist), and only 24% by ω-Conotoxin GVIA (N-type antagonist) (n = 9; p<0.01). This suggests that more of Cav current blocked by the L- on R-type Cav antagonists, and not N-type Cav are likely attributed to the Syn-3 actions, which would be consistent with our protein-binding data (Figs 1 and 2). To confirm that L- and R-type Cavs accounted for most of the increased Cav current caused by the Syn-3 KD, we performed another set of experiments with selective blockade with nifedipine and SNX482 on Syn-3 KD cells (Fig 3H and 3I) and Control cells (Fig 3F and 3G). Nifedipine reduced the Cav current by 55%% (n = 12, p<0.05) in Syn-3 KD cells which was slightly less than the 58% reduction (n = 14; p<0.001) in Control cells. SNX482 reduced the Cav current by 28% (n = 6; p<0.05) in Syn-3 KD cells which was slightly more than the 25% reduction (n = 10; p<0.05) in Control cells.
Fig 3. Depletion of Syntaxin 3 in INS-1 cells increased voltage-gated Ca2+ currents.
(A) Representative traces showing Cav currents recorded in whole-cell mode from control and Syn-3 KD INS-1 cells. (B) Current-voltage relationship of Cavs from control (n = 16) and Syn-3 KD (n = 11) INS-1 cells. Currents were normalized to cell capacitance to yield current density. Values are means±SEM. (C) Bar chart shows the maximum increase in current density under stimulation of 10 mV voltage. *p<0.05 for control vs Syn-3 KD (D) Representative Cav currents from normal INS-1 cells before and after treatment with nifedipine (10 μM Nif; n = 8), SNX482 (400 nM SNX; n = 9) or ω-Conotoxin GVIA (100 nM Conotoxin; n = 10); their summary analysis (E) of the maximum increase in current densities normalized to the percentage of control (Con). ***p<0.001, **p<0.01 and *p<0.05 compared to control. We then performed another set of experiments (different from A-E) to compare the effects of nifedipine and SNX on Syn-3 KD (H and I) and Control INS-1 cells (F and G). (F) Representative Cav currents from control INS-1 cells before (control, n = 25) and after treatment with nifedipine (10 μM Nif) (n = 14) or SNX482 (400 nM SNX) (n = 6); their summary analysis (G) of the maximum increase in current densities normalized to the percentage of control (Con). ***p<0.001; *p<0.05 compared to Control. (H) Representative Cav currents of Syn-3 KD INS-1 cells before (Syn-3 KD, n = 11) and after treatment with nifedipine (n = 12) or SNX482 (n = 6); and their summary analysis (I) of the maximum increase in current densities normalized as percentages of the Syn-3 KD cells. *p<0.05 compared to Syn-3 KD. Here, Syn-3 KD Cav currents were 148% of Control cells, similar to A and B.
The Functional Domain of Syn-3 that Modulates Cav Channel Activity is Different from Syn-1A
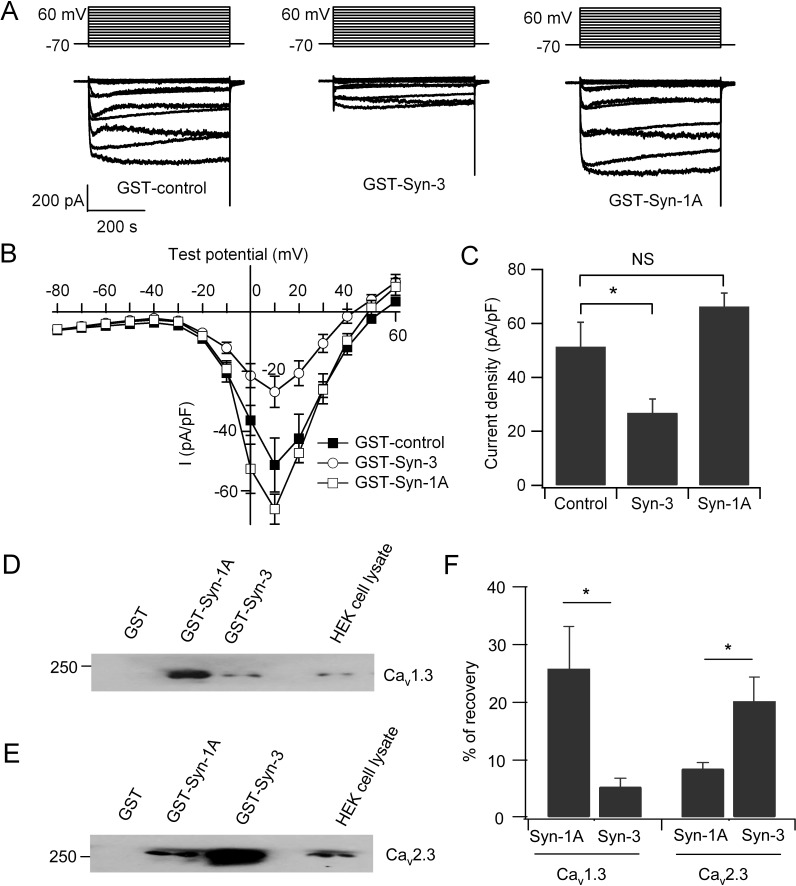
It has been well studied that the putative domain of Syn-1A that modulates Cav activity is the transmembrane domain (amino acid 266–288), particularly the two vicinal cysteines (C271 and C272) [26–28]. Indeed, dialysis of the cytoplasmic domain of Syn-1A, GST-Syn-1A (a.a. 1–265), by patch pipette into INS-1 cells had no significant effects on the Cav current (66.2±5.1 pA/pF; n = 8) compared to GST control (51.4±9 pA/pF; n = 9; Fig 4A–4C). Peculiarly, the two vicinal cysteines in the Syn-1A transmembrane domain are not conserved in the transmembrane domain of Syn-3. Remarkably, dialysis of the cytoplasmic domain of Syn-3, GST-Syn-3 (a.a. 1–263) inhibited Cav current by 48% compared to control cells (26.8±5.2 pA/pF; n = 9; p<0.05; Fig 4A–4C). This result suggests that the Cav-interacting domains of Syn-3 and Syn-1A are different. Syn-3 shares a low 64% amino acid identity to Syn-1A [37], suggesting that it may be the cytoplasmic domains in Syn-3 distinct from Syn-1A that bind the Cavs.
Fig 4. Cytoplasmic Syn-3 domain but not cytoplasmic Syn-1A domain regulates Cav currents.
(A) GST-Syn-3 cytoplasmic domain (a.a. 1–263) or GST-Syn-1A cytoplasmic domain (a.a. 1–265) or GST (control) was dialyzed into INS-1 cells, then Cav currents recorded. Shown are representative traces in the whole-cell mode with stimulation from −80–60 mV. (B) Current-voltage relationship of Cav channels. Currents were normalized to cell capacitance to yield current density. (C) Bar chart showing the maximum current density in INS-1 cells dialyzed with GST control (n = 9), GST-Syn-3 (n = 9), or GST-Syn-1A (n = 8). Values are means±SEM; *p<0.05; NS: no significant difference. (D and E) Representative blots show HEK293 cells transfected with Cav1.3 (D) or Cav2.3 (E) subjected to pull down with 300 pmol of GST-Syn-1A (a.a. 1–265), GST-Syn-3 (a.a. 1–263) or GST. (F) Summary analysis of four separate experiments. Data was expressed as means ± SEM; *p<0.05.
We next employed protein binding and pull down assays of the cytoplasmic domains of Syn-1A (a.a. 1–265) or Syn-3 (a.a. 1–263) with Cav1.3 or Cav2.3 α1 subunit expressed in HEK293 cells. As shown in Fig 4D–4F with transfected HEK293 cells, GST-Syn-1A (a.a. 1–265) preferentially binds to Cav1.3 with an average of 25.7±7.3% versus GST-Syn-3 (a.a. 1–263) of 5.3±1.5%, which is 4.8 times higher (Fig 4D and 4F; n = 4; p<0.05). In contrast, GST-Syn-3 binds more Cav2.3 (20.1±4.1%) than GST-Syn-1A (8.4±1.0%), which is 2.4 times higher (Fig 4E and 4F; n = 4; p<0.05). These results indicate the following. First, whereas Syn-1A cytoplasmic domain can bind Cavα1 subunits, preferentially L-type Cavs, it seems this binding does not significantly regulate the Cav activity. Second, the cytoplasmic domain of Syn-3 binds the Cavα1 subunits, preferring R-type Cav2.3, thus presumably influencing Cav2.3 activity. More work will be required to elucidate the putative functional Cav-binding domain(s) within the Syn-3 cytoplasmic domain.
Discussion
Taken together, these results demonstrate that Syn-3, via its cytoplasmic domain, preferentially binds and regulates R-type/Cav2.3 α1 subunit. This is consistent with Syn-3’s role in mediating fusion of newcomer SGs that account for all of second-phase GSIS [23] and Cav2.3’s role in mediating second-phase GSIS in rodents [21]. In contrast, Syn-1A preferentially binds L-type Cav1.2 and Cav1.3 to direct the PM sites of Ca2+ influx to where fusion of predocked SGs would occur during first-phase GSIS. Nonetheless, both syntaxins are promiscuous in binding both L- and R-type Cavs and could therefore potentially assist each other in regulating various Cavs perhaps when either syntaxin becomes deficient. In pancreatic islets of human type-2 diabetes, Syn-1A levels are severely reduced [38] which may presumably affect β-cell L-type Cav function that may contribute to the reduced efficiency of exocytosis of predocked insulin SGs, with ensuing reduction to absent first phase GSIS [5]. An increased in Syn-3 expression might compensate for the Syn-1A deficiency, perhaps by forming complexes with L-type Cavs which could affect an increase in newcomer SGs exocytosis known to also occur in first-phase GSIS [25]. This could rescue the reduced first-phase secretion in type-2 diabetes islets that has been attributed to a loss of fusion competence of predocked SGs [38].
Syn-3 on insulin SGs [23] likely acts to direct the recruitment of newcomer SGs to PM-bound Cav2.3 by forming an excitosome complex, mimicking the actions of Syn-1A-Cav1.2 and Syn-1A-Cav1.3 excitosomes on predocked SGs [6, 7]. During exocytosis, we postulate that Syn-3 would dissociate from Cav2.3, relieving the inhibition and allowing Ca2+ influx to affect fusion of the newcomer SG. Whereas both syntaxins bind these Cavs, the putative functional domain of Syn-1A is its transmembrane domain [26, 28] whereas the putative functional Cav-binding domain of Syn-3 is within its cytoplasmic domain. It is also possible that the syntaxin-binding domain(s) in Cav2.3 [34] may be different from that reported for L-type Cavs [6, 11], called the synprint site localized to the cytosolic II-III linker connecting the second and third transmembrane domains. Much further work is required to determine the putative interacting domains between Syn-3 and Cav2.3.
Lastly, our data are consistent with newcomer SGs (containing Syn-3) being located further away from the PM-bound R-type Cav2.3 but are recruited to Cav2.3 upon stimulation where they undergo minimal docking time at the PM before fusion. The latter would indicate more rapid priming and a high-affinity Ca2+ sensor (i.e. synaptotagmins) for newcomer SGs [39]. Synaptotagmin 7 has been purported to be the major Ca2+ sensor for β-cells [40], but whether this synaptotagmin or another synaptotagmin is the Ca2+ sensor for newcomer SGs remain to be elucidated. Consistent with this thinking, R-type Cav channel has been shown to recruit synaptotagmins to the PM to form part of the excitosome [34]. We hope that this work will trigger more future study that will lead to the full characterization of the newcomer SG excitosome as has been worked out for the predocked SG excitosome. This is of broad importance to endocrine secretory biology, as newcomer SGs likely also account for the sustained phase of secretion in other endocrine cells. While our work with the INS-1 cell line establishes proof of concept of novel Syn-3-Cav complexes influencing Cav activity, human β-cells do not contain R-type Cav, but rather employ P/Q-type Cav (Cav2.1) to likely mediate second-phase GSIS and consequently newcomer SG exocytosis [41]. Therefore, more exciting work will be required to assess if Syn-3 might similarly form complexes with human P/Q-type Cav (Cav2.1) to mediate exocytosis of newcomer SGs in human β-cells.
Supporting Information
(TIF)
Abbreviations
- Syn
syntaxin
- SG
insulin-containing secretory granule
- GSIS
glucose-stimulated insulin secretion
- PM
plasma membrane
- Cav
voltage-gated calcium channels
- siRNA
small interfering RNA
- KD
knockdown
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP
vesicle-associated membrane proteins
- SNAP25/23
synaptosomal-associated proteins of 25/23 kDa
- Syt
synaptotagmin
- RRP
readily releasable pool
- t-
target-
- GLP-1
glucagon-like peptide-1
- GST
glutathione S-transferase
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants to H. Y. Gaisano from the Canadian Institute of Health Research (MOP 86544 and MOP 69083) and grants to L. Chen from the National Science Foundation of China (81222020). Some of the equipment used in this study was supported by the 3D (Diet, Digestive Tract and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund, project number 19442.
References
- 1.Pfeffer SR. A prize for membrane magic. Cell. 2013; 155(6):1203–6. 10.1016/j.cell.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009; 323(5913):474–7. 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler MB, Sheu L, Ghai M, Bouquillon A, Grondin G, Weller U, et al. Characterization of SNARE protein expression in cell lines and pancreatic islets. Endocrinology. 1996; 137(4):1340–8. [DOI] [PubMed] [Google Scholar]
- 4.Huang XH, Pasyk EA, Kang YH, Sheu L, Wheeler MB, Trimble WS, et al. Ca2+ influx and cAMP elevation overcame botulinum toxin A but not tetanus toxin inhibition of insulin exocytosis. Am J Physiol Cell Physiol. 2001; 281(3):C740–C750. [DOI] [PubMed] [Google Scholar]
- 5.Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, et al. Imaging analysis reveals mechanistic differences between first- and second- phase insulin exocytosis. J Cell Biol. 2007; 177(4):695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, et al. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A. 1999; 96(1):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang SN, Larsson O, Bränström R, Bertorello AM, Leibiger B, Leibiger IB, et al. Syntaxin 1 interacts with the L(D) Subtype of voltage-gated Ca2+ channels in pancreatic beta cells. Proc Natl Acad Sci U S A. 1999; 96(18):10164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji J, Yang SN, Huang X, Li X, Sheu L, Diamant N, et al. Modulation of L-type calcium channels by distinct domains within SNAP-25. Diabetes. 2002; 51(5): 1425–36. [DOI] [PubMed] [Google Scholar]
- 9.Kang Y, Huang X, Pasyk EA, Ji J, Holz GG, Wheeler MB, et al. Syntaxins-3 and –1A inhibit L-type calcium channel activity, insulin biosynthesis and exocytosis in beta-cell lines. Diabetologia. 2002; 45(2):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng ZH, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996; 379(6564):451–4. [DOI] [PubMed] [Google Scholar]
- 11.Atlas D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J Neurochem. 2001; 77(4):972–85. [DOI] [PubMed] [Google Scholar]
- 12.Atlas D. The voltage-gated calcium channel functions as the molecular switch of synaptic transmission. Annu Rev Biochem. 2013; 82:607–35. 10.1146/annurev-biochem-080411-121438 [DOI] [PubMed] [Google Scholar]
- 13.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006; 27(6):621–76. [DOI] [PubMed] [Google Scholar]
- 14.Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009; 19(3):237–44. 10.1016/j.conb.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Nitert MD, Nagorny CL, Wendt A, Eliasson L, Mulder H. Cav1.2 rather than Cav1.3 is coupled to glucose-stimulated insulin secretion in INS-1 832/13 cells. J Mol Endocrinol. 2008; 41(1):1–11. 10.1677/JME-07-0133 [DOI] [PubMed] [Google Scholar]
- 16.Reinbothe TM, Alkayyali S, Ahlqvist E, Tuomi T, Isomaa B, Lyssenko V, et al. The human L-type calcium channel Cav1.3 regulates insulin release and polymorphisms in CACNA1D associate with type 2 diabetes. Diabetologia. 2013; 56(2):340–9. 10.1007/s00125-012-2758-z [DOI] [PubMed] [Google Scholar]
- 17.Davalli AM, Biancardi E, Pollo A, Socci C, Pontiroli AE, Pozza G, et al. Dihydropyridine-sensitive and -insensitive voltage-operated calcium channels participate in the control of glucose-induced insulin release from human pancreatic beta cells. J Endocrinol. 1996; 150(2):195–203. [DOI] [PubMed] [Google Scholar]
- 18.Barg S, Ma X, Eliasson L, Galvanovskis J, Göpel SO, Obermuller S, et al. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001; 81(6):3308–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulla V, Renstrom E, Feil R, Feil S, Franklin I, Gjinovci A, et al. Impaired insulin secretion and glucose tolerance in beta cell-selective Cav1.2 Ca2+ channel null mice. EMBO J. 2003; 22(15):3844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trus M, Corkey RF, Nesher R, Richard AM, Deeney JT, Corkey BE, et al. The L-type voltage-gated Ca2+ channel is the Ca2+ sensor protein of stimulus-secretion coupling in pancreatic beta cells. Biochemistry. 2007; 46(50):14461–7. [DOI] [PubMed] [Google Scholar]
- 21.Jing X, Li DQ, Olofsson CS, Salehi A, Surve VV, Caballero J, et al. Cav2.3 calcium channels control second-phase insulin release. J Clin Invest. 2005; 115(1):146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang SN, Berggren PO. Cav2.3 channel and PKClambda: new players in insulin secretion. J Clin Invest. 2005; 115(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu D, Koo E, Kwan E, Kang Y, Park S, Xie H, et al. Syntaxin-3 regulates newcomer insulin granules and compound fusion. Diabetologia. 2013; 56(2):359–69. 10.1007/s00125-012-2757-0 [DOI] [PubMed] [Google Scholar]
- 24.Xie L, Zhu D, Dolai S, Liang T, Qin T, Kang Y, et al. Syntaxin-4 mediates exocytosis of pre-docked and newcomer insulin granules underlying biphasic glucose-stimulated insulin secretion in human pancreatic beta cells. Diabetologia. 2015; 58(6):1250–9. 10.1007/s00125-015-3545-4 [DOI] [PubMed] [Google Scholar]
- 25.Gaisano HY. Here come the newcomer granules, better late than never. Trends Endocrinol Metab. 2014; 25(8):381–8. 10.1016/j.tem.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 26.Trus M, Wiser O, Goodnough MC, Atlas D. The transmembrane domain of syntaxin 1A negatively regulates voltage-sensitive Ca2+ channels. Neuroscience. 2001; 104(2):599–607. [DOI] [PubMed] [Google Scholar]
- 27.Arien H, Wiser O, Arkin IT, Leonov H, Atlas D. Syntaxin 1A modulates the voltage-gated L-type calcium channel (Cav1.2) in a Cooperative Manner. J Biol Chem. 2003; 278(31):29231–9. [DOI] [PubMed] [Google Scholar]
- 28.Cohen R, Marom M, Atlas D. Depolarization-evoked secretion requires two vicinal transmembrane cysteines of syntaxin 1A. PLoS one. 2007; 2(12):e1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L, Zhu D, Kang Y, Liang T, He Y, Gaisano HY. Exocyst sec5 regulates exocytosis of newcomer insulin granules underlying biphasic insulin secretion. PLoS One. 2013; 8(7):e67561 10.1371/journal.pone.0067561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu D, Zhang Y, Lam PP, Dolai S, Liu Y, Cai EP, et al. Dual Role of VAMP8 in Regulating Insulin Exocytosis and Islet beta Cell Growth. Cell Metab. 2012; 16(2):238–49. 10.1016/j.cmet.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004; 228(1–2):121–8. [DOI] [PubMed] [Google Scholar]
- 32.Lam PP, Ohno M, Dolai S, He Y, Qin T, Liang T, et al. Munc18b is a major mediator of insulin exocytosis in rat pancreatic β-cells. Diabetes. 2013; 62(7):2416–28. 10.2337/db12-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang Y, Leung YM, Manning-Fox JE, Xia F, Xie H, Sheu L, et al. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J Biol Chem. 2004; 279(45):47125–31. [DOI] [PubMed] [Google Scholar]
- 34.Cohen R, Atlas D. R-type voltage-gated Ca2+ channel interacts with synaptic proteins and recruits synaptotagmin to the plasma membrane of Xenopus oocytes. Neuroscience. 2004; 128(4):831–41. [DOI] [PubMed] [Google Scholar]
- 35.Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994; 13(6):1303–13. [DOI] [PubMed] [Google Scholar]
- 36.Taylor JT, Huang L, Keyser BM, Zhuang H, Clarkson CW, Li M. Role of high-voltage-activated calcium channels in glucose-regulated beta-cell calcium homeostasis and insulin release. Am J Physiol Endocrinol Metab. 2005; 289(5):E900–8. [DOI] [PubMed] [Google Scholar]
- 37.Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, et al. The syntaxin family of vesicular transport receptors. Cell. 1993; 74(5):863–73. [DOI] [PubMed] [Google Scholar]
- 38.Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006; 55(2):435–40. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen MG, Sherman A. Newcomer insulin secretory granules as a highly calcium-sensitive pool. Proc Natl Acad Sci U S A. 2009; 106(18):7432–6. 10.1073/pnas.0901202106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008; 105(10):3992–7. 10.1073/pnas.0711700105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, et al. Voltage-gated ion channels in human pancreatic beta-cell: electrophysiological characterization and role in insulin secretion. Diabetes. 2008; 57(6):1618–28. 10.2337/db07-0991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.