Abstract
Aim:
In our study, we aimed to clinically and epidemiologically evaluate respiratory tract infections the viral agents of which were detected by molecular methods and to compare influenza and other respiratory tract viruses in this context.
Material and Methods:
The records of 178 patients aged above 2 years who presented to pediatric emergency outpatient clinic with fever and respiratory tract infection findings between December 2013 and April 2014 were examined retrospectively.
Results:
At least one respiratory tract pathogen was detected by polymerase chain reaction in 78.6% (n=140) of the patients: influenza A 33.5%, influenza B 16.4%, respiratory syncytial virus 9.2%, adenovirus 7.8%, rhinovirus 7.1%, coronavirus 7.1%, human metapneumovirus 5.7%, human bocavirus 5.7%, parainfluenza virus 3.5%, coinfection 2.8%. The mean age of the patients was 6.3±3.6 years. Sixty-nine patients (49.2%) were aged between 2 and 5 years. Seventy-one patients (50.7%) were aged 5 years and above. Upper respiratory tract infection was found with a rate of 65.7% and lower respiratory tract infection was found with a rate of 34.2%. It was observed that the distribution of respiratory tract viruses showed variance by age groups. Influenza A infection was observed with the highest rate in both age groups. Influenza B was the second leading agent (p=0.008) above the age of 5 years and respiratory syncytial virus was the second leading agent in the 2–5 year age group (p=0.003). Influenza viruses were detected in 55.9% of 118 patients who were found to be compatible with the definition of “influenza-like illness” specified in the Center for Disease Control and Prevention guidelines and other viral agenst were detected in 44%. No difference could be found between the clinical pictures and radiological findings caused by influenza and other respiratory tract viruses.
Conclusions:
In this study, it was concluded that influenza and other respiratory viruses can not be differentiated definitely by clinical and radiological findings, though there are some differences.
Keywords: Acute respiratory tract infections, influenza, respiratory viruses
Introduction
Respiratory tract infections are among the most common infectious diseases worldwide. Ability to identify the viruses which are involved in the etiology with a rapid, sensitive and specific method [polymerase chain reaction (PCR)] which have come into use in recent years has drawn attention to respiratory tract viruses (1). Various viruses lead to the clinical picture which is generally defined as viral respiratory tract infection and this picture shows variance depending on the age group, season, underlying disease and upper or lower respiratory tract involvement (2). Viral respiratory tract infections have a significant place in national health spending because of increased hospital admissions, labour loss of parents and school abseteeisms of children and the socioeconomical effects of viral respiratory infections are observed especially in winter (3, 4).
In studies related with respiratory tract infections, influenza A, influenza B, rhinovirus (RV), respiratory syncitial virus (RSV), coronavirus (CV), parainfluenza virus (PIV) and human metapneumovirus (HMPV) and human bocavirus (HBOV) which have been recently identifed are among the viruses commonly found as causative agents (5–7). In our country, there are a limited number of studies evaluating viral agents in respiratory tract infections and generally, hospitalized patients and lower respiratory tract infections have been examined (8, 9). The epidemiology and clinical and radiological findings of respiratory infections with a milder course which do not necessitate hospitalization have been rarely addressed. In addition, there are insufficient data related with respiratory tract infections in older children, because lower respiratory infections in children aged 2 years and younger have generally been evaluated.
Seasonal influenza causes to epidemics in winter months with a severity varying from year to year and may be manifested with variable clinical pictures. Its differentiation from other agents is significant in clinical practice, because it has specific antiviral treatment. The Center for Disease Control and Prevention (CDC) established a definition of ‘influenza-like illness’ and allowed the patients compatible with this definition to be considered influenza in severe epidemics and initiation of treatment when clinically required (10). However, studies showed that other viruses might be causative agents in patients compatible with this definition and the adequacy of this definition in differentiation of influenza cases was questioned (11, 12).
In this study, the epidemiology of respiratory tract viruses found in nasopharyngeal swab samples of patients aged above 2 years who presented to our pediatric emergency outpatient clinic with signs of upper respiratory tract infection in the 2013–2014 influenza season and who were followed up in an inpatient or outpatient setting by clinical status and the clinical reflection of this epidemiology were examined. In addition, influenza and other respiratory tract viruses were compared in terms of clinical and laboratory features during this period when a severe influenza epidemic was experienced.
Material and Methods
The records of 178 patients aged above 2 years who presented to the pediatric emergency outpatient clinic in a tertiary care hospital in Istanbul between December 2013 and April 2014 were examined retrospectively. Since our aim was to demonstrate the types of respiratory tract viruses and their clinical and laboratory differences, 38 patients in whom respiratory tract virus could not be found with the PCR method were not included in the study.
The age, gender, complaints and physical examination findings at presentation, laboratory tests (hemogram and CRP), viral PCR results obtained from respiratory tract swabs, hospitalization states and times were recorded from patient files. Since the patients presented urgently, information related with indoor exposure to smoking, number of siblings, school attendence, contact with upper respiratory tract infection, status of influenza vaccination and disease time which was lacking in the patient files was obtained by phone calls. The lung graphies obtained before were evaluated together with a radiologist who was not aware of the viral assessment result of the patient.
A diagnosis of upper respiratory tract infection (URTI), acute bronchitis, bronchiolitis, bronchopneumonia and pneumonia was made with evaluation of disease symptoms, physical examination findings and radiological data in association. Association of a temperature of ≥37.8°C with cough or sore throat was defined as ‘influenza-like illness’ (9).
This study was approved by the ethics committee of İstanbul University, İstanbul Medical Faculty (2015/688). Verbal consent was obtained from the parents of the patients who were included in the study during phone calls.
Analysis of respiratory tract virus
On the day of presentation at the pediatric emergency outpatient clinic, nasopharyngeal swab samples were obtained by inserting swabs into both nostrils, progressing up to the nasopharyngeal region and rotating the swaps 360°. The swabs were closed in capped boxes which contained transport medium (Virocult, Medical Wire & Equipment, UK). Complete nucleic acid degradation was performed using EZ1 virus mini kit V2.0 (Catalog number: 955134, Qiagen, Germany) in the virology laboratory. FTD® Respiratory Pathogens 21 kit (Fast-track diagnostics Ltd. Malta) which operated with real-time and multiplex PCR method was used to identify respiratory tract pathogens in the RotorGene Q platform (Qiagen®, Germany). This kit can differentiate 21 respiratory tract pathogens (influenza A (H3N2 and H1N1), influenza B, RV, KV NL63, 229E, OC43, HKU1, PIV) type 1, 2, 3, 4, HMPV A/B, HBoV, RSV A/B, adenovirus (AV), enterovirus (EV) and parechovirus) at one time.
Statistical analysis
The analyses were performed using Statistical Package for the Social Sciences version 21, (SPSS, Inc.; Chicago, IL, USA) 21. package program. Analysis of normality was performed using Shapiro Wilk and Kolmogorov Smirnov tests. The data were expressed as mean, standard deviation, median, the lowest-the highest value, frequency and percentage. The data which showed a normal distribution were compared using t-test in independent groups. The other data were compared using Mann-Whitney U test. The categorical data were evaluated using chi-square and Fisher’s exact tests. The limit of significance was accepted as a p value of <0.05 and to be bidirectional.
Results
The records of 178 patients aged above 2 years who presented to our pediatric emergency outpatient clinic between December 2013 and April 2014 were examined retrospectively. The data of 140 (78.6%) of these patients in whom at least one respiratory tract pathogen was found with the PCR method were presented.
The mean age of the patients in whom respiratory tract pathogens were found was found to be 6.3±3.6 years (Median: 5.5 years, range: 2.5–16 years). It was observed that male patients predominated (M:82; 58.5%) / F:58; 41.4%). Sixty nine patients (49.2%) were aged between 2 and 5 years, 71 patients (50.7%) were aged 5 years and above. The following viruses were identified in nasopharyngeal swab samples in order of frequency (n -%): influenza A (H3N2) (47–33.5%), influenza B (23–16.4%), RSV (13–9.2%), AV (11–7.8%), RV (10–7.1%), CV (10–7.1%), HMPV (8–5.7%), HBoV (8–5.7%), PIV (5–3.5%) and multiple infection (4–2.8%). Rhinovirus and EV were identified in one of four patients who had multiple infection and association of RV and HBoV was found in the other three patients. A diagnosis of upper respiratory tract infection (URTI) was made in 92 (65.7%) of the patients and a diagnosis of lower respiratory tract infection (LRTI) was made in 48 (34.2%) of the patients. No difference was found between the viruses in terms of rates of leading to URTI and LRTI (p>0.05 for each virus; Figure 1).
Figure 1.
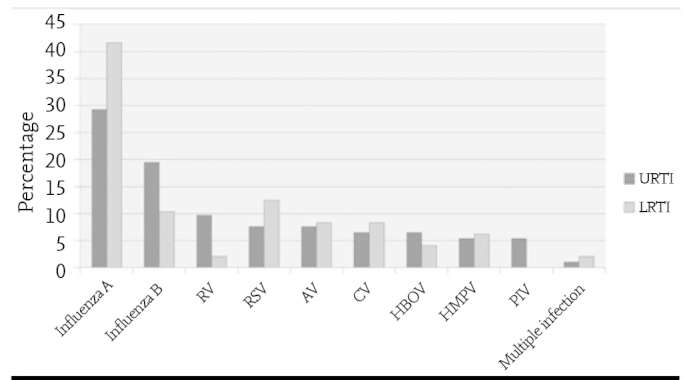
Distribution of respiratory tract viruses in our patients who were diagnosed with upper respiratory tract infection (n=92 and lower respiratory tract infection (n=48)
AV: adenovirüs; İMPV: insan metapnömovirüsü; İBOV: insan bokavirüsü; KV: koronavirüs; PİV: parainfluenza virüslerRV: rinovirüs; RSV: espiratuvar sinsityal virüs
The distribution of the respiratory tract viruses by age groups was found to be statistically different. Although the most common virus was influenza A virus in both groups, the second most common virus was influenza B in the group above 5 years of age (p=0.008) and RSV in the 2–5-year age group (p=0.003, Figure 2).
Figure 2.
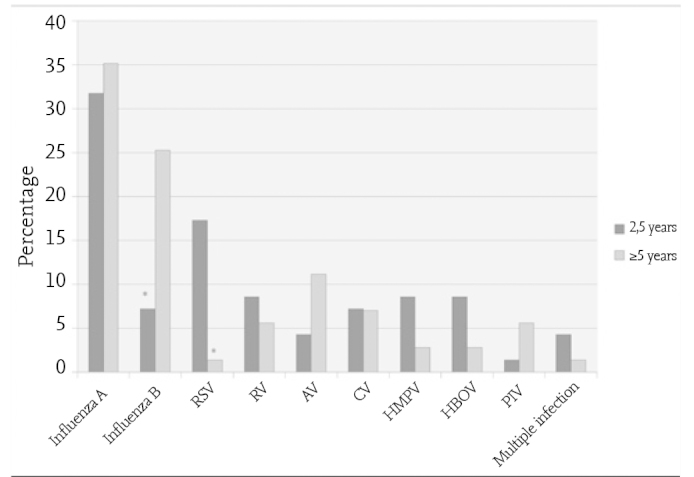
Distribution of respiratory tract viruses by age groups (2–5 years, n=69; ≥5 years, n=71) (*p<0.005)
AV: adenovirüs; İMPV: insan metapnömovirüsü; İBOV: insan bokavirüsü; KV: koronavirüs; PİV: parainfluenza virüslerRV: rinovirüs; RSV: espiratuvar sinsityal virüs
Influenza viruses (A and B) were identified in 50% of the patients. The patients who were found to have influenza and the patients who were found to have other respiratory tract viruses are compared in Table 1 in terms of clinical and laboratory features. Accordingly, the ages of the patients who had influenza infection were found to be older (p<0.001). The symptom period was found to be similar in both groups (the median value was 3 days) (p=0.43). The patients who had influenza presented most frequently in January. Subsequently, the frequency decreased gradually and this infection was lastly observed in March (5 patients). The other respiratory tract viruses made two peaks in January and March (Figure 3). When the signs and symptoms at presentation were evaluated, it was found that headache and malaise-myalgia were markedly more common in cases of influenza (p=0.01, p<0.001, respectively). In addition, cough was also more common in cases of influenza (94.3%) compared to the cases of other viral respiratory tract infections (81.4%) p=0.02). While 95.7% of the patients with influenza had fever, this rate was found to be 78.6% in the other group (p=0.002). Influenza viruses were identified in 55.9% of 118 patients who were compatible with the definition of “influenza-like illness” stated in the Center for Disease Control and Prevention guidelines and other viral agents were identified in 44%. Other than influenza viruses, AV, RSV and HBoV most frequently led to the picture of “influenza-like illness”. No significant difference was found between the clinical diagnoses and radiological findings caused by influenza and other respiratory tract viruses. In terms of laboratory findings, the leukocyte and lymphocyte counts were found to be significantly lower in cases of influenza compared to the other group (p<0.001 and p=0.006, respectively). On the other hand, CRP was found to be higher in respiratory tract infections related with non-influenza viruses (p=0.001). No difference was observed between influenza and other respiratory tract viruses in terms of requirement for hospitalization in hospital and intensive care unit and hospitalization time and disease time. When examined in terms of presence of signs of influenza-like illness defined by the Center for Disease Control and Prevention, these criteria were present in 94.2% of the cases of influenza and in 74.2% of the other respiratory tract infections (p=0.001). Influenza vaccine was administered in only 6 of a total of 140 patients (4.2%).
Table 1.
Comparison of the clinical and laboratory features of influenza and other respiratory tract viruses
| RTI related with influenza viruses (n=70) | RTI related with non-influenza viruses (n=70) | p | |
|---|---|---|---|
| Age, median (the lowest-the highest) | 6.5 (2.5–16.0) | 4.5 (2.5–14.0) | <0.001 |
| Gender, male/female n (%) | 46/24 (65.7/34.3) | 36/34 (51.4–48.6) | 0.086 |
| Age group, n (%) | |||
| 2–5 years | 27 (38.6) | 42 (60) | 0.011 |
| >5 years | 43 (61.4) | 28 (40) | |
| Indoor exposure to smoking, n (%) | 24 (34.3) | 22 (31.4) | 0.719 |
| School/kindergarten, n (%) | 58 (82.9) | 41 (58.6) | 0.002 |
| Number of siblings, ≥2, n (%) | 25 (35.7) | 34 (48.6) | 0.123 |
| Contact with URTI, n (%) | 44 (62.9) | 34 (48.6) | 0.089 |
| Clinical Diagnosis, n (%) | |||
| URTI | 45 (64.3) | 47 (67) | |
| Acute bronchitis | 11 (15.7) | 9 (12.9) | |
| Bronchopneumoniae | 11 (15.7) | 6 (8.6) | 0.382 |
| Pneumoniae | 2 (2.9) | 4 (5.7) | |
| Bronchiolitis | 1 (1.4) | 4 (5.7) | |
| Presence of underlying disease, n (%) | 17 (24.3) | 11 (15.7) | 0.205 |
| Influenza-like illness, n (%) | 66 (94.3) | 52 (74.3) | 0.001 |
| Radiology, n (%) | (s=31) | (s=28) | |
| Normal | 13 (18.6) | 13 (18.6) | |
| Peribronchial infiltration | 12 (17.1) | 11 (15.7) | |
| Consolidation | 5 (7.1) | 3 (4.3) | 0.663 |
| Increased aeration | 1 (1.4) | 1 (1.4) | |
| Atelectasis | - | 1 (1.4) | |
| Laboratory, median (the lowest-the highest) | (n=59) | (n=58) | |
| Leukocytes | 7 500 (3 020–31 390) | 10 250 (3 500–25 500) | <0.001 |
| Neutrophils | 3 910 (700–27 720) | 5 730 (1 400–23 200) | 0.038 |
| Lymphocytes | 1 950 (500–6 100) | 2 450 (450–10 470) | 0.006 |
| CRP | 7 (0–74) | 18 (0–200) | 0.001 |
| Hospitalization, n (%) | 15 (21.4) | 19 (27.1) | 0.430 |
| Hospitalization in ICU, n (%) | 2 (2.9) | 1 (1.4) | 0.559 |
| Hospitalization time, days, median (the lowest-the highest) | 6 (2–12) | 5 (1–18) | 0.767 |
| Disease time, days, median (the lowest-the highest) | 7 (4–14) | 6 (4–21) | 0.078 |
CRP: C reactive protein; ICU: intensive care unit, n: number; RTI: respiratory tract infections
Figure 3.
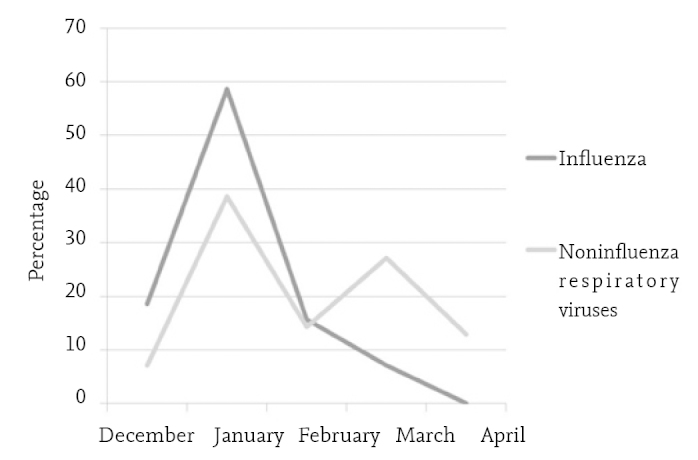
Percentage distribution of respiratory tract viruses in the time period of December 2013 and April 2014
24.2% of 140 patients who were found to have a respiratory tract virus were hospitalized. Three of these patients were followed up in intensive care unit. No difference was found between the patients who were hospitalized and the patients who were not hospitalized in terms of viral agent (Table 2). It was observed that the patients who were hospitalized were younger compared to the patients who were not hospitalized (p=0.045). Hospitalization was required more frequently in the patients who had an underlying disease (p=0.01). The symptom period was found to be longer in the ones who were followed up as outpatients (p=0.027). Rhinorrhea and sore throat were more common in the outpatients (p<0.001) and wheezing and dyspnea were more common in the patients who were hospitalized (p=0.005, p<0.001, respectively). When examined in terms of laboratory values, the leukocyte and neutrophil counts, CRP values and neutrophil/lymphocyte ratios at presentation were found to be markedly higher in the patients who were hospitalized. On the other hand, the eosinophil counts in the patients who required hospitalization were markedly lower compared to the outpatients (Table 2).
Table 2.
Comparison of the demographic, clinical and laboratory features of the patients who were followed up as outpatients and who were hospitalized
| Patients with RTI followed up as outpatients (n=106) | Patients with RTI who were hospitalized (n=34) | p | |
|---|---|---|---|
| Age, median (the lowest-the highest) | 5.5 (2.5–16) | 3.8 (2.5–15) | 0.045 |
| Gender, male/female n (%) | 61/45 (57/42.5) | 21/13 (61.8/38.2) | 0.664 |
| Age group, n (%) | |||
| 2–5 years | 48 (45.3) | 21 (61.8) | 0.094 |
| >5 years | 58 (54.7) | 13 (38.2) | |
| Indoor exposure to smoking, n (%) | 38 (35.8) | 8 (23.5) | 0.183 |
| Presence of underlying disease, n (%) | 16 (15.1) | 12 (35.3) | 0.01 |
| Symptom time, days, median (the lowest-the highest) | 3 (0–10) | 2 (1–7) | 0.027 |
| Virus distribution*, n (%) | |||
| Influenza A | 35 (33) | 12 (35.3) | 0.971 |
| Influenza B | 20 (18.9) | 3 (8.8) | 0.267 |
| RV | 10 (9.4) | 0 | 0.118 |
| RSV | 8 (7.5) | 5 (14.7) | 0.305 |
| HMPV | 8 (7.5) | 0 | 0.199 |
| KV | 6 (5.7) | 4 (11.8) | 0.256 |
| HBoV | 6 (5.7) | 2 (5.9) | 1.00 |
| PIV | 5 (4.7) | 0 | 0.335 |
| AV | 5 (4.7) | 6 (17.6) | 0.25 |
| Multiple infection | 2 (1.9) | 2 (5.9) | 0.248 |
| Laboratory, median (the lowest-the highest) | |||
| Leukocytes | 8 000 (3 020–25 500) | 10 700 (3 500–31 390) | 0.016 |
| Neutrophils | 4 000 (740–23 200) | 7 095 (700–27 720) | 0.006 |
| Lymphocytes | 2 170 (550–10 470) | 2 265 (450–4 600) | 0.782 |
| Neutrophil/lymphocyte | 2.05 (0.29–20.59) | 3.28 (0.15–22.53) | 0.015 |
| Eosinophil | 50 (0–1 000) | 10 (0–200) | 0.004 |
| CRP | 7 (0–71) | 20 (0–200) | <0.001 |
AV: adenovirus; CRP: C reactive protein; CV: coronavirus; HBoV: human bocavirus; HMPV: human metapneumovirus; n: number; PIV: parainfluenza virus; RSV: respiratory syncitial virüs; RV: rhinovirus; RTI: respiratory tract infection
P values in virus distribution were obtained by comparing the numbers of each virus found in the patients who were followed up as outpatients and who were hospitalized using chi-square test.
Discussion
In our study, respiratory tract viruses in children aged above 2 years who presented to our pediatric emergency outpatient clinic between December 2013 and March 2014 with signs of respiratory tract infection and who were followed up as outpatients or hospitalized were examined. At least one viral respiratory tract pathogen was found in 78.6% of our patients with molecular method (multiplex PCR). This rate has been found to range between 41.8% and 67.8% in limited number of studies performed in this area in our country (8, 9, 13, 14). On the other hand, the virus identification rates were reported to be 85.3% and 88.7%, respectively, in two studies performed with multiplex PCR method in Japan and France covering the years of 2010–2011 (15, 16). In our study, patients who were followed up as outpatients and who had upper respiratory tract infection were also included as well as hospitalized patients. This might have contributed to the high virus identification rate we found in our study. In addition, it should also be considered that this study covered the season during which respiratory tract infections are observed most commonly and the multiplex PCR kit used is able to identify 21 respiratory tract pathogens. Multiplex PCR kits which could identify 115 respiratory tract pathogens were used in some other studies conducted in Turkey (8, 9, 14, 17).
In the 2013–2014 winter season, an influenza epidemic was experienced. Therefore, influenza viruses were identified in half of our patients. In one study which was conducted in the 2010–2011 season and included hospitalized patients, influenza viruses were found only with a rate of 12.6% (14). RSV and AV which were in the first two orders in this study ranked after influenza viruses in our study. It can be stated that the frequency of other respiratory tract viruses varies according to the severity of seasonal influenza epidemic, but the distribution generally remains the same.
The distribution and frequency of respiratory tract viruses show variance depending on different factors including age, season, socioeconomical status, coverage of the PCR test used, the study plan and number of patients. In this study, the predominant viruses included influenza A (H3N2) and influenza B. The other respiratory tract viruses found in our patients showed a distribution with similar numbers. This distribution may show difference in different studies, but RSV and RV are generally observed most frequently (8, 9, 13, 16).
We have started to identify viral multiple infections with availability of PCR kits which operate with the multiplex method. It is not yet clear if these multiple viruses identified are all infectious agents. In our study, multiple infections were found with a lower rate (n=4, 2.8%) compared to other studies (10%–43.5%) (14–19). One of these was association of RV and EV which was diagnosed as URTI and the other three were associations of HBoV and RSV which were diagnosed as LRTI. Two patients who had association of human bocavirus and RSV were hospitalized. Although some studies reported that patients with multiple viral infections had a more severe course, some other studies reported that the disease course was not affected or a milder course was observed (16, 20). It is thought that the disease severity may vary depending on the agent viruses. In conclusion, the relation between the disease severity and identification of multiple viral agents is still unclear.
Age has always been a significant factor in prediction of agent microorganisms in childhood infections. Knowledge of the age distribution of respiratory tract viruses is significant in this context. When our patients were classified as “2–5 years” and “above 5 years”, it was observed that the agent viruses showed difference. The age of the patients with influenza viruses was older compared to the patients with other respiratory tract infections (median 6.5 years, 4.5 years; p<0.001). When the viruses were examined one by one, the most prominent difference between the age groups was observed in RSV and influenza B infections. Respiratory syncytial virus was mostly found in the younger age group which was compatible with the literature (21). Influenza B was observed more frequently in children aged above five years compared to the younger age group. In a study conducted in our country, it was reported that patients with influenza B were older compared to patients with influenza A [72.5 months (15–183 months), 55 months (11–68 months, respectively)] (13). In addition, it was noted that HMPV and HBoV infections were mostly observed in the 2–5 year age group and AV infections were mostly observed in children aged above 5 years in our study, though statistical significance was not present. In a study conducted in our country in which 27 cases of AV-related respiratory infection were examined, it was reported that the patients were mostly aged 4 years and older (22). In another study, it was reported that AV infections were found at older ages compared to RSV infections (mean age, 41 months, 23 months) (23). Although human metapneumovirus and HBoV may be observed in any age group, studies have shown that they mostly affect younger children (24, 25).
Differentiation of influenza infections from the other respiratory tract infections is significant in terms of case management, because specific antiviral treatment is available. In addition, it is helpful in terms of guidance and prioritization of large populations especially during epidemic periods. There are many definitions of influenza-like illness made with this objective and none has been found to be sufficiently sensitive and specific to describe influenza infection (10, 26). 84% of our patients were compatible with the definition of influenza-like illness established by the CDC. Influenza viruses were identified in 56% of these patients. In a study conducted in Ankara region, this rate was found to be 43% (27). In our study, AV (primarily), RSV, HBoV, RV, CV, HMPV and PIV were identified as the agents of influenza-like illness. Thus, this study showed that differentiation of cases of influenza based only on clinical definitions was not possible.
It has been observed that the complaints of malaise, headache, fever and cough are more common in cases of influenza compared to other respiratory tract infections. Differences have been found in similar symptoms in various studies (13, 17). However, it was concluded that these differences did not allow to make a diagnosis of clinically specific viral infection considering other mutual signs and symptoms (13, 17). When we examined the final clinical diagnoses of our patients, no difference was found between influenza and other viruses. Again, no difference was found between influenza and other viruses in terms of hospitalization in hospital and intensive care unit and disease time and hospitalization time which can be generally defined as disease severity. In laboratory findings, it was noted that leukopenia, neutropenia and lymphopenia were found more commonly in the patients who were found to have influenza compared to the other patients. There are few studies related with the hematological findings of influenza. One of these was related with pandemic H1N1 and neutropenia was found in 35% of the patients and leukopenia and lymphopenia were found in 26% (28). In a study conducted by Biçer et al. (14), this issue was examined for influenza and other respiratory tract viruses and no difference was found.
The respiratory tract viruses observed commonly in the mild temperate zone which also includes our country have typical seasonal distributions. It has been shown that respiratory syncitial virus and influenza make a peak in winter months and RSV generally emerges earlier (16). In a study conducted in Japan between 2004 and 2011 in which 13 325 nasal samples most of which were obtained from subjects aged below 5 years were examined, it was reported that RSV infections commonly occured at the end of the year, influenza A infections commonly occured between January and March, IMPV infections commonly occured between March and April and parainfluenza type 3 infections commonly occured between May and July (29). Although our study could not give a full seasonal distribution, because it did not cover a whole year, influenza, RSV, IMPV infections were observed most commonly in January during our study period. Influenza B infections occured later compared to influenza A and were also found in March in contrast to influenza A infections which ended in February. After the big wave in January, a second but more limited wave was observed in March. This wave was related with AV and CV and less frequently with RV and influenza B. Rhinovirus and HBoV were found with a similar frequency each month throughout the study period.
24% of our patients in whom a respiratory tract virus was identified required hospitalization. Since most studies included patients who were hospitalized, there is a limited number of studies with a similar design and the hospitalization rates have a wide range (3%–80%) in these studies (13, 17). In our study, it was found that younger patients and patients who had an underlying disease were hospitalized with a higher rate. The most common viral respiratory tract infections which required hospitalization included influenza A, AV and RSV. In the study conducted by Gooskens et al. (17), most patients were aged below 3 years in contrast to our study and the viruses which required hospitalization included RSV, RV and influenza viruses in order of frequency. When the laboratory findings were examined, the frequency of neutrophilic leukocytosis, elevated CRP and elevated procalcitonin were found with a markedly higher rate in the patients who were hospitalized. The neutrophil/lymphocyte ratio which is a practical marker of systemic inflammation was studied as a prognostic factor (30). In our study, the neutrophil/lymphocyte ratio at presentation was also found to be significantly higher in the patients who were hospitalized. On the other hand, studies have shown that eosinopenia may be used as a prognostic marker in differentiating infectious and non-infectious conditions and in identifying the severity of infection (31). The eosinophil counts at presentation in our patients who required hospitalization were found to be markedly lower compared to the patients who were followed up as outpatients and this supported the literature information in this area.
In conclusion, at least one respiratory tract infection was found in the majority (78.6%) of the children who presented to our pediatric emergency outpatient clinic with complaints of respiratory tract infection in this study and information about age and month distributions of viral infections was given. Data about children aged above 5 years who have rarely been studied and about viral pathogens identified in upper respiratory infections in outpatients were presented. In addition, our study showed that many main respiratory tract viruses other than influenza viruses might lead to influenza-like illness. It has been found that influenza and other respiratory tract viruses lead to diseases with similar severity and course and it has been concluded that a definite differentiation can not be made based on clinical and laboratory findings, though malaise-headache and leukopenia/lymphopenia were observed more frequently in cases of influenza. 24% of our patients were hospitalized and the factors which increased the risk of hospitalization included presence of an underlying disease, young age, a high neutrophil/lymphocyte count and a low eosinophil count at presentation.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Istanbul University Istanbul Medical Faculty (23.03.2015-2015/688).
Informed Consent: Verbal informed consent was obtained from parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.A.; Design - H.A., M.S.; Supervision - H.A., S.B., A.S., N.S.; Data Collection and/or Processing - H.A., M.S., S.H.T., O.B.E., A.Ç.; Analysis and/or Interpretation - H.A., M.S., S.H.T., A.S., N.S.; Literature Review - H.A., M.S., O.B.E.; Writing - H.A.; Critical Review - S.B., A.Ç., A.S., N.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Liolios L, Jenney A, Spelman D, Kotsimbos T, Catton M, Wesselingh S. Comparison of a multiplex reverse transcription PCR-enzyme hybridization assay with conventional viral culture immunofluorescence techniques for the detection of seven viral respiratory pathogens. J Clin Microbiol. 2001;39:2779–83. doi: 10.1128/JCM.39.8.2779-2783.2001. http://dx.doi.org/10.1128/JCM.39.8.2779-2783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. http://dx.doi.org/10.1016/S1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 3.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza virüses among young children. Pediatrics. 2004;113:1758–64. doi: 10.1542/peds.113.6.1758. http://dx.doi.org/10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 4.Massin MM, Montesanti J, Gerard P, Lepage P. Spectrum and frequency of illness presenting to a pediatric emergency department. Acta Clin Belg. 2006;61:161–5. doi: 10.1179/acb.2006.027. http://dx.doi.org/10.1179/acb.2006.027. [DOI] [PubMed] [Google Scholar]
- 5.Yeolekar LR, Damle RG, Kamat AN, Khude MR, Simha V, Pandit AN. Respiratory viruses in acute respiratory tract infections in Western India. Indian J Pediatr. 2008;75:341–5. doi: 10.1007/s12098-008-0035-4. http://dx.doi.org/10.1007/s12098-008-0035-4. [DOI] [PubMed] [Google Scholar]
- 6.Allander T, Jartti T, Gupta S, et al. Human bocavirüs and acute wheezing in children. Clin Infect Dis. 2007;44:904–10. doi: 10.1086/512196. http://dx.doi.org/10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloots TP, Whiley DM, Lambert SB, Nissen MD. Emerging respiratory agents: new viruses for old diseases? J Clin Virol. 2008;42:233–43. doi: 10.1016/j.jcv.2008.03.002. http://dx.doi.org/10.1016/j.jcv.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancaklı Ö, Yenigün A, Kırdar S. Alt solunum yolu enfeksiyonunda nazofaringeal örneklerde polimeraz zincir reaksiyonu sonuçları. J Pediatr Inf. 2012;6:84–9. http://dx.doi.org/10.5152/ced.2012.26. [Google Scholar]
- 9.Akçalı S, Yılmaz N, Güler Ö, Şanlidağ T, Anıl M. Alt solunum yolu enfeksiyonu olan çocuklarda solunum yolu viral etkenlerinin sıklığı. Turk Arch Ped. 2013;48:215–20. [Google Scholar]
- 10.Centers for Disease Control and Prevention Overview of influenza surveillance in the United States. 2010. Available from: http://www.cdc.gov/flu/weekly/overview.htm.
- 11.Boivin G, Hardy I, Tellier G, Maziade J. Predicting influenza infections during epidemics with use of a clinical case definition. Clin Infect Dis. 2000;31:1166–9. doi: 10.1086/317425. http://dx.doi.org/10.1086/317425. [DOI] [PubMed] [Google Scholar]
- 12.Thiberville SD, Ninove L, Vu Hai V, et al. The viral etiology of an influenza-like illness during the 2009 pandemic. J Med Virol. 2012;84:1071–9. doi: 10.1002/jmv.23265. http://dx.doi.org/10.1002/jmv.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karadag-Oncel E, Ciblak MA, Ozsurekci Y, Badur S, Ceyhan M. Viral etiology of influenza-like illnesses during the influenza season between December 2011 and April 2012. J Med Virol. 2014;86:865–71. doi: 10.1002/jmv.23747. http://dx.doi.org/10.1002/jmv.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bicer S, Giray T, Çöl D, et al. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013;39:22. doi: 10.1186/1824-7288-39-22. http://dx.doi.org/10.1186/1824-7288-39-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaida A, Kubo H, Takakura K, et al. Associations between co-detected respiratory viruses in children with acute respiratory infections. Jpn J Infect Dis. 2014;67:469–75. doi: 10.7883/yoken.67.469. http://dx.doi.org/10.7883/yoken.67.469. [DOI] [PubMed] [Google Scholar]
- 16.Mengelle C, Mansuy JM, Pierre A, et al. The use of a multiplex real-time PCR assay for diagnosing acute respiratory viral infections in children attending an emergency unit. J Clin Virol. 2014;61:411–7. doi: 10.1016/j.jcv.2014.08.023. http://dx.doi.org/10.1016/j.jcv.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooskens J, van der Ploeg V, Sukhai RN, Vossen A, Claas E, Kroes A. Clinical evaluation of viral acute respiratory tract infections in children presenting to the emergency department of a tertiary referral hospital in the Netherlands. BMC Pediatr. 2014;14:297. doi: 10.1186/s12887-014-0297-0. http://dx.doi.org/10.1186/s12887-014-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonzel L, Tenenbaum T, Schroten H, Schlidgen O, Schweitzer-Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27:589–94. doi: 10.1097/INF.0b013e3181694fb9. http://dx.doi.org/10.1097/INF.0b013e3181694fb9. [DOI] [PubMed] [Google Scholar]
- 19.Marcone DN, Ellis A, Videla C, et al. Viral etiology of acute respiratory infections in hospitalized and outpatient children in Buenos Aires, Argentina. Pediatr Infect Dis J. 2013;32:e105–10. doi: 10.1097/INF.0b013e31827cd06f. [DOI] [PubMed] [Google Scholar]
- 20.Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012;6:71–7. doi: 10.1111/j.1750-2659.2011.00265.x. http://dx.doi.org/10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucion MF, Juarez Mdel V, Viegas M, et al. Respiratory syncytial virus: clinical and epidemiological pattern in pediatric patients admitted to a children’s hospital between 2000 and 2013. Arch Argent Pediatr. 2014;112:397–404. doi: 10.5546/aap.2014.eng.397. [DOI] [PubMed] [Google Scholar]
- 22.Biçer S, Küçük O, Giray T, et al. Evaluation of clinical and laboratory findings of pediatric patients with adenovirus-associated respiratory tract infections. Mikrobiyol Bul. 2013;47:295–304. doi: 10.5578/mb.4970. http://dx.doi.org/10.5578/mb.4970. [DOI] [PubMed] [Google Scholar]
- 23.Kwon JM, Shim JW, Kim DS, Jung HL, Park MS, Shim JY. Prevalence of respiratory viral infection in children hospitalized for acute lower respiratory tract diseases, and association of rhinovirüs and influenza virüs with asthma exacerbations. Korean J Pediatr. 2014;57:29–34. doi: 10.3345/kjp.2014.57.1.29. http://dx.doi.org/10.3345/kjp.2014.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljubin-Sternak S, Mlinaric-Galinovic G, Buntic AM, et al. Seasonal occurrence of human metapneumovirüs infections in Croatia. Pediatr Infect Dis J. 2014;33:165–7. doi: 10.1097/INF.0000000000000026. http://dx.doi.org/10.1097/INF.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 25.Tran DN, Nguyen TQ, Nguyen TA, Hayakawa S, Mizuguchi M, Ushijima H. Human bocavirus in children with acute respiratory infections in Vietnam. J Med Virol. 2014;86:988–94. doi: 10.1002/jmv.23789. http://dx.doi.org/10.1002/jmv.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thursky K, Cordova SP, Smith D, Kelly H. Working towards a simple case definition for influenza surveillance. J Clin Virol. 2003;27:170–9. doi: 10.1016/s1386-6532(02)00172-5. http://dx.doi.org/10.1016/S1386-6532(02)00172-5. [DOI] [PubMed] [Google Scholar]
- 27.Ceyhan M, Karadag-Oncel E, Badur S, et al. Effectiveness of a new bioequivalent formulation of oseltamivir (Enfluvir®) on 2010–2011 seasonal influenza virüses: an open phase IV study. Int J Infect Dis. 2012;16:273–8. doi: 10.1016/j.ijid.2011.12.008. http://dx.doi.org/10.1016/j.ijid.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Unal S, Gökçe M, Aytaç-Elmas S, et al. Hematological consequences of pandemic influenza H1N1 infection: a single center experience. Turk J Pediatr. 2010;52:570–5. [PubMed] [Google Scholar]
- 29.Mizuta K, Abiko C, Aoki Y, et al. Seasonal patterns of respiratory syncytial virus, influenza A virus, human metapneumovirüs, and parainfluenza virus type 3 infections on the basis of virus isolation data between 2004 and 2011 in Yamagata, Japan. Jpn J Infect Dis. 2013;66:140–5. doi: 10.7883/yoken.66.140. http://dx.doi.org/10.7883/yoken.66.140. [DOI] [PubMed] [Google Scholar]
- 30.de Jager CP, Wever PC, Gemen EF, et al. The neutrophil lymphocyte count ratio in patients with community acquired pneumonia. PloS ONE. 2012;7:e46561. doi: 10.1371/journal.pone.0046561. http://dx.doi.org/10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merino CA, Martínez FT, Cardemil F, Rodríguez JR. Absolute eosinophils count as a marker of mortality in patients with severe sepsis and septic shock in an intensive care unit. Crit Care. 2012;27:394–9. doi: 10.1016/j.jcrc.2011.10.010. http://dx.doi.org/10.1016/j.jcrc.2011.10.010. [DOI] [PubMed] [Google Scholar]


