Abstract
In mammals, there is an underlying mechanism that dictates the organism’s biological functions and daily activity schedule, known as circadian rhythms, which play a major role in maintaining steady metabolism, homeostasis, and immunity. Limited research has been done investigating the effects of continuous opiate administration on the circadian rhythm activity pattern. A change in circadian activity pattern is suggested as an experimental model to demonstrate long-term effect of the drug. The objective of this study was to investigate the effects of morphine treatment on the long term activity (24 hour) of the animal as well as the activity after abrupt removal. Since prescribed medication containing morphine is widely use and abused and its long term effects are not known. Male Sprague-Dawley rats were contained in stable conditions with a standard light/dark cycle recordings were taken before, during and after morphine pellet implantation. Cosinor analysis was used to fit a 24-hour curve to the activity pattern. Results indicate that morphine pellet administration alters the mesor, amplitude, the day-time and night-time activity levels, and during the withdrawal period demonstrates a remarkable change in the maximal circadian rhythm timing. The question whether morphine changes the circadian rhythm or a change in circadian rhythm results in tolerance and withdrawal is discussed.
Keywords: morphine pellet, opioids, behavior, circadian rhythm, tolerance, withdrawal
Introduction
Drug dependence has become a significant medical and public health problem in society today, especially in vulnerable populations. The over use and abuse of certain classes of prescription medications can result in drug dependence (Rosenblum, et al., 2003). Aside from the economic and behavioral burden drug dependents place on their families, friends, and society, physicians are faced with the problem of how to treat and ultimately cure this dependence. Today’s physicians face the ever growing issue of chronic pain management while patients encounter a variety of adverse outcomes, such as use of no prescribed pain medication, illicit drug use, or the negative medical and psychosocial effects associated with continued drug-seeking behavior (Savage, 1996). Although a great deal of research has been conducted on both human and laboratory animal models regarding the mechanisms of dependence and withdrawal, our understanding of such is far from complete.
While there may be few studies testing the long-term effects of opiates on circadian rhythms, there is an enormous literature on the long-term effects of opiates on numerous other behavioral and physiological functions (Nestler, 2004). The biology of mammals is intricately organized in time as biological rhythms (Carpenter and Grossberg, 1985). Indeed, most, if not all biological functions and processes exhibit prominent circadian rhythms; (Lowrey and Takahashi, 2004; Reppert and Weaver, 2002), that are driven by an endogenous biological clock system (Minors and Waterhouse, 1986; Menaker et al., 1978), and which are synchronized to the ambient environment by specific time cues. The most prominent clock is the suprachiasmatic nuclei which, under normal circumstances, receive light/dark cues from the eye via the retinohypothalamic projection (Reppert and Weaver 2002). It was reported that there are clock genes that regulate homeostasis (Arjona and Sarkar, 2005) via positive and negative feedback loop that control the circadian output.
Several analgesics are known to exhibit significant circadian rhythm-dependent differences in their pharmacokinetics and dynamics (Asai et al., 2007; Li et al., 2010). For example, in humans the duration of analgesia produced by lidocaine is approximately three-fold greater when administered in the late afternoon and early evening than when administered in the morning (Bruguerolle and Labrecque, 2007). Moreover, the Tmax (when the rate of absorption equals the rate of elimination) is shorter and the Cmax (the maximum plasmatic concentration of the drug) greater when the drug is administered to subjects in the morning than evening (Bruguerolle et al., 2007). Opioids exert a broad range of physiological effects, the most familiar being analgesia (Smith et al., 1995). Pain is associated with functional impairment and self-medication for pain with psychoactive drugs appears especially problematic among substance users (Rosenblum et al., 2003). Because of the state of pleasure they induce, opioids, such as morphine and heroin, have become some of the most widely abused recreational drugs in the United States (Smith et al., 1995). Most reports on the effect of morphine examine the immediate post-morphine administration effect (Ribeiro et al., 2005). To our knowledge, there are limited reports pertaining to the influence of acute and chronic morphine on the locomotor circadian activity patterns. One of the established mechanisms underlying repetitive opioid exposure is the modulation of the cAMP second messenger pathway. This modulation results in activation of several transcription factors such as cAMP response element binding protein (CREB). It was reported that CREB mediate aspects of tolerance to the drug (Chao and Nestler, 2004; Nestler, 2004). We postulate that repetitive morphine treatment that bind to opioid receptors modulate neuronal activity (Dafny et al., 1983) that triggers cascades of molecular events like CREB, and these events would alter the locomotor circadian rhythm activity patterns. Clock genes regulate changes in the locomotor circadian rhythm activity pattern; and a change in clock gene expression is the result of long-term effect on the clock machinery. This leads to the hypothesis that the changes in circadian rhythm activity pattern induced by any drugs, are indicating a long-term effect of these drugs. The present study show that chronic morphine administration alters the circadian rhythm activity pattern, which suggests that, its chronic use can elicit long lasting effects.
Materials and Methods
Animals
Male Sprague-Dawley rats (N = 16) weighing, 180–190 g were initially housed four per cage in an experimental room maintained at 21 ± 2° C and 37–42% relative humidity for adaptation to the experimental room. Animals were maintained on schedule of 12h:12h light/dark (light on: 07:00–19:00 h) for three weeks before and throughout the recording sessions. Water and food were supplied ad libitum throughout the experiment. After acclimation period, the rats were weighed and then individually housed in their test cages that become their home cage. They were allowed at least an additional 48 h of habituation to the test/home cage before non-stop twelve (12) consecutive recording days of their locomotor activities. Housing conditions and experimental procedure were approved by our institutional animal welfare committee and the experiments were carried out in accordance with the National Institute of Health Guide For The Care and Use Of Laboratory Animals. All efforts were made to minimize the number of animals used.
Testing apparatus
The computerized animal activity monitoring (CAAM; AccuScan Instruments, Inc., Columbus, OH) systems are open field systems used to record several different locomotor indices. The activity chambers consisted of clear acrylic open field boxes (40.5×40.5×31.5 cm) with three levels of strip each consisting of 16 infrared beams and their respective motion sensors (Yang et al., 2011). The lower set of 16 infrared beams and their sensors are located 6 cm from the floor of the cage and measure the amount of movement along the floor of the cage. Vertical activity 1 (V1) is the next set of 16 infrared beams and their sensors recording any animal movement occurring vertically 12 cm above the cage floor; i.e. movements associated with so-called ‘wet dog shake’ and grooming movements. Vertical 2 activity (V2) is the highest 16 infrared beams and their sensor located 18 cm from the cage floor. Vertical 2 records movements of the rat fully extending along the wall of the cage trying to find a way to get out from the cage, i.e., climbing behaviors. The activity monitoring system checks each of the beams at a frequency of 100 Hz to determine whether the beams are interrupted. Interruptions of two or more consecutive beams separated by at least a time interval of one second are recorded as a movement score. Cumulative counts are compiled by the AccuScan analyzer and downloaded every 10 minutes into the OASIS data collection program and organized into several locomotor indices (Yang et al., 2011). The 10-mintue counts were summarized as hourly means and standard deviations (± SD) throughout the entire span of recording during the experiment. Three representative locomotor indices were analyzed: horizontal activity (HA) that record the total number of beam interruptions that occurred in the lower sensors during a given time and represents the total activity. Vertical activity1 (V1) that record movement associated with “wet dog shakes”, rearing and grooming. Vertical activity 2 (V2) associated with climbing behavior (Yang et al., 2011).
Procedure
Prior to the recording sessions of all the rats (N=16) they were placed in their test/home cages for an additional two days of acclimatization, and thereafter baseline locomotor recording was done continuously for 6 consecutive days. On experimental Day 7, the 16 animals were randomly divided into two comparable groups: a control and an experimental group, each consisting of 8 animals. The control animals were implanted with a placebo pellet and the experimental animals with the 100 mg morphine pellet simultaneously in the cervical spine region under ether anesthesia. The details of the morphine pellet implantation have been described previously (Dougherty and Dafny, 1988). The animals were then returned to their cage and locomotor activity recording resumed for an additional 3 days (experimental days 7 to 9). On experimental Day 10, the residual pellets were removed from the animals of both groups. The rats were placed back in their cages and locomotor activity recordings continued for an additional 3 days until experimental Day 12 (See Table 1). Pellet implantation and removal were performed at noon time.
Table 1.
Experimental procedures. Morphine pellet containing 100mg morphine sulfate.
| Exp Day | 1–6 | 7 | 7–9 | 10 | 10–12 |
|---|---|---|---|---|---|
| Control Group | Baseline recording | Placebo pellet implantation | Recording | Placebo pellet removal | Recording |
| Experimental Group | Baseline Recording | Morphine pellet implantation | Recording | Morphine pellet removal | Recording |
Statistical analysis
Two calculations were used: (1) 6 0f the 10 min consecutive sections (bins) of the locomotor activity indices were summed and averaged to produce hourly histograms with their standard errors. The histograms were used to analyze and compare the locomotor activity visually between the experimental days. (2) The second evaluation used the 10 min counts (bins) as points in a time series for statistical analysis using the Cosine Curve Statistical Analysis (CCSA) test (Bingham et al., 1982), to perform statistical comparisons by parameterizing the hurly activity pattern. The cosinor analysis technique group the 24 hour data into three model parameters by estimating the mesor (average activity represented by the curve); amplitude (distance from the mesor to the highest point-the peak activity), and acrophase (time at which the maximum amplitude occurs) to model of the cyclic nature of the activity over a 24 hour cycle. The estimates of these three parameters provide the ability to test for statistically significant changes in locomotor activity patterns with respect to time and intensity within the 24 hours of each day. It is therefore possible to determine whether a significant shift in locomotor activity rhythm pattern occurring during the drug exposure and its removal (Bingham et al., 1982). These analyses were exploratory in nature, uncorrected for multiple comparisons. Table 2 reports the days for which Cosinor Analysis of the locomotor data was done and the corresponding findings in terms of circadian rhythm detection (Ho: amplitude=0, P<0.05) and the rhythm parameters of MESOR, amplitude and acrophase. Baseline activity level was evaluated by testing for statistical differences between experimental days 1, 5 and 9 for the control animals (N=8) and pooling the activity with day 6 (non-treatment) of the experimental animals (N=8). Parameter estimates of the Cosinor analysis for circadian rhythm are presented for select days and combinations of days in Table 2, and F-tests comparison of the significance of differences between the MESOR, amplitude and acrophase of the different -- baseline vs. morphine tolerance vs. morphine withdrawal -- phases of the experiment are presented in Tables 3.
Table 2.
Cosinor parameter estimates (24h), for the Mesor (average activity level), Amplitude (distance from mesor to the highest point; Acrophase (time at which the maximum amplitude occurs) and f-statistics, and p-values contrasting the time-period day pairs.
| Day | Mesor | Amplitude* | Acrophase Ref (00:00h) |
Ho: Equal Parameter |
F-statistic (df1,df2) |
p-value | |
|---|---|---|---|---|---|---|---|
| A | |||||||
| HA | |||||||
| 7 | 2575 | 2366 | 21:36h | Mesor | 17.75 (1,234) | <0.001 | |
| Acrophase | 3.98 (1,234) | <0.047 | |||||
| 9 | 1544 | 687 | 23:20h | Mesor | 34.26 (1,234) | <0.001 | |
| 7 | 2575 | 2366 | 21:36h | Mesor | 41.71 (1, 90) | <0.001 | |
| 9 | 1544 | 687 | 23:20h | Amplitude | 55.34 (1, 90) | <0.001 | |
| 10 | 2450 | 1592 | 16:50h | Mesor | 12.18 (1,234) | <0.001 | |
| Acrophase | 9.51 (1,234) | <0.002 | |||||
| 11 | 2938 | 2117 | 01:37 | Mesor | 46.40 (1,234) | <0.001 | |
| Acrophase | 104.3 (1,234) | <0.001 | |||||
| 11 | 2938 | 2117 | 01:37 | Amplitude | 0.58 (1, 90) | <0.447 | |
| Acrophase | 46.74 (1, 90) | <0.001 | |||||
| 9 | 1544 | 687 | 23:20h | Mesor | 55.77 (1, 90) | <0.001 | |
| 11 | 2938 | 2117 | 01:37 | Amplitude | 29.37 (1, 90) | <0.001 | |
| Acrophase | 4.03 (1, 90) | <0.048 | |||||
| 10 | 2450 | 1592 | 16:50h | Mesor | 5.59 (1, 90) | <0.020 | |
| 11 | 2938 | 2117 | 01:37 | Amplitude | 3.24 (1, 90) | <0.075 | |
| Acrophase | 15.16 (1, 90) | <0.001 | |||||
| B | |||||||
| VA1 | |||||||
| 9 | 202 | 152 | 21:45h | Mesor | 22.04 (1,234) | <0.001 | |
| 7 | 296 | 165 | 19:36h | Mesor | 32.74 (1, 90) | <0.001 | |
| Acrophase | 5.67 (1, 90) | <0.019 | |||||
| 10 | 371 | 200 | 18:58h | Mesor | 18.78 (1,234) | <0.001 | |
| 11 | 434 | 125 | 02:35h | Mesor | 57.31 (1,234) | <0.001 | |
| Acrophase | 22.22 (1,234) | <0.001 | |||||
| 7 | 296 | 165 | 19:36h | Mesor | 32.75 (1, 90) | <0.001 | |
| Acrophase | 23.75 (1, 90) | <0.001 | |||||
| 9 | 202 | 152 | 21:45h | Mesor | 122.4 (1, 90) | <0.001 | |
| Acrophase | 18.00 (1, 90) | <0.001 | |||||
| 10 | 371 | 200 | 18:58h | Mesor | 5.10 (1, 90) | <0.026 | |
| Acrophase | 15.38 (1, 90) | <0.001 | |||||
| C | |||||||
| VA2 | |||||||
| 6 | 25 | 24 | 21:28h | Mesor | 5.92 (1,186) | <0.016 | |
| Acrophase | 5.79 (1,234) | <0.017 | |||||
| 9 | 15 | 15 | 22:03h | Mesor | 41.81 (1,234) | <0.001 | |
| 7 | 25 | 21 | 22:38h | Mesor | 27.73 (1, 90) | <0.001 | |
| 9 | 15 | 15 | 22:03h | Amplitude | 4.25 (1, 90) | <0.042 | |
| 10 | 23 | 22 | 16:39h | Mesor | 6.35 (1,234) | <0.012 | |
| Acrophase | 14.09 (1,234) | <0.001 | |||||
| 11 | 59 | 17 | 01:56h | Mesor | 127.9 (1,234) | <0.001 | |
| Acrophase | 5.42 (1,234) | <0.021 | |||||
| 7 | 25 | 21 | 22:38h | Mesor | 96.06 (1, 90) | <0.001 | |
| 9 | 15 | 15 | 22:03h | Mesor | 201.5 (1, 90) | <0.001 | |
| 10 | 23 | 22 | 16:39h | Mesor | 107.6 (1, 90) | <0.001 |
Experimental day 6 (control) was compared with experimental days 7, 9, 10 and 11 (see table 1). Only the significant data are shown A: data of horizontal activity (HA). B: data for vertical 1 activity and C: data for vertical 2 activity. For simplification only the significant data are presented.
The study was conducted in accordance with the declaration of Helsinki and according to the requirement of our local Animal Welfare Committee.
Results
Placebo Pellet (Control) baseline locomotor activity
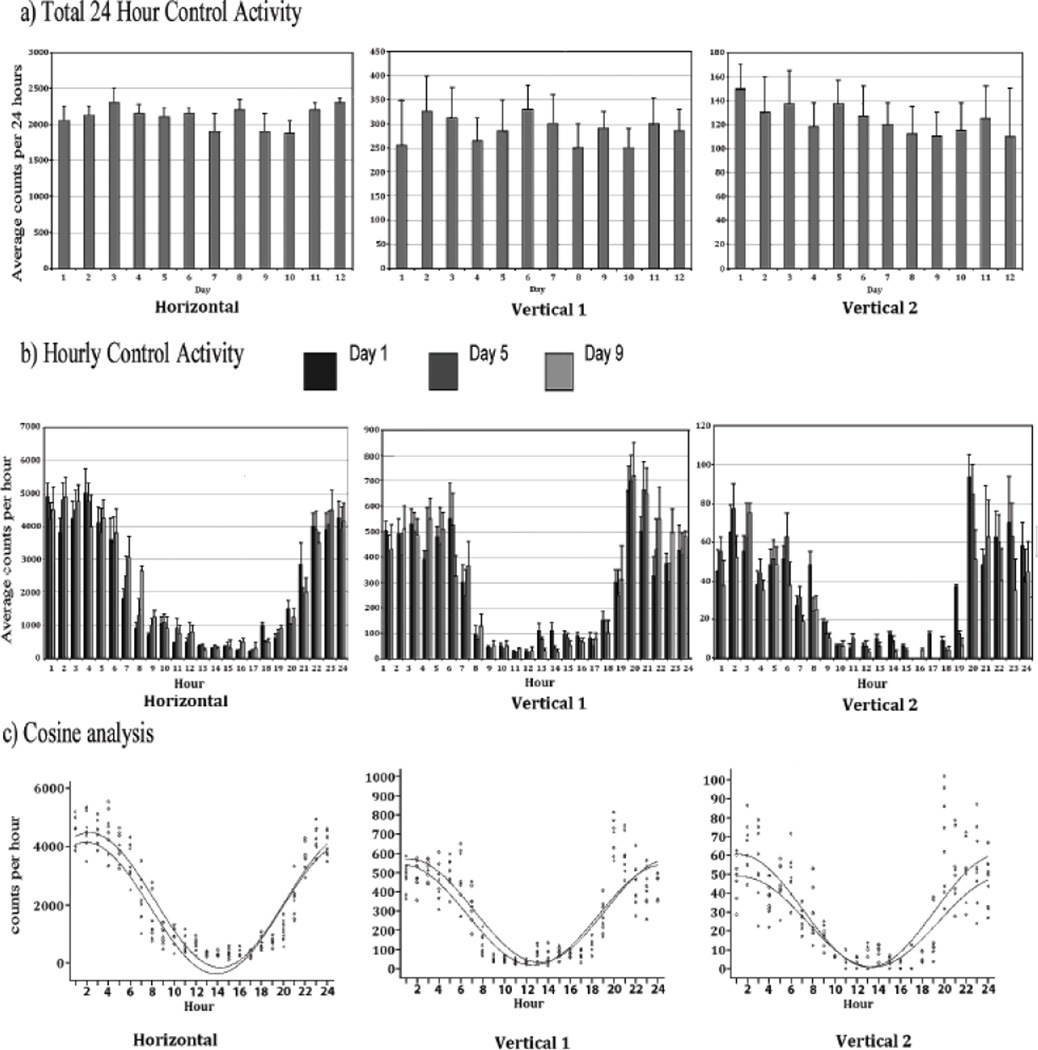
Figure 1a summarizes the mean horizontal 24 hour activity as counts/h. The daily (24 hour) histograms demonstrate that the placebo group exhibits similar activity level for all three locomotor indices, i.e., horizontal, vertical 1, and vertical 2 activity across days with only minor fluctuations over the 12 experimental days. Fig 1b summarizes the mean ± SD of the hourly horizontal, vertical 1, and vertical 2 locomotor activity indices. Hourly activity measurement is indicated relative to lights out (19:00 h). As expected, locomotor activity was greatest in the nocturnally active rats during the night and lowest during the day.
Figure 1.
The figure summarizes the average activity and its standard deviation (SD) of the placebo pellet group (N = 8). The upper histograms (Fig 1a) summarize the total locomotor activity count for 24 hours for the 12 experimental days and shows that the horizontal activity, vertical activity 1 and vertical activity 2 during the 12 experimental days are similar with minor fluctuations. The middle histogram (Fig 1b) summarizes this hourly (60 min) horizontal, vertical 1, and vertical 2 activity of experimental Days 1, 5, and 9 next to each other. These hourly histograms show similarity of the circadian activity pattern on the specified days. The lower data (Fig 1C) show the statistical analysis using the cosine curve statistical analysis test for these days and shows that the rhythmic activity is similar with minor non-significant fluctuations. The symbols 0, + indicate the experimental day 1, and 9, respectively. Experimental day 5 was identical to day 1. The curves demonstrate that the activity patterns of these days were similar.
Experimental Days 1, 5, and 9 of the untreated animals (placebo) were chosen as representatives of the repeated consistent 24-hour pattern of activity seen on all 12 days of the experiment. Since there were no significant differences in the 24-hour horizontal level activity pattern (MESOR = 2159 counts/h, amplitude=2309 counts/h, acrophase=21:13h) contrasting experimental Day 1, 5 and 9, the data of these days were pooled to generate summary parameters for the control group (Fig. 1c).
Activity following morphine pellet implantation
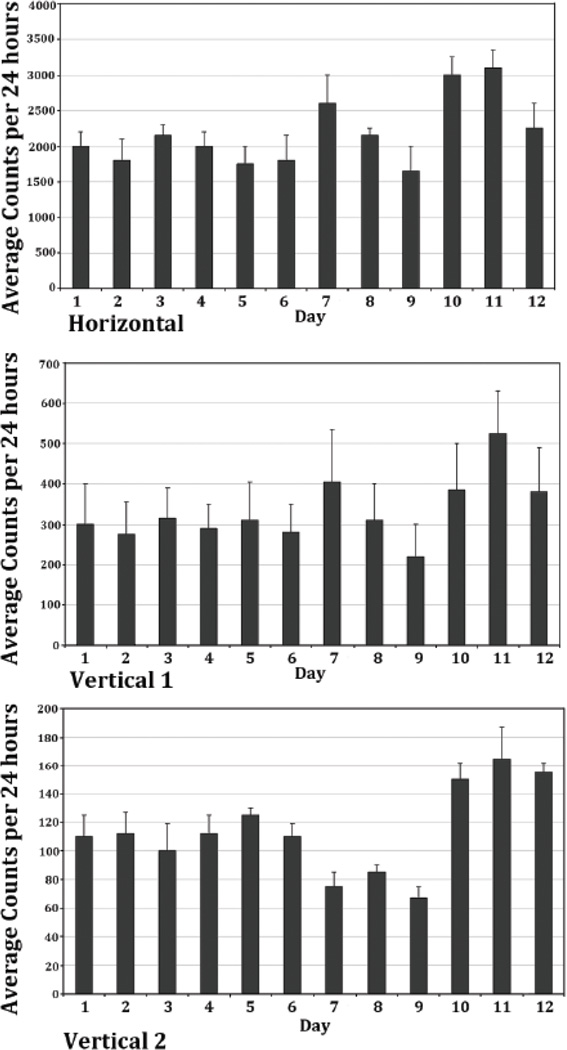
In the morphine experimental group, six baseline recording days prior to morphine pellet implantation were obtained. Experimental Day 6 of the morphine pellet animals was used to represent a control baseline activity of this animal group (N=8). The cosine parameter estimates for Day 6 of the treated animals, were contrasted to the three control days (1.5 and 9) of the placebo-treated control animals and were found to be not significantly different. Thus, the data for Day 6 of the experimental rats was used as the reference control for subsequent contrast comparisons with the treatment days. Figure 2 summarizes the mean ± SD of the 24-hour activity of the three locomotor indices; horizontal, vertical 1, and vertical 2 for the morphine experimental group of rats (N=8) from Days 1 through 12. On experimental Day 7, the first day after morphine pellet implantation, morphine elicited a significant increase in the total amount of 24-hour horizontal activity to 2575 counts/h (p<0.05), as compared to 1875 counts/h in previous days before to morphine treatment, and vertical activity 1 from 296 counts/h (p<0.05) to 410 counts/h after morphine exposure (Figure 2 and Tables 2 and 3). The locomotor activity then slowly returned back to the baseline level the next two days post morphine exposure, i.e., on experimental Days 8 and 9. By experimental Day 9, the 24-hour horizontal activity and vertical 1 activity had returned to the baseline activity level (Figure 2). In contrast, the vertical activity 2 (Figure 2) showed a significant (p<0.05) reduction in counts/h. The vertical 2 activity level remained depressed for the duration of morphine treatment, Days 8 and 9 (Figure 2).
Figure 2.
The figure summarizes the averaged total 24 hour horizontal, vertical 1, and vertical 2 locomotor activity and its SD from the mean (SEM) of the morphine pellet group (N = 8). The initial 6 days represent the 24 hour activity prior to morphine treatment (experimental Days 1 to 6), followed by 3 days after morphine pellet implantation (experimental Days 7 to 9), and followed by an additional 3 recording days after the residual pellet was removed. The histograms show that morphine increases the horizontal and vertical 1 activity in the first day post-morphine and then the total activity per 24 hours returns to baseline activity levels. The vertical 2 activity shows a decrease in this activity (experimental Days 7 to 9). However, following morphine removal, all the locomotor indices show an increase in activity level.
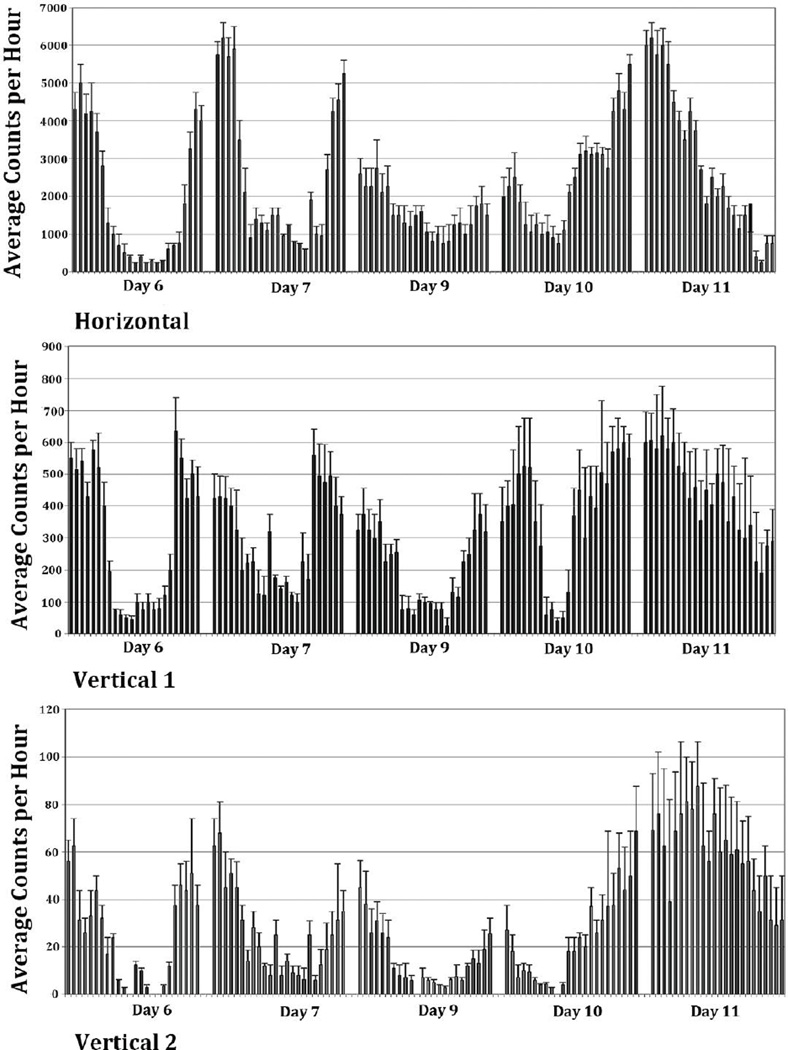
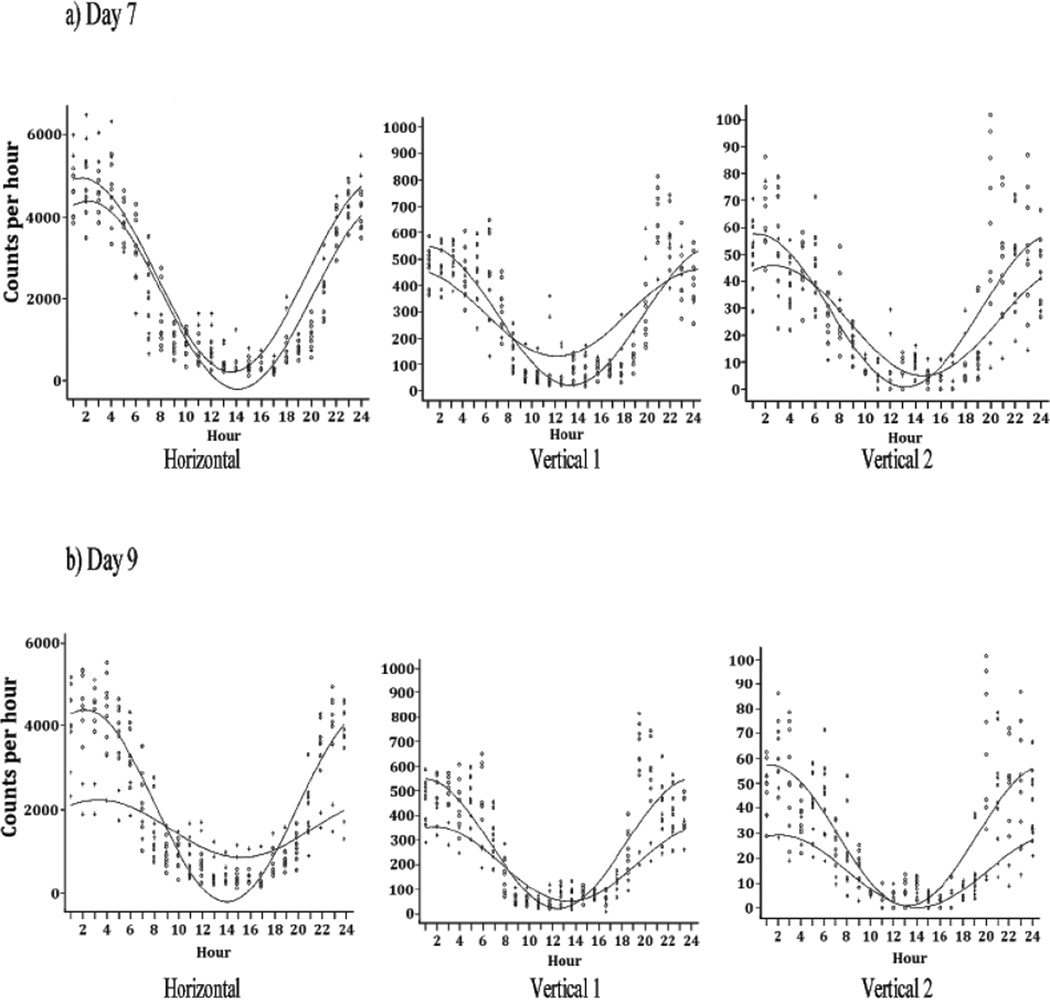
Figure 3 presents the mean ± SD hourly activity of the morphine group and illustrates that morphine treatment changed the pattern of activity on experimental Days 7 and 9 compared to experimental Day 6 (control recording prior to morphine). On experimental Day 7, the pattern of the circadian rhythm of horizontal activity showed a peak activity time (acrophase) of 21:36 h (Table 2, Figure 4a), which was slightly earlier than the acrophase of 22:08 h of the pooled control animals, (p = 0.047, Table 2). The overall magnitude of the daily activity is visually greater than that of controls (Figure 3), although the ratio of the day to night variation remained unchanged. These impressions were statistically validated by the findings, a lack of statistically significant difference between the amplitude values (p = 0.656) (Figure 4a), and by a statistically significant (p< 0.001) difference between the average activity (MESOR) for Day 7 versus the control group (Figure 4a and Table 2). Vertical activity 1 showed no significant difference in the 24-hour mean level of activity (MESOR, p = 0.542) in comparing baseline and morphine dependence values and no shift in the time of peak activity (acrophase, p = 0.517). However, there was a difference (p< 0.001) in the amplitude of vertical 1 activity (Fig. 4a). Vertical activity 2 showed no significant difference in the activity level (MESOR, p = 0.107), but did show significant change in the amplitude (p = 0.018) and acrophase (p = 0.017) (Figure 4a and Table 2).
Figure 3.
The figure summarizes the averaged hourly activity and its SD (N=8) of experimental Days 6, 7, 9, 10, and 11, and shows that in the initial day post-morphine treatment the circadian activity remained in a similar pattern as the control days (experimental Day 6) with some increase in the activity during both the night and day (experimental Day 7 compared to experimental Day 6). On the last day of morphine exposure (experimental Day 9), the circadian activity pattern was altered. After abrupt residual morphine pellet removal, the hourly histogram shows that the circadian activity pattern was altered dramatically (experimental Days 10 and 11).
Figure 4.
The figure shows the averaged (N=8) superimposed cosine analysis curve of experimental Day 7 (first day of morphine pellet treatment) compared to experimental Day 6 (control) (Fig 4A) as well as experimental Day 9 (last day of morphine treatment) compared to experimental Day 6 (Fig 4B). They show that the morphine treatment in these 2 days altered significantly the circadian activity pattern of the 3 locomotor activities (see table 2). The symbol 0 represents experimental day 6 data (Fig. 4a and b) and the symbol + represent experimental day 7 at 4a and experimental day 9 at 4b. See table 2 for the statistical analysis of the cosine curves.
By experimental Day 9, the last day of morphine treatment, the horizontal 24-hour activity rhythm exhibited a remarkable change compared to the control days (MESOR, p<0.001) (Figure 4b). The level of activity at night was reduced, while it was greater during the daytime giving rise to a different activity pattern with a dampened circadian amplitude value of 1544 vs. 2091 for the control days (p<0.001). However, the time of peak activity was comparable (acrophase, p = 0.078, Table 2). Vertical 1 activity and vertical 2 activity on experimental Day 9 (Figure 3) mimics the activity pattern seen on the horizontal axis; vertical activity 1 MESOR, p< 0.001, amplitude, p< 0.001, acrophase, p = 0.099, and vertical activity 2 MESOR, p<0.001, amplitude, p<0.001, acrophase, p = 0.275 (Figure 4b and Table 2). This evaluation indicates that at experimental Day 9, the total activity per 24hours was reduced compared to experimental Day7 (the first day of morphine pellet implantation). However, the activity pattern was changed significantly which suggests that the exposure to chronic morphine treatment modulates the circadian activity pattern.
Activity following morphine pellet removal - morphine withdrawal
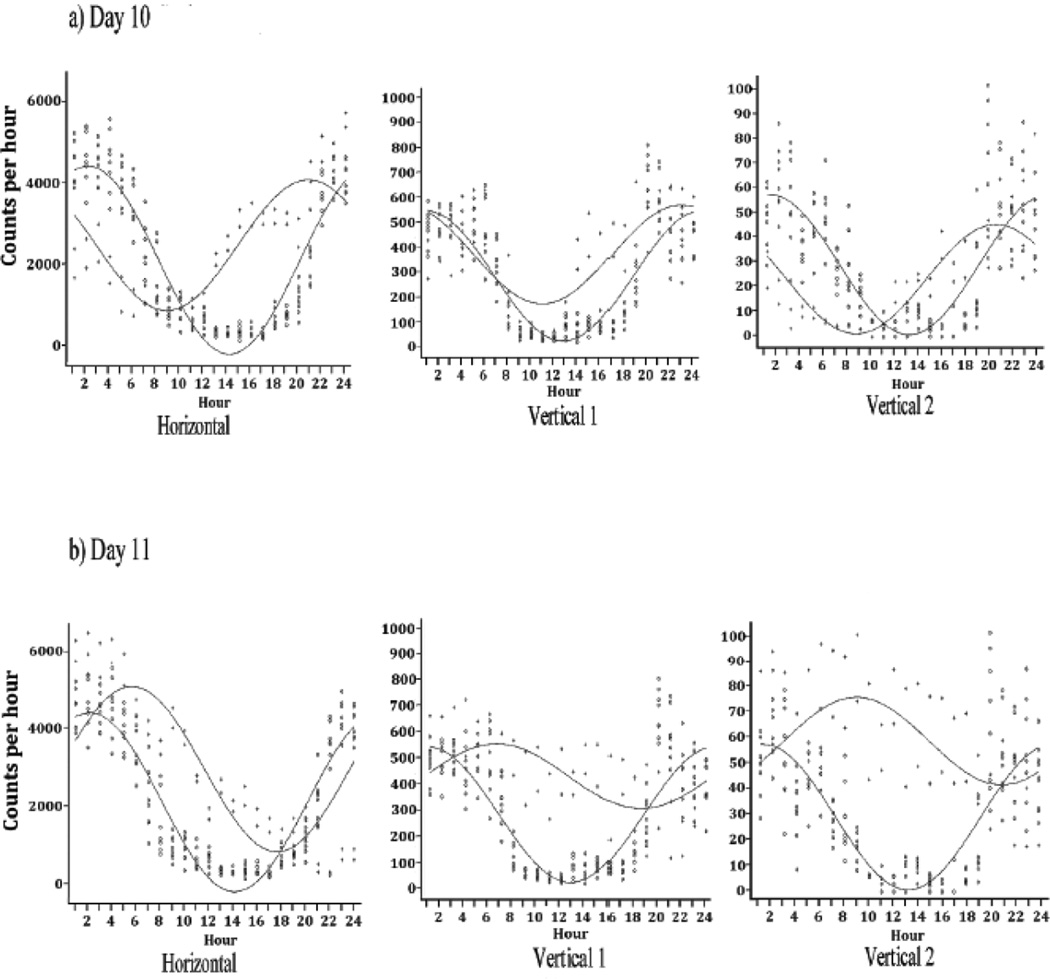
Figure 3 summarizes also the locomotor activity after morphine pellet removal (experimental Days 10 and 11). It indicates that all three locomotor activity levels for experimental Day 10 exhibited an increase in the MESOR and amplitude values (p<0.05) (Figure 2 and Table 2). On experimental Day 10 (Figure 3), there was a change in the pattern of the hourly activity levels, with a mid-day spike in activity of all three locomotor indices, which continued through the night into the next day. The horizontal activity showed a significant difference in the level of activity (MESOR, p<0.001), change in amplitude (p<0.001), and change in the time of peak activity (acrophase, p = 0.002) (Figure 5a). Vertical activity 1 showed a significant difference in MESOR (p< 0.001), change in amplitude (p = 0.024), but no change in acrophase (p = 0.591) (Figure 5a). Vertical activity 2 exhibited a significant difference in all three statistical parameter estimates (MESOR, p = 0.012; amplitude, p = 0.062; acrophase p< 0.001, Table 2).
Figure 5.
The figure shows the superimposed cosine analysis curves of experimental Day 10 (the first day after abrupt residual morphine pellet removal) compared to experimental Day 6 (baseline activity) (Fig 5A) as well as experimental Day 11 (the second day after abrupt morphine removal) compared to experimental Day 6 (Fig 5B). Both curves (Figs 5A and 5B) show that removal of morphine significantly alters the circadian pattern of the horizontal, vertical 1 and vertical 2 activities. The symbol 0 represents experimental day 6 data (Fig. 5a and b) and the symbol + represent experimental 10 at 5a and experimental day 11 at 5b. See table 2 for the statistical analysis of the cosine curves.
The histograms of experimental Day 11 activity (Figure 3) show a complete change in the circadian rhythm pattern. The cosine analysis of the overall horizontal activity level changed (MESOR, p<0.001) and the acrophase markedly phase shifted (acrophase, p<0.001) reflecting high activity levels during the first few hours of the day (01:00 to 04:00 h) and less activity at the end of the day (22:00 to 12:00 h (Figure 5b). Vertical activity 1 followed a similar pattern (Figure 5b), but with a smaller slope of decline (MESOR, p<0.001, amplitude, p<0.001, acrophase, p<0.001). Vertical activity 2 showed great amplification in activity compared to the control (MESOR, p<0.001, amplitude, p = 0.002, acrophase, p = 0.021; Table 2).
Animal weight during the experiment
The first day after the morphine pellet implantation the rats lost 4 ± 2 gm and they regained the weight in the next 2 days. Following removal of the residual morphine pellet, the rats lost 6 ± 3 gm in the first 2 days and then regained the weight, and weight similar to the placebo pellet group 5 days after morphine pellet removal.
Discussion
The circadian activity rhythm is essential for biological function, allowing for the maintenance of homeostasis, metabolism, and overall well-being in organisms (Carpenter et al., 1985; Minors et al., 1986; Reppert et al., 2002). Studies indicate that the master clock that regulate the circadian activity rhythm is located in the hypothalamus i.e., in the suprachiasmatic nucleus (SCN) which regulates and controls the circadian activity and other circadian rhythms (Reppert et al., 2002; Herzog and Schwartz, 2002). It is known that medications as well as opioid can have different effects when administered at the morning compared to night administration (Auvil-Novak, 1999; Bruguerolle et al., 2007; Li et al., 2010). Opioids work through interaction with one of several receptors in the brain, specifically the μ (mu) type receptors, to produce analgesia (Smith et al., 1995). Continual use of opioids can lead to a state of tolerance in which increasing doses of the drug are required to produce the same effect. This can, in turn, lead to a state of dependence. Abrupt removal of morphine in an addictive state produces a set of distinctly characteristic behaviors known as the withdrawal syndrome (Dafny, et al., 1983).
In this experiment, morphine was administered continually from an implanted pellet, removing stress elicited by the animal handling and the injection itself, which can mask the observed drug effect. Thus, the present study investigated the circadian rhythm of behavioral activity pattern during morphine administration without interruption, and thereafter during withdrawal following the removal of the remaining morphine pellet as opposed to precipitating withdrawal by an opiate antagonist as used previously (Dafny et al., 1983). To measure the locomotor activity this study used the open field assay, which contain three levels of strips, each consist of 16 infrared beams, separated 2.5 cm each other, and their respective motion sensors. This device can record the breaking of the beam in three vertical levels which express different locomotor activity but it do not distinguish, for example, between wet dog shakes and grooming or rearing. However it provides a reliable tool to measure the total locomot activity.
In the present study we use the Cosinor Curve Statistical Analysis (CCSA) test (Bingham et al., 1982) because it best evaluate the potential hour to hour, magnitude and timing components of the 24 hour activity with minimal parameter estimation. The fact that numerous days are involved is what is driving the multiple comparison issue.
It was reported that endogenous opioid peptide levels exhibit circadian patterns in the brain, as well as other substances such as the levels of 5-hydroxytryptamine, bradykinin, glutamate, nitrous oxide, substance P, cytokines (IL1, IL6) and prostanoids (Asai et al., 2007; Li et al., 2010). These chemical agents are known to be involved in the activation of nociceptors. Studies show that endogenous opioid peptides are higher at the beginning of the day and lower in the evening (Bruguerolle et al., 2007).
Previous studies have shown that administering chronic morphine causes a change in cAMP-CREB pathway (Chao et al., 2004; Nestler, 2004). This, in turn, changes the level of calcium influx via voltage dependent calcium channels. The new level of calcium modifies the amount of per and clock gene transcription within the cells of the locus coeruleus (Li et al., 2009; Nahm et al., 2005; McClung et al., 2005). The genes Bmal1, Period1 (Per1), Per2, Cryptochrome1 (Cry1), Cry2, and Rev-erb are rhythmically regulated through mutual interactions between their protein products, and this feedback loop forms the core of the circadian clock mechanism (Reppert et al., 2002). It was reported in an earlier study that following morphine treatment the morphine levels in each brain site exhibit different concentrations (Fuller et al., 1988). This may explain why the same drug can have biphasic effects or can affect each brain site differently. Indeed, morphine administration is known to produce unique outcomes in different brain regions (Dafny et al., 1983), such as an increase in locomotor activity and a decrease in sleep time, while withdrawal may produce a more global response in gene regulation (McClung et al., 2005). In addition, chronic morphine administration has been shown to upregulate the cAMP pathway. This upregulation of cAMP results in the change in calcium channels which has been shown to modify the amount of circadian rhythm gene transcription (Kogan et al., 1992). Removal of morphine demonstrates an activation of the locus coeruleus by increasing the tonic pacemaker activity of the neurons (Kogan et al., 1992). The tonic pacemaker activity demonstrates a circadian rhythm-type pattern of activity which could then be modified by various treatments.
This study addressed alterations of the circadian locomotor activity rhythm during “chronic” morphine treatment and its abrupt removal. The main finding of this study is a marked increase in the total levels of locomotor activity between control rats and the rat implanted with morphine pellet as well as after removal of the residual morphine pellet. On the first day post-morphine pellet implantation (experimental Day 7), the locomotor activity level increased and the circadian rhythm activity pattern was significantly modulated. This increase in activity reflects the stimulating effects of morphine upon the initiation of treatment. On the second day (experimental Day 8), the total activity per 24 hours began to decrease, which suggests that tolerance is induced. By the third day post-pellet implantation (experimental Day 9), the total locomotor activity per 24 hours returned to baseline level, i.e. tolerance is expressed (Dafny et al., 1983). A similar observation after 72 hours of morphine administration was reported by Blasig et al., (1973), and Wei and Way (1975).
The circadian activity pattern of the locomotor activity in the morphine pellet animals changed from day to day. It is possible to assume that “chronic” morphine treatment results in up-regulation of cAMP and CREB (Chao et al., 2004; Nestler, 2004) and calcium channels are modified. Fluctuations in intracellular calcium has been shown to modify circadian gene transcription (McClung et al., 2005), and these molecular changes, we hypothesize, result in the modification in locomotor circadian activity pattern seen in this study following “chronic” morphine administration.
The morphine pellet was removed on Experimental Day 10. Changes in the total activity and the hourly activity were observed beginning mid-day after morphine pellet removal. Horizontal activity and Vertical 1 activity on experimental Days 10 and 11 demonstrate a robust increase in total 24-hour mean activity as well as the remarkable increase in the hourly locomotor activity. This change in hourly activity significantly alters the locomotor circadian activity pattern. This circadian rhythm pattern differs from both the control rhythm and the “tolerance” rhythm and seems to be an expression of morphine withdrawal. When comparing the three locomotor indices of experimental Day 10 in the withdrawal state, one notes that withdrawal elicits more vertical 2 activity. The manifestation of withdrawal symptoms, again, simply could be the manifestation of the molecular changes observed with the changing levels of circadian gene transcription elicited by the abrupt removal of chronic morphine. Short term effects of a drug we define as – the initial drug exposure bind to its receptor that results in alteration in neuronal activity that regulate the animal behavior. After limited time the drug is metabolized and the animal behavior returns to its baseline. Long term effect we define that the drug after continue exposure trigger molecular and cellular cascade of events that regulate gene expression (Li et al., 2009 and 2010), that modulate the circadian rhythm activity pattern, these alterations we define as long-term effect. The observation of this study demonstrate that morphine pellet exposure exert long-term effects.
A better understanding of the mechanisms underlying dependence and withdrawal of opioids could enhance the modern physicians’ ability to treat patients. A multidisciplinary approach to the clinical management of chronic pain could benefit from the understanding that opioids act by molecularly changing the level of circadian gene transcription. However, this approach to morphine’s effects along with our data raises the question, does a change in circadian rhythms cause tolerance and withdrawal, or does tolerance and withdrawal to an addictive substance cause a change in the circadian rhythm?
Highlights.
Chronic effects of morphine modulate animal behavior
Chronic effect of morphine alter the circadian activity pattern.
Abrupt morphine cessation elicits behavioral withdrawal and increase animal behavior.
Abrupt morphine cessation modulate the circadian activity pattern.
Acknowledgments
This study was supported in art by NIH DA R01 027222. We also like to thanks Dr. Levine for his manuscript reading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- 2.Asai MA, Lilian Mayagoitia LM, David Garcia DG, Gilberto Matamoros-Trejo GM, Marcela Valdes-Tovar MV, Phillipe Leff PL. Rat brain opioid peptides-circadian rhythm is under control of melatonin. Neuropeptides. 2007;41:389–397. doi: 10.1016/j.npep.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Auvil-Novak SE. The chronobiology, chronopharmacology, and chronoterapeutics of pain. Ann. Rev. Nurs. Res. 1999;17:133–153. [PubMed] [Google Scholar]
- 4.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 5.Blasig J, Herz A, Reinhold K, Zieglgansberger S. Development of physical dependence on morphine in respect to time and dosage and quantification of the precipitated withdrawal syndrome in rats. Psychopharmacologia. 1973;33:19–38. doi: 10.1007/BF00428791. [DOI] [PubMed] [Google Scholar]
- 6.Bruguerolle B, Labrecque G. Rhythmic pattern in pain and their chronotherapy. Adv. Drug Deliv. Rev. 2007;59:883–895. doi: 10.1016/j.addr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter GA, Grossberg S. A neural theory of circadian rhythms: split rhythms, after-effects and motivational interactions. J. Theor. Biol. 1985;113:163–223. doi: 10.1016/s0022-5193(85)80083-7. [DOI] [PubMed] [Google Scholar]
- 8.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu. Rev. Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- 9.Dafny N, Burks TF, Bergmann F. Dose effects of morphine on the spontaneous unit activity recorded from the thalamus, hypothalamus, septum, hippocampus, reticular formation, central gray, and caudate nucleus. J. Neurosci. Res. 1983;9:115–126. doi: 10.1002/jnr.490090203. [DOI] [PubMed] [Google Scholar]
- 10.Dafny N, Zielinksi M, Reyes-Vazquez C. Alteration of morphine withdrawal to naloxone by interferon. Neuropeptides. 1983;3:453–463. doi: 10.1016/0143-4179(83)90036-7. [DOI] [PubMed] [Google Scholar]
- 11.Dougherty PM, Dafny N. Cyclosporine affects central nervous system opioid activity via direct and indirect means. Brain Behav. Immun. 1988;2:242–253. doi: 10.1016/0889-1591(88)90026-8. [DOI] [PubMed] [Google Scholar]
- 12.Fuller GN, Lin SN, Caprioli RM, Wiggins RC, Dafny N. Dose-related differential accumulation of morphine in specific regions of rat brain determined by mass fragmentography. Int. J. Neurosci. 1988;38:31–38. doi: 10.3109/00207458809000479. [DOI] [PubMed] [Google Scholar]
- 13.Herzog ED, Schwartz WJ. A neural clockwork for encoding circadian time. J. Appl. Physiol. 2002;92:401–408. doi: 10.1152/japplphysiol.00836.2001. [DOI] [PubMed] [Google Scholar]
- 14.Kogan JH, Nestler EJ, Aghajanian GK. Elevated basal firing rates and enhanced responses to 8-Br-cAMP in locus coeruleus neurons in brain slices from opiate-dependent rats. Eur. J. Pharmacol. 1992;211:47–53. doi: 10.1016/0014-2999(92)90261-2. [DOI] [PubMed] [Google Scholar]
- 15.Li SX, Liu LJ, Jiang WG, Lu L. Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J. Neurochem. 2009;109:1668–1679. doi: 10.1111/j.1471-4159.2009.06086.x. [DOI] [PubMed] [Google Scholar]
- 16.Li SX, Liu LJ, Jiang WG, Sun LL, Zhou SJ, Le FB, Zhang XY, Kosten TR, Lu L. Circadian alteration in neurobiology during protracted opiate withdrawal in rats. J. Neurochem. 2010;115:353–362. doi: 10.1111/j.1471-4159.2010.06941.x. [DOI] [PubMed] [Google Scholar]
- 17.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J. Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menaker M, Takahashi JS, Eskin A. The physiology of circadian pacemakers. Annu. Rev. Physiol. 1978;40:501–526. doi: 10.1146/annurev.ph.40.030178.002441. [DOI] [PubMed] [Google Scholar]
- 20.Minors DS, Waterhouse JM. Circadian rhythms and their mechanisms. Experientia. 1986;42:1–13. doi: 10.1007/BF01975875. [DOI] [PubMed] [Google Scholar]
- 21.Nahm SS, Farnell YZ, Griffith W, Earnest DJ. Circadian regulation and function of voltage-dependent calcium channels in the suprachiasmatic nucleus. J. Neurosci. 2005;25:9304–9308. doi: 10.1523/JNEUROSCI.2733-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro S, Yang P, Reyes-Vazquez C, Swann A, Dafny N. Sex differences in tail-flick latency of non-stressed and stressed rats. Int. J. Neurosci. 2005;115:1383–1395. doi: 10.1080/00207450590956404. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–2378. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- 26.Savage SR. Long-term opioid therapy: assessment of consequences and risks. J. Pain Symptom. Manage. 1996;11:274–286. doi: 10.1016/0885-3924(95)00202-2. [DOI] [PubMed] [Google Scholar]
- 27.Smith AP, Lee NM, Loh HH. Opioid Analgesics and Antagonists. In: Munson PL, editor. Principles of Pharmacology. New York: Chapman & Hall; 1995. pp. 399–413. [Google Scholar]
- 28.Wei E, Way EL. Application of the pellet implantation technique for the assessment of tolerance and physical dependence in the rodent. In: Ehrenpreis S, Neidle A, editors. Methods in Narcotics Research. New York: Marcel Dekker; 1975. pp. 243–259. [Google Scholar]
- 29.Yang PB, Cuellar DO, III, Swann AC, Dafny N. Age and genetic strain differences in response to chronic methylphenidate administration. Behav. Brain Res. 2011;218:206–217. doi: 10.1016/j.bbr.2010.11.034. [DOI] [PubMed] [Google Scholar]