Abstract
Research during the last decade has significantly advanced our understanding of the molecular mechanisms at the interface between the nervous system and the immune system. Insight into bidirectional neuroimmune communication has characterized the nervous system as an important partner of the immune system in the regulation of inflammation. Neuronal pathways, including the vagus nerve-based inflammatory reflex are physiological regulators of immune function and inflammation. In parallel, neuronal function is altered in conditions characterized by immune dysregulation and inflammation. Here, we review these regulatory mechanisms and describe the neural circuitry modulating immunity. Understanding these mechanisms reveals possibilities to use targeted neuromodulation as a therapeutic approach for inflammatory and autoimmune disorders. These findings and current clinical exploration of neuromodulation in the treatment of inflammatory diseases defines the emerging field of Bioelectronic Medicine.
Keywords: neurons, immunity, inflammation, vagus nerve, cytokines, cholinergic
1. Introduction
The more we know about the molecular mechanisms of disease, the better therapeutic approaches we can design to treat and potentially cure it. This is one of the main principles of clinically oriented research, which has led to successful treatments of many disorders. Still, there are numerous diseases with no adequate treatments despite serious research efforts, employing state of the art technology, to identify new molecular therapeutic targets. There are several reasons for this failure to successfully translate research discoveries into the clinic, including the complexity of mechanisms regulating these targets within the scope of major biological events and the alteration and dysfunction of these regulatory mechanisms in disease settings. While this may sound trivial to many and the need for new multidisciplinary approaches seems obvious, the path forward is not always clear.
Here we outline important aspects of research performed on crossroads between neuroscience and immunology, which holds a great promise to significantly advance the treatment of a broad spectrum of diseases characterized by immune dysfunction. This work has provided important insight into the role of neurons as partners of immune cells in the regulation of inflammation, which has significant implications in disease pathogenesis. It has now become clear that neurons sense inflammatory products and that neuronal activity is modulated in inflammatory conditions. Moreover, neurons can detect molecules associated with pathogen invasion simultaneously, or even before immune cells [1]. The nervous system is positioned to mount fast and directed responses to inflammatory stimuli through reflex-like mechanisms and to regulate immune function and inflammation [2–4]. These neuron-immune cell interactions can be exploited therapeutically in inflammatory and autoimmune conditions, obesity-driven disorders and other diseases characterized by aberrant inflammation [5,6]. A growing body of mechanistic insight into the pathways and mechanisms at the interface of the nervous system and the immune system has also led to a major conceptual switch in designing therapeutic approaches. These new concepts within the scope of the emerging field of Bioelectronic Medicine explore selective neuromodulation directed to specific molecular targets in disease treatment. Although this field is in its infancy, device-generated modulation of vagus nerve activity has already started yielding successful translational applications in human disease [7].
2. Nervous system and immune system features favoring interaction and loci of neuroimmune communication
The nervous and the immune systems evolved to respond to external and internal stimuli. The nervous system integrates physiological processes and controls homeostasis through neuronal networks. The immune system provides defense responses against infection and injury through immunity. A long history of mutual disregard between Neuroscience and Immunology has been followed by research bridging the two disciplines and demonstrating a variety of neuro-immune interactions.
2.1. The nervous system flexible authority
The nervous system regulates a broad spectrum of physiological processes. Constant communication between physiological systems operating in peripheral (abdominal and thoracic) organs and the brain mediates this regulation. A significant part of this communication is based on neural reflex mechanisms such as the neural regulation of blood pressure and gastrointestinal function and takes place through the neurons of the autonomic nervous system. The vagus nerve is a major constituent of the parasympathetic division of the autonomic nervous system. This nerve contains afferent (or sensory) fibers that are specialized in transmitting signals from peripheral organs to the brainstem, and efferent (or motor) fibers that originate in the brainstem medulla oblongata, innervate major peripheral organs, and control heart rate, gastrointestinal motility and other vital physiological functions [6]. Neural networks involving higher, forebrain re[8]gions also participate in the coordinated neural regulation of physiological functions. Vagus and brain neuronal activity is constantly modulated in response to peripheral physiological alterations and their deviations in disease settings. These peripheral alterations also trigger brain-derived responses to maintain homeostasis.
2.2. The immune complex defense
The immune system detects pathogen invasion, tissue injury or self danger signals, mounts responses to contain, neutralize and eliminate these potentially damaging stimuli and forms an immunological memory that facilitates this defense strategy upon encountering similar challenges in the future. These complex regulatory processes represent immunity. Leukocyte/neutrophil and monocyte migration to the site of injury or infection is an early and important event in the immune defense repertoire [9]. Pathogen fragments and molecules associated with tissue injury interact with molecular sensors, including toll-like receptors (TLRs), NOD-like receptors (NLRs) and other pattern recognition receptors on the cell surface or in the cytoplasm of macrophages and other innate immune cells. These interactions trigger immune cell activation through intracellular cascade mechanisms, resulting in activation of NF-κB and other transcription factors with the release of cytokines including TNF, IL-1β, IL-6, and HMGB1. In addition to cytokines, the release of other inflammatory molecules such as prostaglandins and leukotrienes mediates inflammation, which is aimed at neutralizing the invading pathogen and tissue repair. Macrophages and dendritic cells also are antigen-presenting cells, linking innate immune factors with adaptive immunity, characterized by T and B cell responses, which also contribute to containing the inciting stimulus and forming an immunological memory. The release of cytokines is essential in inflammation, which normally is localized and timely resolved. The resolution of inflammation is an active process guided by molecular mediators collectively termed resolvins and protectins, and aimed at restraining leukocyte infiltration and activation phagocytosis of pathogen fragments by macrophages [9–11]. However, due to circumstances that are not yet well understood, inflammation resolution might be compromised and the inflammatory response might become systemic and chronic with deleterious consequences. There are various pathophysiological scenarios in which excessive release of TNF and other pro-inflammatory cytokines in the circulation mediates secondary tissue damage that can be debilitating or lethal. Persistent, non-resolving chronic inflammatory responses are implicated in the pathology of several disorders, including sepsis, rheumatoid arthritis, inflammatory bowel disease, cardiovascular disease, the metabolic syndrome and diabetes.
In the brain, inflammation is predominantly mediated by two types of cells with immune function - microglia and astrocytes [12,13]. At physiological conditions these cells are important modulators of neuronal structure and function; they play a role in neurogenesis, synaptic formation, plasticity and remodeling through the release neurotrophins and complement neurotransmission through the release of gliotransmitters [12–15]. Astrocytes also are important constituents of the blood brain barrier [13]. Microglia provide immune surveillance in the CNS/brain [12,13]. Immune activation in the CNS and the brain in particular occurs through mechanisms similar to peripheral innate immune activation [14]. Brain infection, brain injury, and neurodegenerative processes trigger microglia activation through TLRs and other pattern recognition receptor-mediated signaling with the release of TNF, IL-1β, IL-6, reactive oxygen species and other pro-inflammatory molecules, which mediate neuroinflammation [14,16,17]. Astrocyte activation with the release of inflammatory mediators also contributes to inflammation in the brain [13]. These brain immune responses and inflammation are directed towards repair and restoration of neuronal integrity and function. However, chronic neuroinflammation exacerbates neuronal damage, associated with further neuronal dysfunction [12,17].
2.3. The neuroimmune common ground
Neurons and immune cells interact with each other. Cooperation between neurons and innate immune defense mechanisms is in play even in invertebrates where the immune system and the nervous system have their phylogenetic origins. The simple nervous system of Caenorhabditis elegans, with 302 neurons – contains neurons specialized in controlling a noncanonical unfolded protein response pathway required for innate immunity in this soil nematode [18]. Specific aspects of neuron-immune cell interactions in the periphery and in the CNS have been actively studied. In the periphery these interactions have been highlighted in neurogenic inflammation [19]. In this scenario somatosensory neurons (neuroceptors) detect cytokines and other inflammatory products from activated immune cells in response to bacterial invasion and mediate inflammatory pain. In addition, these neurons - in an antidromic fashion- release substance P and other mediators, which cause vasodilation by acting on endothelial and smooth muscle cells, and serve as immune cell attractants and activators of immune cells, including dendritic cells, mast cells and T lymphocytes [19]. These neuro-immune interactions mediate integrated protective mechanisms. However, constant amplification of neurogenic inflammation may have implications in the pathogenesis of autoimmune diseases and allergic conditions [19]. Important insight into the role of neurons in sensing pathogen-associated molecules was provided by Clifford Woolf and colleagues. They have recently found that the presence of bacterial (S. aureus) infection activates neuroceptors without triggering innate and adaptive immune activation as a mediating event, because it does not involve neutrophils, monocytes, T cells, B cells and signaling through TLR2 and MyD88 [1]. Instead, S. aureus activates these sensory neurons though induction of calcium flux and action potentials in a direct manner through the release of N-formyl peptides and the pore-forming toxin alpha-hemolysin. In addition, these sensory neurons release neuropeptides that inhibit innate immune activation within an axon-reflex mechanism [1]. Specific nociceptor ablation abrogates pain sensation, which is also associated with increased local inflammation and lymphadenopathy, indicating a tonic anti-inflammatory role of these reflex mechanisms [1]. In the CNS/brain, microglial activation and astrogliosis mediate neuroinflammation. Persistent brain inflammatory responses have been associated with promoting neurodegeneration and contributing to neuronal dysfunction in the context of Alzheimer’s disease, multiple sclerosis, Parkinson’s disease, and the sequelae of traumatic brain injury and stroke [20].
3. Bidirectional immune-brain communication and the emergence of the reflex concept
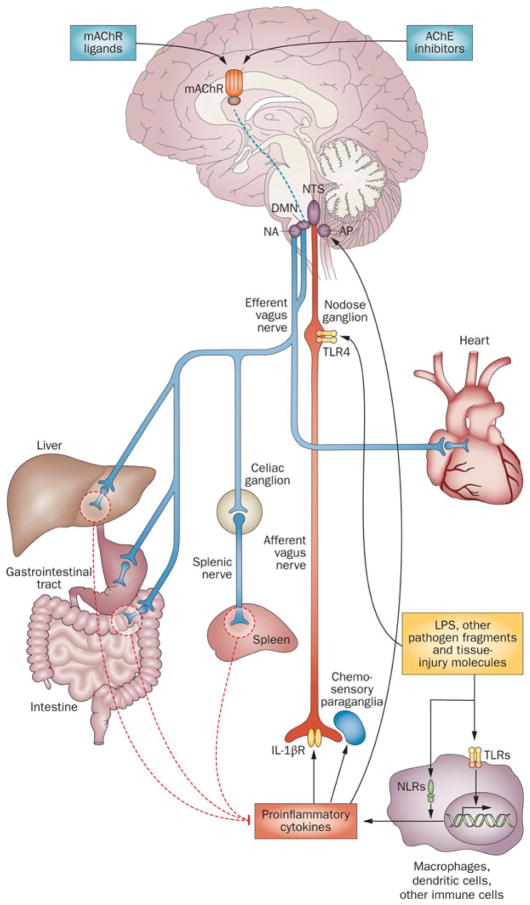
A neuro-immune dialog bridging the brain and the immune system also takes place. Peripheral immune cell activation with the release of cytokines and other immune molecules is communicated to the brain through neural and humoral mechanisms and triggers brain-derived immunoregulatory output through neural and neurohormonal pathways [2,6,21–25]. Infiltration of immune cells, including monocytes, T cells, and neutrophils in the brain has also been described [25]. Linda Watkins and colleagues have demonstrated an important role of afferent vagus neurons in immune-to-brain communication [26]. Cytokines and other inflammatory molecules interact with afferent vagus neurons transmitting the signals to the nucleus tractus solitarius (NTS) in the brainstem, where these signals are integrated with efferent vagus nerve signaling through neurons residing in the dorsal motor nucleus of the vagus (DMN) [8] (Fig. 1). Efferent vagus nerve cholinergic signaling provides a conduit of brain-to-immune communication for inhibition of excessive release of TNF and other pro-inflammatory cytokines [27]. This neuronal circuit is termed “the cholinergic anti-inflammatory pathway” [27] (Fig. 1). This discovery has been shortly followed by the identification of a specific receptor on macrophages and other immune cells - the alpha7 nicotinic acetylcholine receptor (α7nAChR), as a mediator of anti-inflammatory cholinergic output [28]. Together these findings defined a new physiological immunoregulatory mechanism - the inflammatory reflex. [2] (Fig. 1). Afferent and efferent vagus nerve fibers within the inflammatory reflex provide a physiological interface between the immune system and the brain, for communication and control of excessive pro-inflammatory signaling. Recent developments in our understanding of the inflammatory reflex have highlighted that the efferent vagus nerve communicates with the splenic nerve to suppress excessive pro-inflammatory cytokine responses and inflammation [29–31] (Fig. 1). Although splenic nerve fibers are catecholaminergic, vagus nerve stimulation results in increased levels of acetylcholine in spleen, and vagotomy or splenic nerve transection decreases it [32]. Insight into this interesting phenomenon has revealed that a subset of splenic T cells, which contain functional choline acetyltransferase, is a source of acetylcholine in spleen with a mediating role in the inflammatory reflex [31]. This acetylcholine, released under vagus nerve control, mediated through splenic nerve catecholaminergic output, interacts with α7nAChR on macrophages and other immune cells. This interaction causes suppression of macrophage activation and results in lower TNF and other pro-inflammatory cytokine release via intracellular mechanisms, involving suppression of NF-κB nuclear translocation. The vagus nerve-based inflammatory reflex is a prototypical mechanism of neuroimmunomodulation in the context of physiological reflex regulation. Ongoing work has identified other neurons and circuits that regulate immune function. For instance, a neural mechanism controlling autoreactive T cell access to the CNS of mice with autoimmune encephalomyelitis has been recently demonstrated [33]. In this animal model of multiple sclerosis, dorsal vessels of the L5 spinal cord have been identified as an entry point for pathogenic CD4+ T cells to enter the CNS [33]. A complex interaction among sensory neurons innervating the soleus muscles and catecholaminergic (sympathetic) output activates an IL-6-mediated inflammatory amplification in endothelial cells with increased generation of chemokines, which facilitates T cell entry into the CNS [33,34]. Another study has demonstrated the critical importance of neural mechanisms in immunosuppression after stroke and the specific role of catecholaminergic modulation of hepatic invariant NKT cells as a central event in this context, and has suggested implications of neural circuitry in pneumonia development and mortality after stroke [35]. Catecholaminergic signaling was also recently implicated in a neural circuit controlling lymphocyte trafficking [36]. Neuronal catecholaminergic output, mediated through lymphocyte β2 receptor signaling, which is associated with chemokine receptors suppresses lymphocyte egress from lymph nodes. The physiological importance of this neural mechanism, which reduces lymphocyte recruitment into peripheral tissues is related to suppression of T cell mediated tissue damage [37,38]. These reports highlighted the broader scope of neural circuitry that is organized in a reflex manner in controlling immunity by regulating a variety of immune responses at different anatomical locations [4,5].
Fig. 1. The functional anatomy of the inflammatory reflex.
Inflammatory mediators, such as cytokines, are released by activated macrophages and other immune cells when TLRs and NLRs are activated upon immune challenge. These mediators are detected by sensory components of the afferent arm of the inflammatory reflex (red). Neuronal interconnections between the NTS, AP, DMN, NA, and higher forebrain regions (not shown) integrate afferent signaling and efferent vagus nerve-mediated immunoregulatory output. Efferent vagus nerve cholinergic output to the spleen, liver and gastrointestinal tract (blue) regulates immune activation and suppresses pro-inflammatory cytokine release (dotted red lines). Efferent vagus nerve fibers interact with the splenic nerve to suppress pro-inflammatory cytokine release in spleen – a major organ source of TNF and other pro-inflammatory cytokines in endotoxemia and other inflammatory conditions. This efferent cholinergic arm of the inflammatory reflex is termed the cholinergic anti-inflammatory pathway and can be activated in the brain through mAChR-mediated mechanisms triggered by M1 mAChR agonists and other mAChR ligands, and AChE inhibitors, such as galantamine. Abbreviations: AChE, acetylcholinesterase; AP, area postrema; DMN, dorsal motor nucleus of the vagus nerve; LPS, lipopolysaccharide (endotoxin); mAChR, muscarinic acetylcholine receptor; NA, nucleus ambiguus; NLRs, nucleotide-binding oligomerization domain-like receptors; NTS, nucleus tractus solitarius; TLR4, toll-like receptor 4. (Adapted from [6])
4. A close look at the efferent vagus nerve immunoregulatory circuitry
Research during the last 10–15 years utilizing vagus nerve stimulation, surgical transection of the vagus nerve (vagotomy) and state-of-the-art genetic, pharmacological and immunological methodology generated abundant mechanistic insight into vagus nerve neurocircuitry implicated in immune regulation and the neurophysiology of the inflammatory reflex.
4.1. Vagus nerve stimulation
Electrical cervical vagus nerve stimulation (VNS) has been instrumental for demonstrating the efficacy of efferent vagus nerve signaling in suppressing serum and hepatic TNF and other pro-inflammatory cytokine levels and attenuating systemic endotoxemic shock [27]. Acetylcholine, an important mediator of efferent vagus nerve signaling has been found to suppress endotoxin-stimulated macrophage release of TNF, IL-1β, IL-6 and IL-18 [27]. These discoveries have led to numerous subsequent studies that have elucidated immunoregulatory vagus nerve signaling in various conditions. In rats with acute hypovolemic hemorrhagic shock and systemic inflammation, VNS suppresses serum TNF, hepatic TNF gene expression and hepatic NF-κB activation by blocking IκB degradation; these effects parallel alleviated hypotension and an increased survival time [39]. In murine postoperative ileus, VNS suppresses intestinal muscle layer resident macrophage activation and alleviates the condition [40]. Activation of the signal transducer and activator of transcription (STAT) in intestinal macrophages was shown to play a role in mediating VNS effects in this context [40]. VNS suppresses leukocyte trafficking to a subcutaneous site of inflammation, a finding implicating vagus nerve circuitry in the regulation of leukocyte migration across the endothelium during local inflammation [41]. A very recent study reported that single pulse low frequency VNS stimulation is sufficient to suppress TNF release in endotoxemia [42]. This finding is important because it suggests further exploration of a very short duration of VNS in a broader scope of inflammatory conditions, including asthma, where continuous VNS should be avoided because of potential bronchoconstriction effects. In addition to efferent signals, the activation of afferent vagus nerve signaling contributes to anti-inflammatory effects, an observation suggesting that VNS may trigger brain-derived immunoregulatory output [42]. VNS also suppresses peripheral hemorrhage, which suggests important therapeutic implications of VNS in preventing uncontrolled bleeding [43]. In addition to electrical VNS, transcutaneous vagus nerve stimulation suppresses serum TNF levels in murine endotoxemia [44]. The efficacy of this approach of VNS has been shown in preclinical settings of sepsis, a notorious clinical syndrome that is characterized by dysregulation of immune and other physiological responses and which is the number one killer in the ICUs. VNS initiated in a clinically-relevant time frame – 24 hours after the onset of cecal ligation and puncture-induced murine sepsis significantly suppresses serum levels of HMGB1 - a pro-inflammatory molecule and a pivotal cytokine mediator of sepsis lethality and improves survival [44]. VNS by implanted device-generated electrical stimulation at relatively high frequency is clinically approved for the treatment of epilepsy and pharmacoresistant depression [45]. In addition to acute settings, recent studies have evaluated the efficacy of VNS generated by implanted devices in murine models of chronic inflammatory disease. Low frequency VNS through implanted cuff electrode (once daily for 7 days) significantly alleviates the disease score, inflammation and other underlying pathology in rats with collagen-induced arthritis [46]. Implanted VNS (3 hours of stimulation per day for 5 days) in freely moving rats significantly alleviates colonic inflammation and severity of colitis [47]. These studies with implanted VNS in animals with chronic inflammatory conditions have paved the path to ongoing clinical trials in patients with rheumatoid arthritis and inflammatory bowel disease.
4.2. Vagotomy
In contrast to VNS, interruption of vagus nerve signaling through vagotomy results in higher pro-inflammatory cytokine levels in endotoxemic animals, pointing to a tonic anti-inflammatory role of the vagus nerve [27]. Cervical bilateral vagotomy provides an approach for a total interruption of vagus nerve signaling. However, it is associated with high lethality, which limits the use of this approach to acute experiments with anesthetized animals. Therefore, bilateral subdiaphragmatic vagotomy and unilateral cervical vagotomy have also been explored in inflammatory settings. For instance, bilateral subdiaphragmatic vagotomy prior to dextran sodium sulfate (DSS)-induced murine colitis results in higher colonic levels of TNF and other pro-inflammatory cytokines, increased disease activity indices and elevated macroscopic and histological scores as compared to sham-operated mice [48]. Vagotomy does not alter disease severity in M-CSF-deficient mice, which indicates a role for macrophages in mediating these vagus nerve effects [48]. The disease-exacerbating effect of subdiaphragmatic vagotomy in a DSS-induced model of colitis has been recently confirmed and associated with decreased acetylcholine levels in spleen in vagotomized animals [32]. Subdiaphragmatic vagotomy also increases serum pro-inflammatory cytokine levels and worsens the disease severity in a dog model of acute necrotizing pancreatitis, thus indicating a tonic suppressive role of the vagus nerve in the regulation of pancreatitis inflammation and severity [49]. Moreover, unilateral cervical vagotomy results in exacerbated pancreatic damage and increased systemic IL-6 levels in a mouse model of cerulean-induced pancreatitis [50]. Unilateral cervical vagotomy exacerbates the inflammatory response manifested by increased plasma and cardiac TNF and IL-1β levels in endotoxemic rats [51]. This approach results in a decreased survival rate in mice with E. coli pneumonia [52]. Unilateral cervical vagotomy has been instrumental in making the discovery by Charles Serhan and colleagues that the vagus nerve controls the resolution of inflammation [11]. Vagotomy results in lower lung and peritoneum gene and protein expression of the axon guidance molecule netrin-1, which is an important endogenous pro-resolution molecule in zymosan-induced peritonitis [11]. In addition to decreased neutrin-1 levels, vagotomy is associated with lower acetylcholine levels, higher TNF, IL-1β, IL-6, HMGB1, MIP-1α, and KC, and higher leukocyte numbers in inflammatory exudates. These and additional observations indicate that vagus nerve signaling regulates a bidirectional synergistic interaction between netrin-1 and other resolvins, including resolvin D1 to decrease leukocyte migration and stimulate macrophage phagocytosis in facilitating inflammation resolution. These findings importantly broaden the scope of implications of vagus nerve signaling in the regulation of inflammation.
4.3. The vagus nerve - splenic nerve partnership in the inflammatory reflex
The anti-inflammatory effects of VNS are linked to suppression of TNF in the spleen, the organ with a major contribution to systemic TNF during endotoxemia [29,30]. These effects require an interaction between the vagus nerve and the splenic nerve in the efferent arm of the inflammatory reflex (Fig. 1). Neuroanatomical findings have pointed to the celiac plexus as an important site of vagus nerve–splenic nerve communication. Efferent vagus nerve fibers through abdominal subdiaphragmatic anterior and posterior branches innervate the celiac ganglia and the superior mesenteric ganglion in the celiac plexus [53,54]. Recently, by utilizing double-labeling for tyrosine hydroxylase and vesicular acetylcholine transporter (VACh), Hoover and colleagues have demonstrated that cholinergic VAChT-positive nerve fibers surround abundant neuronal populations with catecholaminergic phenotype in the celiac ganglia and the superior mesenteric ganglion of mice [55]. These ganglia are a major source of spleen-projecting catecholaminergic fibers [56–58]. Accordingly, carefully performed transection of the multiple nerve fibers within the splenic nerve (splenic neurectomy) abolishes the anti-inflammatory effect of VNS [30]. Catecholaminergic nerve endings in spleen are in proximity of splenic lymphocytes, including T cells, which contain choline acetyltransferase (ChAT), the acetylcholine biosynthesizing enzyme [31]. VNS results in increased acetylcholine release in spleen and this effect is mediated through splenic nerve catecholaminergic signaling acting on lymphocytes. VNS does not suppress TNF levels in nude mice, indicating that lymphocytes are required [31]. Passive adoptive transfer of ChAT-T lymphocytes into nude mice restores the anti-inflammatory efficacy of VNS, which demonstrates that these acetylcholine-producing cells are necessary [31]. Consequently, acetylcholine acts on the α7 nAChRs on macrophages in the spleen and suppresses TNF production. Vagus nerve–splenic nerve signaling is implicated in the regulation of adaptive immune responses [59]. VNS in Streptococcus pneumonia suppresses the normal migration of B cells to the splenic red pulp, where they become antibody-secreting cells, by retaining the cells in the marginal zone, and decreases B cell antibody production. Splenic nerve transection significantly abolishes the migration arrest and results in reorganization of lymphoid architecture [59]. These and other studies in the context of endotoxemia, sepsis, inflammatory bowel disease [29,30,32] and other inflammatory conditions have triggered a great deal of interest in the vagus nerve-splenic nerve signaling in immune regulation. Of note, a study has questioned the vagus nerve-splenic nerve interaction [60]. Based on a single approach – injection of a retrograde tracer, the suprarenal ganglia were indicated as the main source of spleen-projecting neurons [60]. In addition, no labeled neurons in the right celiac ganglion and only a small percentage of these neurons in the left celiac ganglion were found [60]. No labeled neurons were reported in “superior mesenteric ganglia of either side” [60], a puzzling anatomical designation, considering the fact that the superior mesenteric ganglion is a single formation in a very close proximity to the left celiac ganglion and a part of the celiac-mesenteric ganglionic complex [53–55]. These observations are in clear contrast with other previous studies, utilizing a variety of approaches to identify the celiac ganglia and the superior mesenteric ganglion as the source of spleen-projecting catecholaminergic fibers [56–58]. The superior mesenteric-celiac ganglia have been demonstrated as the site of origin of catecholaminergic/noradrenergic innervations of the rat spleen by using selective ganglionectomy and retrograde tracing combined with immunocytochemical localization of tyrosine hydroxylase [57]. Simultaneously, the celiac-mesenteric gangla have been characterized as a major source of efferent innervations to the spleen in rats by using retrograde tracing, splenic nerve transection and catecholamine histofluorescence in the spleen [56]. The celiac ganglia as a supplier of splenic catecholaminergic innervations has been further demonstrated by using selective celiac ganglionectomy, tyrosine hydroxylase immunohystochemistry and norepinephrine determination [58]. Surprisingly, the authors of this study [60] do not comment on the apparent discrepancy between their observations and these previously published findings [56–58]. Instead, they focus on determining the effect of VNS on the electrical activity of suprarenal ganglia neurons to conclude that there is no effect. They also record whole splenic nerve activity and examine the effect of VNS on it and propose that activation of efferent vagus nerve signaling does not alter splenic nerve activity [60]. As previously demonstrated, splenic nerve catecholaminergic fibers innervate splenic capsule, trabeculae, vasculature, and parenchyma [57]. In addition to vasomotor fibers, whose activity is usually recorded there are other catecholaminergic fibers branching in the parenchyma and associated with T cell and other immune cell interactions [31,61–63]. The lack of adequate methodological description of splenic nerve isolation and recording in this study [60] makes it virtually impossible to assess what splenic neuron activity was recorded. A preponderance of data indicates the importance of efferent vagus nerve-splenic nerve functional integrity in controling spleen pro-inflammatory cytokine production [29–32].
4.4. The interface with, and within the immune component
α7nAChR is an important receptor mediator in the neural vagus nerve control of immune function. This role of the α7nAChR was initially indicated by the observation that in contrast to its suppressive effect in wild type (WT) mice, VNS does not significantly alter TNF levels in α7nAChR KO mice during endotoxemia [28]. In addition, treatment with the centrally-acting acetylcholinesterase inhibitor galantamine, whose brain-mediated mode of anti-inflammatory activity requires an intact vagus nerve as a brain-to periphery conduit, also fails to inhibit systemic TNF levels in endotoxemic α7nAChR KO mice [64]. The classical neuronal homeopentameric α7nAChR is expressed in the brain and the presence of α7 subunit containing forms of the receptor has been reported in peripheral autonomic ganglia and immune cells. Experimental evidence has attributed the cellular origin of the α7nAChR with a role in mediating cholinergic anti-inflammatory output to immune cells. For instance, the release of pro-inflammatory cytokines by macrophages from α7nAChR KO mice or macrophages with α7nAChR anti-sense downregulation is not affected by cholinergic stimulation [28]. Furthermore, α7nAChR deficiency in the immune system generated by adoptive transfer of α7nAChR KO bone marrow cells to irradiated WT mice, abolishes VNS inhibition of serum TNF levels during endotoxemia while α7nAChR neuronal deficiency by transferring WT bone marrow cells to irradiated alpha7 KO mice does not alter this VNS anti-inflammatory effect [65]. This mechanistic insight has allowed studying the anti-inflammatory efficacy of small molecule α7nAChR agonists at various inflammatory conditions. GTS-21 was the first selective α7nAChR agonist whose anti-inflammatory properties were demonstrated in murine models of pancreatitis [50], endotoxemia and severe sepsis [66]. Importantly, treatment with GTS-21 initiated within a clinically relevant time frame (24 hours after the onset of severe sepsis), significantly improves survival and lowers serum levels of HMGB1, a late mediator of the disease [66]. Another selective endogenous α7nAChR agonist is choline, a byproduct of acetylcholine degradation. Choline suppresses serum TNF and HMGB1, and improves survival in endotoxemia and experimental sepsis in a clinically-relevant time frame [67]. The anti-inflammatory effects of choline are mediated through α7nAChR-signaling, as demonstrated by using α7nAChR KO mice [67]. Several other studies have evaluated anti-inflammatory efficacy of α7nAChR agonists in endotoxemia, sepsis, rheumatoid arthritis, mechanical ventilation-induced lung injury, postoperative neuroinflammation, ischemia-reperfusion injury and other inflammatory conditions [68–71]. These studies have also contributed to delineating intracellular mechanisms downstream of the α7nAChR with a role in inhibiting TNF and other pro-inflammatory cytokine production. These mechanisms involve inhibition of the nuclear translocation of the transcription factor NF-κB [21,66,67,72] and activation of the JAK2-STAT-3 pathway [40]. In addition to triggering anti-inflammatory signal transduction through activation of the α7nAChR on the immune cell surface, a very recent report has revealed a role for cholinergic activation of the α7nAChR expressed on the mitochondrial membrane in immune regulation through inhibition of the inflammasome [73]. Immune cell activation is accompanied by intracellular accumulation of acetylcholine, which interacts with the mitochondrial α7nAChR resulting in stabilization of mitochondrial membrane permeability, preventing mitochondrial DNA release and consequently, inhibition of the NLRP3 inflammasome activation [73].
5. The remote immunomodulatory switches in the brain
The brain is an active participant in the regulation of immunity; it receives and integrates signals for altered immune homeostasis and mounts responses to control peripheral immune function through the inflammatory reflex and other pathways. We have begun to reveal the neuromodulatory and neuroreceptor mechanisms with a role in this regulation and evaluate therapeutic anti-inflammatory approaches based on brain cholinergic activation.
5.1. Relevant brain neuroanatomical organization
Neuronal interactions between the NTS, DMN and area postrema within the dorsal vagal complex integrate the vagus nerve-based inflammatory reflex at the level of the brainstem (Fig. 1). The dorsal vagal complex is interconnected with the hypothalamus, thalamus, hippocampus, amygdala, medial prefrontal and other cortex regions. These reciprocal circuits provide neuroanatomical and functional substrates for modulation of vagus nerve activity and the inflammatory reflex, and intersection with brain networks associated with behavioral, endocrine and metabolic regulation [6,74]. Experimental evidence indicates a role for cholinergic signaling in the brain modulation of the inflammatory reflex. The brain cholinergic system is a major neuromodulatory system with widespread innervations, originating from two major locations, the basal forebrain and the mesopontine tegmentum [75,76]. Basal forebrain cholinergic pathways predominantly innervate the cortex (neocortex), hippocampus, olfactory bulb and amygdale [75,76]. Cholinergic innervations from the mesopontine pedunculopontine tegmental and laterodorsal tegmental nuclei innervate, among other brain regions, the thalamus, hypothalamus and cerebellum [75,76]. Acetylcholine, released from cholinergic axons interacts with two types of receptors on cholinoceptive neurons; muscarinic M1 through M5 subtypes of metabotropic, G protein-coupled receptors and nicotinic ionotropic receptors. Muscarinic acetylcholine receptors (mAChRs) and specifically the M1 subtype play a major role in processing cholinergic transmission in the brain [77]. The cortex, hippocampus, hypothalamus and other brain areas innervated by cholinergic pathways interact directly or through multisynaptic pathways with the dorsal motor complex, the brainstem integrative center of the inflammatory reflex [6,74,78–80].
5.2. Brain muscarinic receptor–mediated networks regulate peripheral inflammatory responses
Brain mAChR subtypes have different synaptic locations and function in cholinergic transmission. The M1 mAChR is predominantly postsynaptic on cholinoceptive neurons in the cortex, hippocampus and other brain areas. The M2 mAChR is mostly presynaptic and its role in the cholinergic synapse is inhibition (autoinhibition) of acetylcholine release. Central, intracerebroventricular administration of an M1 mAChR agonist or a M2 mAChR antagonist significantly suppresses serum TNF levels in endotoxemic animals [81]. Central mAChR ligand administration also significantly increases efferent vagus nerve activity indicated by activation of the high frequency component of heart rate variability based on ECG analysis [81]. These observations have provided experimental evidence for a role of brain mAChR- mediated cholinergic signaling in the regulation of peripheral inflammation through functional integration with efferent vagus nerve activity (Fig. 1). They also suggest that brain mAChR signaling may mediate the previously demonstrated central activation of the cholinergic anti-inflammatory pathway by the anti-inflammatory compound CNI-1493 [82], identified as a mAChR ligand. Central administration of the M1 mAChR McN-A-343 and the M2 mAChR antagonist methoctramine have recently been shown to inhibit colonic TNF, IL-6 and other pro-inflammatory cytokines and alleviate disease severity in two models of colitis [32,83]. Increased acetylcholine levels in spleen of mice treated with McN-A-343 mediate anti-inflammatory drug efficacy in colitis and these effects require signaling along the vagus nerve and splenic nerve, because they are abolished in mice with vagotomy and splenic nerve transection [32]. An anti-inflammatory role of brain mAChRs has been demonstrated in experimental models of hemorrhagic shock [84,85] and in mediating the anti-inflammatory efficacy of acupuncture [86]. In addition, peripheral administration of the centrally-acting M1 mAChR agonist xanomeline significantly suppresses serum TNF and improves survival in murine endotoxemia [87]. Anti-inflammatory effects of xanomeline are mediated through brain mAChRs and require an intact vagus nerve-splenic nerve axis [87]. Interestingly, xanomeline provides a long-lasting anti-inflammatory protection, indicated by the observation that drug administration as early as 20 hours prior to LPS is efficient in suppressing serum TNF levels and improving survival in endotoxemic mice [87]. This long-lasting drug efficacy is associated with alterations in splenic leukocyte subpopulations including a proportional increase in T cell numbers in parallel with reduced splenocyte responsiveness to inflammatory stimulation. In addition, current studies indicate that peripheral administration of a centrally-acting compound, which belongs to the latest generation of positive allosteric modulators of the M1 mAChRs suppresses pro-inflammatory cytokine levels in endotoxemia and improves survival in endotoxemia and severe sepsis (unpublished data). Preclinical development of xanomeline and this latest generation of compounds has been mainly driven by the search for mAChR ligands that are more efficient and devoid of side effects in the context of Alzheimer’s disease and schizophrenia [88–92]. Xanomeline treatment of patients with schizophrenia significantly alleviates positive and negative symptoms and cognitive deterioration [90]. Peripheral inflammation reported in the context of schizophrenia has been proposed to have a causative role in disease pathology [93,94]. In light of ameliorating peripheral inflammation by xanomeline [87], one may now ask the intriguing question of whether anti-inflammatory effects of xanomeline contribute to its efficacy in clinical settings of schizophrenia. In contrast to centrally-acting cholinergic modalities, anticholinergic drugs, which are frequently prescribed to elderly have been associated with exacerbated inflammatory responses to endotoxemia in a neurodegenerative tauopathy murine model [95,96]. Importantly, an increase in IL-1β expression in spleen and brain is significantly more pronounced in endotoxemic mice pretreated with trihexyphenidyl - a centrally-acting M1 mAChR antagonist as compared with another compound with a weaker CNS activity [95]. These findings suggest that inhibition of brain cholinergic M1 mAChR signaling exacerbates inflammation. It is interesting and potentially important that while modulation of brain mAChRs and specifically activation of the M1 subtype have been linked to anti-inflammatory effects, cholinergic activation through peripheral mAChR-mediated mechanisms has been associated with pro-inflammatory effects as demonstrated in patients with irritable bowel syndrome [97]. In addition, Kawashima and colleagues have shown that genetic ablation of both M1 and M5 mAChRs results in lower levels of TNF and IL-6 released by splenic mononuclear leucocytes incubated with ovalbumin, suggesting a tonic pro-inflammatory role of these receptors [98].
5.3. Borrowing from Alzheimer’s disease drugs to study inflammation: acetylcholinesterase inhibitors as anti-inflammatory agents
Diminished acetylcholine release in the brain due to neurodegeneration involving basal forebrain cholinergic neurons is an important pathophysiological feature in Alzheimer’s disease. Accordingly, the current treatment of cognitive deterioration associated with Alzheimer’s disease is largely based on the use of centrally-acting acetylcholinesterase inhibitors, which inhibit acetylcholine breakdown, thus potentiating brain cholinergic signaling. These drugs also have powerfull anti-inflammatory properties. Central cholinergic activation by peripheral administration of the centrally-acting AChE inhibitors galantamine and huperzine-A results in significant inhibition of serum TNF and improved survival in endotoxemic mice [64]. The anti-inflammatory effect of galantamine is mediated through brain mAChRs, depends on the integrity of the vagus nerve and is abolished in α7nAChR KO mice [64]. These findings and the reported stimulation of efferent vagus nerve activity by galantamine [99], indicated that CNS mAChR-mediated effects of galantamine are functionally linked to activation of the vagus nerve-based cholinergic anti-inflammatory pathway (Fig. 1). Interestingly, single dose galantamine administration provides at least a 20 hours time window of anti-inflammatory protection, in line with a similar long-lasting effect of the M1 mAChR agonist xanomeline in endotoxemic mice [87]. Galantamine treatment of mice with colitis significantly alleviates colonic inflammation and disease severity, associated with increased acetylcholine release in spleen, interacting with the α7nAChRs on dendritic cells [32]. Pharmacological blockade of brain mAChR receptors, vagotomy and splenic neurectomy abolish these effects and the increased release of acetylcholine in spleen. In addition, vagotomy and splenic neurectomy significantly decreases acetylcholine release in spleen. Together these findings indicate that vagus nerve - splenic nerve interaction mediates galantamine effects in inflammatory bowel disease [32] (Fig. 1). In addition to endotoxemia and inflammatory bowel disease, galantamine exerts anti-inflammatory effects and alleviates the disease progression in a rat model of adjuvant-induced arthritis [100]. Involvement of brain AChE inhibition and peripheral site AChE inhibition has been described in lowering intestinal inflammation by rivastigmine, another centrally-acting AChE inhibitor [101]. Several studies further indicate the primary importance of CNS AChE inhibition in achieving suppression of inflammation at various inflammatory conditions. For instance, treatment with the centrally-acting AChE inhibitor physostigmine results in higher level of suppression of the acute colonic inflammatory response in rats with colitis as compared to treatment with the peripherally-acting neostigmine [102]. In addition, treatment with the peripherally-acting AChE inhibitor neostigmine fails to alleviate organ injury in murine endotoxemia [103] and pro-inflammatory cytokine responses associated with mechanical ventilation [104]. Very recently, treatment with the centrally-acting AChE inhibitor donepezil has been shown to increase vagal tone, suppress plasma TNF, IL-6 and IFN-γ levels, attenuate development of hypertension and prevent cardiac remodeling in spontaneously hypertensive rats [105]. In contrast, treatment with the peripherally-acting AChE inhibitor pyridostigmine fails to suppress plasma pro-inflammatory cytokine levels and reduce high blood pressure [105].
Interestingly, in addition to neuroinflammation, increased levels of peripheral inflammation have been reported in patients with Alzheimer’s disease, a neurodegenerative disorder characteristically affecting basal forebrain cholinergic neurons with a resultant diminished acetylcholine release. There are also reports that treatments with AChE inhibitors of patients with Alzheimer’s disease result in suppression of peripheral inflammatory responses. For instance, treatment of patients with Alzheimer’s disease with the AChE inhibitor donepezil, as compared to untreated patients and healthy controls results in lower gene expression and protein levels of IL-1β and higher expression and levels of the anti-inflammatory IL-4 in both unstimulated and phytohemagglutinin-stimulated peripheral blood mononuclear cells [106]. Furthermore, peripheral blood mononuclear cells isolated from Alzheimer’s disease patients release higher levels of IL-1β and IL-6 as compared to healthy controls, and treatment of these patients with the AChE inhibitor donepezil for one month significantly decreases these levels as compared to prior to treatment conditions [107]. An inverse correlation between duration of treatment with galantamine, rivastigmine or donepezil of patients with Alzheimer’s disease and plasma CRP levels also was reported [108]. These findings highlight a correlation between brain cholinergic hypofunction and elevated markers of peripheral inflammatory responses in humans. In addition, they suggest that a boost in brain cholinergic signaling that compensate for this hypofunction may alleviate peripheral inflammation.
5.4. The “suffering” brain and its cholinergic symptoms in inflammatory and autoimmune conditions
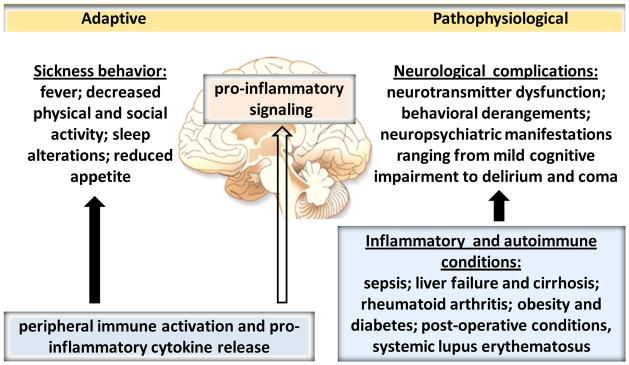
Immune cell activation and inflammation in response to infection or injury can cause a spectrum of alterations in brain function known as sickness behavior, which is regarded as an important adaptive mechanism [23–25] (Fig. 2). These brain alterations occur in parallel with activation of brain-derived neural immunoregulatory pathways. Peripheral administration of LPS or live bacteria has been instrumental in demonstrating that peripheral inflammation causes brain pro-inflammatory signaling [109], which has a mediating role in sickness behavior [23,25] (Fig. 2). Increased systemic cytokine levels in endotoxemia are associated with activation in pro-inflammatory IL-1β and IL-6 gene expression and microglial morphological alterations, indicative for microglia activation as early as 4 hours following peripheral endotoxin administration [110]. These pro-inflammatory alterations affect the hippocampus, cortex, hypothalamus and other brain areas in a region-specific manner with no apparent evidence of neurodegeneration as an underlying event [110]. Excessive and non-resolved forms of peripheral inflammation and maladaptive transformations of sickness behavior can result in deleterious and life-threatening neurological complications described in several conditions with inflammatory and autoimmune etiology, including sepsis, chronic liver disease, inflammatory bowel disease, rheumatoid arthritis, postoperative conditions and obesity [23,69,111–119] (Fig. 2). Many patients with these disorders suffer depression; it is documented that depressive conditions exacerbate pro-inflammatory cytokine levels and inflammation, which can affect brain neurotransmitter systems and brain function [120]. Studying brain cholinergic signaling in the context of neurological complications associated with inflammatory disorders is important for several reasons. Brain cholinergic mAChR–mediated signaling regulate long-term potentiation and neuroplasticity, adult neurogenesis, and cerebral blood flow [76,121]. Brain cholinergic pathways are critically implicated in learning, memory, attention, conscious awareness, sleep, control of movements and peripheral/visceral function [76,77,122]. Many of these functions are impaired in inflammatory conditions (Fig. 2). Brain cholinergic mAChR-mediated signaling also has an important role in the regulation of peripheral cytokine responses and inflammation [6,32,64]. In addition, experimental evidence indicates that brain cholinergic signaling regulates local brain inflammatory responses [123–126] and the brain cholinergic system is affected in peripheral inflammatory and metabolic conditions [110,127–129]. Sepsis is a lethal disorder with peripheral immune and metabolic dysregulation and neuropsychiatric manifestations. This clinical syndrome is the most frequent cause of death in the intensive care units [130]. The spectrum of pathophysiological brain alterations during sepsis is termed sepsis-associated encephalopathy (SAE), or septic encephalopathy, which ranges from delirium to coma [114]. SAE can be defined as brain dysfunction, secondary to infection in the body, and no CNS infection [114]. Delirium is a neurobehavioral syndrome caused by dysregulation of neuronal activity secondary to broad spectrum of systemic disturbances, including increased release of pro-inflammatory cytokines and systemic inflammation [114,127,131]. Important hallmarks in SAE and delirium are altered neurotransmission and cerebral microcirculation [114]. There is experimental evidence for a major role of cholinergic hypofunction in delirium [132]. Considering the importance of brain cholinergic signaling in cerebral blood flow regulation, attention, memory, sleep and the control of voluntary movements, decreased cholinergic tone can have a profound contribution to SAE. This cholinergic hypofunction occurs in parallel, and is interrelated to alterations in other neurotransmitter systems, including dopaminergic and catecholaminergic hyperactivity, and fluctuations in GABA-ergic and glutamatergic systems [132]. SAE is an early event in sepsis, can be developed in upto 80% of the patients and is an independent predictor of mortality and long term morbidity. The saga of the patients with sepsis does not end with their discharge from the hospital. Long-term cognitive impairment, functional disability and increased mortality have been documented in sepsis survivors. Insight into SAE and its long reaching post-sepsis consequences holds promises for an early diagnosis and adequate treatments, which are urgently needed [114]. The pro-inflammatory cytokine HMGB1 was recently identified as an important mediator of pathogenesis and cognitive impairment in murine sepsis survivors and as a potential therapeutic target [133,134]. Another option to be explored in alleviating septic sequelae is the use of galantamine and other AChE inhibitors. These drugs are already in clinical use in the treatment of cognitive impairment in patients with Alzheimer’s disease and myopathies such as myasthenia gravis, and they have powerful anti-inflammatory properties. Another complex and life-threatening neuropsychiatric syndrome is hepatic encephalopathy, which occurs in the context of acute and chronic liver failure in cirrhosis associated with metabolic and immune dysregulation [116,117]. Cognitive impairment, deteriorations in sleep, motor activity and coordination, lethargy, confusion, and ultimate progression into coma define the spectrum of hepatic encephalopathy [117]. The pathogenesis of hepatic encephalopathy is not well understood. Increased ammonia levels are thought to play an important role in astrocyte enlargement and dysfunction in the brain in parallel with microglia activation, oxidative stress and increased pro-inflammatory cytokine levels [116,117]. Brain cholinergic impairment has also been reported in patients with cirrhosis and in models of liver failure [135]. Significant increases in postmortem cortex AChE activity in patients with hepatic encephalopathy and cirrhotic rats, and a marked decrease in acetylcholine levels in these animals are found [135]. In addition, treatment with the centrally-acting AChE inhibitor rivastigmine alleviates memory deficits in a model of liver failure [135]. As summarized by Inouye and colleagues, brain cholinergic pathways overlap with locations of brain abnormalities and abnormal perfusion detected by neuroimaging in patients with hepatic encephalopathy, cardiotomy, and traumatic brain injury [132]. Intriguingly, brain cholinergic impairment coexists with lower vagal tone in patients with cirrhosis [136,137] and the decrease in vagus nerve activity in patients with cirrhosis correlates with the degree of hepatic encephalopathy [137]. These findings provide a rationale for further studies exploring the relationship between altered brain cholinergic signaling, decreased vagus nerve activity and increased inflammatory indices in SAE, hepatic encephalopathy and other inflammatory conditions with a deferent degree of brain impairment for evaluating therapeutic interventions. Promising results from a very recent clinical trial with rivastigmine in cirrhotic patients with hepatic encephalopathy represent one of the first validations of this line of therapeutic explorations [138]. Brain pro-inflammatory signaling, cognitive impairment and behavioral deficiencies have been described in obesity and type 2 diabetes, diseases with metabolic and inflammatory etiology [118,119,139–141] (Fig. 2). An autoimmune disease such as type 1 diabetes is associated with alterations in brain cholinergic signaling and impaired cognitive functions reported in animal models [142,143] and worsened cognitive domains in patients [141]. Another notorious example of an autoimmune disease with characteristic neurological complications is systemic lupus erythematosus (Fig. 2). Betty Diamond and colleagues have provided important insight into the neurological complications of this autoimmune condition pointing to a mediating role of peripheral antibodies targeting the brain glutamatergic system [144–147]. These findings have provided a rationale for developing new therapeutic modalities.
Fig. 2. Peripheral inflammation, autoimmunity and brain function: from sickness behavior to neurological complications.
The impact of peripheral immune activation with the release of cytokines and other inflammatory molecules on the brain is manifested by a spectrum of alterations, known as sickness behavior. This is an important adaptive mechanism of brain function in acute inflammatory conditions. Peripherally released pro-inflammatory cytokines signal the brain through afferent vagus neurons, circumventricular organs, and other mechanisms [8,25,26] (not shown). Peripheral inflammation with the release of cytokines also triggers brain pro-inflammatory signaling, which plays an important mediating role in sickness behavior. Brain function alterations and neurological complications are described in numerous inflammatory and autoimmune disorders. Dysregulations in neurotransmitter systems, cerebral blood flow and microcirculation, brain metabolic activity, and brain pro-inflammatory signaling underlie these neurological complications, which can be manifested by cognitive deterioration, behavioral derangements and characteristic encephalopaties. These neurological conditions can be debilitating and life-threatening and need to be properly diagnosed and treated.
5.5. Device-generated modulation of brain neurocircuitry as a future anti-inflammatory approach
The brain cholinergic system provides widespread innervations to the cortex, other forebrain areas, brainstem and cerebellum and controls a variety of brain functions [75,76]. In addition, brain cholinergic mAChR mediated signaling plays a role in controlling cardiovascular and gastrointestinal function, pancreatic secretion and other peripheral physiological processes via vagus nerve mediated mechanisms [85,148–151]. In addition to pharmacological modalities cholinergic pathways in the brain can be modulated by using electrical or other forms of device-generated output. Device-generated brain modulation, including deep brain electrical stimulation, transcranial magnetic stimulation, and transcranial direct current stimulation has been in clinical or exploratory use for various neurological conditions [152,153]. There is a growing interest in these approaches judging by the increasing number of relevant publications as recently summarized [152]. In parallel, optogenetic modulation has been actively utilized as a highly selective methodology to provide specific mechanistic insight into the physiological role of brain neuronal circuits. Electrical and other forms of device-generated output have been currently used in studying the role of specific cholinergic pathways in the regulation of inflammation. Identifying brain networks with a role in peripheral neuromodulation may indicate future implications of deep brain stimulation or other non-invasive approaches in the treatment of inflammatory and autoimmune conditions.
6. Neurocircuitry and inflammation in obesity and cancer
The prevalence of obesity and the associated metabolic syndrome has reached epidemic proportions. The serious health threat, which obesity and the metabolic syndrome represent, is associated with the increased risk of developing type 2 diabetes, cardiovascular disease, cancer and other debilitating and life-threatening disorders [154]. The metabolic dysregulation in obesity is importantly interrelated with chronic low-grade inflammation as major underlying events in insulin resistance, fatty liver disease and other obesity-associated complications [6,155–157]. The expanded abdominal adipose tissue, infiltrated with macrophages, neutrophils, T cells and other immune cells is considered to be the main source of inflammation in obesity [155,156]. In addition to immune cells, enlarged adipocytes, which are typically a fat storage, become active cytokine- and adipokine-producing cells in this microenvironment [156,158]. Dysregulated release of adipokines, including leptin, resistin, adiponectin, and TNF, IL-1β, IL-6 and other cytokines mediate obesity-associated inflammation [6,155,156,158]. The vagus nerve within reflex mechanisms plays an important role in metabolic homeostasis. In addition to mediating satiety, afferent vagus nerve fibers, through associated mechanoreceptors, chemoreceptors, and specific metabolite receptors transmit alterations in peripheral levels of metabolic molecules, including cholecystokinin, peptide YY, and glucagon-like peptide-1 to the brainstem NTS and then these signals reach the hypothalamus and other brain areas [6]. Efferent vagus nerve cholinergic output regulates pancreatic insulin and glucagon secretion and hepatic gluconeogenesis, the main determinant of fasting blood glucose levels [6]. There is also experimental evidence for compromised vagus nerve regulatory function in obesity [6,159,160]. In addition, lower vagus nerve activity, determined by heart rate variability analysis is documented in autonomic dysfunction reported in obesity, the metabolic syndrome and type 2 diabetes [6,161,162]. VNS, a clinically approved approach for treating depression and epilepsy has been associated with weight loss, which is more substantial in patients with higher degree of obesity [163]. Inflammation in obesity represents an important therapeutic target in alleviating insulin resistance and other obesity-related complications. Exploring vagus nerve-based cholinergic neurocircuitry in metabolic and inflammatory regulation in obesity is an emerging approach that has been experimentally tested by using cholinergic modalities, including the acetylcholinesterase inhibitor galantamine [164] and α7nAChR agonists [165,166]. Galantamine treatment of mice with high fat-induced obesity and the metabolic syndrome prevents further body weight gain and significantly decreases glucose and insulin levels and insulin resistance [164]. In addition, galantamine lowers leptin, resistin and IL-6 levels and attenuates fatty liver disease in these mice [164]. A very recent study significantly substantiated these findings by demonstrating the therapeutic anti-inflammatory and disease-alleviating efficacy of galantamine in an animal model of type 2 diabetes [167]. These beneficial metabolic and anti-inflammatory effects of galantamine, other cholinergic modalities and VNS in experimental settings of obesity and type 2 diabetes provide a platform for further exploration of these approaches in the treatment of obesity-related disorders [6].
There is a growing interest in inflammation in the context of cancer and especially in solid tumors/cancers. Chronic inflammation holds an increased risk of developing cancer. On the other hand, oncogene alterations and tumor progression are associated with forming tumor-associated microenvironment in which immune cells such as tumor associated macrophages and T cells, and cytokines and other inflammatory products are active modulators of tumor-associated angiogenesis, progression and metastases [168]. Therefore, cancer related inflammation has been actively studied in the pursuit of identifying new molecular targets that can be explored to improve diagnosis and treatment. Implications of neurocircuitry and axon guidance molecules, such as netrin-1 in cancer have been indicated [169,170]. A role for the vagus nerve in cancer has also been reported. Unilateral cervical vagotomy has been shown to promote lung, liver, kidney and adrenal metastases in experimental 4THMpc breast carcinoma, suggesting a tonic protective effect of the vagus nerve against metastatic disease in this context [171,172]. In line with these observations, higher baseline high frequency of heart rate variability, which is indicative for higher vagal tone is associated with significantly longer survival of patients with metastatic and recurrent breast cancer [173]. Bilateral subdiaphragmatic vagotomy promotes the development of experimental carcinogen-induced pancreatic [174] and gastric cancer [175,176]. In contrast to these observations, vagotomy has been found to suppress the development of gastric cancer through a mechanism involving signaling through M3 mAChRs [177]. Subdiaphragmatic vagotomy has been clinically used in the treatment of ulcer. Vagotomy in these settings has been associated with increased rate of cancer development and mortality of gastric, colorectal, biliary tract, and lung cancer [178–181]. Lower vagal tone (based on heart rate variability analysis) is associated with higher risk of 7-day mortality in patients with advanced pancreatic and other non-lung cancers [182] and in patients with colorectal, pancreatic, prostate, lung and ovarian cancer as compared to healthy individuals [183]. In addition to a role in immune and metabolic control, reflex neuronal regulation, involving the vagus nerve, of the inflammatory tumor microenvironment has been proposed [184–186]. Further studying this regulation and the role of the vagus nerve and other peripheral nerves in the complex cancer-inflammation relationship hold significant promises for new therapeutic approaches.
7. Translational implications
Ongoing in vitro and in vivo preclinical research has identified neuronal circuits with a role in the regulation of immunity and inflammation, characterized cellular and molecular mechanisms mediating these pathways and examined the therapeutic efficacy of their modulation. Studies translating this insight into clinical settings were also initiated. These studies may validate the therapeutic efficacy of new anti-inflammatory compounds, device-generated neuromodulation or combinations of these two strategies [187].
7.1. Pharmacological interventions
A role for activation of the cholinergic anti-inflammatory pathway by utilizing α7nAChR agonists or centrally-acting acetylcholinesterase inhibitors in ameliorating inflammation and alleviating disease severity has been shown in various disease models, including septic shock, severe sepsis, hemorrhagic shock, rheumatoid arthritis, inflammatory bowel disease, obesity and type 2 diabetes [6,70,188]. These preclinical insights have paved the way to clinical exploration of these modalities. The anti-inflammatory effects of the α7nAChR agonist GTS-21 in doses, previously reported to be safe in humans [189] have been studied in human endotoxemia [190]. GTS-21 anti-inflammatory effects have overall been found not statistically significant as compared to the placebo-controlled group [190]. However, a correlation between higher GTS-21 plasma levels and lower plasma levels of TNF, IL-6 and IL-1 receptor agonist has been found [190]. It is an open question whether a higher dose of GTS-21 will generate pronounced anti-inflammatory effects. The efficacy of the α7nAChR agonist choline has been studied in patients with asthma. Choline treatment significantly lowers TNF and other inflammatory markers, suppresses oxidative stress and alleviates disease scores [191]. An important new area for clinical studies on the efficacy of α7nAChR agonists and galantamine is the spectrum of obesity-driven disorders [6]. An ongoing clinical study evaluates the effects of the selective α7nAChR agonist TC-6987 in type 2 diabetes https://clinicaltrials.gov/ct2/show/NCT01293669?term=Targacept&rank=11). Performing clinical trials with galantamine and other centrally-acting acetylcholinesterase inhibitors can be facilitated by the abundant clinical database available for these drugs and their FDA approval for the symptomatic treatment of Alzheimer’s disease. The efficacy of galantamine in doses, which are clinically approved for Alzheimer’s disease, is currently being examined in individuals with the metabolic syndrome (https://clinicaltrials.gov/ct2/show/NCT02283242?term=cholinergic+obesity&rank=2). Using galantamine and other centrally-acting acetylcholinesterase inhibitors with proven efficacy in improving memory and cognition might address another important aspect in obesity-driven disorders. These diseases and specifically type 2 diabetes are associated with certain degree of cognitive dysfunction [140]. Accordingly, the need for prevention or alleviation of this cognitive deterioration in strategizing new therapeutic approaches for the treatment of type 2 diabetes has been emphasized [140].
7.2. Bioelectronic medicine
The discovery of neuronal circuits in the regulation of immunity and inflammation at the Feinstein Institute and other research centers has importantly contributed to the foundation of Bioelectronic Medicine. This emerging field focuses on studying neuronal networks that target molecular mechanisms of disease, and using neuromodulation by bioelectronic devices as treatment. The need for new therapeutic approaches under the umbrella of Bioelectronic Medicine is dictated by the lack of efficient pharmaceutical options in the treatment of some diseases, significant side effects of currently used drugs, and the astronomical cost of some pharmacological remedies. Biolelectronic medicine brings together Molecular Medicine, Neuroscience, and Bioengineering. Basic and clinical scientists with various background, skills, and different viewpoints are engaged in major team efforts to address challenging questions in translating preclinical findings in bioelectronic approaches to treat diseases. The use of electricity, i.e. VNS has been pivotal in evaluating the therapeutic potential of neuromodulation in preclinical settings of various inflammatory and autoimmune disorders, including rheumatoid arthritis. The therapeutic use of electricity has a long history. It dates back to Scribonius Largus, the court physician of the Roman emperor Claudius. It is intriguing that by using a natural “device”, a live torpedo fish as a source of electricity, he was able to treat gout, a form of inflammatory arthritis. If we fast-forward to the present, we get to the modern era of utilizing electricity in the treatment of rheumatoid arthritis, but this time by electrical VNS through an implanted device in a successful clinical trial [7]. This and other ongoing studies with implanted device VNS in patients with inflammatory bowel disease (https://clinicaltrials.gov/ct2/show/NCT02311660?term=vagus+nerve+stimulation&rank=8, https://clinicaltrials.gov/ct2/show/NCT01569503?term=vagus+nerve+stimulation&rank=7) validate targeted neuromodulation in disease treatment. Continuous research efforts focusing on the neuroanatomical and functional evaluation of neural networks to target specific molecular mechanisms of disease are likely to culminate in new clinical trials. Preclinical research has multiple aspects, including neuroanatomical mapping of peripheral organ innervations, determining patterns of peripheral autonomic and somatic nerve activity associated with certain disease conditions, characterizing the specificity of interaction between nerves and muscle cells, glandular cells, immune cells and other types of cells, and elucidating intracellular and molecular mechanisms of these interactions. These research efforts go hand in hand with developing new technology, including miniaturized devices and electrodes and optogenetic methodology suitable for better interrogation of discrete neuronal circuits, imaging systems, biocompatible materials, and programs for processing and analyzing large sets of data. The therapeutic scope of bioelectronic neuromodulation is not limited to the treatment of inflammatory and autoimmune diseases. Continuous progress in evaluating the role of neuronal circuitry in obesity-driven diseases, cancer and other debilitating and life-threatening disorders and identifying related molecular targets holds a significant promise to provide a rationale necessary for designing new therapeutic strategies based on neurostimulation or neuroinhibition to treat these diseases.
8. Conclusions and future directions
We have summarized here findings highlighting interactions between the nervous system and the immune system. Vagus nerve-mediated and other neural circuits operating on reflex principles regulate multiple aspects of immunity and inflammation. On the other hand, dysregulated immune activation is associated with altered vagus and other peripheral nerve activity and may cause brain neuronal dysfunction. Preclinical research has identified several points of the reciprocal nervous system – immune system relationship and its intersections with metabolism and behavior that can be explored in new therapeutic strategies. Studying the role of cholinergic signaling in neuro-immune interactions in various conditions characterized by adverse inflammation has validated the anti-inflammatory and disease-alleviating properties of α7nAChR agonists, centrally-acting acetylcholinesterase inhibitors and M1 mAChR agonists. Clinical testing of some of these compounds has also been initiated. In parallel, evaluating the anti-inflammatory efficacy of VNS and advanced understanding of neural circuitry controlling inflammation has allowed implementation of conceptually novel platforms of disease treatment based on neuromodulation within the scope of Bioelectronic Medicine. There is more to learn going forward. Continuous research will further elucidate molecular and cellular mechanisms underlying neuro-immune interactions and delineate discrete neuronal circuitry that can be explored for therapeutic benefit. The current clinical testing of VNS in inflammatory and autoimmune disorders promises to be the beginning of the use of bioelectronic technology in safe, inexpensive and efficient treatments of a broad spectrum of diseases.
Acknowledgments
This work was supported by the following grants from the National Institute of General Medical Sciences, National Institutes of Health: R01GM089807 (to VA Pavlov and KJ Tracey) and R01GM057226 (to KJ Tracey). The authors would like to thank Mauricio Rosas-Ballina for critically reading the manuscript.
Reference List
- 1.Chiu IM, Heesters BA, Ghasemlou N, von Hehn CA, Zhao F, Tran J, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 3.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057–1068. doi: 10.1084/jem.20120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koopman F, Miltjko S, Grazio S, Socolovic S, Tracey K, Levine Y, et al. First-in-Human Study of Vagus Nerve Stimulation for Rheumatoid Arthritis: Clinical and Biomarker Results through Day 84. Annals of the Rheumatic Diseases. 2013;172:245. [Google Scholar]
- 8.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015 doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211:1037–1048. doi: 10.1084/jem.20132103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 13.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 15.Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial Transmission. Physiol Rev. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 17.Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 2011;332:729–732. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallowitsch-Puerta M, Pavlov VA. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;80:2325–2329. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley KW, McCusker RH. Getting nervous about immunity. Semin Immunol. 2014 doi: 10.1016/j.smim.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 27.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 29.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arima Y, Harada M, Kamimura D, Park JH, Kawano F, Yull FE, et al. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Tracey KJ. Immune cells exploit a neural circuit to enter the CNS. Cell. 2012;148:392–394. doi: 10.1016/j.cell.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 36.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tracey KJ. Lymphocyte called home: beta2-adreneric neurotransmission confines T cells to lymph nodes to suppress inflammation. J Exp Med. 2014;211:2483–2484. doi: 10.1084/jem.21113insight3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarini S, Altavilla D, Cainazzo MM, Giuliani D, Bigiani A, Marini H, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 40.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 41.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olofsson PS, Levine YA, Caravaca A, Chavan SS, Pavlov VA, Faltys M, et al. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces tumor necrosis factor in endotoxemia. Bioelectronic Medicine. 2015;2:37–42. [Google Scholar]
- 43.Czura CJ, Schultz A, Kaipel M, Khadem A, Huston JM, Pavlov VA, et al. Vagus nerve stimulation regulates hemostasis in swine. Shock. 2010;33:608–613. doi: 10.1097/SHK.0b013e3181cc0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 45.Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208–221. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- 46.Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014;9:e104530. doi: 10.1371/journal.pone.0104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meregnani J, Clarencon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 49.Sun JJ, Chu ZJ, Zhang YM, Qi SF, Chang YC, Xin SY, et al. Beneficial effect of splanchnic nerve transection and harmful effect of vagotomy on acute necrotizing pancreatitis in the dog. Dig Dis Sci. 2015;60:118–126. doi: 10.1007/s10620-014-3315-z. [DOI] [PubMed] [Google Scholar]
- 50.van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Schulte A, Lichtenstern C, Henrich M, Weigand MA, Uhle F. Loss of vagal tone aggravates systemic inflammation and cardiac impairment in endotoxemic rats. J Surg Res. 2014;188:480–488. doi: 10.1016/j.jss.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J Immunol. 2010;184:401–410. doi: 10.4049/jimmunol.0901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 54.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 55.Downs AM, Bond CE, Hoover DB. Localization of alpha7 nicotinic acetylcholine receptor mRNA and protein within the cholinergic anti-inflammatory pathway. Neuroscience. 2014;266:178–185. doi: 10.1016/j.neuroscience.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–290. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 57.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 58.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci. 2010;154:66–73. doi: 10.1016/j.autneu.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Mina-Osorio P, Rosas-Ballina M, Valdes-Ferrer SI, Al Abed Y, Tracey KJ, Diamond B. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol Med. 2012;18:618–627. doi: 10.2119/molmed.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol. 2012;97:1180–1185. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 61.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–21. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 62.Ackerman KD, Felten SY, Bellinger DL, Felten DL. Noradrenergic sympathetic innervation of the spleen: III. Development of innervation in the rat spleen. J Neurosci Res. 1987;18:49–5. doi: 10.1002/jnr.490180109. [DOI] [PubMed] [Google Scholar]
- 63.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 64.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olofsson PS, Katz DA, Rosas-Ballina M, Levine YA, Ochani M, Valdes-Ferrer SI, et al. alpha7 nicotinic acetylcholine receptor (alpha7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol Med. 2012;18:539–543. doi: 10.2119/molmed.2011.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 67.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norman GJ, Morris JS, Karelina K, Weil ZM, Zhang N, Al Abed Y, et al. Cardiopulmonary arrest and resuscitation disrupts cholinergic anti-inflammatory processes: a role for cholinergic alpha7 nicotinic receptors. J Neurosci. 2011;31:3446–3452. doi: 10.1523/JNEUROSCI.4558-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pavlov VA. Cholinergic modulation of inflammation. Int J Clin Exp Med. 2008;1:203–212. [PMC free article] [PubMed] [Google Scholar]
- 71.Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J, et al. alpha7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 2014;20:350–358. doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- 76.Woolf NJ, Butcher LL. Cholinergic systems mediate action from movement to higher consciousness. Behav Brain Res. 2011;221:488–498. doi: 10.1016/j.bbr.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 77.Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 78.Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruit KG, Neafsey EJ. Cardiovascular and respiratory responses to electrical and chemical stimulation of the hippocampus in anesthetized and awake rats. Brain Res. 1988;457:310–321. doi: 10.1016/0006-8993(88)90701-9. [DOI] [PubMed] [Google Scholar]
- 80.AKERT K, GERNANDT BE. Neurophysiological study of vestibular and limbic influences upon vagal outflow. Electroencephalogr Clin Neurophysiol. 1962;14:904–914. doi: 10.1016/0013-4694(62)90141-4. [DOI] [PubMed] [Google Scholar]
- 81.Pavlov VA, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston JM, Czura CJ, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci U S A. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munyaka P, Rabbi MF, Pavlov VA, Tracey KJ, Khafipour E, Ghia JE. Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+ PLoS One. 2014;9:e109272. doi: 10.1371/journal.pone.0109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 85.Lee ST, Chu K, Jung KH, Kang KM, Kim JH, Bahn JJ, et al. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164–171. doi: 10.1016/j.brainres.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 86.Song JG, Li HH, Cao YF, Lv X, Zhang P, Li YS, et al. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology. 2012;116:406–414. doi: 10.1097/ALN.0b013e3182426ebd. [DOI] [PubMed] [Google Scholar]
- 87.Rosas-Ballina M, Valdes-Ferrer SI, Dancho ME, Ochani M, Katz D, Cheng KF, et al. Xanomeline suppresses excessive pro-inflammatory cytokine responses through neural signal-mediated pathways and improves survival in lethal inflammation. Brain Behav Immun. 2015;44:19–27. doi: 10.1016/j.bbi.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 89.Bodick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, van Lier R, et al. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl 4):S16–S22. [PubMed] [Google Scholar]
- 90.Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 91.Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37:16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106:15950–15955. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 94.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoshiyama Y, Kojima A, Itoh K, Uchiyama T, Arai K. Anticholinergics boost the pathological process of neurodegeneration with increased inflammation in a tauopathy mouse model. Neurobiol Dis. 2012;45:329–336. doi: 10.1016/j.nbd.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 96.Yoshiyama Y, Kojima A, Itoh K, Isose S, Koide M, Hori K, et al. Does Anticholinergic Activity Affect Neuropathology? Implication of Neuroinflammation in Alzheimer’s Disease. Neurodegener Dis. 2015;15:140–148. doi: 10.1159/000381484. [DOI] [PubMed] [Google Scholar]
- 97.Dinan TG, Clarke G, Quigley EM, Scott LV, Shanahan F, Cryan J, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 98.Kawashima K, Fujii T, Moriwaki Y, Misawa H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 2012;91:1027–1032. doi: 10.1016/j.lfs.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Waldburger JM, Boyle DL, Edgar M, Sorkin LS, Levine YA, Pavlov VA, et al. Spinal p38 MAP kinase regulates peripheral cholinergic outflow. Arthritis Rheum. 2008;58:2919–2921. doi: 10.1002/art.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gowayed MA, Refaat R, Ahmed WM, El Abhar HS. Effect of galantamine on adjuvant-induced arthritis in Rats. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 101.Shifrin H, Nadler-Milbauer M, Shoham S, Weinstock M. Rivastigmine alleviates experimentally induced colitis in mice and rats by acting at central and peripheral sites to modulate immune responses. PLoS One. 2013;8:e57668. doi: 10.1371/journal.pone.0057668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miceli PC, Jacobson K. Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci. 2003;105:16–24. doi: 10.1016/S1566-0702(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 103.Akinci SB, Ulu N, Yondem OZ, Firat P, Guc MO, Kanbak M, et al. Effect of neostigmine on organ injury in murine endotoxemia: missing facts about the cholinergic antiinflammatory pathway. World J Surg. 2005;29:1483–1489. doi: 10.1007/s00268-005-0073-2. [DOI] [PubMed] [Google Scholar]
- 104.Kox M, Pompe JC, Peters E, Vaneker M, van der Laak JW, van der Hoeven JG, et al. alpha7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-alpha production and lung injury. Br J Anaesth. 2011;107:559–566. doi: 10.1093/bja/aer202. [DOI] [PubMed] [Google Scholar]
- 105.Lataro RM, Silva CA, Tefe-Silva C, Prado CM, Salgado HC. Acetylcholinesterase Inhibition Attenuates the Development of Hypertension and Inflammation in Spontaneously Hypertensive Rats. Am J Hypertens. 2015 doi: 10.1093/ajh/hpv017. [DOI] [PubMed] [Google Scholar]
- 106.Gambi F, Reale M, Iarlori C, Salone A, Toma L, Paladini C, et al. Alzheimer patients treated with an AchE inhibitor show higher IL-4 and lower IL-1 beta levels and expression in peripheral blood mononuclear cells. J Clin Psychopharmacol. 2004;24:314–321. doi: 10.1097/01.jcp.0000125683.74595.2f. [DOI] [PubMed] [Google Scholar]
- 107.Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Exp Gerontol. 2005;40:165–171. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Blasko I, Knaus G, Weiss E, Kemmler G, Winkler C, Falkensammer G, et al. Cognitive deterioration in Alzheimer’s disease is accompanied by increase of plasma neopterin. J Psychiatr Res. 2007;41:694–701. doi: 10.1016/j.jpsychires.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 109.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de BD. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silverman HA, Dancho M, Regnier-Golanov A, Nasim M, Ochani M, Olofsson PS, et al. Brain region-specific alterations in the gene expression of cytokines, immune cell markers and cholinergic system components during peripheral endotoxin-induced inflammation. Mol Med. 2014;20:601–611. doi: 10.2119/molmed.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sankowski R, Mader S, Valdes-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci. 2015;9:28. doi: 10.3389/fncel.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lampa J, Westman M, Kadetoff D, Agreus AN, Le Maitre E, Gillis-Haegerstrand C, et al. Peripheral inflammatory disease associated with centrally activated IL-1 system in humans and mice. Proc Natl Acad Sci U S A. 2012;109:12728–12733. doi: 10.1073/pnas.1118748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diamond B, Tracey KJ. Mapping the immunological homunculus. Proc Natl Acad Sci U S A. 2011;108:3461–3462. doi: 10.1073/pnas.1100329108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 115.Lemstra AW, Groen in’t Woud JC, Hoozemans JJ, van Haastert ES, Rozemuller AJ, Eikelenboom P, et al. Microglia activation in sepsis: a case-control study. J Neuroinflammation. 2007;4:4. doi: 10.1186/1742-2094-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys. 2013;536:189–196. doi: 10.1016/j.abb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 117.Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci. 2013;14:851–858. doi: 10.1038/nrn3587. [DOI] [PubMed] [Google Scholar]
- 118.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stranahan AM. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sato A, Sato Y, Uchida S. Activation of the intracerebral cholinergic nerve fibers originating in the basal forebrain increases regional cerebral blood flow in the rat’s cortex and hippocampus. Neurosci Lett. 2004;361:90–93. doi: 10.1016/j.neulet.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 122.Kolisnyk B, Al Onaizi MA, Hirata PH, Guzman MS, Nikolova S, Barbash S, et al. Forebrain deletion of the vesicular acetylcholine transporter results in deficits in executive function, metabolic, and RNA splicing abnormalities in the prefrontal cortex. J Neurosci. 2013;33:14908–14920. doi: 10.1523/JNEUROSCI.1933-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 124.Terrando N, Yang T, Ryu JK, Newton PT, Monaco C, Feldmann M, et al. Stimulation of the alpha7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol Med. 2014;20:667–675. doi: 10.2119/molmed.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Gool WA, van de BD, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 126.Gnatek Y, Zimmerman G, Goll Y, Najami N, Soreq H, Friedman A. Acetylcholinesterase loosens the brain’s cholinergic anti-inflammatory response and promotes epileptogenesis. Front Mol Neurosci. 2012;5:66. doi: 10.3389/fnmol.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scherer EB, Loureiro SO, Vuaden FC, da Cunha AA, Schmitz F, Kolling J, et al. Mild hyperhomocysteinemia increases brain acetylcholinesterase and proinflammatory cytokine levels in different tissues. Mol Neurobiol. 2014;50:589–596. doi: 10.1007/s12035-014-8660-6. [DOI] [PubMed] [Google Scholar]
- 128.Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. 2010;45:408–417. doi: 10.1016/j.mcn.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 130.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 132.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valdes-Ferrer SI, Rosas-Ballina M, Olofsson PS, Lu B, Dancho ME, Ochani M, et al. HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors. J Intern Med. 2013;274:381–390. doi: 10.1111/joim.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med. 2012;18:930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Garcia-Ayllon MS, Cauli O, Silveyra MX, Rodrigo R, Candela A, Compan A, et al. Brain cholinergic impairment in liver failure. Brain. 2008;131:2946–2956. doi: 10.1093/brain/awn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barron HV, Alam I, Lesh MD, Strunk A, Bass NM. Autonomic nervous system tone measured by baroreflex sensitivity is depressed in patients with end-stage liver disease. Am J Gastroenterol. 1999;94:986–989. doi: 10.1111/j.1572-0241.1999.01000.x. [DOI] [PubMed] [Google Scholar]
- 137.Mani AR, Montagnese S, Jackson CD, Jenkins CW, Head IM, Stephens RC, et al. Decreased heart rate variability in patients with cirrhosis relates to the presence and degree of hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2009;296:G330–G338. doi: 10.1152/ajpgi.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Basu PP, Shah NJ, Aloysius MM, Brown RS. Randomized, placebo-controlled trial of transdermal rivastigmine for the treatment of encephalopathy in liver cirrhosis (TREC trial) Open Journal of Gastroenterology. 2014;4(6):255–264. [Google Scholar]
- 139.Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne) 2014;5:74. doi: 10.3389/fendo.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 141.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 142.Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Correa M, et al. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;610:42–48. doi: 10.1016/j.ejphar.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 143.Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, et al. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220:30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 144.Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009;9:449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Diamond B, Volpe BT. A model for lupus brain disease. Immunol Rev. 2012;248:56–67. doi: 10.1111/j.1600-065X.2012.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annu Rev Immunol. 2013;31:345–385. doi: 10.1146/annurev-immunol-020711-075041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang EH, Volpe BT, Mackay M, Aranow C, Watson P, Kowal C, et al. Selective Impairment of Spatial Cognition Caused by Autoantibodies to the N-Methyl-d-Aspartate Receptor. EBioMedicine. 2015;2:755–764. doi: 10.1016/j.ebiom.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saito K, Yoshioka M, Kohya T, Kitabatake A. Involvement of muscarinic M1 receptor in the central pathway of the serotonin-induced Bezold-Jarisch reflex in rats. J Auton Nerv Syst. 1994;49:61–68. doi: 10.1016/0165-1838(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 149.Matsushita H, Ishikawa K, Shimazu T. Chemical coding of the hypothalamic neurones in metabolic control. I. Acetylcholine-sensitive neurones and glycogen synthesis in liver. Brain Res. 1979;163:253–261. doi: 10.1016/0006-8993(79)90353-6. [DOI] [PubMed] [Google Scholar]
- 150.Shimazu T, Matsushita H, Ishikawa K. Cholinergic stimulation of the rat hypothalamus: effects of liver glycogen synthesis. Science. 1976;194:535–536. doi: 10.1126/science.9692. [DOI] [PubMed] [Google Scholar]
- 151.Li Y, Wu X, Zhu J, Yan J, Owyang C. Hypothalamic regulation of pancreatic secretion is mediated by central cholinergic pathways in the rat. J Physiol. 2003;552:571–587. doi: 10.1113/jphysiol.2003.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cabrera LY, Evans EL, Hamilton RH. Ethics of the electrified mind: defining issues and perspectives on the principled use of brain stimulation in medical research and clinical care. Brain Topogr. 2014;27:33–45. doi: 10.1007/s10548-013-0296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 154.Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 156.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 157.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 158.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 159.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 2009;10:99–109. doi: 10.1016/j.cmet.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 160.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 161.Richter WO, Geiss HC, Aleksic S, Schwandt P. Cardiac autonomic nerve function and insulin sensitivity in obese subjects. Int J Obes Relat Metab Disord. 1996;20:966–969. [PubMed] [Google Scholar]
- 162.Ziegler D, Zentai C, Perz S, Rathmann W, Haastert B, Meisinger C, et al. Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp Clin Endocrinol Diabetes. 2006;114:153–159. doi: 10.1055/s-2006-924083. [DOI] [PubMed] [Google Scholar]
- 163.Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond) 2007;31:1756–1759. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Satapathy SK, Ochani M, Dancho M, Hudson LK, Rosas-Ballina M, Valdes-Ferrer SI, et al. Galantamine alleviates inflammation and other obesity-associated complications in high-fat diet-fed mice. Mol Med. 2011;17:599–606. doi: 10.2119/molmed.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, et al. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332:173–180. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 166.Wang X, Yang Z, Xue B, Shi H. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152:836–846. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ali MA, El Abhar HS, Kamel MA, Attia AS. Antidiabetic Effect of Galantamine: Novel Effect for a Known Centrally Acting Drug. PLoS One. 2015;10:e0134648. doi: 10.1371/journal.pone.0134648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 170.Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Erin N, Akdas BG, Harms JF, Clawson GA. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters substance P level. Regul Pept. 2008;151:35–42. doi: 10.1016/j.regpep.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 172.Erin N, Barkan GA, Clawson GA. Vagus nerve regulates breast cancer metastasis to the adrenal gland. Anticancer Res. 2013;33:3675–3682. [PubMed] [Google Scholar]
- 173.Giese-Davis J, Wilhelm FH, Tamagawa R, Palesh O, Neri E, Taylor CB, et al. Higher vagal activity as related to survival in patients with advanced breast cancer: an analysis of autonomic dysregulation. Psychosom Med. 2015;77:346–355. doi: 10.1097/PSY.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ogawa T, Makino T, Mizumoto K, Nakayama F. Promoting effect of truncal vagotomy on pancreatic carcinogenesis initiated with N-nitrosobis(2-oxopropyl)amine in Syrian golden hamsters. Carcinogenesis. 1991;12:1227–1230. doi: 10.1093/carcin/12.7.1227. [DOI] [PubMed] [Google Scholar]
- 175.Tatsuta M, Yamamura H, Iishi H, Ichii M, Noguchi S, Baba M, et al. Promotion by vagotomy of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1985;45:194–197. [PubMed] [Google Scholar]
- 176.Tatsuta M, Iishi H, Yamamura H, Baba M, Taniguchi H. Effects of bilateral and unilateral vagotomy on gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Int J Cancer. 1988;42:414–418. doi: 10.1002/ijc.2910420318. [DOI] [PubMed] [Google Scholar]
- 177.Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Watt PC, Patterson CC, Kennedy TL. Late mortality after vagotomy and drainage for duodenal ulcer. Br Med J (Clin Res Ed) 1984;288:1335–1338. doi: 10.1136/bmj.288.6427.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Caygill CP, Hill MJ, Hall CN, Kirkham JS, Northfield TC. Increased risk of cancer at multiple sites after gastric surgery for peptic ulcer. Gut. 1987;28:924–928. doi: 10.1136/gut.28.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Houghton PW, Leaper DJ. Gastric cancer following highly selective vagotomy. Postgrad Med J. 1987;63:47–48. doi: 10.1136/pgmj.63.735.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Caygill CP, Knowles RL, Hall R. Increased risk of cancer mortality after vagotomy for peptic ulcer: a preliminary analysis. Eur J Cancer Prev. 1991;1:35–37. doi: 10.1097/00008469-199110000-00007. [DOI] [PubMed] [Google Scholar]
- 182.Chiang JK, Kuo TB, Fu CH, Koo M. Predicting 7-day survival using heart rate variability in hospice patients with non-lung cancers. PLoS One. 2013;8:e69482. doi: 10.1371/journal.pone.0069482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Couck M, Gidron Y. Norms of vagal nerve activity, indexed by Heart Rate Variability, in cancer patients. Cancer Epidemiol. 2013;37:737–741. doi: 10.1016/j.canep.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 184.Mravec B, Gidron Y, Hulin I. Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Semin Cancer Biol. 2008;18:150–163. doi: 10.1016/j.semcancer.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 185.Ondicova K, Mravec B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010;11:596–601. doi: 10.1016/S1470-2045(09)70337-7. [DOI] [PubMed] [Google Scholar]
- 186.Gidron Y, Perry H, Glennie M. Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol. 2005;6:245–248. doi: 10.1016/S1470-2045(05)70096-6. [DOI] [PubMed] [Google Scholar]
- 187.Steinman L. A century of pavlovian experiments forming a circuit from the elucidation of neural reflexes to pharmaceuticals and electroceuticals to treat diseases. Brain Behav Immun. 2015;44:17–18. doi: 10.1016/j.bbi.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 188.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 190.Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Effects of the alpha7 nicotinic acetylcholine receptor agonist GTS-21 on the innate immune response in humans. Shock. 2011;36:5–11. doi: 10.1097/SHK.0b013e3182168d56. [DOI] [PubMed] [Google Scholar]
- 191.Mehta AK, Singh BP, Arora N, Gaur SN. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology. 2010;215:527–534. doi: 10.1016/j.imbio.2009.09.004. [DOI] [PubMed] [Google Scholar]