The (pro)renin receptor [(P)RR] was discovered by virtue of its role in the activation of the renin–angiotensin–aldosterone system (RAAS).1 Clinically, (P)RR is thought to be involved in end-organ damage from diabetes mellitus and hypertension.2,3 Yet despite being named for its ability to bind renin and prorenin, (P)RR has remarkably low affinity for these ligands, suggesting extra-renal functions for this receptor,4 a concept supported by its recently discovered role as a novel regulator of the vacuolar ATPase.5 In this issue, Lu et al.6 report the use of an unbiased proteomics approach to better characterize the extra-renal roles of (P)RR and thereby discover a novel connection between (P)RR and lipoprotein metabolism, specifically that (P)RR regulates two physiologically important lipoprotein receptors, sortilin-1 (SORT1) and the low-density lipoprotein receptor (LDLR).
Lu et al. found through an interactome screen and subsequent co-immunoprecipitation experiments that (P)RR directly interacts with SORT1, a multifunctional cellular trafficking protein that was recently discovered through human genetics to be an important regulator of lipid metabolism. Genome-wide association studies identified DNA variants in a locus on chromosome 1p13 to be strongly linked to both blood low-density lipoprotein (LDL) cholesterol levels and risk for coronary artery disease.7 Initial functional studies in human cells and in mice suggested that a single noncoding DNA variant in the locus, rs12740374, regulates the hepatic expression of the SORT1 gene and that SORT1, out of several candidate proteins expressed from the 1p13 locus, regulates blood cholesterol levels by (1) modulating the hepatic secretion of very-low-density lipoprotein (VLDL) particles (the precursors to LDL particles in the blood) and (2) directly binding and taking up LDL particles into cells, similar to the well-known function of LDLR.7–9
In their follow-up experiments after finding an interaction between (P)RR and SORT1, Lu et al. observed that RNA interference (RNAi)-mediated knockdown of (P)RR reduces both SORT1 and LDLR protein levels without affecting SORT1 and LDLR mRNA levels. Consistent with the roles of both LDLR and SORT1 in the cellular uptake of LDL particles, knockdown of (P)RR also reduces LDL uptake in multiple cell lines. Lu et al. provide evidence that this phenomenon is largely due to the effect on LDLR, with a smaller contribution from the effect on SORT1.
Intriguingly, Lu et al. saw a decrease in LDLR protein with SORT1 knockdown independent of (P)RR and an increase in LDLR with SORT1 overexpression. They also found that SORT1 overexpression is unable to rescue LDLR from the effect of (P)RR knockdown and that reduced (P)RR expression increases the lysosomal degradation of LDLR without increasing its rate of removal from the plasma membrane or its degradation by proprotein convertase subtilisin/kexin type 9 (PCSK9). To place these findings in context, previous studies have reported conflicting findings with respect to the relationship between SORT1 and LDLR. A couple of studies showed no difference in LDLR protein with RNAi-mediated hepatic Sort1 knockdown in mice as well as in Sort1 knockout mice,7,9 and another study reported no difference in LDLR protein with Sort1 deficiency using the same knockout mouse.10 In contrast, one study reported increased LDLR protein in CHO-Trex cells with SORT1 overexpression (consistent with the findings of Lu et al.),11 while yet another study reported increased LDLR protein in a different Sort1 knockout mouse.12
While this work has important implications for our understanding of (P)RR, it also increases our understanding of the role of SORT1 in lipid metabolism and LDL uptake, which has been a subject of debate for the last five years. Overexpression studies in a variety of cultured cell lines as well as in vivo have consistently demonstrated a positive association between SORT1 expression and LDL clearance.9,11,13 Knockdown and knockout studies have been less consistent. One in vivo study reported compromised LDL clearance in Sort1 knockout mice,9 and another in vivo study reported delayed VLDL and chylomicron clearance (LDL clearance was not assessed) in mice with reduced Sort1 expression.14 One in vitro study showed a correlation between reduced cell surface SORT1 and reduced LDL uptake11, a second in vitro study reported compromised LDL uptake in Sort1-deficient primary mouse bone marrow macrophages15, and a third study found no difference in LDL uptake in primary hepatocytes isolated from Sort1 knockout mice.8 In this latest report, Lu et al. found that both indirect SORT1 reduction secondary to (P)RR knockdown and direct RNAi-mediated SORT1 knockdown are associated with reductions in LDL uptake.
The findings of Lu et al. remind us that we do not completely understand how LDLR and SORT1 are regulated at the post-transcriptional level. The LDLR story seems more straightforward: Lu et al. report that RNAi-mediated (P)RR knockdown is associated with increased lysosomal LDLR degradation. One can envision a model in which (P)RR protects LDLR from lysosomal degradation and facilitates its trafficking to the cell surface, and since SORT1 knockdown reduces the (P)RR protein level, it also is associated with a reduction in LDLR protein. This model would explain why SORT1 overexpression does not rescue LDLR levels in the context of (P)RR knockdown. The observation that (P)RR overexpression does not increase LDLR levels suggests that if a (P)RR-SORT1-LDLR pathway exists it is saturable, which is quite plausible for a Golgi-to-plasma membrane trafficking route (Figure).
Figure. Models of the interactions of (P)RR, SORT1, and LDLR.
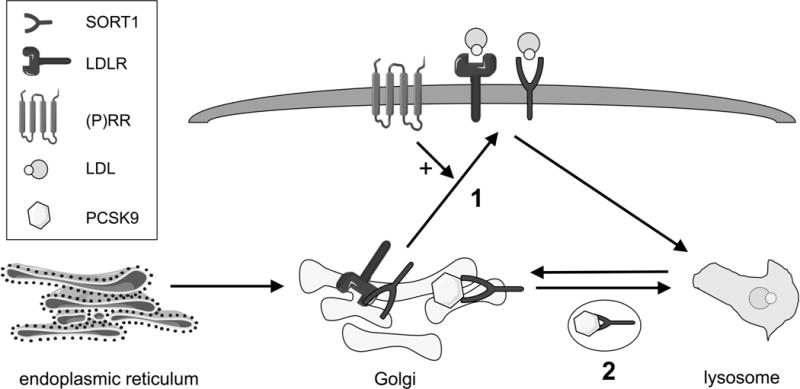
SORT1 and LDLR are synthesized in the endoplasmic reticulum and trafficked to the Golgi apparatus for post-translational processing and maturation. From the Golgi apparatus, LDLR and SORT1 can traffic to the cell surface to serve as endocytic receptors for LDL to facilitate lysosomal LDL degradation, or they can be trafficked to lysosomes themselves for degradation. Lu et al. reported that reduced (P)RR is associated with post-transcriptional downregulation of SORT1 and LDLR and that LDLR levels can be rescued by lysosome inhibition. (1) One potential model is that (P)RR through either direct binding or indirect effects facilitates SORT1 and LDLR trafficking to the cell surface, thereby protecting LDLR and possibly SORT1 from lysosomal degradation. (2) Lu et al. also reported that decreased SORT1 expression is associated with reduced levels of LDLR and (P)RR proteins. SORT1 has been previously reported to bind PCSK9, and it is possible that this interaction sequesters PCSK9 and prevents it from degrading LDLR at the expense of SORT1. SORT1 may also protect (P)RR from lysosomal degradation by trafficking along with it to the cell membrane and preventing binding of receptors that would otherwise traffic it to lysosomes.
The SORT1 story is more difficult to sort out. Lu et al. found that RNAi-mediated (P)RR knockdown is associated with reduced SORT1 protein with no change in SORT1 mRNA, and the reduction is not rescued by lysosome, autophagy, or proteasome inhibition. A similar finding was reported by another group when they demonstrated that overexpression of PCSK9 reduced intracellular SORT1 levels, and though this inhibition was partially rescued by lysosome inhibition, they could not achieve complete rescue with blockade of any pathway.10 SORT1 is known to be palmitoylated,16 ubiquitinated,17 and phosphorylated18 and has been suggested to undergo degradation via proteasomes19 and lysosomes.16,17 The data presented by the two aforementioned studies are consistent with a novel yet uncharacterized pathway of post-transcriptional SORT1 regulation. An initial step would be to determine if the translational efficacy of the SORT1 mRNA is influenced by PCSK9 or (P)RR, but further insight will likely require detailed mechanistic studies to account for the reduced SORT1 protein.
Perhaps the most significant contribution of the work of Lu et al. is uncovering a novel link between the RAAS and atherosclerotic cardiovascular disease. Renin and prorenin are profoundly elevated in diabetes mellitus and hypertension, and this is believed to contribute to the microvascular damage associated with these two disorders.2,3 The notion that the elevations in renin and prorenin may also contribute to hypercholesterolemia via crosstalk through (P)RR and reducing the expression of two proteins that mediate cellular LDL uptake, LDLR and SORT1, is intriguing. Lu et al. have opened a new avenue of inquiry into the interconnections of the RAAS and lipid metabolism, which we expect will be the focus of future investigation and may inform the debate of whether (P)RR would be a good therapeutic target in the treatment of diabetes mellitus and hypertension.
Acknowledgments
SOURCES OF FUNDING
None
ABBREVIATIONS
- (P)RR
(pro)renin receptor
- RAAS
renin–angiotensin–aldosterone system
- SORT1
sortilin-1
- LDLR
low-density lipoprotein receptor
- RNAi
RNA interference
- LDL
low-density lipoprotein
- PCSK9
proprotein convertase subtilisin/kexin type 9
- VLDL
very-low-density lipoprotein
Footnotes
DISCLOSURES
None
Subject Terms: Lipids and Cholesterol; Metabolism
References
- 1.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichihara A, Sakoda M, Mito-Kurauchi A, Itoh H. Activated prorenin as a therapeutic target for diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S63–S66. doi: 10.1016/j.diabres.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension. 2015;65:352–361. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batenburg WW, Lu X, Leijten F, Maschke U, Müller DN, Danser AH. Renin- and prorenin-induced effects in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor: does (pro)renin-(pro)renin receptor interaction actually occur? Hypertension. 2011;58:1111–1119. doi: 10.1161/HYPERTENSIONAHA.111.180737. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schägger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem. 1998;273:10939–10947. doi: 10.1074/jbc.273.18.10939. [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Meima ME, Nelson JK, Sorrentino V, Loregger A, Scheij S, Dekkers DH, Mulder MT, Demmers JA, Dallinga-Thie GM, Zelcer N, Danser AJ. Identification of the (pro)renin receptor as a novel regulator of low-density lipoprotein metabolism. Circ Res. 2016;118:xxx–xxx. doi: 10.1161/CIRCRESAHA.115.306799. [in this issue] [DOI] [PubMed] [Google Scholar]
- 7.Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12:213–223. doi: 10.1016/j.cmet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Strong A, Ding Q, Edmondson AC, et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest. 2012;122:2807–2816. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butkinaree C, Canuel M, Essalmani R, et al. Amyloid precursor-like protein 2 and sortilin do not regulate the PCSK9 convertase-mediated low density lipoprotein receptor degradation but interact with each other. J Biol Chem. 2015;290:18609–18620. doi: 10.1074/jbc.M115.647180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tveten K, Strøm TB, Cameron J, Berge KE, Leren TP. Mutations in the SORT1 gene are unlikely to cause autosomal dominant hypercholesterolemia. Atherosclerosis. 2012;225:370–375. doi: 10.1016/j.atherosclerosis.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsen C, Kjolby M, Nyegaard M, Mattheisen M, Lundhede J, Buttenschøn H, Mors O, Bentzon JF, Madsen P, Nykjaer A, Glerup S. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 2014;19:310–315. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Linsel-Nitschke P, Heeren J, Aherrahrou Z, et al. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis. 2010;208:183–189. doi: 10.1016/j.atherosclerosis.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel KM, Strong A, Tohyama J, Jin X, Morales CR, Billheimer J, Millar J, Kruth H, Rader DJ. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116:789–796. doi: 10.1161/CIRCRESAHA.116.305811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormick PJ, Dumaresq-Doiron K, Pluviose AS, Pichette V, Tosato G, Lefrancois S. Palmitoylation controls recycling in lysosomal sorting and trafficking. Traffic. 2008;9:1984–1997. doi: 10.1111/j.1600-0854.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumaresq-Doiron K, Jules F, Lefrancois S. Sortilin turnover is mediated by ubiquitination. Biochem Biophys Res Commun. 2013;433:90–95. doi: 10.1016/j.bbrc.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Bi L, Hulke M, Li T. Fish oil and fenofibrate prevented phosphorylation-dependent hepatic sortilin 1 degradation in Western diet-fed mice. J Biol Chem. 2014;289:22437–22449. doi: 10.1074/jbc.M114.548933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Matye DJ, Li T. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J Biol Chem. 2015;290:11526–11536. doi: 10.1074/jbc.M115.641225. [DOI] [PMC free article] [PubMed] [Google Scholar]


