Abstract
Hepatic fibrosis is a dynamic process resulting from excessive deposition of extracellular matrix in the liver; uncontrolled progression of fibrosis can eventually lead to liver cirrhosis and/or hepatocellular carcinoma. The fibrogenic process is complex and modulated by a number of both hepatic and extra-hepatic biological factors. Growing evidence indicates that adipokines, a group of cytokines produced by adipose tissue, impart dynamic functions in liver and are involved in modulation of hepatic fibrosis. In particular, two key adipokines, adiponectin and leptin, directly regulate many biological responses closely associated with development and progression of hepatic fibrosis. Leptin acts as a pro-fibrogenic cytokine, while adiponectin possesses anti-fibrogenic and anti-inflammatory properties. Adiponectin, acting via its cognate receptors, adiponectin receptors 1 and 2, potently suppresses fibrosis and inflammation in liver via multiple mechanisms. This review summarizes recent findings concerning the role of adiponectin in fibrogenic process in liver and addresses the underlying molecular mechanisms in modulation of fibrosis.
Keywords: Adiponectin, Fibrosis, Inflammation, Liver
Introduction
Hepatic fibrosis is a dynamic integrated process that occurs in response to liver injury, resulting from a repeated cycle of injury and activation of the wound healing process. Fibrosis is characterized by excessive accumulation of extracellular matrix (ECM), predominantly collagen 1, and can ultimately lead to irreversible cirrhosis [1]. Hepatic stellate cells (HSCs) act as central players in development and progression of liver fibrosis. In quiescent stage, HSCs reside in the Space of Disse and serve as the principal storage of retinyl esters in liver; additional significant biological functions of quiescent HSCs are not well understood. In response to liver injury, HSCs become activated, their morphological features are dramatically changed and they acquire new biological functions. In the activated state, the morphology of the cells is sharply changed from star-like shape to that of fibroblasts, associated with an increase in expression of cytoskeletal proteins, such as α-smooth muscle actin (α-SMA). Activated HSCs are proliferative and become resistant to apoptosis, secrete collagen and other extracellular matrix proteins, migrate to the sites of liver injury and secrete chemotactic factors that recruit immune cells to the sites of injury [2–4]. Therefore, activation of HSCs is considered a central event in the onset and progression of hepatic fibrosis.
It is well established that various cytokines are involved in the pathogenesis of hepatic fibrosis. For example, transforming growth factor (TGF)-β1, the proto-typical pro-fibrotic cytokine, stimulates HSCs to produce ECM [5]. A number of studies have also demonstrated that adipokines, a group of cytokines primarily derived from adipose tissue, exert a role in the regulation of hepatic fibrosis [6]. The two primary adipokines, adiponectin and leptin, are the best characterized adipokines affecting liver disease. Adiponectin is widely known to act as a potent anti-fibrotic cytokine, while leptin acts as a pro-fibrogenic cytokine. Interestingly, adiponectin impacts the development of hepatic fibrosis via multiple mechanisms, with both direct anti-fibrotic effects on HSCs, as well as indirect anti-fibrotic roles via its anti-inflammatory activities [7–9]. Recent evidence also suggests that adiponectin can influence the regulation of hepatocyte proliferation [10], which may also influence the fibrogenic response to liver injury. Herein, we review the recent findings regarding the protective effects of adiponectin in hepatic inflammation and fibrosis, as well as briefly summarizing the contributions of other key adipokines.
Overview of the effects of adiponectin and other adipokines on liver diseases
Adipose tissue acts as a dynamic endocrine organ via secretion of diverse biomolecules, collectively called adipokines. In addition to playing a critical role in the physiology of adipose tissue, adipokines regulate multiple non-adipose cellular and tissue targets and are involved in regulation of a variety of homeostatic functions, including energy metabolism, inflammation and immune function [11]. Once adipokines are secreted from adipose tissue, they circulate in the blood stream to reach their target organ(s). Adipokines then bind their cognate receptors and modulate various physiological functions. Liver is one of the major target organs affected by adipokines; adiponectin and leptin have particularly critical regulatory functions in the liver. Development and progression of various liver diseases, including ALD and NAFLD/NASH, are modulated by adiponectin and leptin. Here, we provide a general overview of the effects of adiponectin in liver disease, particularly focused on its role in fibrosis, as well as a brief description of the contributions of other adipokines to fibogenesis.
ADIPONECTIN
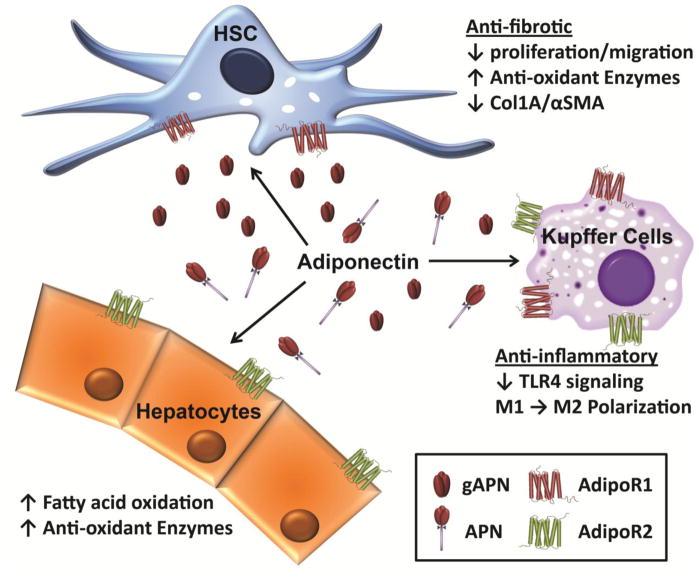
Adiponectin is the most abundant adipokine in the plasma, comprising approximately 0.05% of the total plasma protein [12]. In addition to the crucial role in the lipid and glucose metabolism, adiponectin has multiple beneficial effects in the human body, including potent anti-inflammatory responses and modulation of reactive oxygen species production [13]. The two major receptors for adiponectin (AdipoR1 and AdipoR2) are present in liver and the signaling generated from binding with its receptors plays a protective role against various liver diseases (see Figure 1). In particular, growing evidence from both in vitro and in vivo models suggests that adiponectin suppresses development and progression of hepatic fibrosis. For example, injection of adenovirus producing adiponectin suppressed carbon tetrachloride (CCl4)-induced liver fibrosis [7]. Adiponectin treatment also had a protective effect in acetaldehyde-induced collagen production in HSCs [14]. Moreover, hepatic fibrosis and tumor formation was enhanced by choline-deficient L-amino acid-defined (CDAA) diet in adiponectin knock-out compared to wild-type mice [15]. Progression of high-fat diet-induced liver injury, a common model of NAFL/NASH, to hepatic fibrosis was also significantly increased in adiponectin knock-out compared to wild-type mice [16], while adiponectin-overexpressing transgenic mice were resistant to fibrosis induced by exposure to thioacetamide [17].
Figure 1. Adiponectin receptors in liver cells.
AdipoR1 and AdipoR2 are differentially expressed on hepatocytes, hepatic stellate cells and Kupffer cells. Cellular responses to adiponectin interaction with receptors can have anti-oxidant, anti-inflammatory and anti-fibrotic effects within individual cell types in the liver.
These protective effects of adiponectin are due, at least in part, to its direct effects in regulating the activation state of HSCs. For example, adiponectin prevents proliferation and migration of HSCs [18], as well as decreases ECM deposition via alteration of molecular ratio of MMP-1 to TIMP-1 (by increasing TIMP-1 expression, and/or decreasing MMP-1 expression) [19]. Adiponectin also sensitizes activated HSCs to caspase-mediated apoptosis [20]. Collectively, these data implicate adiponectin in maintenance of HSCs in their quiescent state and promoting the reversal of HSC activation. The detailed molecular mechanisms underlying these anti-fibrotic effects of adiponectin will be discussed in the next sections.
Although adiponectin is predominantly produced from adipocytes, the plasma level of adiponectin is decreased in obesity, in contrast to other adipose tissue-derived hormones, such as leptin. Given this inverse relationship between obesity and the plasma level of adiponectin, lower availability of adiponectin has been implicated in the development of steatosis, inflammation and fibrosis in liver. For example, hypoadiponectinemia predicts the severity of hepatic fibrosis [21] and is associated with advanced fibrosis in patients with NAFLD [22]. Furthermore, lower plasma level of high molecular weight (HMW) adiponectin is a predictor of liver fibrosis in patients with HCV infection [23]. The circulating concentration of adiponectin is also negatively associated with chronic inflammation and accumulation of fat in liver in some rodent models of alcoholic liver disease [24]. Taken together, these data indicate that plasma concentrations of adiponectin might be a useful biomarker of hepatic steatosis, inflammation and fibrosis in liver diseases of varied etiologies.[25].
While many studies have identified adiponectin as an anti-inflammatory and anti-fibrotic cytokine, a few studies have reported that the circulating adiponectin concentration is positively associated with the development of liver diseases. For example, adiponectin concentration in the plasma is enhanced in severe hepatic fibrosis and declines with reduction in fibrosis in chronic hepatitis B patients [26]. Further, plasma adiponectin correlates with the progression of liver disease in HBV infection [27]. These contradictory results are most likely related to the stage of liver injury studied, with more severely impaired hepatic function perhaps impairing clearance of adiponectin from the circulation. Alternatively, the specific type of liver disease, i.e. HBV compared to HCV, may also contribute to these disparate findings. The impact of elevated adiponectin in more severe liver injury is not well understood and should be the focus of future investigations.
Given the predominant inverse correlation between lower adiponectin and progression of liver injury, studies have investigated the impact of restoring adiponectin in halting the progression of disease. For example, treatment with pioglitazone, an agonist of peroxisome proliferator-activated receptor-γ (PPAR-γ), increased plasma level of adiponectin in NASH and also improved associated pathologies in NASH patients, including reducing hepatic steatosis, necro-inflammation and progression to fibrosis [28,29]. In addition, a recent study found that treatment with a synthetic peptide possessing adiponectin properties (ADD355) modulates various biochemical markers for fibrosis and reversed liver fibrosis induced by CCl4 in mice [30]. These studies suggest that there is potential promise in the development of agents to enhance adiponectin production or activation of adiponectin receptor via agonists as interventions for the treatment or reversal of hepatic fibrosis.
Additional adipokines and liver fibrosis
Leptin, originally reported to regulate energy balance and appetite, was the first identified hormone produced from adipose tissue [31]. Leptin is also produced by other tissues, including skeletal muscle, placenta and vascular cells [32], as well as adipose tissue. Leptin is also secreted by activated, but not quiescent, HSCs and can interact with HSCs in an autocrine manner [33]. In contrast to the beneficial effects by adiponectin, leptin has been shown to promote hepatic fibrosis via up-regulation of TGF-β1 and other inflammatory cytokines by Kupffer cells and sinusoidal endothelial cells [34–36], as well as enhancing expression of collagens and facilitating proliferation of HSCs [37]. Moreover, the absence of leptin or leptin receptor causes a marked reduction of hepatic fibrosis in various in vivo models of fibrosis [38], indicating a critical role of leptin in the fibrogenic process in liver. The cognate receptors for leptin, members of the cytokine receptor family, are prevalently expressed in the body, such as brain (hypothalamus), lung, spleen and liver [39]. Upon binding of leptin with its full-length receptor, JAK/STAT signaling pathway is activated; JAK/STAT signaling plays a crucial role in leptin-induced ECM deposition and activation of HSCs [40].
In addition to adiponectin and leptin, a few additional adipokines can modulate liver fibrosis. Resistin, abundantly expressed in adipose tissue, was originally reported to antagonize the action of insulin and the circulating level of resistin is increased during obesity [41]. On the basis of these findings, resistin has been proposed as a link between obesity and insulin resistance. In addition to its role in the regulation of insulin activity, recent studies suggest that resistin is also relevant in the pathophysiology of liver injury. For example, plasma resistin is enhanced in BDL-induced cirrhotic rats [42], as well as in patients with liver cirrhosis [43]. Furthermore, elevated plasma resistin positively correlated with increased mortality of cirrhotic patients [43], consistent with an involvement of resistin in hepatic fibrosis and advanced liver dysfunction. Moreover, resistin exerts pro-inflammatory effects in HSCs, increasing expression of inflammatory cytokines and chemokines by HSCs, including monocyte chemoattractant protein-1 (MCP-1/CCL2) and interleukin-8. These effects of resistin are mediated via NF-κB dependent mechanisms [44]. Resistin also increases expression of TGF-β1 by Kupffer cells, which in turn, enhances expression of collagen in HSCs [45], suggesting that resistin contributes to hepatic fibrosis through both direct and indirect activation of HSCs.
Plasminogen activator inhibitor-1 (PAI-1) inhibits urokinase plasminogen activator (uPA) and prevents production of plasmin. Since plasmin degrades extracellular matrix (ECM) both directly and through activation of matrix metalloproteinases (MMPs), PAI-1 induces accumulation of ECM and is involved in the development of hepatic fibrosis. Adipose tissue synthesizes and secretes PAI-1 into the circulation [46]. Hepatic stellate cells (HSCs) are also an important source of PAI-1, and expression level of PAI-1 is significantly enhanced during fibrosis [47]. Furthermore, knock-down of PAI-1 increases expression of MMPs (in particular, MMP9 and MMP13) and significantly improves hepatic fibrosis induced by dimethyl nitrosamine (DMN) and bile-duct ligation (BDL) models [48], suggesting that regulation of PAI-1 would be a promising strategy for the treatment of liver fibrosis.
Adipokines and inflammation in liver
Development of hepatic fibrosis occurs in response to repeated or sustained hepatocellular injury and is commonly preceded by chronic inflammation in liver, including infiltration of leukocytes and activation of macrophages resident in liver [49]. While activation of inflammatory responses is essential for a normal wound healing response to injury, dysregulated activation and/or resolution of this salutary inflammation can contribute to pathologic fibrogenic responses. Sustained/chronic inflammation contributes to fibrogenesis via multiple mechanisms. For example, chronic inflammation in liver induces necrosis and apoptosis of hepatocytes. Dead and dying hepatocytes stimulate Kupffer cells to secret inflammatory mediators and can also directly initiate the activation process of HSCs when HSCs phagocytose apoptotic hepatocytes [50]. Therefore, while the inflammatory process is a critical step in the response to injury, it can also be a harmful process itself in liver and contribute to progression of fibrosis. In established fibrosis, anti-inflammatory agents, immune-suppressants or anti-viral agents, all of which suppress inflammatory activity, can improve fibrotic status and even reverse fibrotic process [51–53].
In addition to its metabolic and anti-fibrotic effects, adiponectin is widely known to possess potent anti-inflammatory properties. It suppresses expression of inflammatory cytokines, including TNF-α, IL-6 and IL-1, as well as induction of anti-inflammatory signaling molecules, including IL-10, IL-1R antagonist and heme oxygenase-1 (HO-1). Interestingly, there is a significant inverse correlation between plasma adiponectin and TNF-α expression [54]. Adiponectin and TNF-α negatively regulate each other’s expression in adipose tissue, as well as other tissues. Treatment with adiponectin suppresses TNF-α expression in mice liver and decreases the plasma level of TNF-α, associated with the prevention of steatosis and inflammation in various models of chronic liver injury [55,56]. On the other hand, TNF-α also regulates the expression of adiponectin. For example, treatment with TNF-α suppresses adiponectin expression in human white adipose tissue [57] and decreases expression of adiponectin in adipocytes via suppressing transcriptional activity of PPAR-γ and CCAAT/enhancer binding protein (C/EBP), which act as transcriptional inducers of adiponectin [58,59] and preventing secretion of adiponectin through activation of c-Jun N-terminal kinase (JNK) [60].
In contrast to adiponectin, leptin stimulates inflammatory responses. Treatment with leptin enhanced acute CCl4-induced necro-inflammatory responses in mice [61]. Leptin-deficient ob/ob mice are resistant to Concanavalin A (Con A)-induced hepatitis and production of inflammatory cytokines [62]. Taken together, these data suggest that, given the critical role of inflammatory processes in hepatic fibrosis, the modulation of chronic inflammatory responses by adiponectin and leptin could be one of the principal mechanisms underlying anti-fibrotic effects of adiponectin and fibrotic effect of leptin.
Relationship between obesity, hepatic fibrosis and plasma adiponectin
Obesity and associated metabolic syndrome is regarded as one of the key factors responsible for the progression of metabolic liver diseases, from NAFLD to NASH and the eventual progression to hepatic fibrosis. Obesity may contribute to hepatic fibrosis through multiple mechanisms. Abnormal accumulation of fat in liver creates what is considered to be a pro-fibrotic milieu, enhancing the production of reactive oxygen species, inducing cellular death in hepatocytes and dysregulating inflammatory responses [63]. In addition, circulating adiponectin is reduced in obesity, whereas leptin is increased. Given the complex pro/anti-fibrotic and pro/anti-inflammatory effects of leptin and adiponectin, this altered balance of adipokines likely contributes to the enhanced propensity of patients with obesity/metabolic syndrome to an increased progression of liver disease to the stage of fibrosis.
Molecular mechanisms underlying regulation of fibrosis and inflammation in liver by adiponectin
Molecular forms of adiponectin and adiponectin receptors involved in hepatic fibrosis
The adiponectin transcript encodes a 28–30KDa hydrophilic protein, with 247 amino acids and 4 clearly differentiated domains: the N-terminal domain, which contains the secretion signal domain; the variable region (28 aas); the collagenous domain with 22 Gly-X-Tyr (G-X-T) triplets; and the globular domain in the C-terminal region. The native adiponectin structure is a highly associated homo-trimer that forms via association through the collagenous domain. These homo-trimers can then associate into larger structures, including low molecular weight homo-hexamers (LMW), as well as higher order complexes of between 12–18 subunits (HMW). Moreover, a globular fragment is found in human plasma at very low concentrations; this globular adiponectin is thought to be the domain of adiponectin with maximal biological activity. Post-translational modifications, such as O-glycosylation by disialic acid at the collagenous domain, as well as hydroxylation, can contribute to maximal activity. In addition to the role of TNFα in regulating adiponectin secretion discussed above, adiponectin secretion is also controlled by additional factors: Insulin-like Growth Factor 1 (IGF-1) enhances its secretion, while glucocorticoids, β adrenergic agonists, prolactin and insulin decrease secretion from adipose tissue [11].
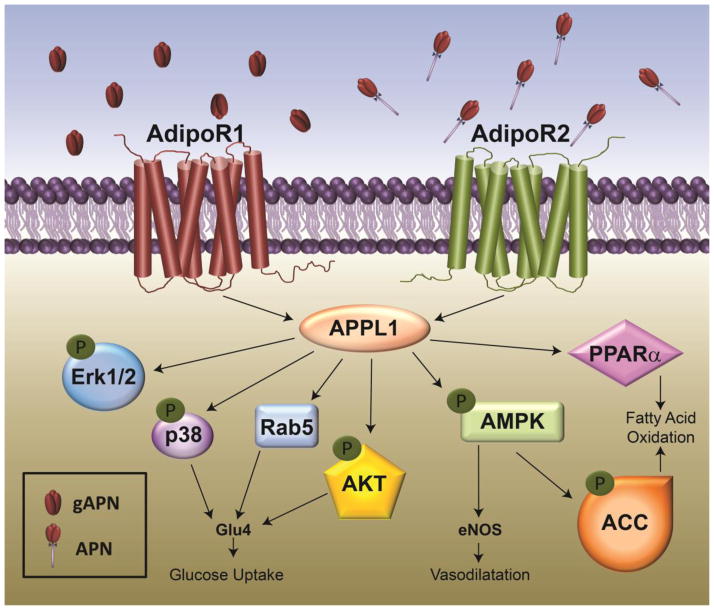
The biological responses by adiponectin are mediated by binding with its receptors. Three different types of receptors for adiponectin have been identified to date. AdipoR1 and AdipoR2, a member of G protein-coupled receptors, are recognized as the cognate receptors for adiponectin. AdipoR1 is abundantly expressed in skeletal muscle and also exists in activated HSCs, while AdipoR2 is predominantly expressed in other types of liver cells [64]. Hepatic macrophages express both AdipoR1 and AdipoR2 [65]. AdipoR1 has a greater affinity for globular adiponectin while AdipoR2 binds full length and multimeric adiponectin more avidly [64].
Accumulating evidence indicates that there are functional differences between AdipoR1 and AdipoR2 signaling (Figure 2). Briefly, activation of AdipoR1 appears to be linked with activation of AMPK, while AdipoR2 signaling is more associated with activation of PPAR-α [66]. It is likely that both AdipoR1 and R2 signaling play important roles in the hepato-protective effects of adiponectin. Overexpression of AdipoR2 suppressed TGFβ-induced ROS production in hepatocytes via enhancing PPAR-α activity and expression of catalase [67], which prevents progression of NASH to the stage of fibrosis. In addition, AdipoR2 signaling plays a crucial role in the modulation of oxidative stress and inflammation in liver [68]. However, recent studies have also indicated that AdipoR1 signaling also plays a critical role in anti-fibrotic effect of adiponectin [69] and is required for disruption of the leptin-induced vascular ECM remodeling [70], suggesting a possibility of AdipoR1 signaling in anti-fibrotic effects of adiponectin. AdipoR1 also contributes to protection from fibrosis due to its potent anti-inflammatory impact on macrophages [71], as well as the ability of adiponectin to shift macrophages to an M2 phenotype [72]. Importantly, specific M2 macrophage subtypes are characterized by increased expression of MMPs and are likely involved in the resolution of fibrosis [73]. While the effect of adiponectin on expression of MMPs has not yet been studied, this could be an important area of future investigation.
Figure 2. Signal transduction pathways activated by adiponect receptors.
Adiponectin R1 and R2 dependent signaling is mediated via APPL; however, activation of down-stream signaling pathways is dependent on cell type and metabolic environment.
T-cadherin, a member of cadherin family, was identified as a co-receptor required for transmission of metabolic signals by adiponectin [74]. Recent studies have demonstrated that T-cadherin could be implicated in adiponectin-mediated biological responses, including revascularization [75], suppression of pulmonary inflammation [76] and protection from stress-induced pathological cardiac remodeling [77]. Although, at this stage, the role of T-cadherin in hepato-protective effects of adiponectin has not been widely investigated, it would be interesting to examine the role of T-cadherin in adiponectin-induced anti-fibrotic and/or anti-inflammatory responses in liver.
AMP-activated protein kinase (AMPK)
The N-terminal domain of both AdipoR1 and AdipoR2 interacts with a pleckstrin homology adaptor protein, APPL1. This protein participates in the activation of different pathways including activation of AMP-activated protein kinase (AMPK), p38 MAPK, as well as ERK1/2 and AKT [78]. In macrophages, activation of ERK1/2/AKT by adiponectin involves Cot/tpl2 signaling [79]. AMPK, a central signaling molecule regulating cellular metabolism, has long been considered as a key molecule mediating the metabolic effects of many hormones, including adiponectin, leptin, insulin, etc. [80]. Apart from the role in metabolism, AMPK signaling plays a critical role in anti-fibrotic effects induced by many natural products [81,82]. Moreover, AMPK signaling plays a key role in inhibiting proliferation of HSCs. Activation of AMPK by adiponectin leads to the increase in the expression of cyclin-dependent kinase inhibitors, including p27 (kip1) and p21 (cip1) and inhibition of AKT pathway [83]. In addition, 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR), a pharmacological activator of AMPK, blocked PDGF-induced phosphorylation of ribosomal S6 kinase (p70S6K) and 4E binding protein-1 (4EBP1), also consistent with a role for adiponectin in the regulation of cell cycle in HSCs [83]. Furthermore, activation of AMPK signaling inhibits TGFβ-induced expression of collagen (COL1A) and the myofibroblast marker, smooth muscle actin (α-SMA), in HSCs by regulating activity of the transcriptional coactivator, p300 [84].
Peroxisome proliferator-activated receptor-γ (PPAR-γ)
Peroxisome proliferator-activated receptor-γ (PPAR-γ), a nuclear receptor family member, plays essential roles in cellular differentiation, proliferation and metabolism through modulating expression of various target genes. It has been shown that expression level of PPAR-γ is decreased during liver fibrosis [85]. PPAR-γ might have inhibitory roles in development of hepatic fibrogenesis and is considered as a potential therapeutic target of hepatic fibrosis [86]. Agonists for PPAR-γ induce expression of adiponectin in adipocytes, indicating a critical role in transcriptional activation of adiponectin. Interestingly, treatment of HSCs with lentivirus encoding adiponectin also increased expression and activity of PPAR-γ. Furthermore, exposure with troglitazone, a PPAR-γ agonist, suppressed mRNA expression of collagen α1 and α-SMA in HSCs [17], suggesting a critical role of PPAR-γ in suppressing activation of HSCs and the fibrogenic process by adiponectin.
Modulation of ROS production
Excessive production of reactive oxygen species (ROS) plays a critical role in initiating inflammatory responses and promoting fibrogenesis in liver. Adiponectin has a negative impact on signaling pathways that generate ROS, which may contribute to its protective effects against inflammation and fibrosis. Adiponectin inhibits NADPH oxidase-dependent ROS production via induction of anti-oxidant enzymes in HSCs [87], associated with a suppression of proliferation of HSCs. Adiponectin also modulates ROS production in Kupffer cells. Adiponectin treatment normalizes LPS-stimulated ROS production in Kupffer cells after chronic ethanol feeding, which is critical for the regulation of TNF-α expression [65]. In addition, globular adiponectin prevents ethanol-induced ROS production via modulation of NADPH oxidase in macrophages [88]. In contrast, other studies have reported that adiponectin treatment increased ROS/RNS production, which induces of apoptosis in RAW 264.7 macrophages [89]. These differential effects of adiponectin on ROS production in macrophages could be due to different experimental conditions. For example, pretreatment with lower concentrations of globular adiponectin (0.1 μg/ml) decreased ethanol-stimulated ROS production [88], while higher concentration (10 μg/ml) increased ROS formation in macrophages [89]. Detailed mechanisms underlying the differential impact of adiponectin in ROS production are not clearly understood. Identification of the mechanisms underlying differential effects of adiponectin on ROS production in different experimental conditions (e.g., different concentrations/activation of different receptors) would be valuable to understand the effects of adiponectin on ROS production, which in turn could impact design of therapeutic strategies to treat inflammatory diseases in liver.
Focal adhesion kinase (FAK)
Focal adhesions (FAs) make a link between extracellular environment and actin cytoskeleton. Assembly of focal adhesions is a critical process for maintaining myofibroblast phenotype of activated HSCs. Focal adhesion kinase (FAK), required for FAs assembly, therefore plays a crucial role in adhesion and migration processes of activated HSCs [90], and also modulates proliferation and collagen expression in HSCs [91,92]. Therefore, FAK signaling plays a crucial role in activation of HSCs and development of hepatic fibrosis. In fact, disruption of FAK signaling decreased mobility of cancer cells [93], sensitized activated HSCs to apoptosis [94] and attenuated synthesis of ECM and promoted ECM degradation [95]. In addition, FAK activation promotes cytoskeletal reorganization and fibrogenic phenotype of HSCs in HCV patients [96]. Taken together, these data suggest that FAK would be a potential therapeutic target for the treatment of fibrosis. A recent study reported that adiponectin modulated FAK activity in the generation of anti-fibrotic effects. Injection of adenoviral-adiponectin suppressed CCl4-induced expression of integrins, collagen and α-SMA in liver. These effects were accompanied with dephosphorylation of focal adhesion kinase (FAK) in activated HSCs [97]. In this study, FAK signaling was required for adiponectin-induced TIMP-1 expression.
Tissue inhibitor of metalloproteinase-1 (TIMP-1)
ECM degradation process is mainly mediated by matrix metalloproteinases (MMPs) and enhanced expression and/or activity of MMPs are regarded as a promising strategy for the resolution of fibrosis. Tissue inhibitors of matrix metalloproteinase (TIMPs) reduce the activity of MMPs and are involved in progression of fibrosis. Among various TIMPs, TIMP-1 plays a crucial role in degradation of collagen and is considered as a promising therapeutic target for the prevention of fibrogenesis in liver [98]. Inhibition of TIMP-1 is a potential mechanism underlying anti-fibrotic effects of adiponectin [99]; however, the data from different studies are not all supportive of this hypothesis. In one study, treatment of HSCs with adiponectin promoted expression of TIMP-1 and binding of TIMP-1 with C63/β1-integrin complex reduced phosphorylation of FAK, which suppressed the migration of HSCs [18]. In addition, treatment with adiponectin increases mRNA level of TIMP-1 in dermal fibroblasts [100] and macrophages via Syk-dependent manner [78]. To date, studies have focused on the ability of adiponectin to regulate TIMP-1 expression by HSCs; however, since TIMP-1 is also expressed by resolution macrophages [73], it will also be important to investigate the effects of adiponectin on expression of TIMP-1 (and MMPs) by macrophages.
Negative regulation of leptin signaling
While both adiponectin and leptin are produced from adipose tissue, the biological actions by these adipokines are exactly opposite in many aspects. It has been suggested that these reciprocal functions of adiponectin and leptin contribute to the homeostatic maintenance of metabolic effects. The expression of adiponectin and leptin in HSCs are also reciprocally regulated. Adiponectin is abundantly present in quiescent HSCs, but the expression is significantly suppressed in activated HSC, while leptin expression is more abundant in activated HSCs, but not in quiescent cells [17]. Furthermore, adiponectin treatment has been shown to disrupt leptin-induced hepatic fibrosis. Adiponectin-induced activation of AMPK suppressed leptin-mediated Stat3 phosphorylation and SOCS-3 induction [19]. In addition, adiponectin treatment increased expression of protein phosphatase-1B (PTP1B), which negatively regulates leptin-induced JAK/STAT3 pathway, and blocked leptin-stimulated formation of TIMP1-MMP1 complexes in HSCs [99].
Conclusion and future perspectives
Hepatic fibrosis is a dynamic process under complex regulation by both intra- and extra-hepatic mediators. Emerging evidence has demonstrated that adipokines are closely associated with the pathogenesis of hepatic fibrosis, acting as one of multiple extra-hepatic factors regulating fibrosis. In particular, adiponectin prevents development of fibrosis via multiple mechanisms and is widely considered as a relevant target for the treatment of hepatic fibrosis, while leptin exerts an opposite role and promotes development of hepatic fibrosis. In liver diseases and obesity, the plasma level of adiponectin is usually decreased; these lower circulating concentrations of adiponectin could contribute to the fibrogenic process in liver. Development of agents enhancing the secretion of adiponectin from adipose tissue or activating adiponectin receptors in liver would be valuable for the prevention and/or treatment of hepatic fibrosis.
Acknowledgments
This work was supported in part by grants to LEN: 1U01AA021890, 5R37 AA011876, R01 AA011975 and PP: Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2013R1A1A4A01011110).
Footnotes
Conflict of Interest
Pil-Hoon Park, Carlos Sanz-Garcia and Laura E Nagy declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
*recent publication
• of importance
- *1.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15–S24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *4.Shen C, Zhao CY, Wang W, Wang YD, Sun H, et al. The relationship between hepatic resistin overexpression and inflammation in patients with nonalcoholic steatohepatitis. BMC Gastroenterol. 2014;14:39. doi: 10.1186/1471-230X-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida K, Matsuzaki K. Differential Regulation of TGF-beta/Smad Signaling in Hepatic Stellate Cells between Acute and Chronic Liver Injuries. Front Physiol. 2012;3:53. doi: 10.3389/fphys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 7.Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Koehler E, Swain J, Sanderson S, Krishnan A, Watt K, et al. Growth hormone, dehydroepiandrosterone and adiponectin levels in non-alcoholic steatohepatitis: an endocrine signature for advanced fibrosis in obese patients. Liver Int. 2012;32:279–286. doi: 10.1111/j.1478-3231.2011.02637.x. [DOI] [PubMed] [Google Scholar]
- 9.Latif HA, Assal HS, Mahmoud M, Rasheed WI. Role of serum adiponectin level in the development of liver cirrhosis in patients with hepatitis C virus. Clin Exp Med. 2011;11:123–129. doi: 10.1007/s10238-010-0108-3. [DOI] [PubMed] [Google Scholar]
- *10.Correnti JM, Cook D, Aksamitiene E, Swarup A, Ogunnaike B, et al. Adiponectin fine-tuning of liver regeneration dynamics revealed through cellular network modelling. J Physiol. 2015;593:365–383. doi: 10.1113/jphysiol.2014.284109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •11.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. thorough review of adipokine physiology. [DOI] [PubMed] [Google Scholar]
- 12.Giannessi D, Maltinti M, Del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacol Res. 2007;56:459–467. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 14.Potter JJ, Mezey E. Acetaldehyde increases endogenous adiponectin and fibrogenesis in hepatic stellate cells but exogenous adiponectin inhibits fibrogenesis. Alcohol Clin Exp Res. 2007;31:2092–2100. doi: 10.1111/j.1530-0277.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556–564. doi: 10.1016/j.jhep.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Asano T, Watanabe K, Kubota N, Gunji T, Omata M, et al. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J Gastroenterol Hepatol. 2009;24:1669–1676. doi: 10.1111/j.1440-1746.2009.06039.x. [DOI] [PubMed] [Google Scholar]
- 17.Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690–2699. doi: 10.1016/j.ajpath.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Ramezani-Moghadam M, Wang J, Ho V, Iseli TJ, Alzahrani B, et al. Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion. J Biol Chem. 2015;290:5533–5542. doi: 10.1074/jbc.M114.598011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handy JA, Saxena NK, Fu P, Lin S, Mells JE, et al. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3) J Cell Biochem. 2010;110:1195–1207. doi: 10.1002/jcb.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, et al. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoneda M, Iwasaki T, Fujita K, Kirikoshi H, Inamori M, et al. Hypoadiponectinemia plays a crucial role in the development of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus independent of visceral adipose tissue. Alcohol Clin Exp Res. 2007;31:S15–21. doi: 10.1111/j.1530-0277.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 22.Savvidou S, Hytiroglou P, Orfanou-Koumerkeridou H, Panderis A, Frantzoulis P, et al. Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J Clin Gastroenterol. 2009;43:765–772. doi: 10.1097/MCG.0b013e31819e9048. [DOI] [PubMed] [Google Scholar]
- 23.Sumie S, Kawaguchi T, Kuromatsu R, Takata A, Nakano M, et al. Total and high molecular weight adiponectin and hepatocellular carcinoma with HCV infection. PLoS One. 2011;6:e26840. doi: 10.1371/journal.pone.0026840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musso G, Gambino R, Biroli G, Carello M, Faga E, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438–2446. doi: 10.1111/j.1572-0241.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 25.Park PH, Thakur V, Pritchard MT, McMullen MR, Nagy LE. Regulation of Kupffer cell activity during chronic ethanol exposure: role of adiponectin. J Gastroenterol Hepatol. 2006;21(Suppl 3):S30–33. doi: 10.1111/j.1440-1746.2006.04580.x. [DOI] [PubMed] [Google Scholar]
- 26.Hui CK, Zhang HY, Lee NP, Chan W, Yueng YH, et al. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol. 2007;47:191–202. doi: 10.1016/j.jhep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Liu CJ, Chen PJ, Lai MY, Liu CH, Chen CL, et al. High serum adiponectin correlates with advanced liver disease in patients with chronic hepatitis B virus infection. Hepatol Int. 2009;3:364–370. doi: 10.1007/s12072-008-9111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Wu R, Zhang F, Xu Y, Liu B, et al. Thiazolidinediones improve hepatic fibrosis in rats with non-alcoholic steatohepatitis by activating the adenosine monophosphate-activated protein kinase signalling pathway. Clin Exp Pharmacol Physiol. 2012;39:1026–1033. doi: 10.1111/1440-1681.12020. [DOI] [PubMed] [Google Scholar]
- 29.Gastaldelli A, Harrison S, Belfort-Aguiar R, Hardies J, Balas B, et al. Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment Pharmacol Ther. 2010;32:769–775. doi: 10.1111/j.1365-2036.2010.04405.x. [DOI] [PubMed] [Google Scholar]
- *30.Kumar P, Smith T, Rahman K, Thorn NE, Anania FA. Adiponectin agonist ADP355 attenuates CCl4-induced liver fibrosis in mice. PLoS One. 2014;9:e110405. doi: 10.1371/journal.pone.0110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DePaoli AM. 20 years of leptin: leptin in common obesity and associated disorders of metabolism. J Endocrinol. 2014;223:T71–81. doi: 10.1530/JOE-14-0258. [DOI] [PubMed] [Google Scholar]
- •32.Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91–101. doi: 10.1016/j.pathophys.2008.05.001. interesting review of the overall contributions of adipokines to hepatic fibrosis. [DOI] [PubMed] [Google Scholar]
- 33.Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 34.Yang YY, Huang YT, Tsai TH, Hou MC, Lee FY, et al. Kupffer cell depletion attenuates leptin-mediated methoxamine-stimulated portal perfusion pressure and thromboxane A2 release in a rodent model of NASH-cirrhosis. Clin Sci (Lond) 2012;123:669–680. doi: 10.1042/CS20110572. [DOI] [PubMed] [Google Scholar]
- 35.Ikejima K, Okumura K, Lang T, Honda H, Abe W, et al. The role of leptin in progression of non-alcoholic fatty liver disease. Hepatol Res. 2005;33:151–154. doi: 10.1016/j.hepres.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- 37.Bethanis SK, Theocharis SE. Leptin in the field of hepatic fibrosis: a pivotal or an incidental player? Dig Dis Sci. 2006;51:1685–1696. doi: 10.1007/s10620-006-9126-0. [DOI] [PubMed] [Google Scholar]
- 38.Marra F, Navari N, Vivoli E, Galastri S, Provenzano A. Modulation of liver fibrosis by adipokines. Dig Dis. 2011;29:371–376. doi: 10.1159/000329799. [DOI] [PubMed] [Google Scholar]
- 39.Fei H, Okano HJ, Li C, Lee GH, Zhao C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem. 2010;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Suhaimi EA, Shehzad A. Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res. 2013;18:12. doi: 10.1186/2047-783X-18-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin SY, Sheu WH, Chen WY, Lee FY, Huang CJ. Stimulated resistin expression in white adipose of rats with bile duct ligation-induced liver cirrhosis: relationship to cirrhotic hyperinsulinemia and increased tumor necrosis factor-alpha. Mol Cell Endocrinol. 2005;232:1–8. doi: 10.1016/j.mce.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Yagmur E, Trautwein C, Gressner AM, Tacke F. Resistin serum levels are associated with insulin resistance, disease severity, clinical complications, and prognosis in patients with chronic liver diseases. Am J Gastroenterol. 2006;101:1244–1252. doi: 10.1111/j.1572-0241.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 44.Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, et al. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042–2053. doi: 10.2353/ajpath.2006.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Dong ZX, Su L, Brymora J, Bird C, Xie Q, et al. Resistin mediates the hepatic stellate cell phenotype. World J Gastroenterol. 2013;19:4475–4485. doi: 10.3748/wjg.v19.i28.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falcao-Pires I, Castro-Chaves P, Miranda-Silva D, Lourenco AP, Leite-Moreira AF. Physiological, pathological and potential therapeutic roles of adipokines. Drug Discov Today. 2012;17:880–889. doi: 10.1016/j.drudis.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LP, Takahara T, Yata Y, Furui K, Jin B, et al. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrogenesis of rats: role of stellate cells. J Hepatol. 1999;31:703–711. doi: 10.1016/s0168-8278(99)80351-1. [DOI] [PubMed] [Google Scholar]
- 48.Hu PF, Chen H, Zhong W, Lin Y, Zhang X, et al. Adenovirus-mediated transfer of siRNA against PAI-1 mRNA ameliorates hepatic fibrosis in rats. J Hepatol. 2009;51:102–113. doi: 10.1016/j.jhep.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515–2532. doi: 10.3748/wjg.v20.i10.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czaja AJ. Drug choices in autoimmune hepatitis: part A--Steroids. Expert Rev Gastroenterol Hepatol. 2012;6:603–615. doi: 10.1586/egh.12.40. [DOI] [PubMed] [Google Scholar]
- 52.Abergel A, Darcha C, Chevallier M, Ughetto S, Henquell C, et al. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus-related severe fibrosis. Eur J Gastroenterol Hepatol. 2004;16:1219–1227. doi: 10.1097/00042737-200411000-00022. [DOI] [PubMed] [Google Scholar]
- 53.Poynard T, McHutchison J, Davis GL, Esteban-Mur R, Goodman Z, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology. 2000;32:1131–1137. doi: 10.1053/jhep.2000.19347. [DOI] [PubMed] [Google Scholar]
- 54.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 55.An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol. 2012;86:1337–1348. doi: 10.1007/s00204-012-0814-6. [DOI] [PubMed] [Google Scholar]
- 56.Morris AM, Sennello JA, Fayad RA, Eckel RH, Dinarello CA, et al. T cell-mediated hepatic inflammation modulates adiponectin levels in mice: role of tumor necrosis factor alpha. Metabolism. 2006;55:555–559. doi: 10.1016/j.metabol.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, et al. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39:250–255. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 58.Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, et al. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 59.Ron D, Brasier AR, McGehee RE, Jr, Habener JF. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP) J Clin Invest. 1992;89:223–233. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, et al. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:460–467. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Ikejima K, Honda H, Yoshikawa M, Hirose M, Kitamura T, et al. Leptin augments inflammatory and profibrogenic responses in the murine liver induced by hepatotoxic chemicals. Hepatology. 2001;34:288–297. doi: 10.1053/jhep.2001.26518. [DOI] [PubMed] [Google Scholar]
- 62.Gove ME, Rhodes DH, Pini M, van Baal JW, Sennello JA, et al. Role of leptin receptor-induced STAT3 signaling in modulation of intestinal and hepatic inflammation in mice. J Leukoc Biol. 2009;85:491–496. doi: 10.1189/jlb.0808508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G697–702. doi: 10.1152/ajpgi.00426.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •64.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. important paper that first identified adiponectin receptors. [DOI] [PubMed] [Google Scholar]
- 65.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 67.Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, et al. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–473. doi: 10.1002/hep.22365. [DOI] [PubMed] [Google Scholar]
- 68.Matsunami T, Sato Y, Ariga S, Sato T, Kashimura H, et al. Regulation of oxidative stress and inflammation by hepatic adiponectin receptor 2 in an animal model of nonalcoholic steatohepatitis. Int J Clin Exp Pathol. 2010;3:472–481. [PMC free article] [PubMed] [Google Scholar]
- 69.Cao T, Gao Z, Gu L, Chen M, Yang B, et al. AdipoR1/APPL1 potentiates the protective effects of globular adiponectin on angiotensin II-induced cardiac hypertrophy and fibrosis in neonatal rat atrial myocytes and fibroblasts. PLoS One. 2014;9:e103793. doi: 10.1371/journal.pone.0103793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z, Wang F, Wang BJ, Chu G, Cao Q, et al. Inhibition of leptin-induced vascular extracellular matrix remodelling by adiponectin. J Mol Endocrinol. 2014;53:145–154. doi: 10.1530/JME-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, et al. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928–4937. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, et al. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288:24886–24897. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasahara DI, Williams AS, Benedito LA, Ranscht B, Kobzik L, et al. Role of the adiponectin binding protein, T-cadherin (cdh13), in pulmonary responses to subacute ozone. PLoS One. 2013;8:e65829. doi: 10.1371/journal.pone.0065829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, et al. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu D, Fukuhara A, Miyata Y, Yokoyama C, Otsuki M, et al. Adiponectin regulates vascular endothelial growth factor-C expression in macrophages via Syk-ERK pathway. PLoS One. 2013;8:e56071. doi: 10.1371/journal.pone.0056071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *79.Sanz-Garcia C, Nagy LE, Lasuncion MA, Fernandez M, Alemany S. Cot/tpl2 participates in the activation of macrophages by adiponectin. J Leukoc Biol. 2014;95:917–930. doi: 10.1189/jlb.0913486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- *81.Li J, Pan Y, Kan M, Xiao X, Wang Y, et al. Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life Sci. 2014;98:24–30. doi: 10.1016/j.lfs.2013.12.211. [DOI] [PubMed] [Google Scholar]
- *82.Zhai X, Qiao H, Guan W, Li Z, Cheng Y, et al. Curcumin regulates peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression by AMPK pathway in hepatic stellate cells in vitro. Eur J Pharmacol. 2015;746:56–62. doi: 10.1016/j.ejphar.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 83.Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, et al. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 84.Lim JY, Oh MA, Kim WH, Sohn HY, Park SI. AMP-activated protein kinase inhibits TGF-beta-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J Cell Physiol. 2012;227:1081–1089. doi: 10.1002/jcp.22824. [DOI] [PubMed] [Google Scholar]
- 85.Yang L, Chan CC, Kwon OS, Liu S, McGhee J, et al. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G902–911. doi: 10.1152/ajpgi.00124.2006. [DOI] [PubMed] [Google Scholar]
- 86.Anty R, Lemoine M. Liver fibrogenesis and metabolic factors. Clin Res Hepatol Gastroenterol. 2011;35(Suppl 1):S10–20. doi: 10.1016/S2210-7401(11)70003-1. [DOI] [PubMed] [Google Scholar]
- 87.Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677–685. doi: 10.1002/hep.21991. [DOI] [PubMed] [Google Scholar]
- *88.Kim MJ, Nagy LE, Park PH. Globular adiponectin inhibits ethanol-induced reactive oxygen species production through modulation of NADPH oxidase in macrophages: involvement of liver kinase B1/AMP-activated protein kinase pathway. Mol Pharmacol. 2014;86:284–296. doi: 10.1124/mol.114.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akifusa S, Kamio N, Shimazaki Y, Yamaguchi N, Nishihara T, et al. Globular adiponectin-induced RAW 264 apoptosis is regulated by a reactive oxygen species-dependent pathway involving Bcl-2. Free Radic Biol Med. 2009;46:1308–1316. doi: 10.1016/j.freeradbiomed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 90.Carloni V, Romanelli RG, Pinzani M, Laffi G, Gentilini P. Focal adhesion kinase and phospholipase C gamma involvement in adhesion and migration of human hepatic stellate cells. Gastroenterology. 1997;112:522–531. doi: 10.1053/gast.1997.v112.pm9024306. [DOI] [PubMed] [Google Scholar]
- 91.Jiang HQ, Zhang XL, Liu L, Yang CC. Relationship between focal adhesion kinase and hepatic stellate cell proliferation during rat hepatic fibrogenesis. World J Gastroenterol. 2004;10:3001–3005. doi: 10.3748/wjg.v10.i20.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083–8090. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- 93.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 94.Liu XJ, Yang L, Wu HB, Qiang O, Huang MH, et al. Apoptosis of rat hepatic stellate cells induced by anti-focal adhesion kinase antibody. World J Gastroenterol. 2002;8:734–738. doi: 10.3748/wjg.v8.i4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dun ZN, Zhang XL, An JY, Zheng LB, Barrett R, et al. Specific shRNA targeting of FAK influenced collagen metabolism in rat hepatic stellate cells. World J Gastroenterol. 2010;16:4100–4106. doi: 10.3748/wjg.v16.i32.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alisi A, Arciello M, Petrini S, Conti B, Missale G, et al. Focal adhesion kinase (FAK) mediates the induction of pro-oncogenic and fibrogenic phenotypes in hepatitis C virus (HCV)-infected cells. PLoS One. 2012;7:e44147. doi: 10.1371/journal.pone.0044147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *97.Kumar P, Smith T, Rahman K, Mells JE, Thorn NE, et al. Adiponectin modulates focal adhesion disassembly in activated hepatic stellate cells: implication for reversing hepatic fibrosis. FASEB J. 2014;28:5172–5183. doi: 10.1096/fj.14-253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 99.Handy JA, Fu PP, Kumar P, Mells JE, Sharma S, et al. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem J. 2011;440:385–395. doi: 10.1042/BJ20102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *100.Nakasone H, Terasako-Saito K, Yamazaki R, Sato M, Tanaka Y, et al. Impact of high-/middle-molecular-weight adiponectin on the synthesis and regulation of extracellular matrix in dermal fibroblasts. Exp Hematol. 2014;42:261–273. doi: 10.1016/j.exphem.2013.12.009. [DOI] [PubMed] [Google Scholar]