Abstract
Genetic, biochemical, pathological, and biomarker data demonstrate that Alzheimer’s disease (AD) pathology, including the initiation and progressive buildup of insoluble forms of beta-amyloid (Aβ), appears to begin ~ 10-15 years prior to the onset of cognitive decline associated with AD. Metabolic dysfunction, a prominent feature of the evolving brain pathology, is reflected in a decline of total glucose utilization. Despite decades of interest in declining glucose use in AD no detailed consideration had been given to the possibility that this decline is not just a decline in energy consumption but rather in glycolysis alone.
Glycolysis is a multi-step process that prepares the glucose molecule for oxidative phosphorylation and the generation of energy. In the normal brain, glycolysis exceeds that required for the needs of oxidative phosphorylation. Because it is occurring in a setting with adequate oxygen available for oxidative phosphorylation it is often referred to as aerobic glycolysis (AG).
AG is a biomarker of a group of metabolic functions broadly supporting biosynthesis and neuroprotection. The distribution of AG in normal young adults correlates spatially with Aβ deposition in AD patients and cognitively normal individuals with elevated Aβ. In transgenic mice extracellular fluid Aβ and lactate, a marker of AG, vary in parallel regionally and with changes in activity. Reducing neuronal activity locally in transgenic mice attenuates plaque formation suggesting that plaque formation is an activity dependent process associated with aerobic glycolysis.
Keywords: aerobic glycolysis, cerebral metabolic rate of glucose, cerebral metabolic rate of oxygen, Alzheimer’s disease, positron emission tomography
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia and the need for effective therapies for AD is great [1]. Genetic, biochemical, pathological, and biomarker data demonstrate that AD is a disorder of protein aggregation in which proteins that are normally soluble, aggregate into oligomers, fibrils, and other structures [2]. This process is linked with toxicity and neurodegeneration. The extracellular aggregation of the beta-amyloid (Aβ) peptide in the brain is a key primary event in AD pathogenesis. There are strong data that AD pathology, including the initiation and progressive buildup of insoluble forms of Aβ and tau, appears to begin ~ 10-15 years prior to the onset of cognitive decline associated with AD [3-6]. By the time even the earliest clinical symptoms of AD are detectable, Aβ accumulation is close to reaching its peak, and there is usually a substantial amount of neurofibrillary tangle pathology. Importantly, there is also significant neuronal and synaptic loss in several brain regions relevant to memory and other cognitive functions [4]. The period in which AD pathology is accumulating during which there is no detectable cognitive decline has been termed preclinical AD. Neuroimaging findings suggest that the major growth in Aβ burden occurs during a preclinical stage of AD, prior to the onset of AD-related symptoms [7-9] (Figure 1).
Figure 1.
Global Aβ deposition as measured by mean cortical binding potential (MCBP) in 146 cognitively normal adults for each of 2 [11C]Pittsburgh compound B scans, plotted by the age of the participant at the time of their scan. Fifty individuals were APOE e4 carriers (black) and 89 individuals were non-carriers (blue). The red arrow denotes the threshold (0.18) for abnormal Aβ levels. Reproduced with permission from Vlassenko et al. [7].
To understand the initiation of the AD process at the pathological level, the factors that influence the onset of AD pathology such as Aβ aggregation need to be understood. Metabolic dysfunction appears to be involved with the decline of total glucose utilization being a prominent feature of the evolving brain pathology. Reduction of glucose utilization (up to 50%) is a well-known pathological feature of AD that is known to occur early in the disease in human patients [10-12]. During early stages of AD, declines in global brain or parieto-temporal glucose utilization (45-50%) are greater than in blood flow and oxygen consumption (20-30%) [13,14]. Only in the later stages of AD do the changes in blood flow and oxygen consumption become similar to the changes in glucose use [15,13,16]. This initial discrepancy between glucose consumption and blood flow/oxygen consumption suggests that changes in AG are dominating the initial metabolic features of AG. In this review, we will discuss the current knowledge on the relation of AG to brain functioning during normal lifespan and along the course of the AD pathology (Figure 2).
Figure 2.
Images (left to right) comparing the cortical distribution of elevated AG and task-induced activity decreases in the DMN in normal with areas of decreased glucose metabolism and Aβ plaque accumulation in patients with AD.
Measurement of Aerobic Glycolysis (AG) using PET
[18F] fluorodeoxyglucose (FDG) positron emission tomography (PET) is a well-established technique for the quantitative measurement of regional brain glucose metabolism in humans. FDG is a chemical analog of glucose, where a hydrogen atom of a glucose molecule is replaced with a positron emitter [18F]. Like glucose, FDG is metabolized by hexokinase during glycolysis in the cytosol of the cell; however, unlike glucose-6-phosphate, FDG-6-phosphate is not metabolized further but accumulates intracellularly thus allowing visualization and measurement of local metabolic activity. The FDG technique is almost universally used in brain research with the tacit assumption that the glucose utilization measures energy metabolism via oxidative phosphorylation.
However, the FDG technique measures only the first step in glucose metabolism, the phosphorylation to glucose-6-phosphate by hexokinase. Because the majority of glucose entering the adult human brain is metabolized ultimately to water and carbon dioxide via oxidative phosphorylation, ignoring the other potential fates of glucose has been accepted as an operational convenience when using FDG PET with little interpretive consequences (however see [17]). Fortunately, PET can provide measurement of not only metabolic rate of glucose (CMRGlu) but also metabolic rate of oxygen (CMRO2) which allows estimation of glucose metabolism outside of oxidative phosphorylation, or aerobic glycolysis (AG) [18].
AG is traditionally assessed in terms of the molar ratio of oxygen consumption to glucose utilization (i.e., the so-called oxygen-glucose index or OGI). When all of the glucose metabolized is converted to carbon dioxide and water the OGI is 6. A number less than 6 indicates that AG is present. In our studies, we estimate AG in this traditional manner by the voxel wise division of relative CMRO2 by relative CMRGlu and scaling the resulting quotient imaging to obtain a whole brain molar ratio of 5.3 based on earlier published work [19].
While the OGI is a straightforward measure based on well-established metabolic principles, OGI images may be noisy in areas of low metabolism because they involve voxel-wise division. Also, the value of the OGI is inversely related to the degree of AG, a relationship sometimes confusing to readers. To overcome these limitations, we have defined a novel measure of AG in the brain: the glycolytic index (GI) [18,20]. The GI is obtained by linear regression of CMRGlu on CMRO2 and exhibiting the residuals scaled by 1000. Positive GI values represent more AG and negative GI values represent less glycolysis than that predicted by the line of regression. The two measures of AG, OGI and GI, are highly correlated in our data (r = −0.913, p<0.001) [18,20].
Functions of AG
It is well established that AG is a “biomarker” of a group of metabolic functions which includes biosynthesis of glycogen, proteins, lipids and nucleic acids [21]; neuroprotection through its role in managing reactive oxygen species (ROS) and apoptosis [22] which, in the context of the normal brain is involved in synaptic remodeling, learning and memory [23]; and the generation of energy for membrane pumps [24,25] (Figure 3). Cell biology studies of neurons suggest that AG may be protective with regard to the neurotoxicity of beta-amyloid (Aβ) [26,27], where high levels of AG protect and low levels do not. AG may have a relationship with cortical myelination [28]. Finally, the functions of AG, particularly those related to biosynthesis and neuroprotection, appear to be under the influence of insulin providing a connection between AG, AD and type 2 diabetes mellitus, a major risk factor for AD [29]. In what follows we provide more details on the functions of AG and illustrated with the experimental and clinical observations.
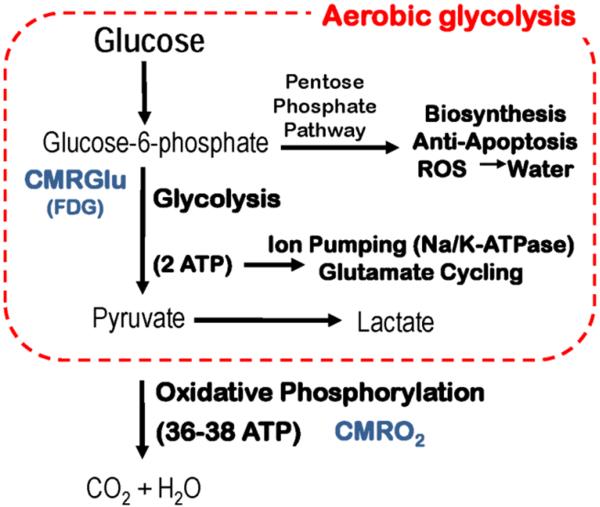
Figure 3.
The figure represents a very simplified schema of AG intended to capture some of the major functions of AG. It should be noted that measurements of glucose utilization with FDG only capture the first step of glycolysis (i. e. phosphorylation of glucose to glucose-6-phosphate) generally with the tacit assumption that AG is not important.
The regional variations in AG in the normal human brain are substantial (Figure 4). In the “resting brain” (a term used to describe the brain when not actively involved in a goal directed task) of healthy young adult, regions with significantly elevated AG are found bilaterally in prefrontal cortex, lateral parietal cortex, posterior cingulate/precuneus, lateral temporal gyrus, gyrus rectus, and caudate nuclei [18]. The highest levels of AG reside within two cortical systems, the default mode network (DMN) [30,31], which has come to be associated with a variety of self-referential functions as well as a more fundamental role in the organization of brain function; and areas in frontal and parietal cortex that have been associated with task control processes [32-34]. The DMN decreases its activity during the performance of a wide variety of goal directed tasks [35,36]. This observation led to the concept of an intrinsically organized default mode of brain function [36] that is hierarchically organized around this group of brain areas subsequently dubbed the brain’s default mode network (DMN) [37-39].
Figure 4.
AG, expressed as the GI, is illustrated here on the lateral and medial brain surface. Computed AG has been geodesically (parallel to the cortical surface) smoothed. The highest levels of AG occur in the medial frontal gyrus, precuneus, and posterior cingulate cortex. Reproduced with permission from Goyal et al. [81].
Buckner and colleagues first observed that regions within the DMN are selectively vulnerable to Aβ deposition by utilizing FDG PET and functional connectivity MRI combined with Aβ imaging [40,39]. Once Aβ deposition occurs, several groups have shown that functional connectivity within this network diminishes even while subjects are still cognitively normal suggesting that Aβ deposition is linked with network disconnection early in AD pathogenesis [41,42]. Importantly, development of brain Aβ deposition in cognitively normal people has now been shown to predict later onset of cognitive decline and AD [43-45].
In contrast to the DMN, significantly low AG is found bilaterally in the inferior temporal gyrus and throughout the cerebellum. A recent meta-analysis of studies on human brain oxygen and glucose consumption identified a similar regional variance of AG [46]. AG correlates with centrality as defined by large-scale, structural, and resting state functional connectivity studies of the human brain [18,47].
For future research the challenge will be to understand why levels of AG vary among brain systems. Although the AG overall correlates to varying degrees with CBF and various metabolic measurements, regional variations in CMRO2, the primary measure of the brain’s energy metabolism, accounted for only 6% of the variance in AG regionally in our recent study, indicating that factors other than energy requirements of brain work are contributing to the regional variations in AG in the normal human brain [18]. An attractive hypothesis is that the reason these centrally connected regions in the human brain are high in AG is that their function requires high levels of new synaptic growth and remodeling.
Energy
One factor responsible for ongoing AG in the brain is likely to be the need to support membrane-bound, ATP-dependent processes. A series of important experiments beginning in the early 1990s by Pierre Magistretti, Luc Pellerin and their colleagues [48] were able to establish that one source of the task-induced increases in AG is the energy demands of the membrane pump Na,K-ATPase in astrocytes [49,50]. Increases in activity in the adult mammalian brain are largely caused by the release of the excitatory neurotransmitter glutamate. Glutamate is removed from the synapse by uptake into astrocytes in a sodium dependent process. Sodium must then be removed from the astrocyte by Na+,K+-ATPase. The energy needed for this process comes from glycolysis which produces a net 2 ATP per molecule of glucose consumed (Figure 3). One might argue that it is inefficient to fuel such a critical pump by glycolysis given such a low yield of ATP for each molecule of glucose used. Moreover, recent studies using fluorescence resonance energy transfer nanosensors for glucose, lactate and ATP combined with a mathematical model of ATP dynamics demonstrated that Na+ pump does not discriminate between ATP generated by glycolysis and ATP generated by oxidative phosphorylation in mitochondria, and that the major part of pump requirements is satisfied by mitochondrial ATP [51]. Nevertheless, the advantage AG has over oxidative phosphorylation is that the ATP is produced at least 2 times faster [52]. Thus, where speed is important, such as at an excitatory synapse, one might posit that glycolysis is the way to go. Regardless of the reason, it is the case that Na+,K+-ATPase is fueled by glycolysis in all membrane systems in which it is found [24,25,53,54] with lactate as a byproduct.
That glycolysis might supply ATP for membrane bound Na+,K+-ATPase is neither new nor restricted to the brain. Data from human red cell membranes [24], skeletal muscle [25], vascular smooth muscle [53], and neurons [54] all provide independent evidence in support of such a possibility. Because of the unique role of the astrocyte in using AG for glutamate cycling, it is interesting to note that the ratio of neurons to non-neuronal cells can vary greatly in the human brain [55]. For example, the cerebral cortex contains 19% of the brain’s neurons and 72% of non-neuronal cells, whereas in the cerebellum the percentages are reversed (i.e., 80% and 19%, respectively) [55]. This observation suggests that one of the factors contributing to the regional variation in AG may be the percentage of non-neuronal cells.
A more extended view of the role of AG in the generation of ATP has emerged from the observation that glycolytic enzymes are found in the postsynaptic density (PSD) [54], a very dynamic complex containing various ion channel proteins, synaptic receptors, and signal transduction pathways [56] that are turning over and being replaced with half-lives of minutes, hours, days, or weeks [57]. In the PSD, Na+,K+-ATPase has been identified as critical for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ( AMPA) receptor turnover [58]. Na+,K+-ATPase dysfunction leads to a loss of cell-surface expression of AMPA receptors and a long-lasting depression in synaptic transmission [58]. If one of the reasons for positioning glycolytic enzymes in the PSD is to fuel the Na+,K+-ATPase pump, then AG assumes a critical role in synaptic plasticity. With regard to the energy needs of the PSD, it should be noted that mitochondria are rarely seen in dendritic spines [59,60] in contrast to axons [61], notwithstanding the fact that lactate transporters designed to import lactate into dendritic spines as mitochondrial fuel are present [62].
Lactate can be converted to pyruvate and enter the Kreb’s cycle where most of the brain’s ATP is produced stimulating much debate as to the importance of lactate as an energy substrate for neurons [63-65]. Some lactate, of course, simply leaves the brain in venous blood, especially when arterial lactate is lower than brain lactate, such as during physical rest [66]. However, lactate may be used in the brain and influence neuronal function in several ways and by different mechanisms. It has been recently suggested that lactate may act not only as a fuel but also as an intercellular messenger helping active brain areas to recruit metabolic resources from neighboring regions [66]. The transfer of lactate from astrocytes to neurons was recently shown to be necessary for the establishment of long-term memory in an inhibitory avoidance paradigm and for the maintenance of long-term potentiation in the rodent hippocampus [67]. It has been reported that lactate can modulate neuronal activity through receptor-mediated pathway [68] and it may stimulate synaptic plasticity-related gene expression in neurons by a mechanism associated with changes in the intracellular redox state and the modulation of the N-methyl-D-Aspartate (NMDA) receptor activity and its downstream signaling cascade Erk1/2 [69]; both publications providing important clues for better understanding the mechanisms and bases of the role of lactate in the establishment of long-term memory.
Neuroprotection
Glucose plays a critical role in protecting the brain against reactive oxygen species (ROS) and avoiding oxidative stress [70], putatively an important factor in the pathophysiology of AD [71,72]. The reduction of peroxides catalyzed by glutathione peroxidase generates glutathione disulfide; however glutathione can be regenerated from glutathione disulfide by the action of glutathione reductase using NADPH as an electron donor. Glutathione in its reduced form is a major scavenger of ROS, therefore constant NADPH supply is critical for the maintenance of cellular redox state. NADPH is mainly provided by the metabolism of glucose through the pentose phosphate shunt (PPP) when glucose-6-phosphate is diverted from glycolysis into the PPP by coordinated changes in the activities of phosphofructokinase-1 (PFK-1), the rate limiting enzyme for glycolysis, and glucose-6-phosphate dehydrogenase (G6PDH) the rate limiting enzyme for the PPP. The protective role of the brain PPP against ROS and apoptosis has been demonstrated in transgenic mice with increased expression of G6PDH when challenged with oxidative stress [73]. Also, up regulation of the PPP is a distinctive feature of surviving brain cells from individuals with DAT [74]. Of note, G6PDH is famous for the most common enzyme deficiency worldwide [75].
Biosynthesis
PPP plays important role not only in maintenance of the cellular redox state and protection against ROS, but also in provision of important components of biosynthesis [76]. Through PPP glucose makes important contributions to anabolic processes in all organs of the body providing intermediates for cellular proliferation including NADPH, nucleotides for DNA replication [77], and intermediates for fatty acid synthesis [78,79] (Figure 3). In animal experiments, estimates of the amount of glucose entering the PPP in the mature brain have been generally low [80].
In the developing nervous system, AG appears to play a substantial role, consistent with its potential to provide the building blocks for cell development and proliferation. In recent meta-analysis of prior studies of whole-brain glucose and oxygen consumption across the human lifespan, we identified several studies of human subjects dating back to 1953 that obtained quantitative measurements of whole-brain or cerebral glucose and oxygen consumption during various stages of life, including premature neonates, growing normal children, young adults, and the elderly [81]. We combined the reported values of whole-brain or cerebral glucose and oxygen consumption from these studies in order to create a summary representation of these metabolic variables as a function of age across the human lifespan.
We demonstrated that during much of adult life, brain glucose consumption is only slightly greater than that expected on the basis of brain oxygen consumption, and whole-brain AG disappears in the elderly (Figure 5A) [81]. However, AG increases dramatically during childhood and accounts for approximately one-third of total glucose consumption at its peak age of ~5 years, when total glucose consumption exceeds adult levels by a factor of two. To compare glucose and oxygen consumption to relative changes in cerebral blood flow, we presented the data in terms of a ratio relative to adult values (Figure 5B). The decrease in AG from young adulthood to a more advanced age has been described previously [82]; in that study, the whole-brain OGI was 4.7 in normal subjects with mean age 21 in comparison to an OGI of 5.9 in older subjects with mean age of 71. Thus, brain AG peaks precisely when synaptic development peaks in the human brain during early childhood.
Figure 5.
A. Whole-brain or cerebral total glucose consumption rates (blue circles) and a fit obtained with loessR (blue line) demonstrate an approximate doubling of CMRglu during early childhood. The expected glucose consumption based on measured oxygen consumption rates is also plotted (red circles and line). This also increases during early childhood, though it did so less than the measured changes in CMRglu, suggesting that approximately 30% of the CMRglu during childhood is in excess of oxygen consumption (i.e., AG). B. CMRglu (blue), CMRO2 (red), and CBF (orange) were plotted across the lifespan as normalized proportions of average adult values. This analysis shows an approximate 2-fold rise in CMRglu and 1.5-fold rise inCMRO2 during early childhood. Interestingly, CBF also increases 2-fold during early childhood, matching the changes in CMRglu, but it then appears to more closely follow changes in CMRO2 during adulthood. Reproduced with permission from Goyal et al. [81].
The next logical suggestion was that, if AG is increased in the developing brain, then regionally high AG in the adult human brain may reflect persistent developmental processes. The persistence of developmental features and processes into adulthood is referred to as ‘‘neoteny’’ [83]. To measure regional transcriptional neoteny in the human brain, we used the Brain Span Study, a publicly available, whole-genome gene expression microarray data set representing 16 different brain regions over 50 human brains across the fetal-adulthood lifespan [84]. To investigate the relationship between this regional neoteny index and AG, we assigned each of the 16 regions an AG value with regional PET imaging data previously obtained by our laboratory in resting, healthy young adults [18]. Across these 16 regions, the neoteny index was significantly correlated with AG. These results support the hypothesis that that AG supports synaptic and neuritic formation as well as turnover, which most likely persists throughout the lifespan [57].
Of note, tasks involving a significant learning component induce prolonged elevations of glycolysis [85]. This observation is of particular interest in that during the performance of the learning task brain blood flow, glucose consumption and lactate production are all elevated, an observation consistent with earlier work by us and others [86,87]. However, during the post-task period Madsen and colleagues [85] demonstrated that when blood flow and lactate production returned to control levels glucose consumption remained elevated. Oxygen consumption did not change at any point during their experiment. Because of the relationship between lactate production, redox potential and blood flow [88,89] it is attractive to posit that glucose has been diverted from glycolysis to serve some other important functions (presumably biosynthetic), and no lactate was produced.
Refining our understanding of how glucose is used in this critical period of development should receive high priority in future research. The necessary data must come from parallel measurements of CMRO2 and CMRGlu that are required for the quantification of AG. Also of interest would be regional studies of the activity of enzymes critical for the control of the brain’s intermediary metabolism during development.
AG and AD pathology
Over the past several years, we explored factors that dynamically regulate Aβ levels in the extracellular space of the brain in vivo. Although molecular events surrounding secretase cleavage of APP are well understood, the cellular processes regulating Aβ production and the subsequent release of Aβ by neurons are poorly characterized. It has been shown that neuronal activity modulates Aβ production via activation of muscarinic, serotonin, and glutamate [90-92]. Modulating neuronal activity alters extracellular soluble Aβ levels in organotypic brain slices [93]. Our colleagues from Washington University demonstrated using APP transgenic and wild-type mice that synaptic activity dynamically modulates steady-state interstitial fluid (ISF) Aβ level in vivo rapidly over minutes to hours [94]. The direct evidence in vivo has been provided that synaptic activity-induced increase in endocytosis also drives more APP into the endocytic compartment, ultimately resulting in increased Aβ production and release [95].
Obsevation that the concentration of ISF Aβ is closely associated with Aβ plaque growth in vivo [96] suggested a hypothesis that the steady-state concentration of ISF Aβ in each brain region early in life would be predictive of the degree of subsequent plaque accumulation. To test this hypothesis, in vivo microdialysis was performed to measure the steady-state concentration of ISF Aβ in multiple brain regions of young, 3 month old Tg2576 (APPswe) Tg mice prior to the onset of plaque deposition. Steady-state levels of ISF Aβx-40 and Aβx-42 in hippocampus, striatum, barrel cortex and piriform cortex of these mice were commensurate with the degree of Aβ plaque deposition in each region later in life at 18 months of age suggesting that regional differences in ISF Aβ levels throughout life mediate the later spatial distribution of plaque deposition.
No evidence was found that Aβ processing from APP or Aβ clearance were related to the regional amount of plaque deposition [97]. It is well established that physiological neural activity stimulates AG in human brain and that the concentration of ISF lactate, a marker of AG, is increased during glutamatergic transmission [86,48]. As it was found increased or decreased neuronal activity co-varied with Aß generation and the concentration of ISF lactate in barrel cortex, piriform cortex, hippocampus, and striatum in Tg2576 mice prior to plaque deposition was proportional to steady-state ISF Aβ levels and subsequent plaque deposition in all brain areas examined [97]. This finding suggests that the spatial distribution of ISF Aβ levels and plaque deposition is closely related to regional differences in chemical synaptic activity and AG.
To determine whether a causal relationship between physiological synaptic activity and ISF Aβ concentration exists in vivo, the mouse whisker-barrel system has been utilized. In rodents, each whisker is somatotopically mapped onto a defined cluster of neurons called a “barrel” in primary somatosensory cortex. Neurons in each barrel respond to stimulation of the whisker to which they are connected. Whisker stimulation increases, while whisker removal decreases, synaptic activity in barrel cortex [98,99]. Synaptic activity in barrel cortex of young Tg2576 mice has been acutely decreased by trimming all mystacial vibrissae on one side of the facial pad while performing in vivo microdialysis in barrel cortex of the contralateral hemisphere. This manipulation significantly reduced ISF Aβ levels by up to 25% by 9 hours after vibrissae removal [97]. ISF lactate levels were also reduced during the same period after vibrissae removal, suggesting that the reduction in ISF Aβ is due to reduced synaptic activity and concomitantly reduced AG. Neuronal activity was increased by stimulating the whiskers and assessed ISF Aβ levels by in vivo microdialysis. Stimulation caused a rapid increase in Aβ levels [97]. In 7 month-old APPswe/ PS1δE9 (APP/PS1) mice, a long-term (28 days) whisker deprivation prevented growth of Aβ plaques in the deprived barrel cortex, while significant plaque growth was observed in the intact hemisphere. Moreover, formation of new plaques was also significantly reduced in the deprived hemisphere compared to the intact hemisphere (Figure 6).
Figure 6.
(a,b) Representative multiphoton micrographs of individual amyloid plaques in barrel cortex in control hemisphere of APP/PS1 mice before (a) and after (b) 28 d of unilateral vibrissal deprivation. (c,d) Representative multiphoton micrographs of amyloid plaques in barrel cortex in vibrissae-deprived hemisphere of APP/PS1 mice before (c) and after (d) 28 d of vibrissal deprivation. (e,f) Quantification of mean plaque growth (e) and new plaque formation (f) in vibrissae-deprived and control hemispheres (n = 6 mice; two-tailed t-test). Arrows, existing amyloid plaques; arrowhead, newly formed amyloid plaque. Scale bar, 50 μm; *P < 0.05; **P < 0.01. Values represent mean ± s.e.m. Reproduced with permission from Bero et al. [97].
In the human brain in preclinical AD and in symptomatic AD, we have demonstrated that when Aβ accumulates it does so in a distribution that closely mirrors that of elevated AG in the resting state of healthy young adults [18,20] (Figure 7). Both the highest levels of AG and Aβ plaque deposition at the preclinical and symptomatic stages of AD are concentrated in the DMN. We would like to stress that these elevated levels of AG are not just the consequence of elevated levels of overall energy metabolism. For example, primary visual cortex shows a very high level of glucose utilization and the highest levels of oxygen utilization (the primary measure of energy consumption) which in the primary visual cortex of the normal adult human is 35% above the brain mean [18]. Yet primary visual cortex accumulates low levels of Aβ and exhibits a minimal decline in glucose utilization in DAT [100,101,18]. The level of AG in primary visual cortex is at the brain mean.
Figure 7.
Maps showing lateral and medial cortical surfaces of the human brain on which are depicted the mean distribution of AG in units of the GI in 33 neurologically normal young adults and 11C-PIB binding potentials in 11 individuals with DAT. Reproduced with permission from Vlassenko et al. [20].
These findings, taken together, strongly suggest that physiological synaptic activity associated with AG regulates ISF Aβ levels and Aβ plaque formation and growth in vivo. Regional differences in such synaptic activity in humans may render the DMN vulnerable to Aβ plaque deposition through lifetime modulation of ISF Aβ levels.
These observations compliment previous experiments and work we have done with PET in humans [36,18,20,81,97] and suggest that the DMN is involved in the early development of AD pathology prior brain atrophy or cognitive impairment. It seems reasonable to suggest that the biology of learning and memory reflected in regional differences metabolism, gene expression and levels of cellular activity not only confer an ability to learn and adapt to an ever changing environment but also carry a potential risk of later developing Alzheimer’s disease later life. The idea that the capacity for synaptic plasticity might carry risks as well as necessary benefits is an idea posed by others some time ago (e.g., see [102]) but rarely voiced in present discussions regarding the pathophysiology of Alzheimer’s disease. We believe that this issue deserves a discussion in more details in light of the exciting new information available to us.
A very important next step in evaluating the role of AG in the pathophysiology of Alzheimer’s disease in humans is obtaining detailed longitudinal quantitative measurements of regional brain circulation, oxygen consumption and glucose use (total as well as the fraction devoted to AG) with PET in individuals at risk for or symptomatic with AD. This should be done in a cohort of well characterized individuals to permit comparison of these measures of brain metabolism with state-of-the-art biomarkers of AD and clinical and neuropsychological assessments in the same individuals. This work is highly significant for many reasons. It may extend significantly our understanding of the role of glucose in brain function beyond supplying energy via oxidative phosphorylation. It will also provide important new insights into the pathophysiology of AD and specifically Aβ plaques accumulation, its relationship with established risk factors for AD and potential for aggravating the development of AD starting from the very early, preclinical stages of AD pathology and continuing throughout the course of the disease until substantial cognitive decline. Characterizing the relationship of changes in clinical assessments and biomarkers of AD to AG, regional circulation and metabolism will provide necessary background for the development of treatment approaches designed to reduce plaque formation by modulating synaptic function and for the establishment of AG not only as a biomarker of synaptic function but also as a useful marker of the efficiency of such treatments.
Footnotes
Compliance with Ethics Guidelines
Conflict of interest The authors, Andrei G. Vlassenko, MD, PhD, and Marcus E. Raichle declare no conflict of interest regarding this article.
Human and animal studies For studies previously published by the authors all institutional and national guidelines for the care and use of laboratory animals were followed; human studies were approved by the Human Research Protection Office and Radioactive Drug Research Committee, and written informed consent was provided by all participants or their caregivers.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Science Translational Medicine. 2011;3(77) doi: 10.1126/scitranslmed.3002369. 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. AnnNeurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. JMolNeurosci. 2001;17(2):101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlassenko AG, Mintun MA, Xiong C, Sheline YI, Goate AM, Benzinger TL, Morris JC. Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [(11) C]Pittsburgh compound B data. Ann Neurol. 2011;70(5):857–861. doi: 10.1002/ana.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Jr., Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, Petersen RC. Brain beta-amyloid load approaches a plateau. Neurology. 2013;80(10):890–896. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 10.Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, Jagust WJ, Reiman EM. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45(4):1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. NEnglJMed. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 12.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. ProcNatlAcadSciUSA. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. JNeural TransmPark DisDementSect. 1991;3(1):1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 14.Fukuyama H, Ogawa M, Yamauchi H, Yamaguchi S, Kimura J, Yonekura Y, Konishi J. Altered cerebral energy metabolism in Alzheimer's disease: a PET study. JNuclMed. 1994;35(1):1–6. [PubMed] [Google Scholar]
- 15.Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. J Alzheimers Dis. 2002;4(3):225–232. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- 16.Lying-Tunell U, Lindblad BS, Malmlund HO, Persson B. Cerebral blood flow and metabolic rate of oxygen, glucose, lactate, pyruvate, ketone bodies and amino acids. Acta Neurol Scand. 1981;63(6):337–350. doi: 10.1111/j.1600-0404.1981.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Rodriguez P, Fernandez E, Bolanos JP. Underestimation of the pentose-phosphate pathway in intact primary neurons as revealed by metabolic flux analysis. J Cereb Blood Flow Metab. 2013;33(12):1843–1845. doi: 10.1038/jcbfm.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol. 1970;23(5):394–403. doi: 10.1001/archneur.1970.00480290014002. [DOI] [PubMed] [Google Scholar]
- 20.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. Proc Natl Acad Sci U S A. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annual review of cell and developmental biology. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 22.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Sheng M. Caspases in synaptic plasticity. Molecular brain. 2012;5:15. doi: 10.1186/1756-6606-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercer RW, Dunham PB. Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. The Journal of general physiology. 1981;78(5):547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto K, Wang W, Rounds J, Chambers EA, Jacobs DO. ATP from glycolysis is required for normal sodium homeostasis in resting fast-twitch rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281(3):E479–488. doi: 10.1152/ajpendo.2001.281.3.E479. [DOI] [PubMed] [Google Scholar]
- 26.Newington JT, Pitts A, Chien A, Arseneault R, Schubert D, Cumming RC. Amyloid beta resistance in nerve cell lines is mediated by the Warburg effect. PLoS One. 2011;6(4):e19191. doi: 10.1371/journal.pone.0019191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newington JT, Rappon T, Albers S, Wong DY, Rylett RJ, Cumming RC. Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid beta and other toxins by decreasing mitochondrial respiration and ROS production. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.366195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasser MF, Goyal MS, Preuss TM, Raichle ME, Van Essen DC. Trends and properties of human cerebral cortex: Correlations with cortical myelin content. Neuroimage. 2014;93P2:165–175. doi: 10.1016/j.neuroimage.2013.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims-Robinson C, Kim B, Rosko A, Feldman EL. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol. 2010;6(10):551–559. doi: 10.1038/nrneurol.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097-1089. [DOI] [PubMed] [Google Scholar]
- 31.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 32.Braver TS, Barch DM. Extracting core components of cognitive control. Trends Cogn Sci. 2006;10(12):529–532. doi: 10.1016/j.tics.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. JCognitNeurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 36.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox MD, Raichle M. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 38.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 44.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 45.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyder F, Fulbright RK, Shulman RG, Rothman DL. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J Cereb Blood Flow Metab. 2013;33(3):339–347. doi: 10.1038/jcbfm.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 48.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belanger M, Allaman I, Magistretti P. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32(7):1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Moncada I, Barros LF. Non-preferential fuelling of the Na(+)/K(+)-ATPase pump. The Biochemical journal. 2014;460(3):353–361. doi: 10.1042/BJ20140003. [DOI] [PubMed] [Google Scholar]
- 52.McGilvery RW, Goldstein GW. Biochemistry: A Functional Approach. Saunders; Philadelphia: 1983. [Google Scholar]
- 53.Campbell JD, Paul RJ. The nature of fuel provision for the Na+,K(+)-ATPase in porcine vascular smooth muscle. J Physiol. 1992;447:67–82. doi: 10.1113/jphysiol.1992.sp018991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu K, Aoki C, Elste A, Rogalski-Wilk AA, Siekevitz P. The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc Natl Acad Sci U S A. 1997;94(24):13273–13278. doi: 10.1073/pnas.94.24.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513(5):532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7(7):563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 58.Zhang D, Hou Q, Wang M, Lin A, Jarzylo L, Navis A, Raissi A, Liu F, Man HY. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J Neurosci. 2009;29(14):4498–4511. doi: 10.1523/JNEUROSCI.6094-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 61.Cai Q, Sheng ZH. Mitochondrial transport and docking in axons. Exp Neurol. 2009;218(2):257–267. doi: 10.1016/j.expneurol.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierre K, Chatton JY, Parent A, Repond C, Gardoni F, Di Luca M, Pellerin L. Linking supply to demand: the neuronal monocarboxylate transporter MCT2 and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor GluR2/3 subunit are associated in a common trafficking process. Eur J Neurosci. 2009;29(10):1951–1963. doi: 10.1111/j.1460-9568.2009.06756.x. [DOI] [PubMed] [Google Scholar]
- 63.Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23(11):1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- 64.Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23(11):1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 65.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 66.Barros LF. Metabolic signaling by lactate in the brain. Trends Neurosci. 2013;36(7):396–404. doi: 10.1016/j.tins.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bozzo L, Puyal J, Chatton JY. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS One. 2013;8(8):e71721. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11(5):388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 71.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7(4):278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31(5):251–256. doi: 10.1016/j.tins.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mejias R, Villadiego J, Pintado CO, Vime PJ, Gao L, Toledo-Aral JJ, Echevarria M, Lopez-Barneo J. Neuroprotection by transgenic expression of glucose-6-phosphate dehydrogenase in dopaminergic nigrostriatal neurons of mice. J Neurosci. 2006;26(17):4500–4508. doi: 10.1523/JNEUROSCI.0122-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron. 2003;39(1):43–56. doi: 10.1016/s0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- 75.Vulliamy T, Mason P, Luzzatto L. The molecular basis of glucose-6-phosphate dehydrogenase deficiency. Trends in genetics : TIG. 1992;8(4):138–143. doi: 10.1016/0168-9525(92)90372-B. [DOI] [PubMed] [Google Scholar]
- 76.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends in biochemical sciences. 2014;39(8):347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janniere L, Canceill D, Suski C, Kanga S, Dalmais B, Lestini R, Monnier AF, Chapuis J, Bolotin A, Titok M, Le Chatelier E, Ehrlich SD. Genetic evidence for a link between glycolysis and DNA replication. PLoS One. 2007;2(5):e447. doi: 10.1371/journal.pone.0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. ProcNatlAcadSciUSA. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaitonde MK, Jones J, Evans G. Metabolism of glucose into glutamate via the hexose monophosphate shunt and its inhibition by 6-aminonicotinamide in rat brain in vivo. ProcRSocLond B BiolSci. 1987;231(1262):71–90. doi: 10.1098/rspb.1987.0036. [DOI] [PubMed] [Google Scholar]
- 80.Dringen R, Hoepken HH, Minich T, Ruedig C, Gibson GE, Dienel GA. Pentose Phosphate Pathway and NADPH Metabolism. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd. Springer; New York: 2007. pp. 41–62. Brain Energetics. Integration of Molecular and Cellular Processes. [Google Scholar]
- 81.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell metabolism. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dastur DK. Cerebral blood flow and metabolism in normal human aging, pathological aging, and senile dementia. J Cereb Blood Flow Metab. 1985;5(1):1–9. doi: 10.1038/jcbfm.1985.1. [DOI] [PubMed] [Google Scholar]
- 83.Bufill E, Agusti J, Blesa R. Human neoteny revisited: The case of synaptic plasticity. American journal of human biology : the official journal of the Human Biology Council. 2011;23(6):729–739. doi: 10.1002/ajhb.21225. [DOI] [PubMed] [Google Scholar]
- 84.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bulow J, Holm S, Wildschiodtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15(3):485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- 86.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 87.Blomqvist G, Seitz RJ, Sjogren I, Halldin C, Stone-Elander S, Widen L, Solin O, Haaparanta M. Regional cerebral oxidative and total glucose consumption during rest and activation studied with positron emission tomography. Acta Physiol Scand. 1994;151(1):29–43. doi: 10.1111/j.1748-1716.1994.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 88.Mintun MA, Vlassenko AG, Rundle MM, Raichle ME. Increased lactate/pyruvate ratio augments blood flow in physiologically activated human brain. Proc Natl Acad Sci U S A. 2004;101(2):659–664. doi: 10.1073/pnas.0307457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vlassenko AG, Rundle MM, Raichle ME, Mintun MA. Regulation of blood flow in activated human brain by cytosolic NADH/NAD+ ratio. Proc Natl Acad Sci U S A. 2006;103(6):1964–1969. doi: 10.1073/pnas.0510632103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 2000;48(6):913–918. [PubMed] [Google Scholar]
- 91.Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49(5):671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 92.Lesne S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci. 2005;25(41):9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 94.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 95.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM. Characterizing the Appearance and Growth of Amyloid Plaques in APP/PS1 Mice. J Neurosci. 2009;29(34):10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melzer P, Van der Loos H, Dorfl J, Welker E, Robert P, Emery D, Berrini JC. A magnetic device to stimulate selected whiskers of freely moving or restrained small rodents: its application in a deoxyglucose study. Brain Res. 1985;348(2):229–240. doi: 10.1016/0006-8993(85)90441-x. [DOI] [PubMed] [Google Scholar]
- 99.Durham D, Woolsey TA. Acute whisker removal reduces neuronal activity in barrels of mouse SmL cortex. J Comp Neurol. 1978;178(4):629–644. doi: 10.1002/cne.901780403. [DOI] [PubMed] [Google Scholar]
- 100.Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Clinical deterioration in probable Alzheimer's disease correlates with progressive metabolic impairment of association areas. Dementia. 1994;5(1):36–41. doi: 10.1159/000106692. [DOI] [PubMed] [Google Scholar]
- 101.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. AnnNeurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 102.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24(3):521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]