Abstract
Multiple sclerosis (MS) is a chronic neurological disorder, often affecting young people. Comorbid disorders such as depression, anxiety and hypertension are common and can affect disease course, treatment, and quality of life (QOL) of people with MS (PwMS). The associations between comorbidities, body mass index (BMI) and health outcomes are not well studied in MS, although research shows most PwMS are overweight. Most data on the prevalence of comorbidities and obesity in PwMS comes from North American populations. This study describes the prevalence of comorbidities, overweight and obesity and associations with modifiable factors in an international sample of PwMS recruited online through social media, MS societies and websites. The online survey consisted of validated and researcher-devised instruments to assess self-reported health outcomes and lifestyle behaviors. Of the 2399 respondents, 22.5% were overweight, 19.4% were obese and 67.2% reported at least one comorbidity, with back pain (36.2%), depression (31.7%), anxiety (29.1%) and arthritis (13.7%) most prevalent and most limiting in daily activities. Obesity and most comorbid disorders were significantly more prevalent in North America. Obese participants were more likely to have comorbidities, especially diabetes (OR 4.8) and high blood pressure (OR 4.5) but also depression (OR 2.2). Being overweight, obese, or a former, or current smoker was associated with an increase in the number of comorbidities; while healthy diet, physical activity (borderline significant) and moderate alcohol consumption were associated with decreased number of comorbidities. Increasing number of comorbidities was related to worse QOL, increased odds of disability and prior relapse. Obese PwMS had higher odds of disability and lower QOL. The associations between BMI, comorbidities and health outcomes are likely to be bi-directional and associated with lifestyle behaviors. Preventing and treating comorbid disorders and obesity in PwMS is warranted, and advice regarding healthy and risky lifestyle may assist in improving health outcomes.
Introduction
Multiple sclerosis (MS) is the most common serious neurological disorder in young people and is often progressively disabling. Recently there has been a growing interest in the influence of comorbidities on the health of people with MS (PwMS); comorbidities have been shown to be associated with increased hospitalization[1], rate of progression to disability [2, 3], and decreased quality of life (QoL)[4]. Comorbidities are associated with increased mortality risk in the general population as well as in PwMS[5]. PwMS may have specific risks for comorbid disorders due to side effects of pharmacological therapies[6] or due to underlying pathology. Further, adverse health behavior including being overweight and obese, smoking and sedentary behaviour, which are known risk factors for adverse health outcomes, are common in PwMS[7]. Additionally, PwMS may have an increased chance of being diagnosed with a comorbid disorder as they make more use of health services[8].
Marrie’s group recently completed a series of systematic reviews on the incidence and prevalence of comorbidities in PwMS worldwide and concluded that findings are inconsistent in Europe and North America, and very little data exist for other regions where MS is less prevalent[9]. The most prevalent comorbidities identified in their meta-analysis were depression, anxiety and hypertension [9]. The prevalence of obesity was not included in this work, but several studies have reported that the majority of people with MS are overweight or obese[7, 10–12]. While being overweight or obese increases the risk for MS and comorbid disorders, body mass index (BMI) does not seem to be a predictor of disability progression[10].
This study examined the prevalence of comorbidities, overweight, and obesity, and their associations with health outcomes in a large international cross-sectional sample of PwMS who self-enrolled in the Health Outcomes and Lifestyle Interventions in a Sample of people with Multiple Sclerosis (HOLISM) study[13].
Methods
The methods of the HOLISM study have been described in detail elsewhere[13] but relevant details are summarised here. The study was approved by the Human Research Ethics Committee of St Vincent’s Hospital Melbourne (LRR 055/12).
Participants
Participants were recruited online in 2012 using social media including Facebook and Twitter, MS society websites and newsletters and other websites and forums all over the world specifically for people with MS. Many of these media had a healthy lifestyle focus and most were in English. A hyperlink took people to a SurveyMonkey webpage detailing participant information where participants consented to participate in the study and confirmed that they were 18 years or older before entering the survey. Analyses were only performed for those completing the Self-administered Comorbidity Questionnaire (SCQ) and reporting a physician confirmed diagnosis of MS.
Tools and instruments
This English-language survey took approximately 40 minutes to complete and comprised validated and researcher-devised or amended questionnaires. All data were self-reported. Several multiple choice items assessed demographic variables of age, gender, country of residence, height and weight. Country of residence was grouped into 4 categories to enable analysis: United States and Canada; Australia and New Zealand; Europe; and Other.
Health outcome measures
Comorbidity was assessed using the SCQ [14], which is an efficient method of assessing the presence of comorbidities when medical record review is impractical, and whether the comorbid condition is treated and whether it limits daily activities. For the purpose of this study, two arthritic conditions were combined into one, and we added anxiety as a listed comorbid condition. The SCQ has previously been used to study PwMS[15] and correlates modestly with the Charlson Comorbidity Index.
Disability was measured by the Patient-Determined Disease Steps (PDDS), a self-administered surrogate tool for the commonly used clinician-administered Expanded Disability Status Scale (EDSS)[16]. For the purpose of data analysis, scores from 0 (normal) to 8 (bed bound) were collapsed into two categories: scores of 0–3 (being able to walk more than 25 feet without assistance) were categorized as no/mild disability; scores of >3 were categorized as moderate/severe disability.
Health related quality of life (HRQOL) was measured by the widely used and validated Multiple Sclerosis Quality Of Life-54 (MSQOL-54)[17], which consists of 54 items, including the 36-item Short Form Health Survey (SF-36), which generates two composite scores: the physical and mental health composites and 12 subscales.
Relapse rate was assessed by self-reporting of how many medically confirmed relapses participants with relapsing-remitting MS had had in the previous 12 months. Participants were categorized as having reported 1 or more relapses in the previous 12 months or no relapses.
Modifiable factors
BMI was calculated by dividing weight (in kilograms) by height2 (in centimetres) and categorized according to World Health Organization (WHO) standards so that those with BMI below 18.5 were classed as underweight, BMI of 18.5 to 25 as normal, BMI of 25 to 30 as overweight and BMI of 30 and over as obese. Physical activity was categorized as low, moderate or high, as assessed with the International Physical Activity Questionnaire (IPAQ)[18], and diet was assessed using the Dietary Health Questionnaire (DHQ), on a 1–100 point scale, and has been previously described for this sample[19]. Alcohol consumption, tobacco smoking, meditation practice, omega-3 fatty acid and vitamin D supplementation were assessed using researcher-devised items and prevalence in this sample has also been previously been described[20–23]. Alcohol consumption was categorized as follows: low (<15g/week), moderate (up to 30g/day for females; up to 45g/day for men), or high for consumption above those amounts; and smoking status was self-reported. Participants were asked to select if they were currently using a disease modifying drug (including glatiramer acetate, interferons, natalizumab, alemtuzumab, daclizumab, rituximab, fingolimod, dimethyl fumarate, teriflunomide, cladribine, laquinimod, and azathioprine) as described in detail previously[24].
Conceptual model
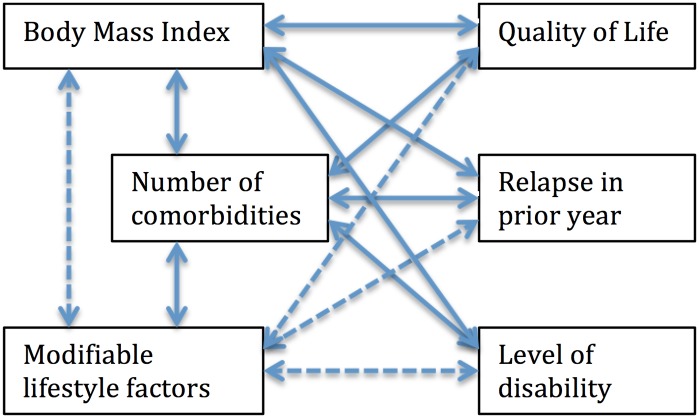
The associative conceptual model used to guide analysis is shown in Fig 1. Hypothesised associations between variables shown in this model were investigated, except for the associations depicted by dashed arrows, as these have been investigated separately [18–24].
Fig 1. Associative conceptual model.
Data analysis
Data were analyzed using SPSS version 23.0 (IBM Corporation). Continuous data were reported using mean and 95% confidence interval (95% CI) or standard error (SE), or median (IQR) and categorical data using number (N) and percentage (%). Pearson’s Chi Square or Fisher’s exact test were used to compare categorical variables, with standardized adjusted residuals indicating over or underrepresentation of groups. Spearman’s rho was used for non-parametric correlation. Analysis of variance was used to compare continuous variables across three or more groups with LSD post-hoc testing or Games-Howell if there was homogeneity of variances. Kruskal Wallis testing was used for comparisons between groups if the variable was not normally distributed with Bonferroni adjustments applied to non-parametric post-hoc tests. Multiple regression (enter method) was used to identify independent variables associated with number of comorbidities and HRQOL. It was ensured that data satisfied the assumptions of normality, linearity, and homoscedasticity by plotting the studentized residuals against the unstandardized predicted values of the dependent variable and assessing the spread. Independence of residuals was assessed by checking the Durbin Watson statistic (values between 1 and 3 were accepted). Variance inflation factor <5 was used as the criterion for absence of multicollinearity. Only correlations of < .70 between independent variables were accepted as inspected from the correlation matrices. Binary logistic regression was used to identify independent variables associated with disability and relapse. Due to participant numbers being low in some regions of the world, regions were collapsed in 4 categories: United States (US) and Canada; Australia and New Zealand; Europe; and Other.
Alpha was set at 0.05 and two tailed tests of significance were used in all instances. Varying denominators are due to varying item completion.
Results
The characteristics of the whole HOLISM sample have been described elsewhere[13]. Here we report data on the 2399 participants who completed the SCQ; 407 (17.7%) men and 1892 (82.3%) women, with an average age of 45.5 years (SD 10.6), diagnosed with MS for a median of 6 years (IQR 3–12), with most reporting having relapsing-remitting MS (1472, 61.6%).
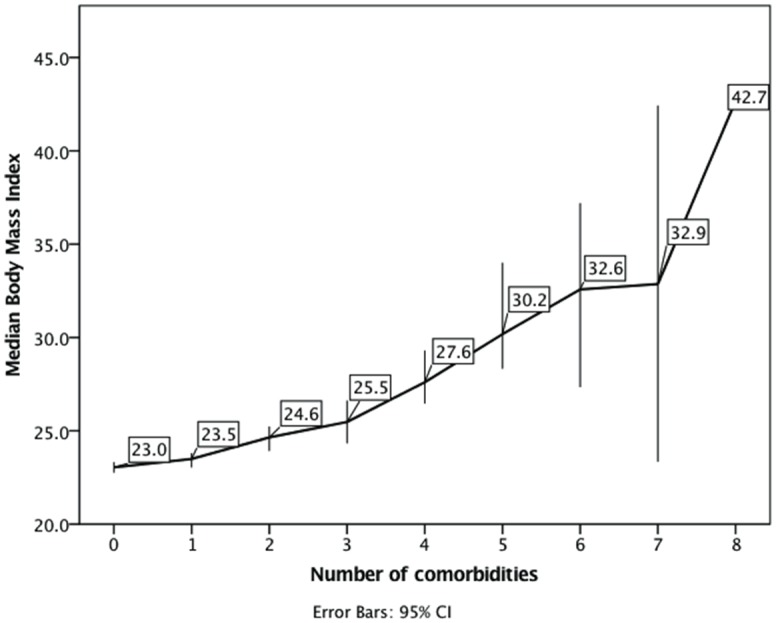
Almost a third (787, 32.8%) reported no comorbidities; one person reported 8 (Fig 2), the median was 1.0 (IQR 0–2). Within this sample, 2366 (98.6%) participants completed data on height and weight. The median BMI was 24.0 (IQR 21.3–28.3); most (53.6% 95%CI 51.5–55.5) had a normal weight, 4.2% (95%CI 3.4–5.1) were underweight, 22.5% (95%CI 21.1–24.4) were overweight and 19.4% (95%CI 18.0–21.2) were obese. BMI was related to the number of comorbidities reported (Spearman’s rho = .23, p < .001) and the association between median BMI and number of comorbidities is shown in Fig 3.
Fig 2. Frequency of comorbidities.
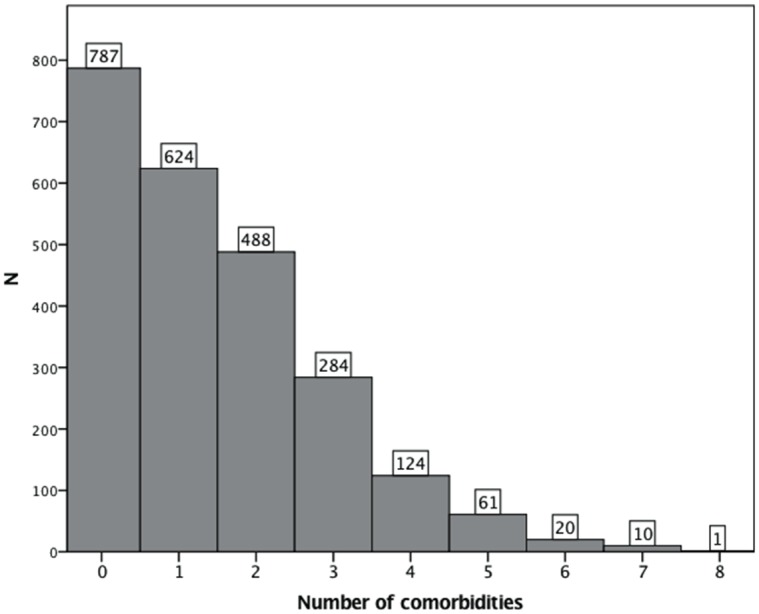
Fig 3. Association of comorbidities with body mass index.
The most commonly reported comorbidity, in more than a third of participants, was back pain, followed by depression and anxiety which were both reported by around 30% of PwMS (Table 1). The four most common comorbidities caused the greatest limitations to activities of daily life. For all comorbidities other than back pain, arthritis, and liver disease the majority were receiving treatment.
Table 1. Prevalence of comorbidities.
| Comorbidities | Has the condition | Receives treatment* | Limits daily activity* | |||
|---|---|---|---|---|---|---|
| N | % (95% CI) | N | % | N | % | |
| Back pain | 869 | 36.2 (34.3–38.1) | 381 | 43.8 | 454 | 52.2 |
| Depression | 760 | 31.7 (29.9–33.6) | 531 | 69.9 | 302 | 39.7 |
| Anxiety | 699 | 29.1 (27.4–31.0) | 351 | 50.2 | 300 | 42.9 |
| Arthritis | 328 | 13.7 (12.3–15.0) | 119 | 36.3 | 165 | 50.3 |
| High blood pressure | 269 | 11.2 (9.8–12.5) | 227 | 84.4 | 13 | 4.8 |
| Anaemia or other blood disease | 154 | 6.4 (5.5–7.4) | 92 | 59.7 | 28 | 18.2 |
| Ulcer or stomach disease | 108 | 4.5 (3.7–5.3) | 79 | 73.1 | 22 | 20.4 |
| Diabetes | 63 | 2.6 (2.0–3.3) | 47 | 74.6 | 7 | 11.1 |
| Lung disease | 61 | 2.5 (1.9–3.2) | 50 | 82.0 | 17 | 27.9 |
| Heart Disease | 54 | 2.3 (1.7–2.9) | 37 | 68.5 | 12 | 22.2 |
| Cancer | 50 | 2.1 (1.5–2.6) | 31 | 62.0 | 5 | 10.0 |
| Kidney disease | 21 | 0.9 (0.5–1.3) | 12 | 57.1 | 8 | 38.1 |
| Liver disease | 15 | 0.6 (0.3–1.0) | 5 | 33.3 | 2 | 13.3 |
Total N = 2399
*Percentage reflects proportion of those with the condition
There was a clear association between obesity and the number of comorbidities (p < .001); obese participants were significantly less likely to have no comorbidities (17.2% vs 36.7% of non-obese participants) and more likely to have 3 or more comorbidities compared to non-obese participants (38.0% vs 16.7%). Those with obesity were more likely, compared to those without obesity, to have back pain (OR 2.0; 95%CI 1.7–2.5, p < .001), depression (OR 2.2; 95%CI 1.8–2.7, p < .001), anxiety (OR 1.7; 95%CI 1.3–2.0, p < .001), arthritis (OR 2.3; 95%CI 1.8–3.0, p < .001), high blood pressure (OR 4.5; 95%CI 3.4–5.8, p < .001), anaemia or other blood diseases (OR 1.5; 95%CI 1.0–2.2, p = .035), ulcer or stomach disease (OR 2.9; 95%CI 1.9–4.3, p < .001), diabetes, (OR 4.8; 2.9–8.0, p < .001), heart disease (OR 2.1; 95%CI 1.2–3.8, p = .014), or liver disease (OR 4.8; 95%CI 1.7–12.3, p = .003), but not cancer, kidney or lung disease.
Sociodemographics
Gender was associated with obesity (p < .001) and number of comorbidities (p < .001); with women more likely to be obese than men (21.3% vs 10.5%), to have any comorbidity (68.7% vs 59.0% of men), and also to have more than 3 comorbidities (22.1% vs 14.7% of men). There was no significant difference in age between obese and non-obese participants, but those with more comorbidities were significantly older (p < .001). Post-hoc testing showed significant differences (all p < .001) in age between those without comorbidities (43.6 years, 95%CI 42.8–44.3 years), 1 comorbidity (45.7 years, 95%CI 44.9–46.5 years), 2 comorbidities (46.7 years, 95%CI 45.7–48.3 years) and 3 or more comorbidities (47.4 years, 95%CI 46.5–48.3 years) as well as a difference between 1 and 3 or more comorbidities (p = .007). Table 2 shows that obesity and most comorbidities were significantly more prevalent in the US and Canada. Mean age was significantly different between regions with the highest mean age in Australia and New Zealand (47.1 years, SE .38), then US and Canada (45.8 years, SE .34), then Europe (43.9 years SE .43) and then Other (39.5 years, SE 1.20) (all post-hoc tests p < .01).
Table 2. Comorbidity and BMI according to region.
| Region | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| United States and Canada | Australia and New Zealand | Europe | Other | P-Value | |||||
| N | % (95%CI) | N | % (95%CI) | N | % (95%CI) | N | % (95%CI) | ||
| Underweight | 37 | 4.2 (3.0–5.6) | 33 | 4.1 (2.8–5.6) | 29 | 4.7 (3.2–6.5) | 1 | 1.5 (0.0–4.6) | |
| Normal weight | 385 | 43.9~ (40.5–47.1) | 461 | 57.3* (53.9–60.6) | 384 | 62.1* (58.1–66.0) | 37 | 55.2 (43.5–67.2) | |
| Overweight | 213 | 24.3 (21.5–27.0) | 183 | 22.8 (19.8–25.4) | 128 | 20.7 (17.5–24.1) | 15 | 22.4 (12.7–32.8) | < .001 |
| Obesity | 242 | 27.6* (24.7–30.7) | 127 | 15.8~ (13.4–18.5) | 77 | 12.5~ (9.9–15.0) | 14 | 20.9 (11.4–31.7) | |
| Back pain | 346 | 39.0* (35.8–42.2) | 289 | 35.4 (32.2–38.6) | 200 | 31.9~ (28.1–35.5) | 34 | 50.0* (38.2–62.7) | .003 |
| Depression | 354 | 39.9* (36.6–43.1) | 221 | 27.1~ (24.1–30.2) | 158 | 25.2~ (21.7–28.5) | 27 | 39.7 (29.0–52.2) | < .001 |
| Anxiety | 309 | 34.8* (31.5–38.0) | 216 | 26.4~ (23.5–29.6) | 148 | 23.6~ (20.0–27.1) | 26 | 38.2 (27.5–50.0) | < .001 |
| Arthritis | 160 | 18.0* (15.4–20.5) | 107 | 13.1 (10.8–15.5) | 49 | 7.8~ (5.7–10.0) | 12 | 17.6 (8.8–27.1) | < .001 |
| High blood pressure | 148 | 16.7* (14.3–19.2) | 78 | 9.5 (7.5–11.7) | 34 | 5.4~ (3.6–7.3) | 9 | 13.2 (5.8–22.4) | < .001 |
| Anaemia or other blood disease | 57 | 6.4 (5.0–8.0) | 53 | 6.5 (4.8–8.2) | 38 | 6.1 (4.3–8.0) | 6 | 8.8 (2.9–16.2) | .851 |
| Ulcer or stomach disease | 51 | 5.7* (4.2–7.4) | 24 | 2.9~ (1.9–4.1) | 25 | 4.0 (2.7–5.5) | 8 | 11.8* (4.4–20.6) | .001 |
| Diabetes | 36 | 4.1* (2.8–5.4) | 19 | 2.3 (1.4–3.4) | 5 | 0.8~ (0.2–1.6) | 3 | 4.4 (0.0–10.3) | .001 |
| Lung disease | 28 | 3.2 (2.1–4.4) | 18 | 2.2 (1.2–3.3) | 13 | 2.1 (1.1–3.2) | 2 | 2.9 (0.0–7.4) | .507 |
| Heart Disease | 31 | 3.5* (2.3–4.8) | 12 | 1.5~ (0.7–2.3) | 7 | 1.1~ (0.3–2.1) | 4 | 5.9* (1.4–11.9) | < .001 |
| Cancer | 23 | 2.6 (1.6–3.6) | 14 | 1.7 (0.9–2.6) | 12 | 1.9 (0.8–3.2) | 1 | 1.5 (0.0–4.6) | .596 |
| Kidney disease | 12 | 1.4 (0.7–2.1) | 5 | 0.6 (0.1–1.2) | 3 | 0.5 (0.0–1.1) | 1 | 1.5 (0.0–4.5) | .221 |
| Liver disease # | 6 | 0.7 (0.2–1.2) | 4 | 0.5 (0.1–1.0) | 3 | 0.5 (0.0–1.1) | 2 | 2.9 (0.0–7.5) | .095 |
* Significantly overrepresented
~ significantly underrepresented
# Chi-square not reliable due to low expected N in >20% of the cells. N = 2366 for BMI and N = 2399 for all comorbidities
Modifiable lifestyle factors
Table 3 shows unadjusted and adjusted regression parameters associated with the number of comorbidities for each modifiable lifestyle factor variable. The variables disease-modifying drugs and meditation did not show significant associations and were therefore not included in the adjusted regression model. A linear regression model including age, gender, BMI, physical activity, diet, alcohol consumption, smoking status, vitamin D and omega-3 supplementation explained 11.7% of the variance in the number of comorbidities. Being overweight, obese, a former or current smoker were associated with an increase in the number of comorbidities; while healthy diet and moderate alcohol consumption were associated with decreased number of comorbidities. A high level of physical activity was borderline significantly associated with a decrease in comorbidity (p = .054).
Table 3. Associations between modifiable lifestyle factors and number of comorbidities,
| Unadjusted regression | Adjusted regression * | |||||||
|---|---|---|---|---|---|---|---|---|
| B | Sig. | 95% CI | B | Sig. | 95% CI | |||
| Body Mass Index | ||||||||
| Underweight | 0.06 | 0.691 | -0.24 | 0.37 | -0.05 | 0.736 | -0.35 | 0.24 |
| Overweight | 0.35 | < .001 | 0.20 | 0.51 | 0.21 | 0.007 | 0.06 | 0.36 |
| Obese | 1.07 | < .001 | 0.91 | 1.24 | 0.81 | < .001 | 0.64 | 0.98 |
| Normal | # | . | . | . | # | . | . | . |
| Physical activity | ||||||||
| High | -0.49 | < .001 | -0.65 | -0.33 | -0.15 | 0.054 | -0.31 | 0.00 |
| Moderate | -0.33 | < .001 | -0.48 | -0.17 | -0.05 | 0.489 | -0.20 | 0.09 |
| Low | # | . | . | . | # | . | . | . |
| Diet score (1–100) | -0.02 | < .001 | -0.03 | -0.02 | -0.01 | 0.003 | -0.02 | 0.00 |
| Alcohol consumption | ||||||||
| High | -0.15 | 0.694 | -0.90 | 0.60 | -0.33 | 0.355 | -1.03 | 0.37 |
| Moderate | -0.44 | < .001 | -0.58 | -0.31 | -0.33 | < .001 | -0.46 | -0.20 |
| Low | # | . | . | . | # | . | . | . |
| Smoking status | ||||||||
| Current | 0.86 | < .001 | 0.65 | 1.06 | 0.71 | < .001 | 0.51 | 0.91 |
| Former | 0.26 | < .001 | 0.13 | 0.40 | 0.21 | 0.002 | 0.08 | 0.34 |
| Never | # | . | . | . | # | . | . | . |
| Vitamin D supplementation | ||||||||
| >5000IU | -0.40 | < .001 | -0.61 | -0.19 | -0.08 | 0.445 | -0.29 | 0.13 |
| 2001-5000IU | -0.41 | < .001 | -0.60 | -0.23 | -0.10 | 0.283 | -0.30 | 0.09 |
| 1-2000IU | -0.17 | 0.098 | -0.36 | 0.03 | -0.07 | 0.445 | -0.26 | 0.12 |
| None | # | . | . | . | # | . | . | . |
| Omega-3 supplementation | ||||||||
| Yes | -0.36 | < .001 | -0.49 | -0.22 | -0.08 | 0.271 | -0.22 | 0.06 |
| No | # | . | . | . | # | . | . | . |
* Covariates not displayed were age and gender
# Reference category. N = 1864
Outcomes measures
Disability
The sample consisted of 1212 (55.6%) people with mild or no disability (able to walk unassisted) and 967 (44.4%) people with moderate or severe disability. A binary logistic regression was carried out to assess the association between BMI, comorbidities and disability, while controlling for time since diagnosis, age and gender. The regression model was significant and showed that obese participants had 1.38 (1.07–1.78, p = .013) the odds of being in the moderate/severe disability category compared to those with normal BMI. Compared to having no comorbidities, the odds of being in the moderate/severe disability category for those with 1 comorbidity was 1.38 (95%CI 1.11–1.72 p = .003), for those with 2 comorbidities it was 1.59 (95% 1.18–2.15, p = .002), and for those with 3 or more comorbidities it was 1.89 (1.28–2.77, p < .001).
Health Related Quality of life (HRQOL)
The mean physical HRQOL score was 59.3 (95%CI 58.3–60.3) and the mean mental HRQOL score was 67.1 (95%CI 66.2–68.1). A multiple linear regression model predicting both the mental and physical HRQOL showed that an increasing number of comorbidities was associated with decreasing mental and physical HRQOL, and overweight or obesity was associated with lower mental and physical HRQOL, while controlling for age, gender and level of disability (Table 4).
Table 4. Health related quality of life regression model.
| Physical Health Related Quality of Life # | Mental Health Related Quality of Life * | |||||
|---|---|---|---|---|---|---|
| Parameter | B | P | 95% CI | B | P | 95% CI |
| Comorbidities | ||||||
| None | 16.3 | <0.001 | 14.5–18.1 | 24.0 | <0.001 | 21.8–26.3 |
| 1 | 11.5 | <0.001 | 9.6–13.3 | 16.3 | <0.001 | 14.0–18.6 |
| 2 | 6.3 | <0.001 | 4.4–8.2 | 8.9 | <0.001 | 6.5–11.3 |
| 3 or more | Reference | Reference | ||||
| Body Mass Index | ||||||
| Underweight | 0.6 | 0.728 | -2.7–3.9 | 1.0 | 0.610 | -2.9–4.9 |
| Overweight | -2.5 | 0.002 | -4.0–0.9 | -2.7 | 0.005 | -4.6–0.8 |
| Obese | -5.5 | <0.001 | -7.2–3.9 | -2.1 | 0.051 | -4.1–0.0 |
| Normal | Reference | Reference | ||||
Covariates were age, gender, level of disability.
# Adjusted R Squared = .61
* Adjusted R Squared = .32
Relapse
Of those with relapsing-remitting MS, 53.1% (703) did not experience a relapse in the previous year while 46.9% (620) experienced one or more relapses. A binary logistic regression was carried out to assess the association between BMI, comorbidities and relapse, while controlling for age and gender. The regression model was significant and showed that BMI was not a significant correlate, while having comorbidities was significantly associated with increased risk for relapse. Compared to having no comorbidities, the odds of having had a relapse for those with 1 comorbidity was 1.68 (95%CI 1.30–2.18, p < .001), for those with 2 comorbidities it was 1.66 (95% 1.14–2.42, p = .008), and those with 3 or more comorbidities it was 2.60 (1.56–4.31, p < .001)
Discussion
Body Mass Index
Our results from a large international sample of PwMS showed that overall, 22.5% were overweight and an additional 19.4% were obese. There is currently no consensus on whether the prevalence of obesity is higher among PwMS compared to the general population[2]. However, the prevalence of obesity in our sample is considerably lower than the prevalence of obesity in the general population, in 2014 of the US (32.6% of men and 34.7% of women), Canada (32.6% of men and 34.7% of women), United Kingdom (26.9% of men and 29.2% of women), New Zealand (27.7% of men and 30.8% of women) and Australia (28.4% of men and 28.8% of women)[25], where most of our participant reside. Within our sample, the prevalence of obesity was significantly higher in US and Canada (27.6%) compared to Europe (12.5%) and Australia and New Zealand (15.8%). North American data from 2006 from the North American Research Committee on Multiple Sclerosis (NARCOMS) study showed that 55.8% were overweight or obese[7], comparable to the 51.9% of overweight or obese PwMS from US and Canada in our sample. However, other studies from North America report prevalence rates above 60%[26, 27]. One Spanish study reported a 39% prevalence rate of overweight (they did not specify obese) PwMS, higher than the 33.2% reported by European PwMS in our sample[28]. The relatively low prevalence of overweight and obesity may be due to the nature of the study as we recruited people with MS from websites and other online sources of which many were promoting healthy lifestyle, to complete a lifestyle survey.
Health outcomes for PwMS in our sample were better for those with lower BMI. Our data showed that overweight and obese PwMS reported lower mental and physical health HRQOL compared to those with normal weight while controlling for age, gender and level of disability and number of comorbidities. However, only obesity was associated with a clinically significant decrease of >5 points in physical HRQOL. A very small proportion in our sample was underweight (4.2%), and there were no differences in HRQOL from those with normal weight. Spanish data has shown that overweight PwMS had lower general and mental health scores compared to those with normal weight, however did not distinguish between overweight and obese, and found no differences in other quality of life scales of the SF-36, possibly due to the small number of people in the study[28]. Another study showed that obese PwMS scored lower on several QOL domains compared with non-obese PwMS but this study did not distinguish between overweight and normal weight[29]. In the general population, both underweight and obese people report lower QOL scores[30].
In our sample, BMI was significantly related to levels of disability, with obese participants 1.4 times more likely to have moderate/severe disability while controlling for age, gender, time since diagnosis and number of comorbidities. This association has been shown previously[31] but was not found in other studies[7, 10]. Current BMI was not associated with having had a relapse in the year prior to the study, similar to another study that did not find an association between BMI and hazard of relapse[32]. There may be a bi-directional association between obesity and health outcomes, and prospective and longitudinal data are needed to further assess this association[2].
Comorbidities
There was a clear association between BMI and number of comorbidities, in line with expectations[33]. Obese participants were more likely to report comorbidities, with odds ratios up to 4.8 (diabetes). More than two thirds of PwMS reported at least one comorbid disorder, similar to previous North America data [34]. Women were more likely to report any comorbidity, while North American data that did not include mental comorbidities have previously shown women are less likely to have physical comorbid disorders[34]. The association found between gender and comorbidities may be mediated by BMI, as women were also more likely to be obese in our sample.
The most commonly reported comorbidity was back pain. Back pain is not commonly specifically studied in MS, but general pain and muscle or joint problems are common in PwMS (over 50%)[4]. Both depression (31.7%) and anxiety (29.1%) were common and more prevalent in our study than in a recent systematic review by Marrie et al. (summary prevalence of 23.7% and 21.9% respectively)[35]. Conversely, a recent summary prevalence estimate of hypertension of 18.6%[36], was higher than the 11.2% reported here. Arthritis was reported by a recent review to range between 3.0–26%[37], here reported to be 13.7%. Lung disease has been recently reported to be reasonably common, with a summary prevalence of 10%, but here only reported in our sample to be 2.5%[37]. Diabetes, here reported in 2.6% of PwMS, has been reported by several studies with inconsistent prevalence rates ranging between 0 and 27%[36]. Cancer, heart, kidney and liver disease were reported by less than 5%, in line with previous data[36–38]. Similarly, the prevalence of ulcer or stomach disease in our study (4.5%) was within a recently reported (albeit wide) range (1.8–18.4%)[37]. Discrepancies between prevalence in comorbidities may be caused by the international nature of our sample, whereas many other studies included mostly PwMS from Canada and the US[9]. The majority of participants received treatment for their comorbidities, except for back pain (44%), arthritis (36%) and liver disease (38%). The most common comorbidities: back pain, depression, anxiety and arthritis, were also the most limiting in daily activities.
Mental and physical health-related HRQOL scores decreased with increasing number of comorbidities, in line with findings from NARCOMS[39] and in other chronic diseases[40, 41]. Further, among those with relapsing-remitting MS, increasing number of comorbidities was associated with higher odds of having had a relapse in the year prior to the study, while controlling for BMI, age and gender. An increasing number of comorbidities was also associated with increasing odds to have moderate/severe disability, adjusted for age, time since diagnosis, gender and BMI. In our sample, prevalence of obesity, and most comorbidities was higher in PwMS residing in US and Canada compared to other regions in the world.
The relationship between weight, comorbidities and MS health outcomes is complex and difficult to disentangle and is likely to be affected by adverse health behaviors. Adjusted regression analysis showed that being overweight, obese or a former or current smoker were associated with an increase in the number of comorbidities; while healthy diet, physical activity (borderline significant) and moderate alcohol consumption were associated with decreased number of comorbidities. While we are unable to draw conclusions on the temporality or causality of these associations, due to the design of the study, these results suggest that further studies should assess these variables over time to elucidate causality. These relationships between healthy lifestyle, obesity and comorbidities may be expected, however, the impact of increasing risk of comorbidities may be of greater significance in PwMS who are already at risk of developing progressive physical disability. And while obesity and comorbidities including back pain, depression and anxiety may lead directly to decreased quality of life, obesity may also interfere with health and wellbeing indirectly through decreased physical activity[42] and quality of sleep[43]. Preventing or treating comorbidities and obesity in PwMS should be an important goal in MS management[44]. Advice regarding healthy lifestyle including diet[19], smoking[20, 45], and physical activity[18, 42] may be helpful in decreasing the risk for comorbidity, as well as improving health and wellbeing in its own right[46].
Limitations
All data were self-reported and it is therefore not possible to assess accuracy or reliability. Measures of waist circumference or fat distribution may have added accuracy but due to the international nature of the study were not feasible. In another study from this sample, 19.3% screened positive for risk for depression as measured by the PHQ-2[47]. Here, 31.7% (760) reported depression including 9.5% (229) who are not currently treated for depression, 12.5% of the entire sample (302) reported that depression limits their daily activity, which indicates that for most, treatment seems effective and they may therefore currently not screen positive on the risk for depression. For some comorbidities, it can be difficult to distinguish between MS symptoms and a comorbidity. Participants in this study were English speaking and with the ability to complete this survey online, and most were women between the ages of 38 and 53 years. This sample may be less representative of the heterogeneous spectrum of PwMS, it may therefore not be generalizable to all PwMS, despite the size of the sample and the variety of backgrounds of participants. Due to the number of countries, we grouped these together in 4 broad regions for the purpose of analysis, but we recognize that variability may exist between countries grouped together. Due to the cross-sectional design of the study, and the complex interactions between the factors assessed, we cannot infer causality or temporality. This is further complicated by the different time frames used in the measures included in the survey. Relapses were assessed for the past 12 months and exercise over the previous 7 days, while only current comorbid disorders were assessed.
Conclusion
In a large international sample of PwMS, those who were overweight or obese and had multiple comorbidities had worse health outcomes. Our study design precludes determination of causality, however there were clear associations of increasing BMI and increasing number of comorbidities with higher odds for disability and prior relapse and lower HRQOL. Similarly, lifestyle factors, including diet, smoking, and physical activity were associated with number of comorbidities. Further research, particularly longitudinal data, is required to better elucidate direction of causality. However, while further research is undertaken, preventing and treating comorbid disorders and obesity in PwMS is warranted especially in PwMS from North America and Canada where obesity and comorbidities were significantly more prevalent.
Acknowledgments
We thank all participants for their contribution.
Data Availability
Data are available from the Neuroepidemiology Unit, University of Melbourne for researchers who meet the criteria for access to confidential data. Claudia Marck and George Jelinek can be contacted and will liaise with interested researchers to obtain permission from the Human Research Ethics Committee for sharing these data.
Funding Statement
The study was funded by philanthropic funders the Bloom Foundation and the Horne Family Charitable Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marrie RA, Elliott L, Marriott J, Cossoy M, Tennakoon A, Yu N. Comorbidity increases the risk of hospitalizations in multiple sclerosis. Neurology. 2015;84(4):350–8. 10.1212/WNL.0000000000001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tettey P, Simpson S Jr., Taylor BV, van der Mei IA. Vascular comorbidities in the onset and progression of multiple sclerosis. J Neurol Sci. 2014;347(1–2):23–33. 10.1016/j.jns.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 3.Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–7. 10.1212/WNL.0b013e3181d6b125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turpin KV, Carroll LJ, Cassidy JD, Hader WJ. Deterioration in the health-related quality of life of persons with multiple sclerosis: the possible warning signs. Mult Scler. 2007;13(8):1038–45. [DOI] [PubMed] [Google Scholar]
- 5.Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Leung S, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240–7. 10.1212/WNL.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Midaglia L, Rodriguez Ruiz M, Munoz-Garcia D. Severe haematological complications during treatment with natalizumab. Mult Scler. 2012;18(11):1644–6. 10.1177/1352458512442262 [DOI] [PubMed] [Google Scholar]
- 7.Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1):105–13. 10.1177/1352458508096680 [DOI] [PubMed] [Google Scholar]
- 8.Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet neurol. 2010;9(8):820–8. 10.1016/S1474-4422(10)70135-6 [DOI] [PubMed] [Google Scholar]
- 9.Marrie RA, Cohen J, Stuve O, Trojano M, Sorensen PS, Reingold S, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21(3):263–81. 10.1177/1352458514564491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilutti LA, McAuley E, Motl RW. Weight status and disability in multiple sclerosis: An examination of bi-directional associations over a 24-month period. Mult Scler Relat Disord. 2012;1(3):139–44. 10.1016/j.msard.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 11.Khurana SR, Bamer AM, Turner AP, Wadhwani RV, Bowen JD, Leipertz SL, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83–91. [DOI] [PubMed] [Google Scholar]
- 12.Pinhas-Hamiel O, Livne M, Harari G, Achiron A. Prevalence of overweight, obesity and metabolic syndrome components in multiple sclerosis patients with significant disability. Eur J Neurol. 2015;22(9):1275–9. 10.1111/ene.12738 [DOI] [PubMed] [Google Scholar]
- 13.Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. Methodology of an International Study of People with Multiple Sclerosis Recruited through Web 2.0 Platforms: Demographics, Lifestyle, and Disease Characteristics. Neurol Res Int. 2013;2013:580596 10.1155/2013/580596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63. [DOI] [PubMed] [Google Scholar]
- 15.Holper L, Coenen M, Weise A, Stucki G, Cieza A, Kesselring J. Characterization of functioning in multiple sclerosis using the ICF. J Neurol. 2010;257(1):103–13. 10.1007/s00415-009-5282-4 [DOI] [PubMed] [Google Scholar]
- 16.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45(2):251–5. [DOI] [PubMed] [Google Scholar]
- 17.Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4(3):187–206. [DOI] [PubMed] [Google Scholar]
- 18.Marck CH, Hadgkiss EJ, Weiland TJ, van der Meer DM, Pereira NG, Jelinek GA. Physical activity and associated levels of disability and quality of life in people with multiple sclerosis: a large international survey. BMC Neurol. 2014;14:143 10.1186/1471-2377-14-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. 2015;18(3):125–36. 10.1179/1476830514Y.0000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiland TJ, Hadgkiss EJ, Jelinek GA, Pereira NG, Marck CH, van der Meer DM. The association of alcohol consumption and smoking with quality of life, disability and disease activity in an international sample of people with multiple sclerosis. J Neurol Sci. 2014;336(1–2):211–9. 10.1016/j.jns.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 21.Jelinek GA, Marck CH, Weiland TJ, Pereira N, van der Meer DM, Hadgkiss EJ. Latitude, sun exposure and vitamin D supplementation: associations with quality of life and disease outcomes in a large international cohort of people with multiple sclerosis. BMC Neurol. 2015;15:132 10.1186/s12883-015-0394-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jelinek GA, Hadgkiss EJ, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. Association of fish consumption and Omega 3 supplementation with quality of life, disability and disease activity in an international cohort of people with multiple sclerosis. Int J Neurosci. 2013;123(11):792–800. 10.3109/00207454.2013.803104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin AB, Hadgkiss EJ, Weiland TJ, Marck CH, van der Meer DM, Pereira NG, et al. Can meditation influence quality of life, depression, and disease outcome in multiple sclerosis? Findings from a large international web-based study. Behav Neurol. 2014;2014:916519 10.1155/2014/916519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelinek GA, Weiland TJ, Hadgkiss EJ, Marck CH, Pereira N, van der Meer DM. Medication use in a large international sample of people with multiple sclerosis: associations with quality of life, relapse rate and disability. Neurol Res. 2015;37(8):662–73. 10.1179/1743132815Y.0000000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. World Health Statistics 2015. 2015.
- 26.Pilutti LA, Dlugonski D, Pula JH, Motl RW. Weight status in persons with multiple sclerosis: implications for mobility outcomes. J Obes. 2012;2012:868256 10.1155/2012/868256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slawta JN, Wilcox AR, McCubbin JA, Nalle DJ, Fox SD, Anderson G. Health behaviors, body composition, and coronary heart disease risk in women with multiple sclerosis. Arch Phys Med Rehabil. 2003;84(12):1823–30. [DOI] [PubMed] [Google Scholar]
- 28.Cambil-Martin J, Galiano-Castillo N, Munoz-Hellin E, Diaz-Rodriguez L, Laguarta-Val S, Fernandez-de-Las-Penas C, et al. Influence of body mass index on psychological and functional outcomes in patients with multiple sclerosis: a cross-sectional study. Nutr Neurosci. 2014. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Bamer AM, Alschuler KN, Johnson KL, Amtmann D. Obesity and symptoms and quality of life indicators of individuals with disabilities. Disabil Health J. 2014;7(1):124–30. 10.1016/j.dhjo.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 30.Renzaho A, Wooden M, Houng B. Associations between body mass index and health-related quality of life among Australian adults. Qual Life Res. 2010;19(4):515–20. 10.1007/s11136-010-9610-z [DOI] [PubMed] [Google Scholar]
- 31.Tettey P, Simpson S Jr., Taylor B, Blizzard L, Ponsonby AL, Dwyer T, et al. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult Scler. 2014;20(13):1737–44. 10.1177/1352458514533162 [DOI] [PubMed] [Google Scholar]
- 32.Tettey P, Simpson S Jr., Taylor B, Blizzard L, Ponsonby AL, Dwyer T, et al. Adverse lipid profile is not associated with relapse risk in MS: results from an observational cohort study. J Neurol Sci. 2014;340(1–2):230–2. 10.1016/j.jns.2014.02.038 [DOI] [PubMed] [Google Scholar]
- 33.Booth HP, Prevost AT, Gulliford MC. Impact of body mass index on prevalence of multimorbidity in primary care: cohort study. Fam Pract. 2014;31(1):38–43. 10.1093/fampra/cmt061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler. 2008;14(8):1091–8. 10.1177/1352458508092263 [DOI] [PubMed] [Google Scholar]
- 35.Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305–17. 10.1177/1352458514564487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Cutter G, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler. 2015;21(3):318–31. 10.1177/1352458514564485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrie RA, Reider N, Stuve O, Trojano M, Sorensen PS, Cutter GR, et al. The incidence and prevalence of comorbid gastrointestinal, musculoskeletal, ocular, pulmonary, and renal disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):332–41. 10.1177/1352458514564488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Sorensen PS, et al. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler. 2015;21(3):294–304. 10.1177/1352458514564489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrie RA, Horwitz R, Cutter G, Tyry T. Cumulative impact of comorbidity on quality of life in MS. Acta Neurol Scand. 2012;125(3):180–6. 10.1111/j.1600-0404.2011.01526.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprangers MA, de Regt EB, Andries F, van Agt HM, Bijl RV, de Boer JB, et al. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol. 2000;53(9):895–907. [DOI] [PubMed] [Google Scholar]
- 41.Sundh J, Johansson G, Larsson K, Linden A, Lofdahl CG, Janson C, et al. Comorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:173–83. 10.2147/COPD.S74645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motl RW, Fernhall B, McAuley E, Cutter G. Physical activity and self-reported cardiovascular comorbidities in persons with multiple sclerosis: evidence from a cross-sectional analysis. Neuroepidemiology. 2011;36(3):183–91. 10.1159/000327749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merlino G, Fratticci L, Lenchig C, Valente M, Cargnelutti D, Picello M, et al. Prevalence of 'poor sleep' among patients with multiple sclerosis: an independent predictor of mental and physical status. Sleep Med. 2009;10(1):26–34. 10.1016/j.sleep.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 44.Culpepper WJ 2nd, Wallin MT. Comorbidity increases the risk of hospitalizations in MS: prevention opportunities. Neurology. 2015;84(4):335–6. [DOI] [PubMed] [Google Scholar]
- 45.Marrie RA, Horwitz RI, Cutter G, Tyry T, Vollmer T. Smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases. Neuroepidemiology. 2011;36(2):85–90. 10.1159/000323948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadgkiss EJ, Jelinek GA, Taylor KL, Marck CH, van der Meer DM, Pereira NG, et al. Engagement in a program promoting lifestyle modification is associated with better patient-reported outcomes for people with MS. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2015;36(6):845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor KL, Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, et al. Lifestyle factors, demographics and medications associated with depression risk in an international sample of people with multiple sclerosis. BMC Psychiatry. 2014;14:327 10.1186/s12888-014-0327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Neuroepidemiology Unit, University of Melbourne for researchers who meet the criteria for access to confidential data. Claudia Marck and George Jelinek can be contacted and will liaise with interested researchers to obtain permission from the Human Research Ethics Committee for sharing these data.