Abstract
Objective
The primary goal of this study was to test the disease-modifying effect of blocking a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-5 with a neutralizing monoclonal antibody (mAb) starting 4 weeks after destabilization of the medial meniscus (DMM) in the mouse. We also investigated whether ADAMTS-5 blockade reversed mechanical allodynia and decreased monocyte chemoattractant protein (MCP)-1 production by dorsal root ganglia (DRG) cells.
Methods
Ten-week old male C57BL/6 mice underwent DMM surgery and were either left untreated or treated with anti-ADAMTS-5 mAb or IgG2c isotype control mAb starting 4 weeks after surgery. Knees were collected for histopathology 4 or 12 weeks later. Mechanical allodynia was monitored biweekly in the ipsilateral hind paw through 16 weeks. DRG were collected and cultured 8 weeks after DMM for analysis of MCP-1 production.
Results
By 4 weeks after DMM, mild cartilage degeneration was evident in the medial compartment, small osteophytes were present, and subchondral bone sclerosis was established. By 16 weeks after surgery, significant cartilage deterioration was apparent on the medial tibial plateaux and medial femoral condyles, osteophyte size had increased, and subchondral bone sclerosis was maintained.
Treatment with ADAMTS-5 mAb from week 4-16 after surgery slowed cartilage degeneration and osteophyte growth but did not affect subchondral bone sclerosis. Moreover, ADAMTS-5 blockade resulted in temporary reversal of mechanical allodynia, which correlated with decreased MCP-1 production by cultured DRG cells.
Conclusions
This study suggests therapeutic efficacy of an ADAMTS-5 mAb in the DMM model, when therapy starts early in disease.
Keywords: Osteoarthritis, Pain, ADAMTS-5, Mouse model
Introduction
Osteoarthritis (OA) is characterized by increased turnover of joint tissues, most notably articular cartilage and subchondral bone, and extracellular proteases play a key role in this pathological process. Based on extensive research in human tissues and in animal models, including genetically modified mice, a range of proteases has been identified as promising targets for the development of disease-modifying OA drugs (DMOADs), including ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)-4 and ADAMTS-5, matrix metalloproteinase (MMP)-13, and cathepsin K [1-3].
The key enzymes responsible for aggrecan degradation in OA cartilage are the aggrecanases, ADAMTS-4 and ADAMTS-5 [4]. Inhibition of either ADAMTS-4 or ADAMTS-5 by siRNA reduced aggrecan loss from human OA cartilage explants [5]. Adamts5-/- mice demonstrate long-term protection from cartilage degeneration in experimental OA induced by destabilization of the medial meniscus (DMM) [6] and in antigen-induced arthritis (AIA) [7].
Mechanical allodynia, defined as pain in response to a normally innocuous stimulus, is a behavioral measure of nervous system sensitization. We have previously shown that following DMM, but not sham surgery, mice develop secondary mechanical allodynia in the ipsilateral hind paw [8, 9]. This mechanical allodynia can be alleviated with morphine or acetaminophen [8], indicating that it is a pain-related behavior. Adamts5-/- mice are protected from secondary mechanical allodynia through 8 weeks after DMM [8].
Recently, a fully selective anti-ADAMTS-5 monoclonal antibody (mAb) was developed and characterized by GlaxoSmithKline [10]. In vivo target engagement was confirmed by intraperitoneal administration of a one-time dose of an IR800 dye-labeled antibody, 6 weeks after DMM surgery. Four days later, the antibody was detected in the superficial cartilage zone and pericellular region of articular chondrocytes. In vivo efficacy was tested in a prophylactic protocol, where mice were pre-dosed 3 days before DMM and once weekly through 8 weeks after surgery. Mice treated preventatively with ADAMTS-5 mAb had attenuated joint damage and were protected from mechanical allodynia through 8 weeks following DMM [10], essentially mimicking findings in Adamts5-/- mice [6, 8]. These findings demonstrate the potential beneficial effect of continued ADAMTS-5 blockade on both structural damage and pain-related behavior in experimental OA. This was corroborated by recent studies in the rat medial meniscal tear (MMT) model, which showed that prophylactic administration of small molecule aggrecanase inhibitors that inhibit both ADAMTS-4 and ADAMTS-5 resulted in protection against development of cartilage damage [11, 12] and weight-bearing changes (an indicator of pain) [12].
The majority of preclinical OA studies aiming to test disease-modifying effects have focused on the prophylactic efficacy of drugs (i.e., treatment begins prior to induction of disease) [13]. While these types of studies are useful for establishing efficacy, they do not mimic the clinical situation, where treatment would presumably begin after onset of joint pathological changes and associated symptoms. Moreover, while OA patients prioritize pain and function, most preclinical studies monitor joint damage alone [13].
Therefore, the goal of the current study was two-fold. First, we sought to test whether ADAMTS-5 blockade starting 4 weeks after DMM surgery affected progression of structural joint damage through 16 weeks. Secondly, we investigated whether ADAMTS-5 blockade altered mechanical allodynia and associated production of the pro-algesic chemokine, monocyte chemoattractant protein (MCP)-1, by dorsal root ganglia (DRG) neurons [9]. We chose to start treatment 4 weeks after DMM surgery since, by this time, mild structural damage is evident [14-16] and mechanical allodynia has fully developed [8, 9], reflecting an early stage of disease.
Materials and Methods
Animals and surgery
For these studies, a total of 76 mice was used. All animal experiments were approved by the Institutional Animal Care and Use Committee at Rush University Medical Center. Animals were housed in a specific pathogen free facility, with food and water ad libitum and kept on 12-hour light cycles. All experiments were performed during the light cycle. Wild-type C57BL/6 mice bred at Rush or ordered from Charles River Laboratories were used in these studies. DMM surgery was performed as previously described [9, 14] in the right knee of 10-week old male mice while mice were anesthetized using xylazine (5 mg/kg) and ketamine (100 mg/kg). Briefly, after medial parapatellar arthrotomy, the anterior fat pad was dissected to expose the anterior medial meniscotibial ligament, which was severed. The knee was flushed with saline and the incision closed.
Antibody
An ADAMTS-5-specific mAb (12F4.1H7) was developed and characterized by GlaxoSmithKline, as described in detail in [10], with a KD = 0.035 nM and IC50 = 1.46 nM. An IgG2c isotype control mAb was also obtained from GlaxoSmithKline.
Treatment groups
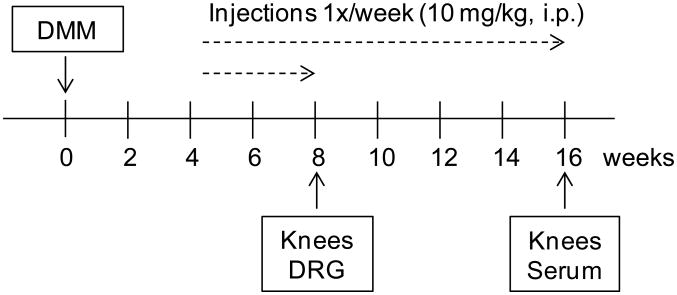
Three treatment groups were included in this study: untreated, ADAMTS-5 mAb-treated, or IgG2c isotype control mAb-treated mice (Fig. 1). Ab treatment commenced 4 weeks after DMM surgery. Mice were injected once per week (10 mg/kg in 100 μL i.p.) until the time of sacrifice (either 8 or 16 weeks after surgery). This dosage regimen has been shown previously to provide sufficient antibody in circulation [10]. Animals were randomized to treatment groups based on their week 4 withdrawal thresholds in order to ensure that all three groups had developed similar levels of allodynia prior to start of treatment. Four independent studies were conducted over a course of two years. Treatment from 4 to 8 weeks after surgery was repeated in 2 independent experiments (study 1: n=4 untreated mice, n=6 IgG2c, n=6 anti-ADAMTS-5; study 2: n=3 untreated, n=3 IgG2c, n=3 anti-ADAMTS-5). Treatment from 4 to 16 weeks after DMM was repeated in 2 independent experiments (study 3: n=6 untreated, n=4 IgG2c, n=5 anti-ADAMTS-5; study 4: n=6 untreated, n=7 IgG2c, n=7 anti-ADAMTS-5) (Fig 1). The number of mice required for study 4 was based on the histopathology results of study 3 in order to ensure 80% power with α = 0.05 comparing untreated mice to mice treated with anti-ADAMTS-5. Mice were weighed weekly, and no weight loss or other ill effects were observed in mice receiving either treatment.
Figure 1.
Experimental design. Treatment groups: ADAMTS-5 mAb, IgG2c isotype control mAb, or no injection. Mice received injections 1×/week (10 mg/kg, i.p.) beginning at week 4 and continuing to week 8 or 16 after DMM surgery. DRG = dorsal root ganglion.
Histopathology of the knee
Four (n=11 mice), eight (n=7-9 mice/treatment group), or sixteen (n=11-12 mice/treatment group) weeks after DMM surgery, histopathology of the knee was evaluated based on a modified OARSI score [17] (Alison Bendele, Bolder BioPATH, Inc., Boulder CO). Joints were fixed in 10% formalin for 48 h, and decalcified for 2 days in 10% formic acid. Knee joints were trimmed of extraneous tissue, embedded in the frontal plane and sectioned. One 8-μm section was taken from each joint at the approximate mid-point of the frontal plane and stained with Toluidine blue. Scoring was performed by an evaluator blinded to the treatment groups, using the following criteria:
Cartilage degeneration
Four joint surfaces, medial and lateral femoral condyles and tibial plateaux, were scored for severity of cartilage degeneration. For each cartilage surface, scores were assigned individually to each of 3 zones (inner, middle, outer) on a scale of 0-5, with 5 representing the most damage. Maximal score for femoral + tibial cartilage degeneration on either the medial or lateral side = 30. Therefore, the maximum possible total cartilage degeneration score for the whole joint is 60.
Osteophyte measurement
The largest osteophyte (medial tibia or femur) was measured using an ocular micrometer.
Bone score
The extent of subchondral bone sclerosis/reduction in bone marrow area was scored from 0 to 5, where 0=no increase; 1=minimal (1-10% increase in bone mass/trabecular widths); 2=mild (11-25% increase in bone mass/trabecular widths); 3=moderate (26-50% increase in bone mass/trabecular widths); 4=marked (51-75% increase in bone mass/trabecular widths); and 5=severe (>75% increase in bone mass/trabecular widths).
Bioavailability of antibody testing
Sixteen weeks after DMM surgery, serum was collected from a subset of mice (n=2-3/treatment group) in order to test for bioavailability of antibody using two assays on an Octet platform. Assay 1 - Free drug assessment: Streptavidin-coated sensors were loaded with 5 μg/mL biotinylated ADAMTS-5 protein, sera was applied (diluted 1:100), followed by a challenge with goat anti-mouse IgG H+L antibody (10 μg/mL). Assay 2 -Immunogenicity/neutralization: Streptavidin-coated sensors were loaded with 10 μg/mL biotinylated ADAMTS-5 mAb (12F4.1H7), sera were applied (diluted 1:100), followed by a challenge with purified human ADAMTS-5 (5 μg/mL) and a subsequent challenge with goat anti-mouse IgG H+L antibody (10 μg/mL). Real-time binding is calculated as relative intensity units (nm shift) using ForteBio Data Analysis Software v8.0.
von Frey testing
Mice were tested using the up-down staircase method of Dixon [18, 19] by an observer blinded to treatment. The threshold force required to elicit withdrawal of the paw (median 50% withdrawal) was determined twice on each hind paw (and averaged) on each testing day, with sequential measurements separated by at least 5 min. Baseline thresholds were assessed prior to surgery, and thresholds were assessed at weeks 4 (prior to beginning therapy), 5, 7, 9, 11, 13, and 16 after DMM surgery.
DRG cell culture following DMM surgery
Eight weeks after surgery, DRGs (L3-L5) were harvested and cells were isolated and pooled from 3-4 mice per treatment group via collagenase 4 (1 mg/mL) and papain (30 U/mL, Worthington Biochemical Corp, Lakewood, NJ) digestion. Cells were plated on poly-L-lysine and laminin (20 μg/mL) coated glass coverslips (25-mm diameter in 6-well plates), and cultured at 37°C with 5% CO2 in adult neurogenic medium: F12 with L-glutamine, 0.5% FBS, l × N2 (Life Technologies), penicillin and streptomycin (100 μg/ml and 100 U/mL) [9]. On day 2, medium was changed, and on day 4, supernatants were collected and concentrated using 3-kDa molecular weight cutoff centrifugal filters (EMD Millipore) for determination of protein levels. Two independent experiments were performed. In one experiment, DRG cells were pooled from 3 mice per treatment group. The second experiment pooled DRG cells from 4 mice per treatment group.
Protein analysis of supernatant
Total protein levels were determined by BCA assay (Thermo Fisher Scientific, Inc., Rockford, IL), and levels of monocyte chemoattractant-1 (MCP-1) protein were determined via ELISA (R&D Systems Inc, Minneapolis, MN), following manufacturer recommendations.
Statistics
For knee histopathology, data were analyzed using the Kruskal-Wallis one-way analysis of variance (ANOVA). Within each treatment group, the 4, 8, and 16-week time points were compared. At the 16-week time point, the 3 treatment groups were compared to one another. When one-way ANOVA results were significant (p<0.05), post-hoc analysis was performed via Dunn's multiple comparison test. For von Frey testing, a two-way ANOVA with Bonferroni post-tests was used to compare mice treated with ADAMTS-5 mAb to either untreated mice or to mice treated with IgG2c isotype control mAb at each time point. For MCP-1 analyses, a one-way ANOVA was performed with Bonferroni post-tests to compare all groups. All analyses were carried out using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA).
Results
Progression of joint histopathology after DMM surgery
None of the 76 mice included in these studies showed cartilage damage or other histological changes on the lateral femoral or lateral tibial surfaces (not shown). Therefore, only histological scoring of the medial compartment is shown. Mild cartilage degeneration was evident on the medial femoral and tibial surfaces by 4 weeks after DMM surgery (Fig 2A-C). Additional degeneration from 4 to 8 weeks after surgery was minimal. By 16 weeks after DMM, significant cartilage deterioration was apparent on the medial tibial plateaux as well as on the medial femoral condyles (Fig 2A-C,F).
Figure 2.
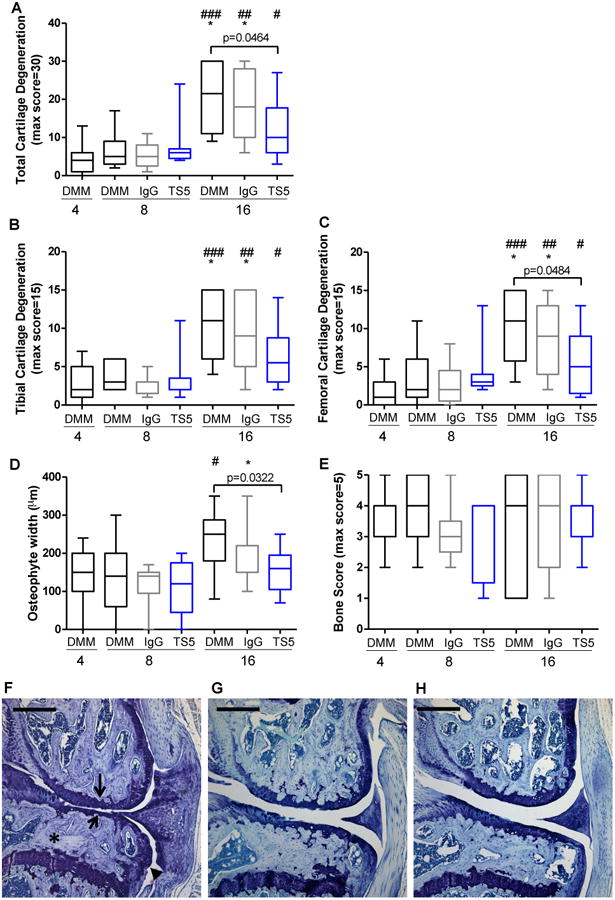
A) Total cartilage degeneration score in the medial compartment, #p=0.015 DMM+4 vs DMM+TS5+16; ##p=0.0014 DMM+4 vs DMM+IGG+16; *p=0.0116 DMM+IGG+8 vs DMM+IGG+16; ###p=0.0002 DMM+4 vs DMM+16; *p=0.0149 DMM+8 vs DMM+16; B) Medial tibial cartilage degeneration score, #p=0.0319 DMM+4 vs DMM+TS5+16; ##p=0.0046 DMM+4 vs DMM+IGG+16; *p=0.0119 DMM+IGG+8 vs DMM+IGG+16; ###p=0.0007 DMM+4 vs DMM+16; *p=0.013 DMM+8 vs DMM+16; C) Medial femoral cartilage degeneration score, #p=0.0188 DMM+4 vs DMM+TS5+16; ##p=0.002 DMM+4 vs DMM+IGG+16; *p=0.0299 DMM+IGG+8 vs DMM+IGG+16; ###p=0.0003 DMM+4 vs DMM+16; *p=0.0333 DMM+8 vs DMM+16; D) Osteophyte width, *p=0.0208 DMM+IGG+8 vs DMM+IGG+16; #p=0.0496 DMM+4 vs DMM+16; and E) Subchondral bone score were assessed 4, 8, and 16 weeks post DMM surgery in mice that were untreated (DMM), treated with IgG2c isotype control mAb (IgG), or treated with ADAMTS-5 mAb (TS5). Whiskers = 5-95 percentile; n=7-12 (details in Materials and Methods). Bar shows p-value for comparisons among treatment groups at a particular time point. F-H) Representative histology images of the medial knee joint compartment from mice 16 weeks after DMM surgery receiving F) no treatment, G) IgG isotype control Ab, or H) ADAMTS5 mAb starting 4 weeks after DMM. Images were chosen for mice representing the median histology scores from each treatment group based on the measures quantified in Figure 2A,D,E. In part F, arrows indicate medial femoral and tibial cartilage degeneration, an asterisk indicates tibial subchondral bone sclerosis, and the arrowhead indicates a tibial osteophyte. Scale bar = 0.5 mm.
Small osteophytes were present 4 weeks after DMM surgery (Figs 2D) and remained a similar size through 8 weeks after DMM. Osteophyte size increased from 4 to 16 weeks (Fig 2D).
Subchondral bone sclerosis was established by the four-week time point and remained relatively unchanged through 16 weeks (Fig 2E).
Effect of ADAMTS-5 mAb on joint histopathology
Treatment from 4-8 weeks (Fig 1) with ADAMTS-5 mAb or with IgG2c isotype control mAb had no effect on cartilage degeneration (Fig 2A-C), osteophyte width (Fig 2D), or subchondral bone sclerosis (Fig 2E) assessed at the 8-week time point.
Treatment with ADAMTS-5 mAb from 4-16 weeks slowed progression of cartilage degeneration on both the medial femoral and tibial surfaces (Fig 2B,C,H) as well as osteophyte formation (Fig 2D), compared to untreated mice or to mice treated with IgG2c isotype control mAb. As a result, mice treated with ADAMTS-5 mAb had significantly less total cartilage degeneration (Fig 2A) and smaller osteophytes (Fig 2D) compared to untreated mice at the 16-week time point. In contrast, mice treated with IgG2c isotype control mAb developed similar cartilage degeneration (Fig 2A,G) and osteophyte formation (Fig 2D) compared to untreated mice by the 16-week time point. Subchondral bone sclerosis was similar among all groups 16 weeks after surgery (Fig 2E).
Mechanical allodynia
We have previously shown that mice develop mechanical allodynia in the ipsilateral hind paw by 4 weeks after DMM, and this allodynia is maintained through 16 weeks after surgery, unlike in sham-operated mice [8, 9]. Here, we confirmed these results, demonstrating again that in untreated mice, mechanical allodynia had fully developed by 4 weeks after DMM surgery and was maintained through week 16 (Fig 3A-DMM). Following the 4-week test for mechanical allodynia, weekly treatment with either ADAMTS-5 mAb or with IgG2c mAb was started. A subset of mice was tested 24 hours after the first injection, and no acute effect of treatment was observed (Fig 3B). Treatment with ADAMTS-5 mAb resulted in decreased mechanical allodynia from 7 through 11 weeks after DMM surgery (Fig 3A-DMM+anti-ADAMTS-5), compared to mice receiving IgG2c mAb (Fig 3A-DMM+IgG2c) or to untreated mice. However, the antiallodynic effect was not sustained, and by 16 weeks after surgery, mice receiving ADAMTS-5 mAb had mechanical allodynia equivalent to the other treatment groups.
Figure 3.
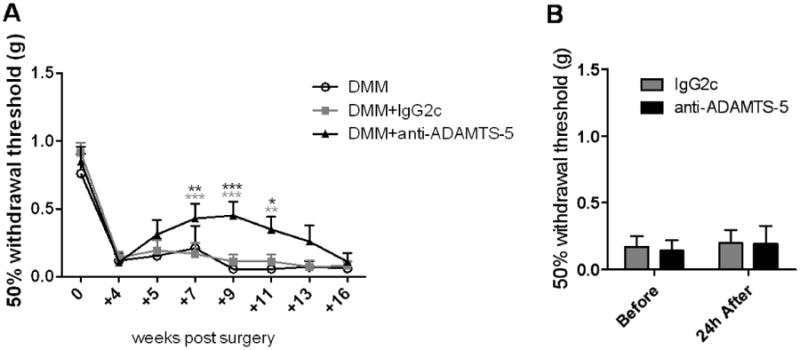
Mechanical allodynia was assessed in mice that were untreated (DMM), treated with IgG2c isotype control mAb (+IgG2c), or treated with ADAMTS-5 mAb (+anti-ADAMTS-5) through 16 weeks after surgery (A). A decrease in withdrawal threshold from the baseline at time 0 indicates development of mechanical allodynia. A subset of mice (n=5) was tested 24 hours after the first injection to rule out an acute analgesic effect (B). mean+95%CI. DMM+anti-ADAMTS-5 vs DMM: +7 weeks, p=0.0051; +9 weeks, p=0.0001; +11 weeks, p=0.0109. DMM+anti-ADAMTS-5 vs DMM+IgG2c: +7 weeks, p<0.0001; +9 weeks, p<0.0001; +11 weeks, p=0.0024; black vs DMM; grey vs DMM+IgG2c.
MCP-1 production by DRG cells
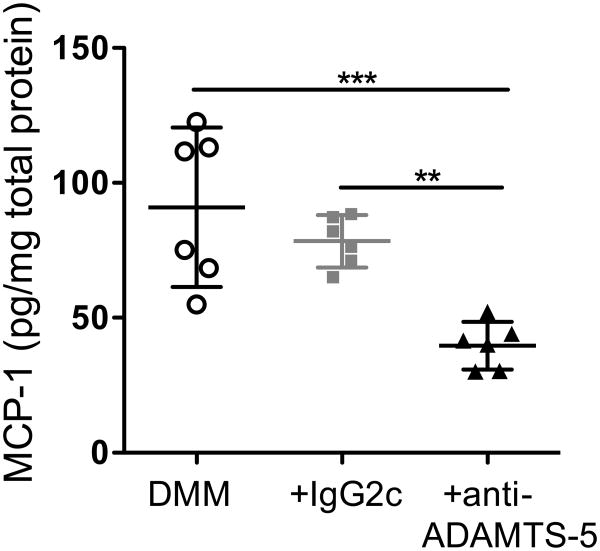
We have previously shown that DRG cells harvested from mice 8 weeks after DMM surgery produce increased amounts of MCP-1 compared to DRG cultures from sham and from age-matched naïve mice [9]. Here, DRG cells were harvested 8 weeks after DMM surgery from the 3 treatment groups (Fig 1), and MCP-1 produced in culture was compared among the groups. DRG cultures from mice treated with ADAMTS-5 mAb produced significantly less MCP-1 compared to mice treated with IgG2c mAb and to untreated mice (Fig 4). This was repeated in two independent experiments.
Figure 4.
Eight weeks after surgery, DRG cells were cultured from mice that were untreated (DMM), treated with IgG2c isotype control mAb (+IgG2c), or treated with ADAMTS-5 mAb (+anti-ADAMTS-5), and supernatants were analyzed for MCP-1 protein. mean±95%CI. **p=0.0056, ***p=0.0005. Dots represent individual wells. Plot shows the result of one out of two independent experiments (Expt 1).
Bioavailability of antibody 16 weeks after surgery
Since the protective effect of ADAMTS-5 mAb treatment on mechanical allodynia had faded by 16 weeks post surgery, serum was collected at this time point in order to test for the presence of free ADAMTS-5 mAb and for the presence of neutralizing antibodies against ADAMTS-5 mAb. Using an Octet platform, sera from mice treated with ADAMTS-5 mAb bound immobilized ADAMTS-5, while sera from untreated mice or from mice treated with IgG2c mAb did not bind, indicating that free ADAMTS-5 mAb was still present at the 16-week time point (Table 1; Supplemental Fig 1A). In contrast, serum samples from all groups did not differentially bind to immobilized ADAMTS-5 mAb or impede subsequent binding of ADAMTS-5 to the immobilized mAb, suggesting that mice treated with ADAMTS-5 mAb had not developed ADAMTS-5 mAb neutralizing antibodies by the 16-week time point (Table 2; Supplemental Fig 1B).
Table 1. Free Drug Assessment in Serum 16 weeks after DMM.
| Sensor Loading Step | Serum Binding Step | anti-Mouse Binding Step | ||
|---|---|---|---|---|
| Sensor | Biotin ADAMTS-5 Binding (nm Shift) | Serum Sample ID (Mouse # / Treatment) | Serum Binding (nm Shift) | anti-Mouse IgG Binding (nm Shift) |
| A1 | 5.71894 | 1425/IgG2c mAb | 0.10265 | 0.05875 |
| B1 | 5.81748 | 1426/ADAMTS-5 mAb | 2.15856 | 2.39165 |
| C1 | 5.67592 | 1464/No Injection | 0.18539 | 0.04822 |
| D1 | 5.60177 | 1465/No Injection | 0.17637 | 0.04432 |
| E1 | 5.0251 | 1466/ADAMTS-5 mAb | 2.15137 | 2.35766 |
| F1 | 5.46195 | 1467/IgG2c mAb | 0.28938 | 0.03224 |
| G1 | 5.70817 | 1505/ADAMTS-5 mAb | 2.94853 | 1.88211 |
| H1 | 5.61472 | 1507/IgG2c mAb | 0.20515 | 0.04857 |
Table 2. Immunogenicity/Neutralization Assessment of Serum 16 weeks after DMM.
| Sensor Loading Step | Serum Binding Step | ADAMTS-5 Binding Step | anti-Mouse Binding Step | ||
|---|---|---|---|---|---|
| Sensor | mAb Binding (nm Shift) | Serum Sample ID (Mouse # / Treatment) | Serum Binding (nm Shift) | ADAMTS-5 Binding (nm Shift) | anti-Mouse IgG Binding (nm Shift) |
| A1 | 4.56144 | 1425/IgG2c mAb | 0.7295 | 2.07617 | 1.65979 |
| B1 | 4.47963 | 1426/ADAMTS-5 mAb | 0.48773 | 2.21794 | 1.67662 |
| C1 | 4.71362 | 1464/No Injection | 0.38654 | 2.3458 | 1.69864 |
| D1 | 4.633 | 1465/No Injection | 1.10738 | 2.03582 | 1.49423 |
| E1 | 4.72779 | 1466/ADAMTS-5 mAb | 0.47894 | 2.21242 | 1.64249 |
| F1 | 4.67933 | 1467/IgG2c mAb | 0.67129 | 2.18379 | 1.56537 |
| G1 | 4.47408 | 1505/ADAMTS-5 mAb | 0.89388 | 2.01044 | 1.60488 |
| H1 | 4.54082 | 1507/IgG2c mAb | 0.65863 | 2.09008 | 1.64701 |
Discussion
We have demonstrated that early therapeutic intervention with an ADAMTS-5 mAb slows progression of joint damage over 16 weeks following DMM surgery. Specifically, ADAMTS-5 blockade attenuated cartilage degeneration and osteophyte growth, but it did not affect subchondral bone sclerosis. Moreover, treatment with ADAMTS-5 mAb resulted in a temporary reversal of mechanical allodynia, which correlated with decreased MCP-1 production by cultured DRG cells. Together, these findings are the result of four independent studies.
The protection against cartilage degradation reported here is concordant with a recent study, which used a different ADAMTS-5 mAb in STR/Ort mice, a strain that develops spontaneous OA [20]. In that study, two intra-articular injections of ADAMTS-5 mAb administered at 5 months (at which time mice have mild-to-moderate OA [21]) and 6.5 months of age were shown to slow progression of cartilage degradation by 8 months of age [20]. Effects on other joint tissues or on behavioral changes were not assessed as part of that study. Together with our data, this suggests that blocking ADAMTS-5 with Abs starting in an early stage of OA may represent a promising method of slowing cartilage destruction associated with OA.
In addition to inhibiting cartilage degradation, early therapeutic intervention with an ADAMTS-5 mAb also slowed osteophyte growth when evaluated by histology 16 weeks after DMM. Data on osteophytes in Adamts5 null mice are scarce, but one study showed a trend of protection (p=0.09) against development of osteophytes when evaluated by μ-computed tomography (μ-CT) 8 weeks after DMM [22].
We found that by 4 weeks after DMM surgery, subchondral bone sclerosis – as assessed by histology - had been fully established, remaining relatively constant through 16 weeks. Jackson et al. recently reported similar subchondral bone histological findings in the DMM model up to 8 weeks after surgery [15]. Adamts5 null mice were protected from subchondral bone changes, measured via μ-CT, through 8 weeks after DMM [22]. We demonstrate here that therapeutic treatment with ADAMTS-5 mAb had no effect on subchondral bone sclerosis, as determined histologically, although this method is less sensitive and less quantitative compared to μ-CT. Together, these studies suggest that treatment to prevent subchondral bone changes may be more effective prior to the 4-week time point in the DMM model.
Synovitis was not included as part of the histopathological scoring in this study due to the fact that a recent report in the DMM model shows that synovitis peaks 4 weeks after surgery and returns to sham levels by 16 weeks [15].
Taken together, these data suggest that treatments targeted at one specific tissue, in this case cartilage, can have effects on other aspects of the joint as well, since complex interrelationships exist among the various joint tissues in OA [23]. It should also be recognized that these effects may change depending on the time of intervention in the disease course.
Mechanical allodynia was temporarily alleviated with ADAMTS-5 mAb treatment. By 16 weeks after surgery, however, allodynia levels in the treated mice had returned to levels similar to untreated mice, even though antibody was still bioavailable at that time (Tables 1,2). One explanation for the temporary reversal of mechanical allodynia is that despite the slowing of joint damage in mice treated with ADAMTS-5 mAb, the antibody treatment did not fully prevent progression. This may suggest that a certain amount or rate of joint damage may be sufficient to promote mechanical allodynia. Mechanical allodynia in the ipsilateral hind paw is an evoked measure of sensitization of the nervous system. This mechanical allodynia can be alleviated with morphine or acetaminophen in the DMM model [8], indicating that it is a pain-related behavior, but additional behaviors should be analyzed in future studies in order to better understand the relationship between structural changes and pain.
In addition to behavioral changes, molecular alterations in the DRG have been associated with the development of pain. In particular, we have shown that 8 weeks after DMM surgery, increased production of MCP-1 by DRG neurons is associated with the persistence of mechanical allodynia [9]. Here, treatment with an ADAMTS-5 mAb was associated with a decrease in DRG MCP-1 production, which suggests that changes in the peripheral nervous system may be delayed by slowing joint deterioration.
An alternative explanation for the observed effects on mechanical allodynia and on MCP-1 production may be direct effects of ADAMTS-5 mAb on the peripheral nervous system. Indeed, ADAMTS-5 can be expressed by DRG neurons [24, 25] and chondroitin sulfate proteoglycans have been shown to interact with peripheral nerves [26]. We demonstrated that there was no acute effect of ADAMTS-5 treatment on mechanical allodynia (Fig 3B), but long-term effects cannot be ruled out and future work will seek to understand this in greater detail.
Whether halting structural progression results in pain alleviation remains unknown. In addition to the current study, a literature search identified just 4 other studies that have attempted to address this question. One study used an antibody against GM-CSF to target inflammation in the mouse collagenase-induced instability model of OA [28]. Treatment beginning at an intermediate point in the model was able to inhibit weight-bearing deficits (an indicator of pain) and cartilage damage over a three-week period. The bisphosphonate, zoledronate, was tested in two different rat models, the medial meniscal tear (MMT) and monoiodoacetate (MIA) models [29, 30]. In both models, early treatment was effective in inhibiting subchondral bone changes, cartilage degeneration, and weight-bearing deficits. In contrast, treatment started late in the model was ineffective in the MMT study and only partially effective in the MIA study. Another study targeted subchondral bone changes using osteoprotegerin (OPG) in rat MIA [31]. OPG treatment starting at an intermediate point in the model partially inhibited weight-bearing deficit and osteoclast number, but it had no effect on mechanical allodynia, cartilage damage, synovitis, or osteophyte score. Together with the current study, these experiments suggest that therapeutically treating bone (zoledronate and OPG), inflammation (GM-CSF Ab), or cartilage (ADAMTS-5 mAb) may be beneficial in slowing both structural progression and development of pain, when treatment is started early in the disease course. Future experiments must be performed to evaluate the therapeutic effect when ADAMTS-5 blockade begins in late-stage experimental OA, particularly after the 8-week time point since that is when disease progression rapidly increases in the DMM model. In addition, it may be interesting to test efficacy of combination therapies that target multiple tissues in order to evaluate whether it is possible to extend the period of time that mechanical allodynia is alleviated.
Despite the promising preclinical results obtained by ADAMTS-5 blockade, no inhibitors have made it to the clinic. One problem hindering the development of effective therapies may be that by the time of diagnosis, the majority of patients have pain and advanced structural damage [32]. Overall, our study suggests therapeutic efficacy of a potent and selective ADAMTS-5 mAb in the DMM model, when therapy starts at an early stage of disease. This is consistent with the recently proposed paradigm that improved identification of early OA patients may allow for treatment before irreversible structural changes have occurred and before chronic pain develops, resulting in improved efficacy [32].
Supplementary Material
Supplementary Figure 1. Sixteen weeks after surgery, serum was collected from a subset of mice (n=2-3/treatment group) in order to test for bioavailability of antibody using two assays on an Octet platform. A) Assay 1 - Free drug assessment: Streptavidin-coated sensors were loaded with 5 μg/mL biotinylated ADAMTS-5 protein, sera was applied (diluted 1:100), followed by a challenge with goat anti-mouse IgG H+L antibody (10 μg/mL). B) Assay 2 -Immunogenicity/neutralization: Streptavidin-coated sensors were loaded with 10 μg/mL biotinylated ADAMTS5 mAb (12F4.1H7), sera was applied (diluted 1:100), followed by a challenge with purified human ADAMTS5 (5 μg/mL) and a subsequent challenge with goat anti-mouse IgG H+L antibody (10 μg/mL). Real-time binding is calculated as relative intensity units (nm shift) using ForteBio Data Analysis Software v8.0.
Acknowledgments
Rachel Miller was supported by an Arthritis Foundation Postdoctoral Fellowship and by the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (F32AR062927). Anne-Marie Malfait was supported by NIAMS (R01AR064251 and R01AR060364).
Role of the funding source: The funding sources were not involved in the study design, collection, analysis and interpretation of data, or in the writing and submission of the manuscript.
Footnotes
Conflict of interest: JL is a current employee of, and shareholder in, GlaxoSmithKline which holds patent application WO2011002968A2.
Author contributions: REM: Conception and design, acquisition of data, analysis and interpretation of data, drafting of the article, final approval of the article
PBT: Acquisition of data, analysis and interpretation of data, critical revision of the article, final approval of the article
SI: Acquisition of data, analysis and interpretation of data, critical revision of the article, final approval of the article
JL: Provision of study materials, acquisition of data, analysis and interpretation of data, critical revision of the article, final approval of the article
AMM: Conception and design, acquisition of data, analysis and interpretation of data, drafting of the article, final approval of the article
Anne-Marie Malfait takes responsibility for the integrity of the work as a whole, from inception to finished article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flannery CR. Novel therapies in OA. Curr Drug Targets. 2010;11:614–619. doi: 10.2174/138945010791011884. [DOI] [PubMed] [Google Scholar]
- 2.Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–1401. doi: 10.1016/j.joca.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Miller RE, Lu Y, Tortorella MD, Malfait AM. Genetically Engineered Mouse Models Reveal the Importance of Proteases as Osteoarthritis Drug Targets. Curr Rheumatol Rep. 2013;15:350. doi: 10.1007/s11926-013-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little CB, Fosang AJ. Is cartilage matrix breakdown an appropriate therapeutic target in osteoarthritis--insights from studies of aggrecan and collagen proteolysis? Curr Drug Targets. 2010;11:561–575. doi: 10.2174/138945010791011956. [DOI] [PubMed] [Google Scholar]
- 5.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 6.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 7.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 8.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18:572–580. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Lohr TA, Elefante L, Shearin J, Matico R, Su JL, et al. Translational Development of an ADAMTS-5 Antibody for Osteoarthritis Disease Modification. Osteoarthritis Cartilage. 2015 doi: 10.1016/j.joca.2015.02.778. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durham TB, Klimkowski VJ, Rito CJ, Marimuthu J, Toth JL, Liu C, et al. Identification of potent and selective hydantoin inhibitors of aggrecanase-1 and aggrecanase-2 that are efficacious in both chemical and surgical models of osteoarthritis. J Med Chem. 2014;57:10476–10485. doi: 10.1021/jm501522n. [DOI] [PubMed] [Google Scholar]
- 12.Glasson SS, Bendele A, Sum PE, Tam S, Tejada J, Rivera-Bermudez M, et al. 2009 World Congress on Osteoarthritis. Vol. 17. Montreal: Osteoarthritis Cartilage; 2009. Selective Aggrecanase Inhibition is Disease Modifying and Pain Alleviating in a Rat Meniscal Tear Model of Osteoarthritis; p. S56. [Google Scholar]
- 13.Malfait AM, Little CB. On the Predictive Utility of Animal Models of Osteoarthritis. Arthritis research & therapy. doi: 10.1186/s13075-015-0747-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Jackson MT, Moradi B, Zaki S, Smith MM, McCracken S, Smith SM, et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis Rheumatol. 2014;66:3337–3348. doi: 10.1002/art.38876. [DOI] [PubMed] [Google Scholar]
- 16.Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan M, Ferguson C, et al. Disease progression and phasic changes in gene expression in a mouse model of osteoarthritis. PLoS One. 2013;8:e54633. doi: 10.1371/journal.pone.0054633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 19.Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 20.Chiusaroli R, Visentini M, Galimberti C, Casseler C, Mennuni L, Covaceuszach S, et al. Targeting of ADAMTS5's ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthritis Cartilage. 2013;21:1807–1810. doi: 10.1016/j.joca.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- 22.Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, et al. ADAMTS5-/- mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–645. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCulloch DR, Le Goff C, Bhatt S, Dixon LJ, Sandy JD, Apte SS. Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns. 2009;9:314–323. doi: 10.1016/j.gep.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhold AK, Batti L, Bilbao D, Buness A, Rittner HL, Heppenstall PA. Differential transcriptional profiling of damaged and intact adjacent dorsal root ganglia neurons in neuropathic pain. PLoS One. 2015;10:e0123342. doi: 10.1371/journal.pone.0123342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall S. The response to injury in the peripheral nervous system. J Bone Joint Surg Br. 2005;87:1309–1319. doi: 10.1302/0301-620X.87B10.16700. [DOI] [PubMed] [Google Scholar]
- 27.Snow DM, Brown EM, Letourneau PC. Growth cone behavior in the presence of soluble chondroitin sulfate proteoglycan (CSPG), compared to behavior on CSPG bound to laminin or fibronectin. Int J Dev Neurosci. 1996;14:331–349. doi: 10.1016/0736-5748(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 28.Cook AD, Pobjoy J, Steidl S, Durr M, Braine EL, Turner AL, et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14:R199. doi: 10.1186/ar4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strassle BW, Mark L, Leventhal L, Piesla MJ, Jian Li X, Kennedy JD, et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthritis Cartilage. 2010;18:1319–1328. doi: 10.1016/j.joca.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Yu DG, Yu B, Mao YQ, Zhao X, Wang XQ, Ding HF, et al. Efficacy of zoledronic acid in treatment of teoarthritis is dependent on the disease progression stage in rat medial meniscal tear model. Acta Pharmacol Sin. 2012;33:924–934. doi: 10.1038/aps.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagar DR, Ashraf S, Xu L, Burston JJ, Menhinick MR, Poulter CL, et al. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann Rheum Dis. 2014;73:1558–1565. doi: 10.1136/annrheumdis-2013-203260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felson DT, Hodgson R. Identifying and treating preclinical and early osteoarthritis. Rheum Dis Clin North Am. 2014;40:699–710. doi: 10.1016/j.rdc.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Sixteen weeks after surgery, serum was collected from a subset of mice (n=2-3/treatment group) in order to test for bioavailability of antibody using two assays on an Octet platform. A) Assay 1 - Free drug assessment: Streptavidin-coated sensors were loaded with 5 μg/mL biotinylated ADAMTS-5 protein, sera was applied (diluted 1:100), followed by a challenge with goat anti-mouse IgG H+L antibody (10 μg/mL). B) Assay 2 -Immunogenicity/neutralization: Streptavidin-coated sensors were loaded with 10 μg/mL biotinylated ADAMTS5 mAb (12F4.1H7), sera was applied (diluted 1:100), followed by a challenge with purified human ADAMTS5 (5 μg/mL) and a subsequent challenge with goat anti-mouse IgG H+L antibody (10 μg/mL). Real-time binding is calculated as relative intensity units (nm shift) using ForteBio Data Analysis Software v8.0.