Abstract
Introduction
In rheumatoid arthritis (RA) immune activation and presence of autoantibodies may precede clinical onset of disease, and joint destruction can progress despite remission. However, the underlying temporal changes of such immune system abnormalities in the inflammatory response during treat-to-target strategies remain poorly understood. We have previously reported low levels of the soluble form of CD18 (sCD18) in plasma from patients with chronic RA and spondyloarthritis. Here, we study the changes of sCD18 before and during treatment of early RA and following arthritis induction in murine models of rheumatoid arthritis.
Methods
The level of sCD18 was analyzed with a time-resolved immunoflourometric assay in 1) plasma from early treatment naïve RA patients during a treat-to-target strategy (the OPERA cohort), 2) plasma from chronic RA patients, 3) serum from SKG and CIA mice following arthritis induction, and 4) supernatants from synovial fluid mononuclear cells (SFMCs) and peripheral blood mononuclear cells (PBMCs) from 6 RA patients cultured with TNFα or adalimumab.
Results
Plasma levels of sCD18 were decreased in chronic RA patients compared with early RA patients and in early RA patients compared with healthy controls. After 12 months of treatment the levels in early RA patients were similar to healthy controls. This normalization of plasma sCD18 levels was more pronounced in patients with very early disease who achieved an early ACR response. Plasma sCD18 levels were associated with radiographic progression. Correspondingly, the serum level of sCD18 was decreased in SKG mice 6 weeks after arthritis induction compared with healthy littermates. The sCD18 levels in both SKG and CIA mice exhibited a biphasic course after arthritis induction with an initial increase above baseline followed by a decline. Shedding of CD18 from RA SFMC and RA PBMC cultures was increased by TNFα and decreased by adalimumab.
Conclusions
The plasma sCD18 levels were altered in patients with RA, in mice with autoimmune arthritis and in cell cultures treated with TNFα and adalimumab. Decreased levels of plasma sCD18 could reflect autoimmunity in transition from early to chronic disease and normalization in response to treatment could reflect autoimmunity in remission.
Introduction
Rheumatoid arthritis (RA) is characterized by swollen and painful joints caused by immune system abnormalities [1]. However, seropositivity for autoantibodies like rheumatoid factor and anti-citrullinated protein antibodies may precede clinical onset of disease [2], and joint damage can progress despite clinical remission [3]. This indicates, that immune system activation may be present in preclinical RA and in RA in clinical remission. Therefore, early and aggressive suppression of synovitis and overactive immune system pathways are principal goals in current treat-to-target strategies [4]. However, not much is known about the temporal course of immune system activation during disease development and immune system resetting during treatment.
The inflammatory response includes many different components. The family of β2 (CD18) integrins (comprising LFA-1 (CD11a/CD18), complement receptor 3 (CD11b/CD18 or Mac-1), complement receptor 4 (CD11c/CD18 or p150,95), and CD11d/CD18) is central in the inflammatory response and in RA. E.g., LFA-1 permits leukocytes to bind ICAM-1 and migrate to inflammatory foci [5]. Blocking this interaction between β2 integrins and their ligands ameliorates arthritis in both animal models of RA and RA [6–10]. β2 integrin small molecule antagonists are under evaluation for the treatment of other autoimmune diseases.[11] We and others have demonstrated a soluble form of CD18 (sCD18) resulting from sheddase activity [12–18]. Shedding of CD18 is increased during chemotaxis and following stimulation with TNFα [12,16,17], and the sCD18 complexes compete with the cell-expressed CD18 integrins for binding to ICAM-1 [12,19]. The plasma concentration of sCD18 seems to be a result of a balance between production by shedding and depletion by ligand binding, and plasma sCD18 may function as a regulatory factor by limiting leukocyte adhesion. In chronic RA and chronic spondyloarthritis, the plasma levels of sCD18 are decreased and associate inversely with disease activity [12,19].
Here, we study changes in plasma sCD18 levels (1) in patients with early RA before and during a treat-to-target strategy (patients from the OPERA cohort), (2) in chronic RA patients, (3) following arthritis induction in murine models of rheumatoid arthritis (the SKG and CIA models) and (4) in RA synovial fluid mononuclear cell (SFMC) and peripheral blood mononuclear cell (PBMC) cultures.
Methods
Patients and healthy controls
Serial plasma samples were obtained from RA patients participating in the OPtimized treatment algorithm in Early RA (OPERA) study (n = 152) (Table 1). The 152 patients used in this study were randomly selected among the 180 patients included in the trial. Detailed study design and outcome measures have been published elsewhere [20,21]. Briefly, the patients were treatment naïve early RA patients with median disease duration before diagnosis of 84 days (IQR 43–132 days). Upon inclusion the patients were randomized to a step-up methotrexate protocol in combination with either adalimumab (Humira, Abbvie, Illinois, USA) or placebo. Patients in either treatment arm received intra-articular glucocorticoid injections when presenting with synovitis as assessed clinically. In this study, we used plasma samples from the initiation of treatment (0 months) and after 3, 6, and 12 months of treatment. Clinical assessments were obtained at baseline (0 months) and after 3, 6, 12, and 24 months of treatment. Conventional radiographs of hands and forefeet were scored according to the Sharp/van der Heijde method at baseline (0 months) and after 6, 12, and 24 months (Table 1). The intraobserver intraclass correlation coefficient for total Sharp score (TSS) change was 0.88 (95% CI 0.72 to 0.95).
Table 1. Characteristics of the early treatment naïve RA patients.
| Early RA patients (n = 152) | |||||
|---|---|---|---|---|---|
| 0 months | 3 months | 6 months | 12 months | 24 months | |
| Age (years) | 56 (43–63) | - | - | - | - |
| Gender (% female) | 69 | - | - | - | - |
| Baseline characteristics | |||||
| Disease duration (days) | 84 (43–130) | - | - | - | - |
| RF (% positive) | 71 | - | - | - | - |
| Anti-CCP (% positive) | 65 | - | - | - | - |
| Treatment (% placebo) | 50 | - | - | - | - |
| Disease activity | |||||
| CRP (mg/L) | 15 (7–40) | 7 (7–11) | 7 (7–10) | 7 (7–8) | 7 (7–7) |
| Patient global (1–100) | 67 (42–83) | 10 (3–29) | 14 (3–41) | 15 (2–30) | 10 (2–29) |
| Physician global (1–100) | 56 (43–73) | 4 (0–12) | 4 (0–11) | 2 (0–11) | 2 (0–5) |
| HAQ (0–3) | 1.1 (0.8–1.8) | 0.1 (0–0.6) | 0.1 (0–0.6) | 0.1 (0–0.5) | 0.1 (0–0.5) |
| DAS28CRP (0–10) | 5.6 (4.9–6.3) | 2.2 (1.8–3.1) | 2.4 (1.8–3.0) | 2.0 (1.8–2.8) | 2.0 (1.8–2.7) |
| ACR response (% responders) | |||||
| ACR20 | - | 88 | 85 | 86 | 84 |
| ACR50 | - | 69 | 73 | 75 | 75 |
| ACR70 | - | 54 | 57 | 59 | 59 |
| ACR90 | - | 32 | 25 | 34 | 32 |
| Radiographic progression (% progressors) | |||||
| TSS | - | - | 32 | 41 | 48 |
| Erosions | - | - | 27 | 32 | 37 |
| JSN | - | - | 20 | 23 | 31 |
Data are expressed as median with IQR. Months indicate time after inclusion (treatment initiation). RF, rheumatoid factor. Anti-CCP, anti-cyclic citrullinated peptide antibody. CRP, C-reactive protein. HAQ, health assessment questionnaire. DAS28CRP, disease activity score 28 based on C-reactive protein. ACR, American College of Rheumatology improvement score. TSS, total Sharp score. JSN, joint space narrowing.
Plasma from patients with chronic RA was obtained from another study population (n = 30). At the time of inclusion, patients presented with disease flare with at least one swollen joint. Median age was 58.5 years (IQR 44.8–66.3 years) and the percentage of females was 66.7%.No disease activity or prognosis scores or test results were available. A subgroup of this population was randomly selected for use in PBMC and SFMC culture stimulation and inhibition experiments (n = 6).
Plasma was also collected from age- and gender-matched healthy controls (HCs) from the Donor Bank at Aarhus University Hospital (n = 88). Median age was 53 years (IQR 46–61 years) and the percentage of females was 68%. All plasma samples were collected in EDTA tubes and kept at -80°C until use. PBMC and SFMC were isolated by conventional Ficoll-Paque (GE Healthcare) density-gradient centrifugation and cryopreserved at -135°C until time of analysis.
SKG mice
Serum samples from a total of 45 SKG mice were included from a previous study [22]. Detailed description of animals and arthritis induction has been described previously [22,23]. Briefly, female 11- to 12-week-old SKG mice were equally randomized into five groups with nine mice in each group. Three groups of animals had arthritis induced by intraperitoneal injection of 20 mg mannan (M7504, Sigma-Aldrich, USA), the animals in another group had arthritis induced with intraperitoneal injection of 2 mg zymosan A (Z4250, Sigma-Aldrich USA), and the animals in the last group served as age-matched controls. The arthritis was scored as described by Sakaguchi et al by an observer blinded for group distribution [24]. After 14, 28, and 42 days the mice were anesthetized by inhalation with isoflorane (IsoFlo vet, Abbott Laboratories Ltd. Kent, UK), serum was collected from the orbital sinus, and the mice were sacrificed by cervical dislocation.
CIA mice
Longitudinal serum samples from a total of 4 female C57BL/6 mice with CIA were included. Mice were 11–12 weeks of age (Taconic, Ry, Denmark) and kept in Scantainers under SPF conditions (21–25°C, 30–60% humidity and 12-hour light/dark cycle). For development of CIA, the mice were immunized with chick collagen II and Complete Freund’s Adjuvant at baseline and after 21 days. Arthritis scores were done in accordance with previously described methods [25]. After 56 days the mice were sacrificed.
PBMC and SFMC in vitro cultures
SFMC and PBMC were thawed and cultured in culture medium (RPMI medium supplemented with 10% (v/v) FCS, penicillin, streptomycin, and glutamine) at a density of 2×106 cells/ml. Cells were grown in either medium alone or medium supplemented with either TNFα at 40 ng/ml (300-01A, Peprotech) or adalimumab at 5 μg/ml (Humira) for 48 h at 37°C in a humidified incubator 5% (v/v) CO2 without changing of medium. After incubation, supernatants were stored at -80°C for later sCD18 analysis.
Measuring human sCD18
The concentration of sCD18 in human plasma and culture supernatants was carried out with a time resolved immunofluorescence assay (TRIFMA) as described previously [12,26]. In brief, microtiter plates were coated with KIM185 capture antibody (hybridoma cell line CRL-2839, GenScript). Following incubation with plasma diluted 1:10 or supernatants diluted 1:2 the detection of sCD18 was achieved by incubation with biotinylated KIM127 (hybridoma cell line CRL-2838, GenScript) and Eu3+-conjugated streptavidin and read in a fluorescence plate reader. A human plasma sample defined to contain 1,000 mU/ml was used to make a standard curve and two internal controls were used to correct for interassay variations. Buffers contained bovine and human immunoglobulins to block interference from anti-antibodies such as rheumatoid factor in the patient samples [19,26]. Also, signals were read in plates coated with isotype mouse IgG1 monoclonal antibody (M7894, Sigma-Aldrich) with no known reactivity as a negative control.
Measuring mouse sCD18
The measurement of sCD18 concentration in mouse serum was carried out with a TRIFMA as described previously [18]. Briefly, microtiter plates were coated with a rat anti-mouse CD18 IgG2a capture antibody (clone 313903) at a concentration of 1 μg/ml in PBS pH 7.4 at 4°C overnight. Samples were diluted 1:10 in TBS/Tween/azid assay buffer containing 1 mM CaCl2, 1 mM MgCl2, and 100 μg/ml heat aggregated human IgG, 100 μg/ml bovine IgG 100 μg/ml rat IgG, and 100 μg/ml mouse IgG and added to the plates. After incubating the plates overnight at 4°C and following three washes with TBS/Tween/azide, a biotinylated rat anti-mouse CD18 detection antibody (clone C71/16) was added at a concentration of 1 μg/ml in TBS/Tween/azid assay buffer with 1 mM CaCl2 and 1 mM MgCl2 for 1 hour at room temperature. Following three washes, signal was developed by incubation with Eu3+-conjugated streptavidin in TBS/Tween with 25 μM EDTA for 1h at room temperature and read in a fluorescence plate reader. A mouse serum sample defined to contain 1,000 mU/ml was used to make a standard curve. Also, signals were read in plates coated with isotype rat IgG2a monoclonal antibody (11021D, BD biosciences) as a negative control.
Ethics
All human samples were obtained after informed written consent according to the Declaration of Helsinki. The Danish Data Protection Agency and the Ethics Committee at Region Midt approved the OPERA study (20070008)[20] and the collection of synovial fluid and peripheral blood from chronic RA patients for isolation of plasma, SFMCs and PBMCs (20121329).
The Danish Animal Experiment Inspectorate under the Danish Ministry of Environment and Food approved both the study using SKG mice (2007/561-1317) and the experimental protocol using CIA mice (2009/561-1667).
Statistics
Patient characteristics were described by the median and interquartile range (IQR). Comparisons of the plasma sCD18 levels between groups of unpaired samples were made using the t-test on log-transformed data. Comparisons of the plasma sCD18 levels between groups of paired samples were made with the paired t-test on log-transformed data. Comparisons of changes in plasma sCD18 levels between ACR non-responders and responders were made using the t-test. Correlation analyses between sCD18 levels and patient clinical scores and test results were performed with the Spearman correlation. Comparisons of the mouse serum sCD18 levels between groups of unpaired samples were made using the t-test on log-transformed data. Cell culture experiments were analyzed with nonparametric statistics. Thus, the Mann-Whitney U test was used for unpaired comparisons and the Wilcoxon signed rank test was used for paired data. A two-tailed p-value below 0.05 was considered significant. Calculations and graphs were made with Stata version 11.1 (StataCorp, College Station, USA) and GraphPad Prism version 5 (GraphPad Software, San Diego, CA).
Results
The plasma levels of sCD18 were decreased in established RA and exhibited a biphasic course during a treat-to-target strategy
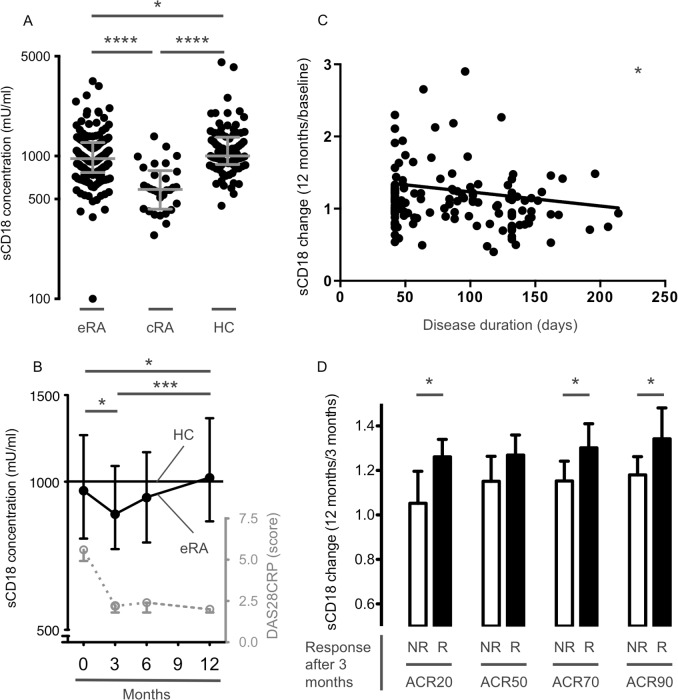
Plasma samples from early RA, chronic RA patients and HCs were used to establish differences depending on disease duration. Plasma samples from patients with early RA before and during a treat-to-target treatment strategy (samples from the OPERA cohort) were used to describe longitudinal changes in systemic sCD18 levels. The levels of sCD18 in plasma from patients with chronic RA were significantly lower than in plasma from both early RA patients and HCs (both P<0.0001). Although the levels of sCD18 in plasma from early RA patients were significantly lower than in plasma from HCs (P<0.05) a clear overlap was seen between the two groups (Fig 1A). The sCD18 levels exhibited a biphasic course during a treat-to-target strategy with an initial decline (P<0.05) followed by a gradual increase to HC levels (P<0.005) (Fig 1B).
Fig 1. Plasma levels of sCD18 in 152 early treatment naïve RA (eRA) patients during 12 months of treatment, 30 chronic RA (cRA) patients, and 88 healthy controls (HC).
(A) Plasma levels of sCD18 in early RA patients at the time of inclusion, in chronic RA patients, and in HCs. Lines indicate median and whiskers indicate IQR. Data were analyzed using the student’s t-test on log-transformed data. (B) Plasma levels of sCD18 in RA patients during 12 months of treatment. Symbols and lines indicate median and IQR. DAS28CRP score serves as a measure of clinical disease (symbols and lines indicate median and IQR). Data were analyzed with the paired t-test on log-transformed data. (C) Association between ratio of the change in plasma sCD18 levels from baseline to 12 months after treatment and disease duration. Data were analyzed using the Spearman correlation. (D) Ratio of the change in plasma sCD18 levels from 3 to 12 months after treatment in ACR non-responders (NR) and ACR responders (R) after 3 months of treatment. The mean increase in plasma levels of sCD18 from 3 to 12 months after treatment was greater in ACR responders compared with ACR non-responders. Boxes and error bars indicate mean and 95% CI. Data were analyzed using the student’s t-test. Months indicate time after inclusion (treatment initiation). * P < 0.05, *** P < 0.001, and **** P < 0.0001.
The increase in plasma sCD18 levels was greater in patients with very early disease rapidly achieving an ACR response
We further analyzed associations between the changes in sCD18 levels and disease duration and clinical treatment response in the early RA patients. The increase in plasma sCD18 levels from baseline to 12 months of treatment was inversely associated with disease duration (ρ = 0.19, P<0.05) (Fig 1C). Further, patients achieving an ACR20, ACR70, and ACR90 response after 3 months had a greater increase in plasma sCD18 levels in the subsequent time period from 3 to 12 months of treatment (all P<0.05) (Fig 1D). Thus, ACR20 non-responders had no increase in plasma sCD18 levels (mean ratio 1.05 (95% CI 0.909–1.20)) while ACR90 responders had the greatest increase in plasma sCD18 levels (mean ratio 1.34 (95% CI 1.20–1.48)). The changes in plasma sCD18 level did not differ between patients randomized to methotrexate in combination with adalimumab and patients receiving methotrexate in combination with placebo (S1 Fig).
The plasma levels of sCD18 associated inversely with joint space narrowing
Based on the measurements made above, potential associations were analyzed between the plasma sCD18 levels and disease activity scores. The levels of plasma sCD18 after 3, 6 and 12 months of treatment were associated with progression in radiographic joint space narrowing after 12 months (Table 2), and the change in plasma sCD18 during the first 12 months of treatment tended to correlate with radiographic progression the subsequent 12 months (S1 Table). There were no significant associations between either baseline plasma sCD18 levels or changes in plasma sCD18 levels and age, gender, RF positivity, anti-CCP positivity, patient global, physician global, HAQ or DAS28CRP (S2 Table).
Table 2. Correlations of sCD18 with radiographic progression.
| Radiographic progression | (Score12 months−ScoreBaseline) | |||
|---|---|---|---|---|
| sCD18 | TSS | JSN | Erosions | |
| 0 months | ρ | -0.09 | -0.09 | -0.06 |
| P | 0.27 | 0.27 | 0.46 | |
| 3 months | ρ | -0.26 | -0.28 | -0.11 |
| P | 0.0030 | 0.0013 | 0.23 | |
| 6 months | ρ | -0.19 | -0.20 | -0.12 |
| P | 0.026 | 0.021 | 0.17 | |
| 12 months | ρ | -0.12 | -0.18 | -0.042 |
| P | 0.19 | 0.034 | 0.63 | |
Data were analyzed using the Spearman correlation. ρ, Spearman’s rho. Months indicate time after inclusion (treatment initiation). Radiographic progression is measured as the difference in TSS, total Sharp score. JSN, joint space narrowing.
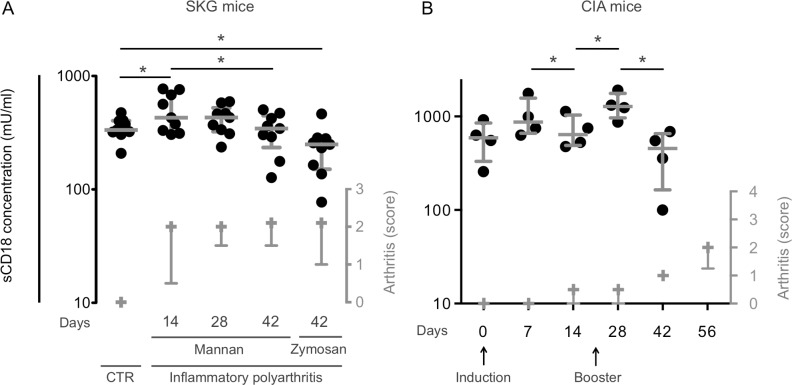
The serum levels of sCD18 were lower in mice with established autoimmune arthritis and exhibited a biphasic course during arthritis development
We used two murine models of rheumatoid arthritis (the SKG model and the CIA model) to assess the changes in systemic sCD18 levels before and during arthritis induction. The serum level of sCD18 showed a biphasic course after arthritis induction with mannan with an initial increase and a secondary decline and was decreased in SKG mice 42 days after arthritis induction with zymosan compared with control SKG mice without arthritis (all P<0.05) (Fig 2A). There was no significant difference between SKG mice 42 days after arthritis induction with mannan compared with control SKG mice without arthritis. Following the induction of CIA the serum levels of sCD18 showed an initial increase after each injection of collagen with a secondary decline (P<0.05) (Fig 2B).
Fig 2. Serum levels of sCD18 in SKG and CIA mice.
(A) The median value of serum sCD18 in healthy SKG mice was lower compared with mice 14 days after arthritis induction with mannan but higher compared with mice 42 days after arthritis induction with zymosan. n = 9. (B) The median value of serum sCD18 in CIA mice was increased after each collagen injection followed by a secondary decrease. n = 4. Lines indicate median and whiskers indicate IQR. Data were analyzed using the student’s t-test on log-transformed data. Arthritis score serves as a measure of clinical disease (symbols and lines indicate the median and IQR). * P < 0.05.
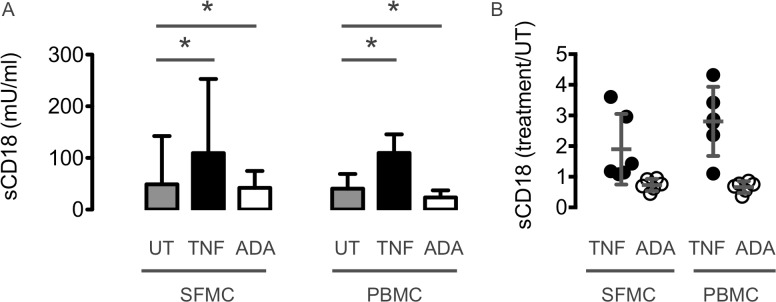
The in vitro CD18 shedding was increased by TNFα and decreased by adalimumab
In vitro cell cultures with SFMC and PBMC from chronic RA patients with disease flare were used to study the regulation of CD18 shedding from leukocytes. Shedding of CD18 from RA SFMC and PBMC was increased by adding recombinant human TNFα and decreased by neutralizing TNFα with adalimumab (all P<0.05) (Fig 3A and 3B).
Fig 3. In vitro CD18 shedding from SFMC and PBMC from 6 chronic RA patients with disease flare after stimulation with TNFα or inhibition with adalimumab (ADA).
The concentration of sCD18 from RA SFMC and PBMC was increased by TNFα and decreased by adalimumab compared with untreated cultures (UT). n = 6. (A) The concentration of sCD18 measured as mU/ml. (B) The relative change in sCD18 compared with untreated cultures. Boxes and error bars indicate median and interquartile range. Data were analyzed using non-parametric statistics. * P < 0.05.
Discussion
In early RA, clinical disease characterized by swollen and painful joints is caused by synovitis. However, presence of autoantibodies may precede the clinical onset of RA by several years, and joint damage can progress despite clinical remission. In this way, clinical disease can be seen as the “tip of the iceberg” with immune activation even in patients without symptoms. Therefore, early and aggressive synovitis suppression has become the principal goal in “treat-to-target” strategies. However, the temporal changes of immune system abnormalities during disease development and therapy and their significance remain poorly understood.
In our previous studies, plasma sCD18 levels were decreased in chronic RA and SpA patients [12,19]. The circulating sCD18 is shed by leukocytes and can bind ICAM-1. Thus, the plasma concentration of sCD18 is a result of a balance between production by sheddase activity and depletion by ligand binding. In this way, decreased levels of plasma sCD18 in chronic arthritis seems to result from insufficient shedding of CD18 from monocytes and binding of sCD18 to ICAM-1 [19,27,28]. The findings in this study support that changes in systemic sCD18 accompanying clinical disease occur in biphasic patterns.
The initial phase of arthritis immediately after induction seems to be characterized by increased sCD18 levels. Thus, there was an initial increase in serum sCD18 levels 14 days after arthritis induction in the SKG mice. Also, the serum levels of sCD18 increased immediately after collagen injection in the CIA mice. This initial phase of arthritis induction in SKG and CIA mice could be simulated in vitro. The pro-inflammatory cytokine TNFα thus increased the concentration of sCD18 in both SFMC and PBMC cultures.
The later phase of arthritis with established and chronic disease seems to be characterized by progressively decreasing sCD18 levels. Thus, plasma sCD18 levels were markedly decreased in chronic RA patients compared with both early RA patients and HCs, while the decrease in plasma sCD18 levels observed in early RA patients compared with HCs was less pronounced. Accordingly, in the SKG mice the serum levels of sCD18 were decreased after 42 days and in the CIA mice the increase in serum levels of sCD18 after collagen injection were followed by a secondary decrease. This indicates that decreased levels of sCD18 reflect inflammation in transition from early to chronic disease.
The decreased plasma sCD18 levels in patients with early RA could be normalized during a treat-to-target strategy showing a biphasic pattern. There was an initial decrease after 3 months of treatment followed by a gradual increase to HC levels after 12 months of treatment. The initial phase of treatment could be simulated in vitro. Anti-inflammatory treatment with adalimumab thus decreased the concentration of sCD18 in both SFMC and PBMC cultures. The late increase in sCD18 was particularly pronounced in patients with very early disease who quickly achieved an ACR response. This suggests that immune system abnormalities are only restored in the early phase of disease and after several months of clinical remission. We speculate that the secondary increase in systemic sCD18 during remission is caused by a slowly adjusted equilibrium between production and depletion. This is supported by previous studies showing changes in monocyte subsets and suppression of endothelial cell activity during treatment of RA [19,27,28].
Our findings support that changes in systemic sCD18 accompanying clinical disease could be important for future radiographic progression in early RA patients. The plasma levels of sCD18 were only associated with JSN and not bone erosions. This is surprising as the two processes are often connected. However, the two radiographic measures are not always coupled as demonstrated by the protective effect of the anti-receptor activator of nuclear factorκB ligand denosumab on bone erosion and not JSN.[29] JSN is thus believed to be caused by the degradation of surface cartilage by matrix metalloproteinases from fibroblast like synovial cells and myeloid cells rather than osteoclasts. In this way, infiltration of myeloid cells could be the link between low sCD18 plasma levels resulting in low ICAM-1 buffer capacity and progression of JSN.
Notably the biphasic changes in systemic sCD18 accompanying clinical disease occur with latency. Thus, there is an unmet need for markers that can guide withdrawal of anti-TNFα treatment in patients with RA [30]. Using normalization of plasma sCD18 as a marker of immune system restoration could potentially be helpful. Thus, this would enable anti-TNFα withdrawal at the time of immune system remission and not just guided by longstanding clinical remission. However, further studies are needed to elucidate whether sCD18 has a role as a marker of immunological changes.
It is also possible that a recombinant form of sCD18 could be used as a drug to inhibit inflammation in RA. Currently, there are no available drugs targeting the interaction between β2 integrins and their ligands. The monoclonal antibody against ICAM-1 (enlimumab) could not be administered repeatedly because of its mouse origin.[31], and the monoclonal antibody blocking LFA-1 (efalizumab) had serious side effects due to its strong immunosuppressive actions [32]. In this context, sCD18 appear to be a possible inhibitor because it is found in both health and disease and does not block interaction completely. Investigations with LFA-1 small-molecule antagonists (e.g. BMS-688521) or endogenous antagonists (e.g. Del-1) to treat autoimmune disease are ongoing [33–35].
One shortcoming of the animal data is that they do not necessarily reflect human disease. We used SKG and CIA mice because we did not have plasma samples from the RA patients before they developed clinical disease. The SKG and CIA mouse models seem to be feasible for studying immune system alterations before and during arthritis development. The SKG and collagen-induced arthritis (CIA) mouse model of arthritis share important features with human RA. SKG mice present with symmetric involvement of small joints, elevated serum levels of pro-inflammatory cytokines, presence of rheumatoid factor and joint destruction [36]. Mouse CIA is characterised by symmetrical joint involvement with synovitis, pannus formation and cartilage and bone erosion [37]. Another weakness is that the difference in serum sCD18 levels between SKG mice with arthritis and SKG mice without arthritis only reached significance when using zymosan as arthritis inducer, despite that there was no difference in arthritis score between the mannan and Zymosan groups [22]. Another obvious weakness of potentially using systemic sCD18 as a marker of immunological changes is the rather small changes observed.
Conclusion
The plasma sCD18 levels were altered in patients with early RA, in mice with autoimmune arthritis and in cell cultures treated with TNFα and adalimumab. Although the changes in circulating sCD18 reported here could represent temporal immunological changes during treatment of RA and arthritis induction in animals, the clinical utility of sCD18 as a biomarker of treatment response and disease progression seems to be limited due to the numerically small oscillations.
Supporting Information
Symbols indicates median. Line indicate HC median.
(TIFF)
Data were analyzed using the Spearman correlation. ρ, Spearman’s rho. Months indicate time after inclusion (treatment initiation). TSS, total Sharp score. JSN, joint space narrowing.
(DOCX)
Data were analyzed using the Spearman correlation. Spearman’s ρ and P value in parenthesis.
(DOCX)
Acknowledgments
We thank Bettina Grumsen (Department of Biomedicine, Aarhus University) for excellent technical assistance concerning the TRIFMA assays and professor Julia Johansen (Department of Rheumatology, Herlev Hospital) for assistance concerning handling of plasma samples from the OPERA cohort.
Abbreviations
- ACR
American College of Rheumatology improvement score
- CCP
cyclic citrullinated peptide
- CD
cluster of differentiation
- CIA
collagen-induced arthritis
- CRP
C-reactive protein
- DAS
disease activity score
- EDTA
ethylenediamine tetraacetic acid
- FCS
fetal calf serum
- HAQ
health assessment questionnaire
- HC
healthy control
- ICAM-1
intercellular adhesion molecule 1
- Ig
immunoglobulin
- IQR
interquartile range
- LFA-1
lymphocyte function-associated antigen 1
- Mac-1
macrophage-1 antigen
- OPERA
OPtimized treatment algorithm in Early Rheumatoid Arthritis
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate buffered saline
- R
receptor
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- S
soluble
- SFMC
synovial fluid mononuclear cell
- TBS
tris buffered saline
- TNFα
tumor necrosis factor alpha
- TRIFMA
time-resolved immunfluorometric assay
- UT
untreated
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by the Danish Multiple Sclerosis Association, the Novo Nordisk Foundation and the Danish Council for Independent Research Medical Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365: 2205–2219. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 2.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8: 573–586. 10.1038/nrrheum.2012.134 [DOI] [PubMed] [Google Scholar]
- 3.Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376: 1094–1108. 10.1016/S0140-6736(10)60826-4 [DOI] [PubMed] [Google Scholar]
- 4.Solomon DH, Bitton A, Katz JN, Radner H, Brown EM, Fraenkel L. Review: Treat to Target in Rheumatoid Arthritis: Fact, Fiction, or Hypothesis? Arthritis Rheumatol. 2014;66: 775–782. 10.1002/art.38323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346: 425–434. [DOI] [PubMed] [Google Scholar]
- 6.Jasin HE, Lightfoot E, Davis LS, Rothlein R, Faanes RB, Lipsky PE. Amelioration of antigen-induced arthritis in rabbits treated with monoclonal antibodies to leukocyte adhesion molecules. Arthritis Rheum. 1992;35: 541–549. [DOI] [PubMed] [Google Scholar]
- 7.Bullard DC, Hurley LA, Lorenzo I, Sly LM, Beaudet AL, Staite ND. Reduced susceptibility to collagen-induced arthritis in mice deficient in intercellular adhesion molecule-1. J Immunol. 1996;157: 3153–3158. [PubMed] [Google Scholar]
- 8.Kavanaugh AF, Davis LS, Nichols LA, Norris SH, Rothlein R, Scharschmidt LA, et al. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994;37: 992–999. [DOI] [PubMed] [Google Scholar]
- 9.Lowin T, Straub RH. Integrins and their ligands in rheumatoid arthritis. Arthritis Res Ther. 2011;13: 244 10.1186/ar3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanaugh AF, Davis LS, Jain RI, Nichols LA, Norris SH, Lipsky PE. A phase I/II open label study of the safety and efficacy of an anti-ICAM-1 (intercellular adhesion molecule-1; CD54) monoclonal antibody in early rheumatoid arthritis. J Rheumatol. 1996;23: 1338–1344. [PubMed] [Google Scholar]
- 11.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9: 804–820. 10.1038/nrd3266 [DOI] [PubMed] [Google Scholar]
- 12.Gjelstrup LC, Boesen T, Kragstrup TW, Jørgensen A, Klein NJ, Thiel S, et al. Shedding of large functionally active CD11/CD18 Integrin complexes from leukocyte membranes during synovial inflammation distinguishes three types of arthritis through differential epitope exposure. J Immunol. 2010;185: 4154–4168. 10.4049/jimmunol.1000952 [DOI] [PubMed] [Google Scholar]
- 13.Vaisar T, Kassim SY, Gomez IG, Green PS, Hargarten S, Gough PJ, et al. MMP-9 sheds the beta2 integrin subunit (CD18) from macrophages. Mol Cell Proteomics. 2009;8: 1044–1060. 10.1074/mcp.M800449-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans BJ, McDowall A, Taylor PC, Hogg N, Haskard DO, Landis RC. Shedding of lymphocyte function-associated antigen-1 (LFA-1) in a human inflammatory response. Blood. 2006;107: 3593–3599. [DOI] [PubMed] [Google Scholar]
- 15.Shin HY, Simon SI, Schmid-Schönbein GW. Fluid shear-induced activation and cleavage of CD18 during pseudopod retraction by human neutrophils. J Cell Physiol. 2008;214: 528–536. [DOI] [PubMed] [Google Scholar]
- 16.Zen K, Guo Y-L, Li L-M, Bian Z, Zhang C-Y, Liu Y. Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood. 2011;117: 4885–4894. 10.1182/blood-2010-05-287722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez IG, Tang J, Wilson CL, Yan W, Heinecke JW, Harlan JM, et al. Metalloproteinase-mediated Shedding of Integrin β2 promotes macrophage efflux from inflammatory sites. J Biol Chem. 2012;287: 4581–4589. 10.1074/jbc.M111.321182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen GK, Vorup-Jensen T. Detection of soluble CR3 (CD11b/CD18) by time-resolved immunofluorometry. Methods Mol Biol. 2014;1100: 355–364. 10.1007/978-1-62703-724-2_30 [DOI] [PubMed] [Google Scholar]
- 19.Kragstrup TW, Jalilian B, Hvid M, Kjærgaard A, Østgård R, Schiøttz Christensen B, et al. Decreased plasma levels of soluble CD18 link leukocyte infiltration with disease activity in spondyloarthritis. Arthritis Res Ther. 2014;16: R42 10.1186/ar4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hørslev-Petersen K, Hetland ML, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: an investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann Rheum Dis. 2014;73: 654–661. 10.1136/annrheumdis-2012-202735 [DOI] [PubMed] [Google Scholar]
- 21.Hørslev-Petersen K, Hetland ML, Ørnbjerg LM, Junker P, Pødenphant J, Ellingsen T, et al. Clinical and radiographic outcome of a treat-to-target strategy using methotrexate and intra-articular glucocorticoids with or without adalimumab induction: a 2-year investigator-initiated, double-blinded, randomised, controlled trial (OPERA). Ann Rheum Dis. 2015. 10.1136/annrheumdis-2015-208166 [DOI] [PubMed] [Google Scholar]
- 22.Keller KK, Lindgaard LM, Wogensen L, Dagnæs-Hansen F, Thomsen JS, Sakaguchi S, et al. SKG arthritis as a model for evaluating therapies in rheumatoid arthritis with special focus on bone changes. Rheumatol Int. 2013;33: 1127–1133. 10.1007/s00296-012-2500-7 [DOI] [PubMed] [Google Scholar]
- 23.Keller KK, Stengaard-Pedersen K, Dagnaes-Hansen F, Nyengaard JR, Sakaguchi S, Hauge E- M. Histological changes in chronic autoimmune SKG-arthritis evaluated by quantitative three-dimensional stereological estimators. Clin Exp Rheumatol. 2011;29: 536–543. [PubMed] [Google Scholar]
- 24.Kobayashi K, Suda T, Nan-Ya K, Sakaguchi N, Sakaguchi S, Miki I. Cytokine production profile of splenocytes derived from zymosan A-treated SKG mice developing arthritis. Inflamm Res. 2006;55: 335–341. [DOI] [PubMed] [Google Scholar]
- 25.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kragstrup TW, Vorup-Jensen T, Deleuran B, Hvid M. A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. Springerplus. 2013;2: 263 10.1186/2193-1801-2-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chara L, Sánchez-Atrio A, Pérez A, Cuende E, Albarrán F, Turrión A, et al. Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res Ther. 2012;14: R175 10.1186/ar3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu Q, Amin MA, Ruth JH, Campbell PL, Koch AE. Suppression of endothelial cell activity by inhibition of TNFα. Arthritis Res Ther. 2012;14: R88 10.1186/ar3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58: 1299–1309. 10.1002/art.23417 [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y. Next stage of RA treatment: is TNF inhibitor-free remission a possible treatment goal? Ann Rheum Dis. 2013;72: 124–7. [DOI] [PubMed] [Google Scholar]
- 31.Kavanaugh AF, Schulze-Koops H, Davis LS, Lipsky PE. Repeat treatment of rheumatoid arthritis patients with a murine anti-intercellular adhesion molecule 1 monoclonal antibody. Arthritis Rheum. 1997;40: 849–853. [DOI] [PubMed] [Google Scholar]
- 32.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61: 35–47. 10.1146/annurev.med.080708.082655 [DOI] [PubMed] [Google Scholar]
- 33.Suchard SJ, Stetsko DK, Davis PM, Skala S, Potin D, Launay M, et al. An LFA-1 (alphaLbeta2) small-molecule antagonist reduces inflammation and joint destruction in murine models of arthritis. J Immunol. 2010;184: 3917–3926. 10.4049/jimmunol.0901095 [DOI] [PubMed] [Google Scholar]
- 34.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim J- H, Liang S, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13: 465–473. 10.1038/ni.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watterson SH, Xiao Z, Dodd DS, Tortolani DR, Vaccaro W, Potin D, et al. Small Molecule Antagonist of Leukocyte Function Associated Antigen-1 (LFA-1): Structure−Activity Relationships Leading to the Identification of 6-((5 S,9 R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]nonan-7-yl)nicotinic Acid (BMS-688521). J Med Chem. American Chemical Society; 2010;53: 3814–3830. 10.1021/jm100348u [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426: 454–460. [DOI] [PubMed] [Google Scholar]
- 37.Holmdahl R, Andersson ME, Goldschmidt TJ, Jansson L, Karlsson M, Malmström V, et al. Collagen induced arthritis as an experimental model for rheumatoid arthritis. Immunogenetics, pathogenesis and autoimmunity. APMIS. 1989;97: 575–584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Symbols indicates median. Line indicate HC median.
(TIFF)
Data were analyzed using the Spearman correlation. ρ, Spearman’s rho. Months indicate time after inclusion (treatment initiation). TSS, total Sharp score. JSN, joint space narrowing.
(DOCX)
Data were analyzed using the Spearman correlation. Spearman’s ρ and P value in parenthesis.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.