Abstract
Using multilocus sequence typing (MLST), Candida albicans can be subdivided into 18 different clades. Farnesol, a quorum-sensing molecule secreted by C. albicans, is thought to play an important role in the development of C. albicans biofilms and is also a virulence factor. This study evaluated whether C. albicans bloodstream infection (BSI) strains belonging to different MLST clades secrete different levels of E,E-farnesol (FOH) and whether they have different clinical characteristics. In total, 149 C. albicans BSI isolates from ten Korean hospitals belonging to clades 18 (n = 28), 4 (n = 23), 1 (n = 22), 12 (n = 17), and other clades (n = 59) were assessed. For each isolate, the FOH level in 24-hour biofilms was determined in filtered (0.45 μm) culture supernatant using high-performance liquid chromatography. Marked differences in FOH secretion from biofilms (0.10–6.99 μM) were observed among the 149 BSI isolates. Clade 18 isolates secreted significantly more FOH than did non-clade 18 isolates (mean ± SEM; 2.66 ± 0.22 vs. 1.69 ± 0.10 μM; P < 0.001). Patients with isolates belonging to clade 18 had a lower mean severity of illness than other patients, as measured using the “acute physiology and chronic health evaluation” (APACHE) III score (14.4 ± 1.1 vs. 18.0 ± 0.7; P < 0.05). This study provides evidence that C. albicans BSI isolates belonging to the most prevalent MLST clade (clade 18) in Korea are characterized by increased levels of FOH secretion and less severe illness.
Introduction
Candida albicans is a commensal organism in healthy individuals, but it often causes nosocomial bloodstream infections (BSIs) [1]. Although BSI due to C. albicans is a major cause of morbidity and mortality in hospital patients worldwide [1,2], the pathogenesis of C. albicans BSI is complex and is not completely understood. C. albicans isolates can be assigned by multilocus sequence typing (MLST) to subsets of closely related strain types, referred to as clades [3,4]. To date, there are over 2,000 diploid sequence type (DST) profiles in the C. albicans MLST database from isolates recovered in multiple locations throughout the world, and C. albicans can be divided into 18 distinct clades (clades 1 to 18) [4,5]. Property differences among C. albicans clades have been described, exemplified for instance in the context of antifungal susceptibility, adhesion molecule structure, gene expression, or association with higher mortality of candidemia [3,6–8]. These previous reports suggest the possibility that clade-specific associations extend to properties of potential relevance to the role of C. albicans as a human commensal and pathogen [6,7].
Farnesol, a quorum-sensing molecule secreted by C. albicans, is considered to play an important role in the development of C. albicans biofilms [1,9–11]. Farnesol also plays a role in increasing the susceptibility of mice to systemic candidiasis, as well as decreasing the expression of T-cell and macrophage cytokines, indicating its role as a virulence factor [11]. Weber et al. [9] reported that C. albicans produces significant amounts of E,E-farnesol (FOH), compared with other Candida species, and FOH production differs markedly within C. albicans strains. Although C. albicans BSI is frequently associated with biofilm growth of Candida organisms on medical devices such as central venous catheters (CVC) [12–14], FOH production by BSI isolates of C. albicans and its genotype- or clade-specific differences have scarcely been assessed. In the current study, we aimed to evaluate whether C. albicans BSI strains belonging to different MLST clades or genotypes display different degrees of FOH secretion, and whether they exhibit different clinical characteristics.
Materials and Methods
Candida albicans BSI isolates and patient data
A total of 149 BSI isolates of C. albicans belonging to MLST clades 18 (n = 28), 4 (n = 23), 1 (n = 22), 12 (n = 17), and others (n = 59) were evaluated [5]. All isolates were obtained from blood cultures of 149 patients of 10 Korean university hospitals between September 2006 and August 2007. The 149 isolates yielded 108 DSTs: DST 727 belonging to clade 18 (12 isolates) was the most common MLST type, followed by 732 belonging to clade 18 (seven isolates), 69 belonging to clade 1 (six isolates), and 601 belonging to clade 12 (six isolates) (Table 1). Clinical information was collected retrospectively and included patient demographics, underlying diseases, clinical status at positive blood culture, antifungal therapy, and outcome of fungemia [15]. The Charlson comorbidity index was used to evaluate the patients’ comorbidities [16]. The severity of illness was assessed using the Acute Physiology and Chronic Health Evaluation III [APACHE III] score [17]. The APACHE III score is the sum of the acute physiology, age, and chronic health scores, ranging from 0 to 299, and is used to estimate the relative risk of hospital death [17]. To compare the clinical outcomes between groups, 14-day and 30-day mortalities after the initial diagnosis of candidemia were assessed. The outcome variable was defined as survival or death within 30 days of the first documented candidemia episode. This study was approved by the institutional review board of Chonnam National University Hospital (IRB CNUH-2014-290). A waiver of consent was granted given the retrospective nature of the project. The patient information was anonymized and de-identified prior to analysis, and no information was used that could lead to patient identification.
Table 1. E,E-farnesol secretion and biofilm formation by 149 bloodstream isolates of Candida albicans according to multilocus sequence typing (MLST) clade.
| MLST | No. of isolates tested | Mean (SEM) of biofilmb | Mean (SEM) of E,E-farnesolc | ||||
|---|---|---|---|---|---|---|---|
| Clade | Diploid sequence type (DST) | XTT reduction (OD492) | Dry weight (mg) | Per culture (μM) | Per metabolic basis | Per weight basis | |
| Clade 18 | DST 727 | 12 | 0.297 (0.030) | 2.78 (0.16) | 2.90 (0.38) | 10.91 (2.07) | 1.04 (0.12) |
| DST 732 | 7 | 0.261 (0.028) | 2.46 (0.10) | 2.52 (0.50) | 11.24 (3.74) | 1.07 (0.23) | |
| Other 9 DSTs | 9 | 0.236 (0.039) | 2.51 (0.29) | 2.46 (0.28) | 11.80 (1.53) | 1.04 (0.13) | |
| Total | 28 | 0.269 (0.019) | 2.61 (0.12) | 2.66 (0.22) | 11.28 (1.32) | 1.05 (0.08) | |
| Clade 4 | All 20 DSTs | 23 | 0.261 (0.017) | 2.50 (0.09) | 1.29 (0.20) | 5.34 (1.00) | 0.53 (0.09) |
| Clade 1 | DST 69 | 6 | 0.241 (0.032) | 3.05 (0.48) | 1.78 (0.36) | 7.18 (1.05) | 0.59 (0.09) |
| Other 16 DSTs | 16 | 0.303 (0.038) | 2.72 (0.19) | 2.04 (0.27) | 8.87 (2.07) | 0.79 (0.10) | |
| Total | 22 | 0.286 (0.029) | 2.81 (0.19) | 1.97 (0.22) | 8.41 (1.52) | 0.74 (0.08) | |
| Clade 12 | DST 601 | 6 | 0.266 (0.038) | 2.87 (0.24) | 1.41 (0.52) | 6.53 (2.88) | 0.52 (0.20) |
| Other 10 DSTs | 11 | 0.247 (0.030) | 2.86 (0.23) | 1.89 (0.33) | 7.73 (1.24) | 0.68 (0.12) | |
| Total | 17 | 0.254 (0.023) | 2.86 (0.17) | 1.72 (0.28) | 7.30 (1.25) | 0.63 (0.10) | |
| Othersa | DST 365 | 4 | 0.189 (0.056) | 3.40 (0.39) | 0.94 (0.25) | 6.10 (2.02) | 0.29 (0.10) |
| Other 48 DSTs | 55 | 0.264 (0.012) | 2.71 (0.10) | 1.78 (0.17) | 7.56 (0.80) | 0.67 (0.06) | |
| Total | 59 | 0.259 (0.012) | 2.76 (0.10) | 1.73 (0.16) | 7.46 (0.75) | 0.65 (0.06) | |
| Total, non-clade 18 | 121 | 0.263 (0.009) | 2.73 (0.07) | 1.69 (0.10) e | 7.21 (0.53)e | 0.64 (0.04)e | |
| Total, all isolates | 149 | 0.264 (0.008) | 2.71 (0.06) | 1.87 (0.10)d | 7.97 (0.51)d | 0.72 (0.04)d | |
a Includes clade 8 (12 isolates), clade 11 (5 isolates), clade 5 (5 isolates), clade 15 (5 isolates), clade 6 (4 isolates), clade 9 (4 isolates), clade 11b (4 isolates), clade 10 (2 isolates), 14 (1 isolate), 16 (1 isolate), and singletons (16 isolates).
b Biofilm-forming abilities were determined by measuring XTT activity reduction and dry weight.
c Quantification of E,E-farnesol from 149 bloodstream isolates of C. albicans was performed using high-performance liquid chromatography and FOH results (μM) obtained from each culture were also normalized on a metabolic basis or a per-weight basis by dividing the values by the biofilm results determined by XTT reduction (OD492) or dry weight (mg) assays, respectively.
d P <0.05, significant difference of E,E-farnesol secretion among 5 different groups by one-way ANOVA.
e P <0.05, significant difference of E,E-farnesol secretion between clade 18 and total non-clade 18 by independent sample t-test.
Biofilm formation
Biofilm formation was assessed using a denture strip model and 12-well tissue culture plates, as described previously for Candida species [18,19]. Briefly, a standard inoculum of 107 cells/ml from an overnight culture of the C. albicans strain was applied to the surface of a 1.5-cm2 denture strip (Nunc 174969 Thermanox Coverslips; diameter, 15 mm; Naperville, IL, USA), placed in a 12-well tissue culture plate with 4 ml phosphate-buffered saline (PBS). The cells were then allowed to adhere for 90 min at 37°C. Nonadherent cells were subsequently removed from the strips by gentle washing with PBS. The strips were then submerged in 4 ml yeast nitrogen base medium supplemented with 50 mM dextrose (YNBD) in 24-well tissue culture plates and incubated at 37°C for 24 h under continuous rotation at 125 rpm. Two milliliters of sterile-filtered (0.45 μm) culture supernatant was used for the determination of FOH [9], and the denture strips containing biofilm were used for the quantitation of biofilm formation [19]. Biofilm was quantified using both the colorimetric 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5((phenyl amino) carbonyl)-2H-tetrazolium hydroxide (XTT) assay and dry weight (DW) [19]. For XTT assay, strips containing biofilm were transferred to individual wells of a new 12-well plate containing 4 ml PBS/well. The plate was covered with aluminum foil after adding 50 μl XTT salt solution (1 mg/ml in PBS) and 4 μl menadione solution (1 mM in acetone; Sigma). The plates were incubated at 37°C for 5 h, after which the media were removed and centrifuged at 3,500 × g for 5 min at 4°C. XTT formazan in the supernatant was measured at 492 nm using a spectrophotometer [19]. For DW measurement, biofilms were scraped off the surface of the strips using a cell scraper, and both strips and scrapers were rinsed with PBS to remove residual biofilms. The material was filtered using a pre-weighed 0.22-μm-pore size filter under vacuum, dried in an incubator at 37°C for 48 h, and weighed (mg) [19].
Determination of E,E-Farnesol
For quantification of FOH, 3 ml n-hexane/ethanol (9:1, v/v) were added to 1 ml sterile-filtered (0.45 μm) culture supernatant, and the derivatization of farnesol with 9-anthroylnitrile was performed as described previously [20]. The farnesol extracts were supplemented sequentially with 250 μl 0.08% 9-anthroylnitrile ethylacetate solution, 10 μl 0.2% 1-butanol ethylacetate solution as an internal standard, and 250 μl 0.2% quinuclidine ethylacetate solution. Sample analysis was performed using a Shim-Pack Vp-ODS column (5 μm, 150 × 4.6 mm; Shimadzu) equipped with a C18 guard cartridge (4.0 × 3.0 mm; Phenomenex) on a Shimadzu HPLC system (Kyoto, Japan). A mixture of acetonitrile and water (87:13, v/v) was used as the mobile phase at a flow rate of 1.5 ml/min. The farnesol derivative was detected using a fluorescence detector at an excitation wavelength of 363 nm and emission wavelength of 470 nm. Standard concentrations ranged from 0.1 to 2.0 μM. A weighted 1/concentration linear regression was used to obtain calibration curves from the standards. The regression equations of the calibration curves were used to calculate the concentrations of the samples. FOH results obtained from each culture were also normalized on a metabolic basis or a per-weight basis by dividing the values (μM) by the biofilm results determined by XTT reduction or DW assays, respectively.
Statistical analysis
Statistical analysis was performed using SPSS version 20. Continuous variables were expressed as medians and ranges or means ± standard deviations. The independent t-test was used to compare continuous variables between two groups, the Mann-Whitney U test to compare continuous variables with a non-normal distribution, and one-way analysis of variance (ANOVA) with Tukey’s post hoc test to compare variables among more than three groups. Comparisons of categorical variables between groups were carried out using the χ2 or Fisher's exact t-test. The correlation between two continuous variables was assessed using Pearson’s test. To assess the relationship between 30-day mortality and a set of variables, a multiple logistic regression model was used. The results of these logistic regression analyses were reported as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). P values < 0.05 were considered statistically significant.
Results and Discussion
Recently, it has become clear that C. albicans BSIs are frequently associated with biofilm formation on CVCs [12–14, 21]. C. albicans is the third leading cause of catheter-related BSIs, with the second highest catheter colonization-to-infection rate [21]. Several reports have demonstrated that certain C. albicans BSI strains are more highly concentrated in particular geographic locales, and that established BSI strains are endemic in certain hospitals [22,23]. Also, our recent MLST study identified a new clade specific to Asia (clade 18) that contained the greatest proportion of BSI isolates from Korean hospitals (18.6%), followed by clades 4 (15.4%), 1 (14.7%), and 12 (11.5%) [5]. DST 727 and its single-locus variant (DST 732), which belong to clade 18, represented the most common MLST types among BSI isolates of C. albicans in Korea [5]. Because increasing use of CVC and the resultant biofilm is an important reason why the C. albicans BSI incidence continues to increase, we hypothesized that the prevalent clonal BSI strains of C. albicans possess higher biofilm-associated virulence traits to cause BSIs than do other isolates, or different clinical characteristics. In the current study, we investigated the secretion of FOH under biofilm conditions as well as the clinical characteristics of C. albicans BSI isolates, compared with those of known MLST types, which were recovered from 10 Korean hospitals over 1 year [5].
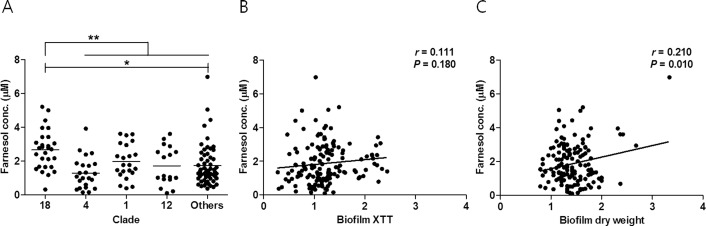
The FOH secretion and biofilm formation by C. albicans BSI isolates of various MLST clades (clades 18, 4, 1, 12, and other clades) are presented in Table 1. All 149 C. albicans BSI isolates produced biofilms, and marked differences in FOH secretion (0.10–6.99 μM) were observed among the 149 BSI isolates under biofilm conditions (S1 Table), which confirms a previous report that individual C. albicans isolates varied remarkably in their ability to produce FOH [9]. The mean ± SEM levels of FOH (μM per culture basis) secreted by the isolates belonging to clades 18 (n = 28), 4 (n = 23), 1 (n = 22), 12 (n = 17), and others (n = 59) were 2.66 ± 0.22, 1.29 ± 0.20, 1.97 ± 0.22, 1.72 ± 0.28, and 1.73 ± 0.16, respectively; the differences among the five groups were found to be significant by one-way ANOVA with Tukey’s post hoc test (P = 0.001). C. albicans BSI isolates belonging to clade 18 showed significantly higher mean FOH secretion levels (2.66 ± 0.22 μM) than those of all other 121 isolates (1.69 ± 0.10 μM) (P < 0.001) (Fig 1A). The mean FOH secretion levels (μM) from isolates belonging to DST 727 (12 isolates) and DST 732 (7 isolates) were 2.90 ± 0.38 and 2.52 ± 0.50, respectively; both DSTs exhibited significantly higher FOH secretion levels than those of the other 130 BSI isolates (2.76 ± 0.30 vs. 1.74 ± 0.10 μM) (P < 0.001). The FOH values, which were normalized on a per weight basis (clade 18, 1.05 ± 0.08; non-clade 18, 0.64 ± 0.04, P < 0.001) or a metabolic basis (clade 18, 11.28 ± 1.32; non-clade 18, 7.21 ± 0.53, P = 0.002), also showed that clade 18 isolates produced greater levels of FOH. These results revealed that C. albicans BSI isolates belonging to the most prevalent MLST clade (clade 18) or the most prevalent DST types (DSTs 727 and 732) in Korea showed higher FOH production under biofilm conditions.
Fig 1. Secretion of E,E-farnesol (μM) of 149 bloodstream isolates of Candida albicans according to multilocus sequence typing clade (A) and their correlation of biofilm formation determined by measuring XTT activity reduction (B) and dry weight (C).
Each symbol represents individual isolate. Horizontal lines indicate the mean farnesol values of the dataset at each clade. The biofilm results by both methods were normalized to those for C. albicans ATCC 90028 (set as 1.0). * Statistically significant (p < 0.05) compared among 5 different groups by one-way ANOVA. ** Statistically significant (p < 0.05) compared between clade 18 and total non-clade 18 by independent sample t-test.
The mean biofilm-forming abilities of C. albicans BSI isolates determined by both XTT reduction and DW assays were similar among isolates from different MLST clades (both, P > 0.05) (Table 1). The levels of FOH secretion by C. albicans showed a slight correlation with the amount of biofilm formation determined by DW (r = 0.210, P = 0.010) (Fig 1B), while it did not show a significant correlation with the amount of biofilm formation by the XTT method (r = 0.111, P = 0.180) (Fig 1C), which is consistent with a previous report [9,24]. There was no correlation between the amount of biofilm determined by the XTT method and that by the DW method (r = -0.146, P = 0.075). The reasons for these discrepant results are unclear. One possible explanation is that some strains that produce abundant biofilm may be shifting metabolism away from routine functions, while strains producing low levels of biofilm or no biofilm might in fact be expected to show a higher level of metabolic activity as determined by XTT assay [25]. In addition, farnesol can be produced under biofilm conditions, but it can inhibit biofilm formation [26]. If the interior of biofilms is anaerobic, then larger biofilms should produce less farnesol, since anaerobic C. albicans does not produce farnesol [27] and thus farnesol production may be correlated with the surface area of the biofilm, rather than the total mass.
Table 2 lists the clinical characteristics of the 149 patients with candidemia according to clade. There were no significant differences among patients harboring different C. albicans clades with respect to age, sex, or underlying diseases. However, some findings showed the possibility of clade-specific or genotype-specific differences in clinical characteristics among C. albicans BSI isolates. Patients receiving total parenteral nutrition were infected more commonly by clade 12 than all non-clade 12 (132 isolates) groups (82.4% vs. 47.7%, P = 0.007). Patients chronic lung disease were infected more commonly by clade 1 than all non-clade 1 (127 isolates) groups (31.8% vs. 12.6%, P = 0.048). Notably, the APACHE III score was lower in patients infected with clade 18 than all non-clade 18 (130 isolates) groups (14.4 ± 1.1 vs. 18.0 ± 0.7, P = 0.024). In addition, patients infected with C. albicans BSI isolates belonging to DST 727 and DST 732 showed lower APACHE III scores than those of patients infected with C. albicans BSI isolates belonging to other DSTs (13.8 ± 1.3 vs. 17.8 ± 0.7, P = 0.033). These results show that patients with clade 18 strains had a lower mean severity of illness than that of other patients, as measured by the APACHE III score.
Table 2. Clinical characteristics of Candida albicans bloodstream isolates from different MLST clades.
| Characteristics a | Clade 18 (N = 28) | Non-clade 18 (N = 121) | ||||
|---|---|---|---|---|---|---|
| Clade 4 (N = 23) | Clade 1 (N = 22) | Clade 12 (N = 17) | Others (N = 59) | Total (N = 121) | ||
| Demographic characteristics | ||||||
| Age, years, mean ± SEM | 63.6 ± 3.9 | 64.6 ± 2.9 | 59.9 ± 4.8 | 67.4 ± 3.1 | 54.9 ± 3.1 | 59.4 ± 1.9 |
| Age ≤48 years, no. (%) | 3 (10.7) | 3 (13.0) | 4 (18.2) | 1 (5.9) | 13 (22.0) | 21 (17.4) |
| Male sex, no. (%) | 21 (75.0) | 14 (60.9) | 12 (54.5) | 14 (82.4) | 29 (49.2) | 69 (57.0) |
| Underlying disease, no. (%) | ||||||
| Malignant tumor | 11 (39.3) | 8 (34.8) | 11 (50.0) | 6 (35.3) | 22 (37.3) | 47 (38.8) |
| Diabetes mellitus | 6 (21.4) | 9 (39.1) | 4 (18.2) | 5 (29.4) | 11 (18.6) | 29 (24.0) |
| Cerebrovascular disease | 2 (7.1) | 3 (13.0) | 4 (18.2) | 4 (23.5) | 11 (18.6) | 22 (18.2) |
| Chronic lung disease | 4 (14.3) | 4 (17.4) | 7 (31.8)b | 3 (17.6) | 5 (8.5) | 19 (15.7) |
| Moderate or severe kidney disease | 1 (3.6) | 2 (8.7) | 2 (9.1) | 2 (11.8) | 13 (22.0) | 19 (15.7) |
| Charlson comorbidity index, mean ± SD | 6.6 ± 6.1 | 6.3 ± 5.4 | 6.1 ± 5.4 | 7.1 ± 6.1 | 5.8 ± 5.2 | 6.2 ± 5.3 |
| Clinical status at positive blood culture, no. (%) | ||||||
| Neutropenia | 2 (7.1) | 3 (13.0) | 2 (9.1) | 0 (0.0) | 3 (5.1) | 8 (6.6) |
| Immunosuppressive therapy | 4 (14.3) | 2 (8.7) | 2 (9.1) | 1 (5.9) | 3 (5.1) | 8 (6.6) |
| Total parenteral nutrition | 14 (50.0) | 9 (39.1) | 12 (54.5) | 14 (82.4)b | 28 (48.3) | 63 (52.5) |
| Surgery within 30 days | 10 (35.7) | 8 (34.8) | 8 (36.4) | 5 (29.4) | 13 (22.0) | 34 (28.1) |
| ICU admission at positive culture | 10 (35.7) | 9 (39.1) | 9 (40.9) | 6 (35.3) | 22 (37.3) | 46 (38.0) |
| Previous use of antifungal agent | 2 (7.1) | 3 (13.0) | 2 (9.1) | 0 (0.0) | 5 (8.5) | 10 (8.3) |
| Concomittant bacateremia | 5 (17.9) | 9 (39.1) | 2 (9.1) | 4 (23.5) | 15 (25.4) | 30 (24.8) |
| Indwelling urinary catheter | 17 (60.7) | 13 (56.5) | 14 (63.6) | 12 (70.6) | 37 (62.7) | 76 (62.8) |
| Presence of CVC | 21 (75.0) | 17 (73.9) | 17 (77.3) | 11 (64.7) | 39 (66.1) | 84 (69.4) |
| CVC-related candidmia | 10 (35.7) | 11 (47.8) | 9 (40.9) | 7 (41.2) | 16 (27.1) | 43 (35.5) |
| APACHE III score, mean ± SEM | 14.4 ± 1.1 | 18.0 ± 1.7 | 18.1 ± 1.3 | 16.9 ± 1.8 | 18.3 ± 1.1 | 18.0 ± 0.7c |
| Therapy, no. (%) | ||||||
| Catheter non-removal | 5/21 (23.8) | 2/17 (11.8) | 6/17 (35.3) | 4/11 (36.4) | 8/39 (20.5) | 20/84 (23.8) |
| Antifungal therapy after diagnosis | 20 (71.4) | 19 (82.6) | 16 (72.7) | 9 (52.9) | 46 (78.0) | 90 (74.4) |
| Outcomes, no. with indicated result / total no. (%) | ||||||
| 14-day mortality | 8/28 (28.6) | 9/23 (39.1) | 8/21 (38.1) | 8/16 (50.0) | 15/54 (27.8) | 40/114 (35.1) |
| 30-day mortality | 9/26 (34.6) | 11/23 (47.8) | 9/20 (45.0) | 9/16 (56.2) | 22/52 (42.3) | 51/111 (45.9) |
a CVC, central venous catheter; ICU, intensive care unit; APACHE III score, “acute physiology and chronic health evaluation” III score.
b P <0.05, significant difference between a given clade and the others (clade 12 vs. all non-clade 12; clade 1 vs. all non clade 1) by χ2 or Fisher's exact t-test.
cP <0.05, significant difference in APACHE III score between clade 18 and all non-clade 18 by independent sample t-test.
In the current study, >70% (105/149) of all patients were using CVC at the time of positive blood cultures, and only 35.6% (53/149) were diagnosed as having CVC-related fungemia (Table 2); these findings are consistent with previous reports [14,28]. The overall 30-day mortality rate for C. albicans candidemia was 43.7% (60/137), which is consistent with a recent report [29]. The lowest 30-day mortality rate was 34.6% for patients with clade 18 isolates, followed by 45.0% with clade 1 isolates, 47.8% with clade 4 isolates, and 56.2% with clade 12 isolates, but the differences were not statistically significant (P = 0.714). Initially, we supposed that C. albicans strains of the clade 18 genotype may be associated with a different frequency of CVC-related candidemia or different mortality rate than other strains because of their higher production of FOH under biofilm conditions. However, we did not find any clade-specific differences in the presence of CVC (clade 18, 75.0%; non-clade 18, 69.4%), CVC-related candidemia (clade 18, 35.7%; non-clade 18, 35.5%), or overall 30-day mortality rate (clade 18, 34.6%; non-clade 18, 45.9%).
Table 3 shows the multiple logistic regression analysis performed to identify risk factors for 30-day mortality. Neither the MLST clade nor FOH concentration showed an association with 30-day mortality, suggesting that higher levels of farnesol were not responsible for clinical outcome. Only two factors, the APACHE III score and surgery within 30 days prior to candidemia, showed an association with 30-day mortality. The APACHE III score increased 30-day mortality (OR, 1.073, 95% CI, 1.014–1.137, P = 0.015), suggesting it to be a predictive factor for 30-day mortality, which is also consistent with previous reports [29,30]. In addition, surgery within 30 days prior to candidemia decreased 30-day mortality (OR, 0.360, 95% CI, 0.151–0.858, P = 0.021). Several previous reports [1,31] have also shown that candidemic patients who had undergone prior surgery showed better outcomes than did those who had not, because the source of candidemia caused by the surgically induced violation of the gastrointestinal mucosa might be easily controlled by appropriate management, in contrast with candidemic patients with underlying medical conditions such as cirrhosis, neutropenia, or steroid use [31].
Table 3. Predictive factors for 30-day mortality by multiple logistic regression analysisa.
| Variable | Category | Adjusted OR (95% CI) | P value |
|---|---|---|---|
| Gender | Male | 1.537 (0.686–3.445) | 0.296 |
| Female | 1.000 | ||
| Age (year) | 1.016 (0.996–1.036) | 0.112 | |
| Clade | Non-clade 18 | 1.000 | 0.409 |
| Clade 18 | 0.648 (0.231–1.815) | ||
| Surgery within 30 days prior to candidemia | No | 1.000 | 0.021 |
| Yes | 0.360 (0.151–0.858) | ||
| ICU admission at positive culture (ref = No) | No | 1.000 | 0.329 |
| Yes | 1.593 (0.625–4.056) | ||
| Indwelling urinary catheter (ref = No) | No | 1.000 | 0.411 |
| Yes | 1.427 (0.612–3.330) | ||
| APACHE III | 1.073 (1.014–1.137) | 0.015 | |
| E,E-farnesol secretion (μM) | 1.050 (0.751–1.470) | 0.774 |
a OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
To date, no substantial study has compared various clinical characteristics among different clades of C. albicans BSI isolates, except for one recent study [8]. That report showed that BSI isolates belonging to the “general-purpose genotype” (GPG; corresponding to clade 1) are associated with increased mortality in patients with candidemia, albeit only in younger (<48 years) patients [8]. In the current study, only 16.1% (24/149) of the total patients were of young age (<48 years), and we did not find any clade-specific differences in age or mortality among the patients. Clade 1 accounted for the largest proportion of C. albicans isolates [3,6], but it contained a lower proportion of blood isolates than commensal isolates [3]. Our MLST study of oral commensal C. albicans isolates from healthy individuals in Korea found that clade 1 comprised the greatest proportion of isolates (21.6%), followed by clades 12 (18.9%), 11 (13.5%), 4 (10.8%), and 18 (5.4%) (data not shown), suggesting that in comparison, clade 18 was particularly enriched in BSI isolates. When only clade 18 and clade 1 strains were considered in the current study, the patients with clade 1 showed significantly higher mean APACHE III scores than those of patients with clade 18 strains (P = 0.032), and they tended to be associated with higher 30-day mortality rates (clade 1, 45.0%; clade 18, 34.6%, P = 0.550), suggesting that clade 1 strains may possess certain virulence factors associated with severe illness or mortality, which may be different from those of clade 18 strains. Several comparative studies between clade 1 strains and other clade strains have identified several virulence determinants, such as increased resilience to chemicals, increased adhesion, GPG-specific alleles of DNA tandem repeat-containing genes, and genes involved in dimorphism [8].
High farnesol-producing biofilms may correlate with the tendency of that biofilm to shed free yeast cells [26], which may or may not correlate with more severe clinical symptoms. In the present study, clade 18 BSI isolates showed higher FOH production, and the patients with clade 18 strains showed a lower mean severity of illness than that of other patients, as measured by the APACHE III score. However, FOH secretion was not correlated with a decreased APACHE III score (r = –0.136, P = 0.099), and similar results were obtained from the FOH values, which were normalized on a per weight basis or a metabolic basis. These results suggest that higher FOH levels may not to predict less severe illness, and that increased FOH secretion and the propensity to cause less severe illness are two independent properties of clade 18 strains.
The pathologic role of FOH remains an open question. Initially, it was thought that farnesol could be manipulated to treat invasive candidiasis as a fungistatic agent [1,32], but the discovery that endogenous farnesol actually contributed to C. albicans virulence has redirected recent research toward understanding quorum-sensing molecules as an important virulence factor of systemic candidiasis [1,11,33]. A recent study showed that secretion of farnesol may promote C. albicans dissemination through macrophages which have an important role in early detection and elimination of C. albicans in the host [34]. Considering the report that FOH may play an important virulence role in destruction of the host epithelial cell layer as the initial invasion process [24], farnesol may act as a virulence factor before, rather than after, C. albicans reaches the bloodstream. Biofilm formation is associated with an enhanced capacity of C. albicans to colonize indwelling CVCs, thus providing a reservoir from which the organism may enter the bloodstream [21]. Therefore, FOH produced under biofilm conditions, as a virulence factor, may be involved in the initial BSI invasion process. In the present study, we showed for the first time that clade 18 isolates produced a greater quantity of FOH under biofilm conditions, as compared with non-clade 18 isolates, in candidemic patients. The high prevalence of clade 18 strains as BSI pathogens in Korea suggests that specific microbial factors, including FOH, secreted under biofilm conditions could contribute to the increased ability of C. albicans clade 18 strains to cause BSIs, which have an impact on the epidemiology of C. albicans BSIs in Korea.
Supporting Information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2A10058555). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lim CS, Rosli R, Seow HF, Chong PP. Candida and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis. 2012; 31: 21–31. 10.1007/s10096-011-1273-3 [DOI] [PubMed] [Google Scholar]
- 2.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003; 37: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 3.Odds FC, Jacobsen MD. Multilocus sequence typing of pathogenic Candida species. Eukaryot Cell. 2008; 7: 1075–1084. 10.1128/EC.00062-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McManus BA, Coleman DC. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect Genet Evol. 2014; 21: 166–178. 10.1016/j.meegid.2013.11.008 [DOI] [PubMed] [Google Scholar]
- 5.Shin JH, Bougnoux ME, d'Enfert C, Kim SH, Moon CJ, Joo MY, et al. Genetic diversity among Korean Candida albicans bloodstream isolates: assessment by multilocus sequence typing and restriction endonuclease analysis of genomic DNA by use of BssHII. J Clin Microbiol. 2011; 49: 2572–2577. 10.1128/JCM.02153-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odds FC. Molecular phylogenetics and epidemiology of Candida albicans. Future Microbiol. 2010; 5: 67–79. 10.2217/fmb.09.113 [DOI] [PubMed] [Google Scholar]
- 7.MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJ, Gow NA, et al. Property differences among the four major Candida albicans strain clades. Eukaryot Cell. 2009; 8: 373–387. 10.1128/EC.00387-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid J, Tortorano AM, Jones G, Lazzarini C, Zhang N, Bendall MJ, et al. Increased mortality in young candidemia patients associated with presence of a Candida albicans general-purpose genotype. J Clin Microbiol. 2011; 49: 3250–3256. 10.1128/JCM.00941-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber K, Sohr R, Schulz B, Fleischhacker M, Ruhnke M. Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob Agents Chemother. 2008; 52: 1859–1861. 10.1128/AAC.01646-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. Plos Pathog. 2010; 6: e1000828 10.1371/journal.ppat.1000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarathna DH, Nickerson KW, Duhamel GE, Jerrels TR, Petro TM. Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model. Infect Immun. 2007; 75: 4006–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andes D, Nett J, Oschel P, Albrecht R, Marchillo K, Pitula A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun. 2004; 72: 6023–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004; 17: 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One. 2012; 7: e33705 10.1371/journal.pone.0033705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MN, Shin JH, Sung H, Lee K, Kim EC, Ryoo N, et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis. 2009; 48: e57–61. 10.1086/597108 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991; 100: 1619–1636. [DOI] [PubMed] [Google Scholar]
- 18.Joo MY, Shin JH, Jang HC, Song ES, Kee SJ, Shin MG, et al. Expression of SAP5 and SAP9 in Candida albicans biofilms: comparison of bloodstream isolates with isolates from other sources. Med Mycol. 2013; 51: 892–896. 10.3109/13693786.2013.824623 [DOI] [PubMed] [Google Scholar]
- 19.Chandra J, Mukherjee PK, Ghannoum MA. In vitro growth and analysis of Candida biofilms. Nat Protoc. 2008; 3: 1909–1924. 10.1038/nprot.2008.192 [DOI] [PubMed] [Google Scholar]
- 20.Saisho Y, Morimoto A, Umeda T. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal Biochem. 1997; 252: 89–95. [DOI] [PubMed] [Google Scholar]
- 21.Crump JA, Collignon PJ. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000; 19: 1–8. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller MA, Lockhart SR, Pujol C, Swails-Wenger JA, Messer SA, Edmond MB, et al. Hospital specificity, region specificity, and fluconazole resistance of Candida albicans bloodstream isolates. J Clin Microbiol. 1998; 36: 1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escribano P, Rodríguez-Créixems M, Sánchez-Carrillo C, Muñoz P, Bouza E, Guinea J. Endemic genotypes of Candida albicans causing fungemia are frequent in the hospital. J Clin Microbiol. 2013; 51: 2118–2123. 10.1128/JCM.00516-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber K, Schulz B, Ruhnke M. The quorum-sensing molecule E,E-farnesol—its variable secretion and its impact on the growth and metabolism of Candida species. Yeast. 2010; 27: 727–739. 10.1002/yea.1769 [DOI] [PubMed] [Google Scholar]
- 25.Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002; 70: 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramage G, Saville SP, Wickes BL, López-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002; 68: 5459–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitru R, Hornby JM, Nickerson KW. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob Agents Chemother. 2004; 48: 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almirante B, Rodríguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005; 43: 1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014; 40:839–845. 10.1007/s00134-014-3310-z [DOI] [PubMed] [Google Scholar]
- 30.Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012; 54: 1110–1122. 10.1093/cid/cis021 [DOI] [PubMed] [Google Scholar]
- 31.Charles PE, Doise JM, Quenot JP, Aube H, Dalle F, Chavanet P, et al. Candidemia in critically ill patients: difference of outcome between medical and surgical patients. Intensive Care Med. 2003; 29: 2162–2169. [DOI] [PubMed] [Google Scholar]
- 32.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001; 67: 2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarathna DH, Hornby JM, Krishnan N, Parkhurst A, Duhamel GE, Nickerson KW. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. Infect Immun. 2007; 75: 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hargarten JC, Moore TC, Petro TM, Nickerson KW, Atkin AL. Candida albicans Quorum Sensing Molecules Stimulate Mouse Macrophage Migration. Infect Immun. 2015; 83: 3857–3864. 10.1128/IAI.00886-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.