Abstract
Objective
This pilot study aimed to inform future research evaluating the effectiveness of Platelet Rich Plasma (PRP) injection for tendinopathy.
Design
Randomized control trial (RCT) and synchronous observational cohort studies. For the RCT, consecutive consenting patients treated at an academic sports medicine clinic were randomly assigned to either a PRP or placebo control group.
Setting
The Glen Sather Sport Medicine Clinic, Edmonton, Canada.
Patients
The RCT included 9 participants with rotator cuff tendinopathy. The cohort study included 178 participants with a variety of tendinopathies.
Interventions
Patients receiving PRP were injected with 4 ml of platelets into the supraspinatus and/or infraspinatus, while patients in the placebo group were injected with 4ml of saline. All participants undertook a 3-month standardized, home-based, daily exercise program.
Main Outcome Measures
Participants in the RCT were re-evaluated 3, and 6 months post-injection. Change scores before and after injection on pain, disability and MRI-documented pathology outcomes were compared. In the cohort study, pain and disability were measured at 1, 2 and 3 months post-injection.
Results
For the RCT, 7 participants received PRP and 2 received placebo injections. Patients receiving PRP reported clinically important improvements in pain (>1.5/10 on VAS), disability (>15 point DASH change), and tendon pathology while those receiving placebo injections did not. In the observational cohort, statistically and clinically significant improvements in pain and disability were observed.
Conclusion
This pilot study provides information for planning future studies of PRP effectiveness. Preliminary results indicate intratendinous, ultrasound-guided PRP injection may lead to improvements in pain, function, and MRI-documented tendon pathology.
Trial Registration
Controlled-Trials.com ISRCTN68341698
Introduction
Tendinopathy is frequently a painful condition that defines the structural and compositional change in a tendon as a result of cumulative overload leading to a failed healing response.[1, 2] Tendinopathy is associated with pain, disability, depressive symptomology and anxiety.[3–5] The rotator cuff tendons are commonly affected, particularly in individuals over the age of 50.[6–8] Despite mixed and limited evidence, several non-surgical treatment approaches have been clinically used, including non-steroidal anti-inflammatory drugs, eccentric strengthening exercises, corticosteroid injections, Extracorporeal Shock Wave Therapy and platelet rich plasma (PRP) injections.[1]
PRP injections are an emerging therapeutic approach that have been used in a variety of clinical contexts, including dermatology, plastic surgery, sport medicine, dentistry and orthopedic surgery.[9–11] Platelets are cells that contain over 300 bioactive proteins and growth factors that control cell growth and differentiation, synthesis of connective tissue, and revascularization. In tissues that are aging and do not repair or regenerate well, growth factors may help to improve healing of the degenerate tissue by stimulating angiogenesis, epithelialisation, cell differentiation, replication, proliferation and formation of extracellular matrix.
PRP injections have been used as a treatment for tendinopathy and have been shown to positively influence health-relevant outcomes, such as pain and disability.[12–18] Mautner et al. found that following PRP injection, 82% of individuals reported an improvement of greater than 50% in pain symptoms measured with a visual analogue scale (VAS).[15] A recent systematic review of tendinopathy found that pain was significantly reduced up to 1-year following PRP injections.[12] However, methodological quality has varied across the studies, with several limitations commonly observed such as lack of a control group, high attrition rate or small sample size.[12–15, 18]
Research has started to examined the effect of PRP injections on clinical outcomes in individuals with rotator cuff tendinopathy.[6, 15, 16, 18] Of these studies, findings and methodological quality have also varied, making it difficult to conclude if PRP injections are an effective treatment for this population. Rha et al found that individuals who received PRP injections had reduced pain and disability compared to those who received dry needling.[16] A later study by Kesikburun et al. found that PRP injections had no effect on such variables.[6] Prior to conducting a full scale randomized controlled trial to clarify the effectiveness of this treatment for rotator cuff tendinopathy, it is important that pilot studies be conducted to provide background information to help overcome limitations of previous studies. For example, it is unknown why previous PRP studies have had such high attrition rates or small samples, or how best to overcome these problems in future studies.
This pilot project aimed to provide background information to assist in planning large-scale studies aimed at determining the effectiveness of PRP injection on clinical and functional outcomes. Two separate studies were conducted that will be described separately. This included a randomized controlled trial (RCT) aimed at evaluating the effectiveness of a single intratendinous, ultrasound-guided Platelet Rich Plasma (PRP) injection into the degenerate rotator cuff. The second study was an observational cohort study that evaluated patients who received PRP treatment for a variety of tendon disorders.
Methods and Results
Study One
Design
A pilot prospective, RCT was conducted. This was a parallel design with 1–1 allocation ratio. The study recruitment and follow-up took place between 1 July 2011 and 31 July 2014. The study registration number was ISRCTN68341698 (due to the nature of this study as a pilot study, it was not registered prior to enrolment). The authors confirm that all ongoing and related trials for this intervention are registered.
Sample
We aimed to enrol 50 participants, balancing adequate statistical power with the feasibility and expense of collecting Magnetic Resonance Imaging (MRI) data. Thirty consecutive patients with rotator cuff tendinopathy and meeting inclusion criteria treated at the Glen Sather Sport Medicine Clinic (GSSMC) in Alberta, Canada, were assessed for eligibility. Inclusion criteria were: 1) patients between 35 and 60 years of age; 2) minimum of 3-month history of non-traumatic shoulder pain that was unresponsive to previous conservative treatment (including analgesia, subacromial steroid injection and physical therapy); and 3) tendinosis/tendinopathy or partial thickness tears in the supraspinatus and/or infraspinatous tendons confirmed using MRI. All participants had MRI of the affected shoulder prior to referral for consideration of enrollment in the study. No important changes were made to the methods after trial commencement.
Patients were excluded if they had: 1) a history of rotator cuff repair; 2) traumatic injury to the shoulder inducing pathology; 3) MRI findings showing full thickness rotator cuff tear; 4) previous PRP treatment for the rotator cuff; 5) shoulder pain due to a work-related injury/complaint; or 6) diabetes.
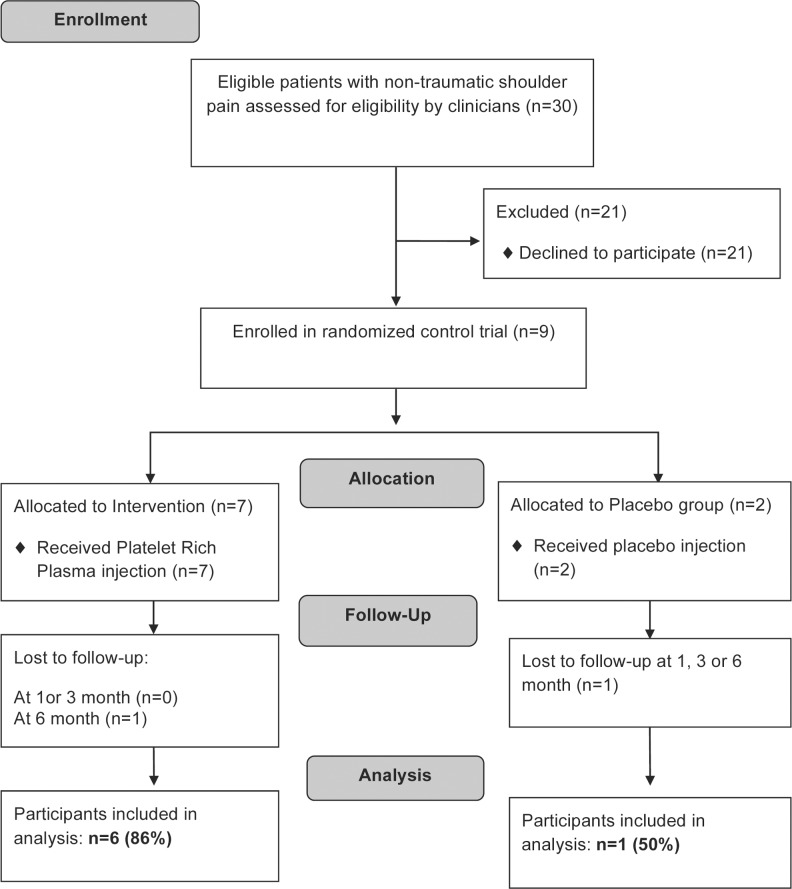
Nine eligible patients agreed to participate. These patients were predominantly middle-aged (mean 49 years) males (67%), with moderate levels (4.2/10) of chronic pain (median duration 19 months). Twenty-one patients declined to participate, stating they would prefer to pay for the PRP treatment out of pocket rather than risk receiving placebo treatment. Due largely to this low participation rate, enrolment was stopped.
Intervention and Data Collection
Patients enrolled in the trial were randomly assigned to either a PRP or placebo control group by pulling group assignment out of a box without replacement (randomization was done by author LP who prepared the injections but was not otherwise involved in patient care). All physicians and the treating physiotherapist were blind to group allocation. At the time of enrolment, all participants had a repeat clinical and ultrasound assessment of the rotator cuff by one of the two physician investigators to correlate ultrasound findings to those demonstrated on MRI. Using the Harvest™ system (Plymouth, Massachusetts) for generating PRP, 4 ml of platelets were fenestrated to the degenerative area of the tendon under ultrasound guidance for each patient in the study group. Patients in the placebo group were injected in the same manner with 4ml of saline. Following the injection, all patients undertook a 3-month standardized, home-based daily exercise program under the supervision of a physical therapist. Patients were clinically re-evaluated at 3, and 6 months post-injection. (See Fig 1 for study flowchart).
Fig 1. Study Flow Diagram for Platelet-Rich Plasma Injection Pilot Randomized Controlled Trial.
Study 1 Flow Diagram.
Measures
Since PRP treatment aims to facilitate tendon healing, reduce pain and increase functional ability, we measured self-report pain intensity and disability using self-report outcome measures as described below, as well as objective clinical evaluations that included assessment of the pre- and post-injection MRI findings. Musculoskeletal radiologists evaluated the MRI results qualitatively and quantitatively where possible using size of the tear in millimeters. MRI was conducted before the injection then repeated 6 months following the study PRP injection. Post-injection findings were compared to pre-injection findings by the assessing radiologist.
Three 10-point VAS measures were used in all patients to measure pain intensity, ability to do daily activities, and physical activity/exercise.[19] These were anchored at 0 with ‘no pain’ and 10 ‘worst imaginable’. These scales were completed before injection and at each follow-up. The minimum clinically important difference for the 10-point VAS was considered to be 1.5/10.[20, 21]
We also measured disability and health-related quality of life using the Disabilities of the Arm Shoulder and Hand (DASH) and Western Ontario Rotator Cuff (WORC) Index questionnaires. These were completed before injection and at each follow-up assessments. The DASH questionnaire is a commonly used, self-report, region-specific outcome instrument developed as a measure of self-rated upper-extremity disability and symptoms.[22] The DASH consists mainly of a 30-item disability/symptom scale, scored from 0 to 100.[23] The Western Ontario Rotator Cuff (WORC) Index questionnaire is a self-report questionnaire consisting of 21 items representing 5 domains to assess disease-specific quality-of-life.[24, 25] There are 6 questions in the physical symptoms domain, 4 in the sports and recreation domain, 4 in the work domain, 4 in the lifestyle domain, and 3 in the emotions domain. All DASH and WORC scores were converted into a standardized score out of 100.[26, 27] Lower DASH scores indicate less disability while higher WORC scores indicate higher life quality. The minimum clinically important difference for the DASH and WORC were considered to be 15/100.[22, 28, 29]
No changes were made to the trial outcomes after the trial commenced.
Data analysis
Descriptive statistics were calculated for demographic information and pain and disability scores for all patients. To determine the effectiveness of PRP, we examined overall mean change scores of the VAS, WORC and DASH within subjects and between groups. Data were handled using intention to treat, however statistical testing was not conducted due to limited sample size (originally group comparisons were planned on all of the main outcome measures). Instead, mean change scores were compared to known clinically important differences for the measures. Since only 9 participants enrolled in the trial and the numbers in each group were imbalanced, we considered participants individually and created a comprehensive table of individual results on the various pain and disability measures. All analyses were performed using SPSS version 22.0 (Armonk, New York).
Results
Of the 9 participants enrolled in the trial, 7 were randomly assigned to PRP and 2 were assigned to placebo (see Table 1). All participants returned for the 3-month follow-up, however, 1 participant in the placebo group did not return for the 6 month follow-up and did not undertake follow-up MRI. Additionally, 1 participant in the PRP group did not complete the follow-up WORC scale but data were available for all other measures.
Table 1. Characteristics and Outcomes of Patients in the Randomized Control Trial.
| Patient | Age (years) | Sex | Group | Pain Duration (month) | Follow-up Duration (months) | Pre-Injection VAS* Scores (out of 10) | Mean 6-month VAS Change (positive scores indicate improvement) | Pre-DASH** | Post-DASH | DASH Change | Pre-WORC# | Post-WORC | WORC Change | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS 1 | VAS 2 | VAS 3 | VAS 1 | VAS 2 | VAS 3 | ||||||||||||
| 1 | 48 | M | PRP | 19 | 6 | 5 | 2 | 7 | 3 | 0 | 1 | 23 | 8 | -15 | 52 | 73 | 21 |
| 2 | 56 | M | PRP | 12 | 6 | 5 | 8 | 7 | 3 | 5 | 5 | 36 | 17 | -19 | 52 | - | - |
| 3 | 52 | F | Placebo | 13 | 3 | 4 | 8 | 8 | -3 | -1 | -1 | 67 | 77 | 10 | 25 | 27 | 2 |
| 4 | 37 | M | PRP | 18 | 6 | 0 | 2 | 10 | -1 | 1 | 5 | 26 | 13 | -13 | 56 | 82 | 26 |
| 5 | 48 | F | PRP | 60 | 6 | 6 | 3 | 3 | 5 | 2 | 2 | 34 | 14 | -20 | 52 | 84 | 32 |
| 6 | 56 | M | PRP | 36 | 6 | 0 | 1 | 4 | -1 | 1 | 2 | 26 | 8 | -18 | 68 | 88 | 20 |
| 7 | 45 | M | PRP | 360 | 6 | 9 | 8 | 8 | 7 | 6 | 5 | 45 | 9 | -36 | 22 | 89 | 67 |
| 8 | 47 | M | Placebo | 18 | 6 | 5 | 6 | 6 | 1 | 2 | 2 | 36 | 11 | -25 | 39 | 84 | 45 |
| 9 | 56 | F | PRP | 24 | 6 | 4 | 3 | 6 | 3 | 1 | 2 | 29 | 29 | 0 | 29 | 95 | 66 |
* Visual Analogue Scales: VAS 1 = Pain intensity, VAS 2 = ability to do daily activities, VAS 3 = physical activity/exercise
** DASH = Disabilities of the Arm, Shoulder, and Hand Questionnaire
# Western Ontario Rotator Cuff Index
Five PRP and 1 placebo participant demonstrated clinically important DASH improvements. Mean change before and after injection on the DASH score was -17.3 (10.7 SD) for the PRP group and -7.5 (24.7 SD) for the placebo group. Six PRP and 1 patient in the placebo group demonstrated clinically important WORC improvements. Mean change on the WORC score was 38.7 (22.0 SD) for the PRP group and 23.5 (30.4 SD) for the placebo group. Mean improvement on the VAS scales was clinically important for the PRP group (2.6, 2.2, 3.0 and 4.1) but not for the placebo group (-1.0, 0.5, 0.5 and 2).
MRI Findings
Analysis of the radiologist reporting of the MRI changes identified that 2 participants experienced worsening of the cuff pathology, one participant experienced equivocal change, and 5 of the 8 participants had improvement of the pathoanatomy of the rotator cuff following the treatment (See Table 2). Five out of 7 participants in the PRP group (71%) experienced improved MRI findings after PRP injection, while the participant in the placebo group did not experience improvement. However, the structural changes to the rotator cuff on MRI did not correlate to the pain or functional improvements demonstrated on self-report questionnaires (see Table 1).
Table 2. Comparison of MRI Findings Before and After Platelet-Rich Plasma Injections.
| Pre-PRP Findings Reported by Radiologist | 6 months Post-PRP Findings Reported by Radiologist | MRI Change Interpretation | |
|---|---|---|---|
| Participant 1 (PRP group) | 7x4 mm Supraspinatus Tear, 1x3 mm Infraspinatus Tear | 3x3 mm Supraspinatus Tear, 1x3 mm Infraspinatus Tear | Better |
| Participant 2 (PRP group) | Tendinosis of Supraspinatus, Intact Infraspinatus | 13 mm Supraspinatus tear, Fraying Infraspinatus | Worse |
| Participant 3 (Placebo) | Tendinosis of Supraspinatus and Infraspinatus | Lost to follow up | Lost to follow up |
| Participant 4 (PRP group) | 9x2 mm Supraspinatus tear, Intact Infraspinatus | 7x2 mm Supraspinatus tear, Intact Infraspinatus | Better |
| Participant 5 (PRP group) | Calcific Tendonitis Supraspinatus, Normal Infraspinatus | Normal Supraspinatus, Normal Infraspinatus | Better |
| Participant 6 (PRP group) | 2x10 mm Supraspinatus tear, Normal Infraspinatus | Normal Supraspinatus, Minor Tendinosis of Infraspinatus | Better |
| Participant 7 (PRP group) | Delamination of Supraspinatus, Normal Infraspinatus | Improvement in Delamination | Better |
| Participant 8 (Placebo) | 3 mm Supraspinatus tear, Normal Infraspinatus | 11x2 mm Supraspinatus tear, Normal Infraspinatus | Worse |
| Participant 9 (PRP group) | 8 mm Supraspinatus tear, Normal Infraspinatus | 8 mm Supraspinatus tear, Questionably More Prominent Tendinosis of Infraspinatus | Equivocal |
Study Two
Design
A retrospective observational cohort study was conducted. As mentioned above, over the course of the pilot RCT, we observed that a high proportion (70%) of patients refused to participate in the RCT and preferred to pay out-of-pocket for treatment to ensure they received PRP instead of chance receiving placebo. Therefore, we conducted a retrospective cohort study to observe outcomes in all patients treated at the clinic with PRP over the course of the original trial.
Sample
Participants included 178 consecutive patients presenting to the clinic between April 2011 and July 2014 for treatment of tendinopathy-related pain complaints of any body parts. Inclusion criteria included being treated with PRP to any body part and followed-up by physicians at the GSSMC. Patients in this study were predominately males (58%) with a mean age of 44.7 years and moderate pain intensity (5.6/10). Most commonly injected body parts were the Achilles tendon (25%), elbow common extensor tendon (20%), supraspinatus and/or infraspinatus (19%) and patellar tendon (19%).
Intervention and Data Collection
Patients in the observational cohort were treated in a similar manner to the RCT protocol described above. Three to 8ml of PRP were fenestrated to the relevant tendon and patients received a daily, structured home exercise program. These patients were re-evaluated at 1, 2 and 3 months post-injection (See Fig 2 for study flowchart).
Fig 2. Study Flow Diagram for the Retrospective Observational Study.
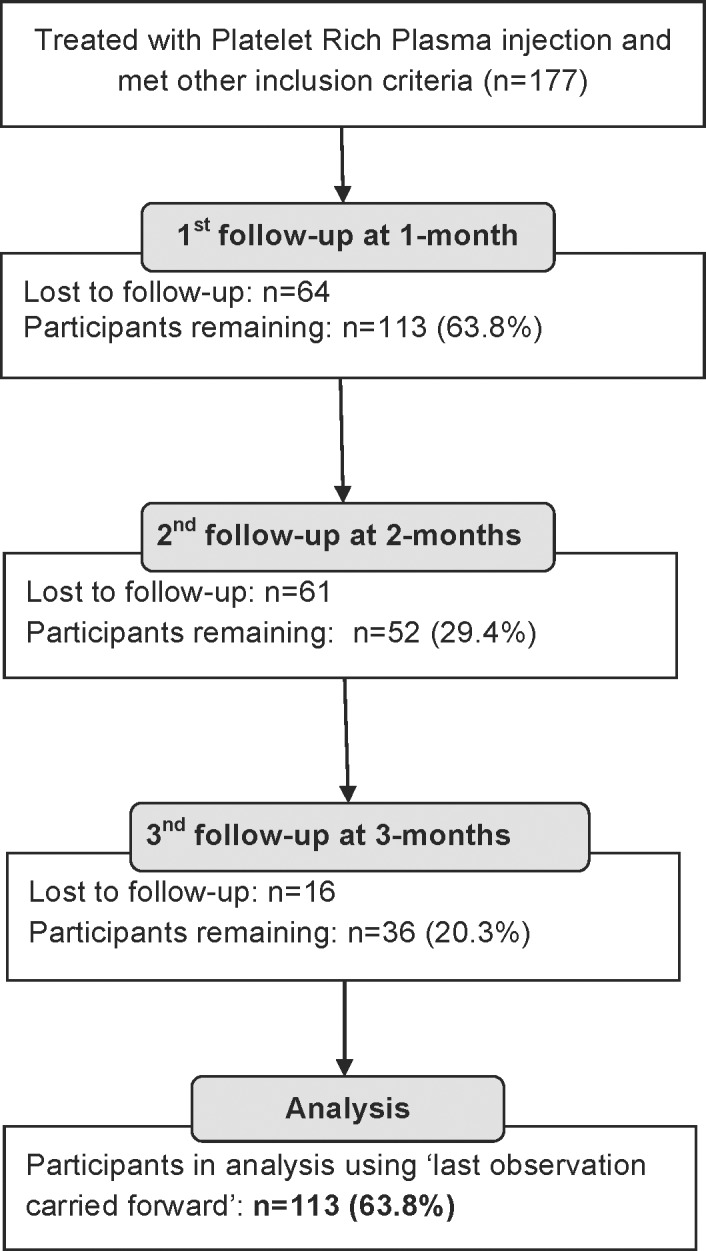
Study 2 Flow Diagram.
Measures
Some basic descriptive information was collected including sex, age group, treating doctor, and number of PRP injections received. The number of injections was dependent on the treating doctor, but was typically repeated if response to the initial injection was not of an expected magnitude. In addition to this descriptive information, three 10-point VAS measures were used in all patients to measure pain intensity, ability to do daily activities, and physical activity/exercise.[19] These were anchored at 0 with ‘no pain’ and 10 ‘worst imaginable’. These scales were completed before injection and at each follow-up. The minimum clinically important difference for the 10-point VAS was considered to be 1.5/10.
Data analysis
Descriptive statistics were calculated for demographic information and pain and disability scores for all patients. Participants who had incomplete or missing data at baseline were considered missing and were not included in subsequent analysis. Those with partial follow-up were analyzed with listwise deletion as well as with a last observation carried forward strategy.[30] Last observation carried forward provides an unbiased estimate of the magnitude of an effect, if data are missing at random.[30] A comparison was made between those with and without missing data using independent t tests for continuous data and chi-square statistics for nominal data. Pre-injection VAS measurements (pain, function and sports activities) were compared to follow-up VAS scores separately using paired-sample t tests. Additionally, MANOVA was used to determine if there were significant differences in change scores between sex, age groups, number of injections and treating doctor. All analyses were performed using SPSS version 22.0 (Armonk, New York). Alpha level for statistical significance was set at α = 0.05.
Results
Of the 178 patients in the observational cohort, 65 participants were excluded due to incomplete data at either baseline or follow-up measures. There were no statistically significant differences between those with and without complete data (See Table 3). The 113 participants (63%) with complete data were predominately male (58%) with a mean age of 44.7 years and had moderate pain intensity (5.6/10). The most commonly injected body parts were the Achilles tendon (25%), elbow (20%), patella tendon (19%) and shoulder (19%). Eighty percent of participants received a single injection while 20% received multiple (2 or 3 injections).
Table 3. Characteristics of Patients in the Observational Cohort Study.
| Characteristics | Complete Data | Missing Data | |
|---|---|---|---|
| Mean (SD) / Number (Percent %) | P Value | ||
| N = 113 | N = 65 | ||
| Age (years) | 44.7 (13.5) | 44.1 (12.9) | 0.75 |
| Gender (male) | 66 (58%) | 35 (54%) | 0.55 |
| Body Part of Injection | 0.96 | ||
| Achilles | 28 (25%) | 18 (28%) | |
| Elbow | 23 (20%) | 14 (22%) | |
| Knee | 21 (19%) | 11 (17%) | |
| Shoulder | 21 (19%) | 13 (20%) | |
| Other | 20 (18%) | 9 (14%) | |
| Number of Injections | 0.37 | ||
| 1 | 90 (80%) | 55 (85%) | |
| 2 | 20 (18%) | 10 (15%) | |
| 3 | 3 (3%) | 0 (0%) | |
Statistically significant and clinically important differences were observed between baseline and follow-up on all VAS measures (See Table 4). Mean improvements on the VAS measures were 2.3 (2.5 SD) for pain intensity VAS (p <0.001), 1.8 (2.5 SD) for limited ability for daily activities VAS (p < 0.001), and 2.7 (2.7 SD) for limited physical activity/exercise VAS (p < 0.001).
Table 4. Clinical Outcomes for Patients in the Observational Cohort Study.
| Visual Analogue Scale for Pain Intensity | Mean (SD) | P Value |
| Pre-injection (N = 113) | 5.6 (2.4) | |
| 1 month post-injection (N = 113) | 3.8 (2.5) | p < 0.001 |
| 2 month post-injection (N = 52) | 3.6 (2.3) | p < 0.001 |
| 3 month post-injection (N = 36) | 3.3 (2.2) | p < 0.001 |
| 3 month post-injection (LOCF*) | 3.3 (2.4) | p < 0.001 |
| Visual Analogue Scale for Daily Activities | ||
| Pre-injection (N = 113) | 4.9 (2.5) | |
| 1 month post-injection (N = 113) | 3.5 (2.5) | p < 0.001 |
| 2 month post-injection (N = 52) | 3.4 (2.2) | p < 0.001 |
| 3 month post-injection (N = 36) | 3.1 (2.6) | p < 0.001 |
| 3 month post-injection (LOCF) | 3.1 (2.4) | p < 0.001 |
| Visual Analogue Scale for Physical Activity/Exercise | ||
| Pre-injection (N = 113) | 7.2 (1.6) | |
| 1 month post-injection (N = 113) | 5.0 (2.6) | p < 0.001 |
| 2 month post-injection (N = 52) | 4.4 (2.1) | p < 0.001 |
| 3 month post-injection (N = 36) | 4.3 (2.7) | p < 0.001 |
| 3 month post-injection (LOCF) | 4.5 (2.6) | p < 0.001 |
All post-injection results were statistically compared to pre-injection scores separately.
* LOCF = Results using Last Observation Carried Forward technique.
MANOVA analysis evaluating differences across covariates found that pain intensity VAS and daily activity ability VAS were significantly different between genders (p < 0.05, See Table 5). Females improved more on both pain (2.8 vs. 1.8) and function (2.5 vs. 1.4) compared to males. No other variables showed statistically significant differences among/between other classifications.
Table 5. MANOVA Analysis of the Observational Cohort Study Assessing Change Scores Between Pre-Injection and 3-month Post-Injection.
| VAS Pain | VAS Function | VAS Sports Activities | ||||
|---|---|---|---|---|---|---|
| Mean (Standard Deviation) (N = 113) | ||||||
| Gender | P Value | P Value | P Value | |||
| Male (N = 66) | 1.8 (2.5) | 0.04* | 1.4 (2.5) | 0.02* | 2.7 (2.7) | 0.996 |
| Female (N = 47) | 2.8 (2.4) | 2.5 (2.5) | 2.7 (2.6) | |||
| Age | ||||||
| Under 30 (N = 18) | 2.1 (2.8) | 0.14 | 1.1 (2.2) | 0.33 | 2.5 (2.3) | 0.31 |
| Between 30 and 60 (N = 84) | 2.1 (2.5) | 1.9 (2.6) | 2.7 (2.7) | |||
| Beyond 60 (N = 11) | 3.0 (2.5) | 2.9 (2.5) | 3.2 (2.7) | |||
| Body Part | ||||||
| Achilles (N = 28) | 2.3 (2.7) | 0.24 | 2.4 (2.9) | 0.96 | 2.7 (2.6) | 0.45 |
| Elbow (N = 23) | 2.3 (2.2) | 2.0 (2.3) | 2.6 (3.0) | |||
| Knee (N = 21) | 1.8 (2.7) | 1.1 (2.5) | 2.6 (2.5) | |||
| Shoulder (N = 21) | 2.6 (2.9) | 1.9 (2.7) | 2.4 (3.0) | |||
| Other (N = 20) | 2.2 (2.2) | 1.8 (2.2) | 3.3 (2.4) | |||
| Number of Injections | ||||||
| 1 (N = 90) | 2.2 (2.5) | 0.82 | 1.8 (2.5) | 0.73 | 2.8 (2.6) | 0.72 |
| 2 (N = 20) | 2.6 (2.9) | 1.9 (2.5) | 2.3 (2.7) | |||
| 3 (N = 3) | 2.2 (2.5) | 3.0 (4.4) | 2.5 (3.3) | |||
| Physician | ||||||
| A (N = 40) | 2.1 (2.3) | 0.36 | 2.0 (2.4) | 0.42 | 2.7 (2.5) | 0.83 |
| B (N = 37) | 3.1 (2.5) | 2.2 (2.6) | 3.3 (2.6) | |||
| C (N = 22) | 1.5 (2.8) | 1.3 (2.6) | 1.8 (2.9) | |||
| Other Doctors (N = 14) | 1.6 (2.4) | 1.5 (2.7) | 2.6 (2.6) | |||
* Indicates statistically significant differences observed between/among groups.
Ethical Considerations
The University of Alberta Health Research Ethics Board approved this research (File Numbers: Pro00019481 and Pro00049954, representing separate protocols for the RCT and observational cohort study respectively). There were no modifications to the pilot RCT.
Discussion
Several observations were made during this pilot study to inform future large-scale RCTs of PRP treatment. Patients considered for this study had very high expectations of PRP treatment, with 70% willing to pay out-of-pocket for the therapy to avoid the possibility of receiving a placebo injection. This presented a serious challenge for recruitment and will likely limit the ability of future investigators to enrol sufficiently large and representative samples of patients for PRP trials. High patient expectations and the placebo effect on clinical outcomes also cannot be discounted.[31] One of the 2 patients in our placebo group demonstrated clinically important VAS scores. This could have been due to a placebo response to the injection, or due to the structured rehabilitation and home exercise program they participated in. Alternative designs that guarantee that patients will receive PRP while still rigourously evaluating effectiveness of the therapy should be considered, such as randomized crossover trials [32] or use of a wait-list control group. Crossover designs also have inherent challenges, such as the duration of acceptable washout period, but they may be more suitable to educated and informed patients who want to experience PRP therapy.
The direction and magnitude of therapeutic effect observed for PRP in this study was quite consistent across patients in the RCT and observational cohort. Clinically important, yet modest improvements were consistently observed. These findings suggest that single intratendinous, ultrasound-guided PRP injection may be effective in improving pain and function in patients with rotator cuff tendinopathy. Interestingly, we observed high rates of MRI-documented improvement in rotator cuff pathology in patients receiving PRP, which was not seen in the patient in the placebo group. However, the improved pathology did not clearly correlate with improved symptoms, a finding that requires further investigation. A moderating variable to be accounted for in future research is gender, as we observed in the cohort study that females demonstrated larger improvements than males. The estimates and standard deviations provided here can be used to estimate sample sizes for future studies.
We found that at one busy academic sports medicine clinic, the number of eligible patients seen meeting our strict inclusion/exclusion criteria was quite low. Over a nearly 3-year enrolment period, we assessed only 30 eligible patients. For the randomized control study, our stringent study criteria focused on patients with confirmed rotator-cuff tendinopathy who had ‘failed’ prior appropriate conservative management. Future studies will either need to have less stringent criteria or have multi-site enrolment to increase sample size.
The current pilot study has several limitations. The sample size for the RCT was too small to conduct inferential statistics. The original study design planned to recruit 50 participants. A large number of patients were screened for potential inclusion in the study, however the vast majority did not meet our strict inclusion criteria and had not attempted or completed what we considered to be appropriate conservative treatment. When conservative treatment was initiated, nearly all patients recovered limiting the number of eligible patients for our study. Nine participants agreed to volunteer in our trial, but 21 other patients meeting the inclusion criteria were not willing to chance receiving a placebo injection and paid out-of-pocket for PRP treatment. Despite not being able to conduct statistical testing with this small sample size, clinically significant results were demonstrated, which was also confirmed in the observational cohort study. Missing data could possibly lead to lack of power and data inconsistency, but we observed little differences across those with complete and incomplete data. Future studies will need to consider providing incentives to improve data collection methods or use different methodological strategies such as a wait-list control group.
Conclusion
This pilot study provides important information for planning future studies of PRP effectiveness. High patient expectations for PRP are likely to influence enrollment and alternative designs such as crossover studies should be considered. Preliminary results from this study indicate a single, intratendinous, ultrasound-guided PRP injection lead to improvements in pain, function, and MRI-documented tendon pathology in patients with rotator cuff tendinopathies.
Supporting Information
(DOCX)
(PDF)
(PDF)
Acknowledgments
The Glen Sather Sports Medicine Clinic facilitated data collection. We would like to thank Nicole Morin, Robyn Gross and Mitchell Reif who assisted with data extraction.
Data Availability
Since these data relate to patient care, the authors have legal and ethical restrictions on making the minimal data set publicly available. These arise from Alberta’s Freedom of Information and Protection of Privacy Act and are a requirement of the authors' ethical approval from the University of Alberta’s Health Research Ethics Board. Data will be available upon request to interested researchers by contacting Doug Gross at dgross@ualberta.ca.
Funding Statement
This research was funded by the Workers' Compensation Board of Alberta Research Grants Program. Project number was RES0012970 [http://www.wcb.ab.ca/public/approved_projects.asp]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–54. 10.1007/s11999-008-0260-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1998;14(8):840–3. . [DOI] [PubMed] [Google Scholar]
- 3.Alizadehkhaiyat O, Fisher AC, Kemp GJ, Frostick SP. Pain, functional disability, and psychologic status in tennis elbow. Clin J Pain. 2007;23(6):482–9. 10.1097/AJP.0b013e31805f70fa . [DOI] [PubMed] [Google Scholar]
- 4.Kettunen JA, Kvist M, Alanen E, Kujala UM. Long-term prognosis for jumper's knee in male athletes. A prospective follow-up study. Am J Sports Med. 2002;30(5):689–92. . [DOI] [PubMed] [Google Scholar]
- 5.MacDermid JC, Ramos J, Drosdowech D, Faber K, Patterson S. The impact of rotator cuff pathology on isometric and isokinetic strength, function, and quality of life. J Shoulder Elbow Surg. 2004;13(6):593–8. 10.1016/S1058274604001247 . [DOI] [PubMed] [Google Scholar]
- 6.Kesikburun S, Tan AK, Yilmaz B, Yasar E, Yazicioglu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41(11):2609–16. 10.1177/0363546513496542 . [DOI] [PubMed] [Google Scholar]
- 7.Miranda H, Viikari-Juntura E, Heistaro S, Heliovaara M, Riihimaki H. A population study on differences in the determinants of a specific shoulder disorder versus nonspecific shoulder pain without clinical findings. Am J Epidemiol. 2005;161(9):847–55. 10.1093/aje/kwi112 . [DOI] [PubMed] [Google Scholar]
- 8.van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54(12):959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anitua E, Sanchez M, Nurden AT, Nurden P, Orive G, Andia I. New insights into and novel applications for platelet-rich fibrin therapies. Trends in biotechnology. 2006;24(5):227–34. 10.1016/j.tibtech.2006.02.010 . [DOI] [PubMed] [Google Scholar]
- 10.Cole BJ, Seroyer ST, Filardo G, Bajaj S, Fortier LA. Platelet-rich plasma: where are we now and where are we going? Sports health. 2010;2(3):203–10. 10.1177/1941738110366385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618–9. 10.1136/bjsm.2009.067108 . [DOI] [PubMed] [Google Scholar]
- 12.Andia I, Latorre PM, Gomez MC, Burgos-Alonso N, Abate M, Maffulli N. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110(1):99–115. 10.1093/bmb/ldu007 . [DOI] [PubMed] [Google Scholar]
- 13.Charousset C, Zaoui A, Bellaiche L, Bouyer B. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes? a prospective study. Am J Sports Med. 2014;42(4):906–11. 10.1177/0363546513519964 . [DOI] [PubMed] [Google Scholar]
- 14.Dallaudiere B, Pesquer L, Meyer P, Silvestre A, Perozziello A, Peuchant A, et al. Intratendinous injection of platelet-rich plasma under US guidance to treat tendinopathy: a long-term pilot study. Journal of vascular and interventional radiology: JVIR. 2014;25(5):717–23. 10.1016/j.jvir.2014.01.026 . [DOI] [PubMed] [Google Scholar]
- 15.Mautner K, Colberg RE, Malanga G, Borg-Stein JP, Harmon KG, Dharamsi AS, et al. Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM & R: the journal of injury, function, and rehabilitation. 2013;5(3):169–75. 10.1016/j.pmrj.2012.12.010 . [DOI] [PubMed] [Google Scholar]
- 16.Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27(2):113–22. 10.1177/0269215512448388 . [DOI] [PubMed] [Google Scholar]
- 17.Rowan TL, Drouin JL. A multidisciplinary approach including the use of platelet-rich plasma to treat an elite athlete with patellar tendinopathy—a case report. The Journal of the Canadian Chiropractic Association. 2013;57(4):301–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Scarpone M, Rabago D, Snell E, Demeo P, Ruppert K, Pritchard P, et al. Effectiveness of Platelet-rich Plasma Injection for Rotator Cuff Tendinopathy: A Prospective Open-label Study. Global advances in health and medicine: improving healthcare outcomes worldwide. 2013;2(2):26–31. 10.7453/gahmj.2012.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finch E, Brooks D, Stratford P, Mayo N. Physical rehabilitation outcome measures: A guide to enhanced clinical decision making 2nd ed. Toronto: Canadian Physiotherapy Association; 2002. [Google Scholar]
- 20.Flynn T. Clinical prediction rules for the lumbar spine. New Zealand Journal of Physiotherapy. 2009;37(3):145–. . Language: English. Entry Date: 20100205. Revision Date: 20100226. Publication Type: journal article. [Google Scholar]
- 21.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emergency medicine journal: EMJ. 2001;18(3):205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaton DE, Davids AM, Hudak PL, McConnell S. The DASH (Disabilities of the Arm, Shoulder and Hand) Outcome Measure: What Do We Know About It Now? British Journal of Hand Therapy. 2001;6(4):109–18. [Google Scholar]
- 23.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC musculoskeletal disorders. 2003;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raman J, Macdermid JC. Western Ontario Rotator Cuff Index. Journal of physiotherapy. 2012;58(3):201 10.1016/S1836-9553(12)70115-7 . [DOI] [PubMed] [Google Scholar]
- 25.Kirkley A, Alvarez C, Griffin S. The development and evaluation of a disease-specific quality-of-life questionnaire for disorders of the rotator cuff: The Western Ontario Rotator Cuff Index. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2003;13(2):84–92. . [DOI] [PubMed] [Google Scholar]
- 26.Raman J, Macdermid JC. Western Ontario Rotator Cuff Index. Journal of physiotherapy. 2012;58(3):201 10.1016/S1836-9553(12)70115-7 [DOI] [PubMed] [Google Scholar]
- 27.Malzahn DE, Fernandez JE, Kattel BP. Design-oriented functional capacity evaluation: the available motions inventory—a review. Disabil Rehabil. 1996;18(8):382–95. . [DOI] [PubMed] [Google Scholar]
- 28.de Witte PB, Henseler JF, Nagels J, Vliet Vlieland TP, Nelissen RG. The Western Ontario rotator cuff index in rotator cuff disease patients: a comprehensive reliability and responsiveness validation study. Am J Sports Med. 2012;40(7):1611–9. 10.1177/0363546512446591 . [DOI] [PubMed] [Google Scholar]
- 29.Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18(6):920–6. 10.1016/j.jse.2008.12.015 . [DOI] [PubMed] [Google Scholar]
- 30.Mallinckrodt CH, Clark SW, Carroll RJ, Molenbergh G. Assessing response profiles from incomplete longitudinal clinical trial data under regulatory considerations. Journal of biopharmaceutical statistics. 2003;13(2):179–90. 10.1081/BIP-120019265 . [DOI] [PubMed] [Google Scholar]
- 31.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. Jama. 1994;271(20):1609–14. . [PubMed] [Google Scholar]
- 32.Jones B, Kenward MG. Design and analysis of cross-over trials 2nd ed Boca Raton, FL: Chapman & Hall/CRC; 2003. xxv, 382 p. p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(PDF)
Data Availability Statement
Since these data relate to patient care, the authors have legal and ethical restrictions on making the minimal data set publicly available. These arise from Alberta’s Freedom of Information and Protection of Privacy Act and are a requirement of the authors' ethical approval from the University of Alberta’s Health Research Ethics Board. Data will be available upon request to interested researchers by contacting Doug Gross at dgross@ualberta.ca.