Abstract
Host Defence Peptides and derived peptides are promising classes of antimicrobial and immunomodulatory lead compounds. For this purpose we examined whether chicken cathelicidin-2 (CATH-2)-derived peptides modulate the function and inflammatory response of avian immune cells. Using a chicken macrophage cell line (HD11) we found that full-length CATH-2 dose-dependently induced transcription of chemokines CXCLi2/IL-8, MCP-3 and CCLi4/RANTES, but not of pro-inflammatory cytokine IL-1β. In addition, CATH-2 efficiently inhibited IL-1β and nitric oxide production by HD11 cells induced by different sources of lipopolysaccharides (LPS). N-terminal truncated CATH-2 derived peptides maintained the capacity to selectively induce chemokine transcription, but despite their high LPS affinity several analogs lacked LPS-neutralizing capacity. Substitution of phenylalanine residues by tryptophan introduced endotoxin neutralization capacity in inactive truncated CATH-2 derived peptides. In contrast, amino acid substitution of phenylalanine by tyrosine abrogated endotoxin neutralization activity of CATH-2 analogs. These findings support a pivotal role for aromatic residues in peptide-mediated endotoxin neutralization by CATH-2 analogs and were shown to be independent of LPS affinity. The capacity to modulate chemokine production and dampen endotoxin-induced pro-inflammatory responses in chicken immune cells implicates that small CATH-2 based peptides could serve as leads for the design of CATH-2 based immunomodulatory anti-infectives.
Introduction
Worldwide, antibiotics are extensively used by the livestock industry in veterinary therapy and as a feed additive to promote animal growth, especially in the poultry and swine industry, and it is estimated to exceed 50% of the total antibiotics use in some countries [1]. The antibiotics used in veterinary medicine are structurally related to antibiotics commonly used for human therapy [2]. Micro-organisms have demonstrated to develop resistance against conventional antibiotics [3] and infections with multi-drug resistant bacteria are an increasingly common challenge in hospitals while the number of new antimicrobials in development is low. Hence, to reduce the use of conventional antibiotics in veterinary medicine, alternative strategies are needed that suppress outbreaks of infectious diseases in farm animals.
A promising approach is the use of host defense peptides (HDPs) such as cathelicidins and defensins. HDPs are capable of killing a wide variety of micro-organisms, including multidrug-resistant bacteria, yeast, fungi, protozoa and viruses. In contrast to conventional antibiotics, HDP-mediated killing of micro-organisms occurs via multiple mechanisms attacking both the cell membrane and intracellular targets [4] and despite millions of years of co-evolution, most micro-organisms remain highly susceptible to HDP-mediated killing [5]. Moreover, HDPs exhibit a diversity of immune-related functions. Some HDPs, such as LL-37 inhibit LPS-induced pro-inflammatory cytokine production [6,7] and protect against development of endotoxin shock in vivo [8]. HDPs may act directly [9] or indirectly by selectively inducing chemokine production in immune cells [8] and recruitment of other immune cells to the site of infection. At the same time, HDPs may enhance antigen uptake and presentation [10] and inhibit apoptosis of neutrophils and macrophages [11,12]. Thus, in addition to direct antimicrobial activities, HDP-derived peptides may boost the innate and adaptive immune system leading to prevention and improved resolution of infectious diseases [13].
Cathelicidin-2 (CATH-2) is a highly cationic (11+) chicken heterophil-derived peptide with antibacterial and antifungal activities [14]. We previously demonstrated that CATH-2 inhibits LPS-induced production of TNFα, IL-6, IL8 and IL-10 and induces expression of Monocyte Chemotactic Protein 1 (MCP-1) in human peripheral blood mononuclear cells (PBMCs) [13]. The immunomodulatory effects of CATH-2 derived peptides on avian immune cells have not been studied.
CATH-2 has the potential to serve as a paradigm for the development of anti-infectives in poultry with immunomodulatory and/or antibacterial activities. Therefore, we examined the immunomodulatory and anti-inflammatory effects of CATH-2 derived peptides on the avian macrophage-like HD11 cell line. We demonstrate that CATH-2 and several truncated analogs thereof selectively induce chemokine transcription in HD11 cells and inhibit LPS-induced IL-1β and nitric oxide production. We further show that the property to neutralize LPS can be modulated by aromatic amino acid substitution.
Materials and Methods
Peptide synthesis
Peptides (Table 1) were synthesized by Pepscan (Lelystad, The Netherlands) and at ACTA (Amsterdam, The Netherlands). The peptides were purified by reverse-phase HPLC and their purity (≥95%) was confirmed by mass spectrometry.
Table 1. Amino acid sequences and minimal inhibitory concentrations of CATH-2 analogs.
| Peptide | Amino acid sequence | Length /Charge | MIC (μM) |
|---|---|---|---|
| C(1–15) | RFGRFLRKIRRFRPK | 15, +8 | 16.7 ± 5.8 |
| CATH-2 | RFGRFLRKIRRFRPKVTITIQGSARF-NH2 | 26, +10 | 5.0 ± 0.0 |
| C(1–21) | RFGRFLRKIRRFRPKVTITIQ-NH2 | 21, +9 | 13.3 ± 5.8 |
| C(4–21) | RFLRKIRRFRPKVTITIQ-NH2 | 18, +8 | 6.7 ± 2.9 |
| C(5–21) | FLRKIRRFRPKVTITIQ-NH2 | 17, +7 | 8.3 ± 5.8 |
| C(7–21) | RKIRRFRPKVTITIQ-NH2 | 15, +7 | 8.3 ± 2.9 |
| C(8–21) | KIRRFRPKVTITIQ-NH2 | 14, +6 | 11.7 ± 7.6 |
| C(9–21) | IRRFRPKVTITIQ-NH2 | 13, +5 | 8.3 ± 2.9 |
| C(10–21) | RRFRPKVTITIQ-NH2 | 12, +5 | 6.7 ± 2.9 |
| C(11–21) | RFRPKVTITIQ-NH2 | 11, +4 | 8.3 ± 2.9 |
| C(1–21)W3 | RWGRWLRKIRRWRPKVTITIQ-NH2 | 21, +9 | 13.3 ± 5.8 |
| C(4–21)W2 | RWLRKIRRWRPKVTITIQ-NH2 | 18, +8 | 8.3 ± 2.9 |
| C(7–21)W | RKIRRWRPKVTITIQ-NH2 | 15, +7 | 13.3 ± 5.8 |
| C(10–21)W | RRWRPKVTITIQ-NH2 | 12, +5 | 11.7 ± 7.6 |
| C(1–21)Y3 | RYGRYLRKIRRYRPKVTITIQ-NH2 | 21, +9 | 10.0 ± 0.0 |
| C(4–21)Y2 | RYLRKIRRYRPKVTITIQ-NH2 | 18, +8 | 10.0 ± 0.0 |
| C(7–21)Y | RKIRRYRPKVTITIQ-NH2 | 15, +7 | 11.7 ± 7.6 |
| C(10–21)Y | RRYRPKVTITIQ-NH2 | 12, +5 | 16.7 ± 5.8 |
Length is measured in amino acids and substituted amino acid residues are underlined. Antibacterial activity of CATH-2 analogs against avian pathogenic E. coli in colony count assays. Means ± SEM for 3 independent experiments.
Antibacterial activity
Avian pathogenic Escherichia coli O78 (field isolate, Zoetis, Kalamazoo, MI) was cultured in Mueller-Hinton broth at 37°C. Overnight grown cultures were diluted to a density of 2×106 CFU/ml in Mueller-Hinton broth (non cation-adjusted, Oxoid Ltd., Hampshire, UK) and mixed in a 96 wells polypropylene microwell plate at a 1:1 ratio with peptide (final concentration: 1.25 to 40 μM). After 3 h incubation samples were serially diluted in medium, plated on trypton soy agar (Oxoid Ltd., Hampshire, UK) media and counted after 24 h incubation at 37°C.
Lipopolysaccharide isolation
Neisseria meningitidis strain H44/76 was cultured on GC agar plates (Difco, Basingstoke, United Kingdom) supplemented with Vitox (Oxoid Ltd., Hampshire, UK) in an atmosphere of 5% CO2 in air at 37°C. Campylobacter jejuni ATCC11168 was cultured on blood agar base II (Oxoid) plates containing 5% saponized horse blood (Biotrading, Mijdrecht, The Netherlands) at 37°C under microaerophilic conditions (5% O2, 10% CO2 and 85% N2). Escherichia coli O111:B4 was grown on Luria-Bertani agar plates (Biotrading) at 37°C. A wild type Salmonella enteritidis 706 strain was grown in Todd-Hewitt broth at 37°C. LPS from was isolated by hot-phenol extraction as described by Westphal and Jann [15]. LPS was quantified using the 3-deoxy-d-manno-2-octulosonic acid assay and 3-deoxy-d-manno-2-octulosonic acid-NH3 as standard [16]. Ultrapure Salmonella minnesota R595 LPS was obtained from InvivoGen (Toulouse, France).
LPS binding affinity
Dansyl-polymyxin B was prepared and quantitated as described by Schindler and Teuber [17]. Subsequently, the capacity of peptides to displace dansyl-labeled polymyxin B complexed to S. minnesota LPS was determined according to Moore et al. [18].
Cell culture
HD11 cells [19] were a kind gift of dr. Jos van Putten (department of Infectious diseases and Immunology, Utrecht University, The Netherlands). HD11 cells were seeded in 96 wells tissue culture treated plates (1×105 cell/mL) and incubated for 18 h at 37°C (5% CO2) before treatment. For peptide-induced effects cells were treated with RPMI-1640 medium containing 20 μM peptide during 4 h or 24 h. In LPS neutralization experiments, final concentrations of 50–100 ng/ml LPS were pre-incubated with or without 20 μM peptide for 30 min at 37°C (5% CO2), applied to the cells and incubated for 4 h. To examine neutralization of LPS-primed cells HD11 cells were exposed for 30 min to 100–1000 ng/ml S. minnesota LPS, washed once and incubated during 4 h in the absence or presence of 20 μM Peptide. Supernatants were collected for the determination of nitric oxide production (24 h incubations only). Remaining cells were lysed in lysis buffer and total RNA was isolated and purified using a High Pure RNA isolation kit (Roche, Mannheim, Germany) according the manufacturer's recommendations. RNA quantity and purity were tested using a Nanodrop ND-1000 spectrophometer (Nanodrop Technologies, Wilmington, DE). Cytotoxicity of peptides was determined using the cell viability reagent WST-1 (Roche) as described previously [13], and by measuring LDH release after 24 h exposure. LDH activities in supernatant cell fractions were measured using the Cytoxicity Detection Kit PLUS (Roche) according the manufacturers recommendations and expressed as the % of released LDH relative to the total LDH activity.
Real time PCR
RNA (250 ng) was reverse transcribed using an iScript cDNA synthesis kit (Bio-rad laboratories (Hemel Hempstead, UK) according to the manufacturers’ instructions. Primers and probes were designed and produced by Eurogentec (Seraing, Belgium) (Table 2). Quantitative real time PCR was performed on a Bio-rad MyiQ system using a qPCR Mastermix (Eurogentec) and 400 nM of each primer and probe. Reactions were performed as follows: 3 min at 95°C; 40 cycles: 10 s at 95°C, 30 s at 60°C and 30 s at 72°C. Relative gene expression levels were normalized against the expression levels of the house keeping genes GAPDH and 28S.
Table 2. Primers used for quantitative real time PCR.
| Gene | Reference | Primer sequence | Product (bp) |
|---|---|---|---|
| 28S | X59733 | F: 5’-GGCGAAGCCAGAGGAAACT-3’ | 62 |
| R: 5’-GACGACCGATTTGCACGTC-3’ | |||
| P: 5’-(FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3’ | |||
| GAPDH | NM 204305 | F: 5’-GCCGTCCTCTCTGGCAAAG-3’ | 73 |
| R: 5’-TGTAAACCATGTAGTTCAGATCGATGA-3’ | |||
| P: 5’-(FAM)AGTGGTGGCCATCAATGATCCC-(BHQ1)-3’ | |||
| IL-1β | NM 204524 | F: 5’-GCTCTACATGTCGTGTGTGATGAG-3’ | 80 |
| R: 5’-TGTCGATGTCCCGCATGA-3’ | |||
| P: 5’-(FAM)CCACACTGCAGCTGGAGGAAGCC-(BHQ1)-3’ | |||
| CXCLi2 | NM 205498 | F: 5’-GCCCTCCTCCTGGTTTCA-3’ | 68 |
| R: 5’-CGCAGCTCATTCCCCATCT-3’ | |||
| P: 5’-(FAM)-TGCTCTGTCGCAAGGTAGGACGCTG-(BHQ1)-3’ | |||
| MCP-3 | Kaiser et al., | F: 5’-CTGCTGCTTCTCCTATGTTCAAC-3’ | 126 |
| 2005 [20] | R: 5’-ACACATATCTCCCTCCCTTTCTTG-3’ | ||
| P: 5’-(FAM)-ACCTCATTGCCTCCGCCTACATCACCA-(BHQ1)-3’ | |||
| CCLi4 | AY037859 | F: 5’-CCCTCTCCATCCTCCTGGTT-3’ | 66 |
| R: 5’-TATCAGCCCCAAACGGAGAT-3’ | |||
| P: 5’-(FAM)-CCGCCCTCTTCCCTCAAGCCTC-(BHQ1)-3’ |
Nitric oxide production
Nitric oxide production was assessed as the accumulation of nitrite (NO2−) in cell supernatants during a 24 h incubation period. Nitrite concentrations were determined using a colorimetric reaction with the Griess reagent using (from 1 to 50 μM) sodium nitrite dissolved in water as standards. Briefly, cell culture supernatants were mixed with an equal volume of 1% sulfanilamide (dissolved in 2.5% phosphoric acid) and incubated for 5 min. The same volume of 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride was added and incubated for 5 min. The absorbance was measured at 520 nm using a 96-well microplate reader (FLUOstar Omega, BMG labtech).
Statistical analysis
Statistical analyses were performed using SPSS version 16.0 statistical software (SPSS inc., Chicago, IL) with one-way analysis of variance (ANOVA) and Dunnett post hoc tests.
Results
Antibacterial and cytotoxic activities of CATH-2-derived peptides
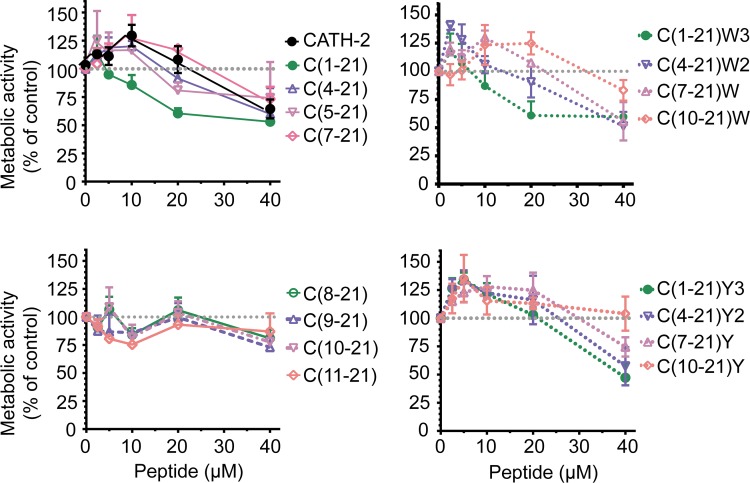
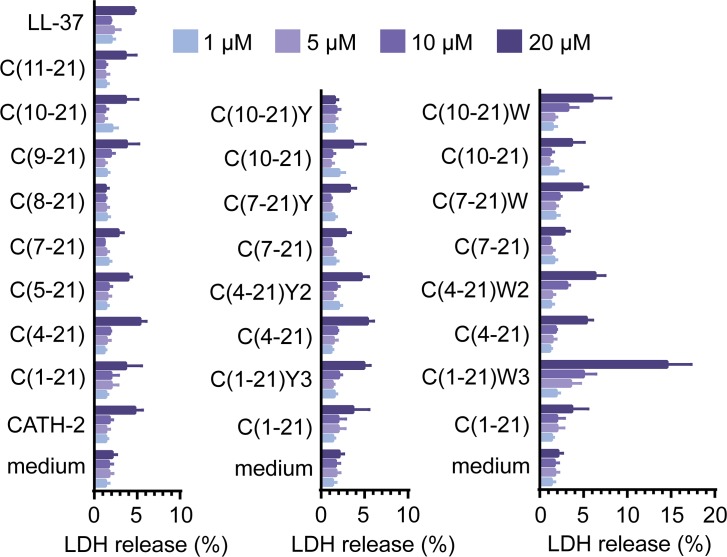
To determine the effect of truncation and amino acid substitution on the antibacterial activity minimal inhibitory concentrations (MIC) were determined using colony count assays. All truncated and substituted peptides exhibited antibacterial activity against avian pathogenic E. coli O78 (Table 1); bacterial survival was reduced to below the detection limit in the presence of 5–20 μM peptide. To determine the toxicity of CATH-2 analogs HD11 cells were exposed for 24 h to different peptide concentrations. Most peptides did not affect HD11 metabolic activity up to peptide concentrations of 20 μM (Fig 1). At 40 μM, HD11 metabolic activities decreased considerably for all peptides except peptide C(10–21)[F12Y]. The peptide-induced LDH release from HD11 cells during 24 h exposure corresponded well with the metabolic activities of HD11 cells (Fig 2); less than 5% LDH release occurred for most peptides at 20 μM.
Fig 1. Metabolic activity of CATH-2 derived peptide treated HD11 cells.
Metabolic activity of HD11 determined by WST-1 assays after 24 h stimulation with CATH-2 derived peptides (2.5–20 μM). Shown are means ± SEM for at least 3 independent experiments.
Fig 2. Peptide-induced LDH release from HD11 cells.
The percentage of lactate dehydrogenase (LDH) released from HD11 cells during 24 h exposure to medium containing CATH2 derived peptides (1–20 μM). Shown are means ± SEM for at least 3 independent experiments.
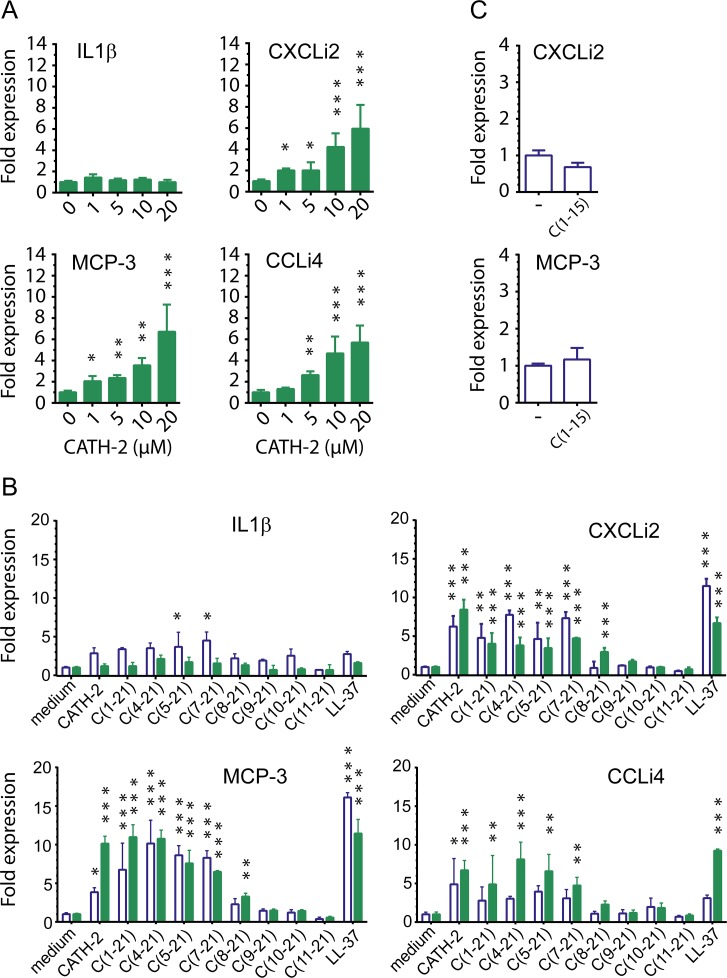
Full-length CATH-2 peptide selectively induces chemokine transcription in HD11 cells
To examine if CATH-2 analogs induce chemokine production in chicken macrophages, HD11 cells were exposed to different doses of peptide. CATH-2 was found to dose-dependently increase transcription of chemokines CXCLi2/IL-8, MCP-3 and CCLi4/RANTES in HD11 cells, whereas pro-inflammatory cytokine IL-1β was not induced (Fig 3A). Induced transcription of CXCLi2 and MCP-3 was observed with CATH-2 concentrations as low as 1 μM. To determine the minimal molecular size required for the immunomodulatory activity of CATH-2, truncated analogs were examined for their capacity to induce chemokine transcription in HD11 cells. Brief stimulation (4h) of HD11 cells with 20 μM of N-terminally truncated C(1–21) based peptides induced CXCLi2 and MCP-3 transcription, with a minimal size of 15 amino acid residues (Fig 3B). Prolonged stimulation was needed to induce expression of CCLi4. Further truncation diminished chemokine induction. Peptide C(1–15) comprising the first 15 N-terminal amino acids did not induce chemokine expression (Fig 3C).
Fig 3. Induction of chemokine transcription in HD11 cells by truncated CATH-2 analogs.
CXCLi2, MCP-3, CCLi4 and IL-1β transcription in HD11 cells treated with CATH-2 derived peptides (1 to 20 μM). Transcription was measured by real time QPCR analysis after 4 h (open bars) or 24 h (closed bars) of stimulation. a CATH-2 dose-dependently induced transcription of chemokines chCXCLi2/IL-8, MCP-3 and chCCLi4/RANTES, but not of pro-inflammatory cytokine IL-1β. b The capacity to induce chemokine transcription was maintained for N-terminally truncated C(1–21) analogs up to 14 amino acid residues during 24 h exposure. c No chemokine induction was observed when cells were exposed (4 h) to peptide C(1–15). Data from 3 to 4 independent experiments; means ± SEM * p<0.05, ** p<0.01, *** p<0.001.
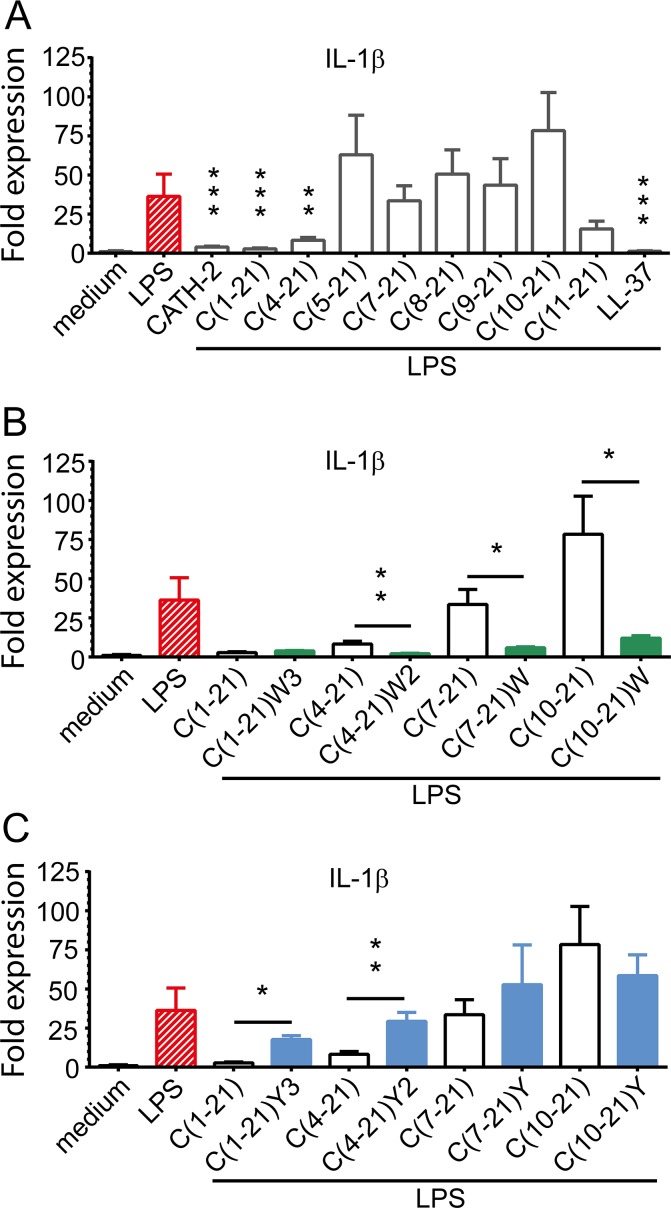
CATH-2 analogs neutralize LPS-induced pro-inflammatory cytokine expression in HD11 cells
To examine the capacity of CATH-2 analogs to neutralize lipopolysaccharide-induced pro-inflammatory cytokine expression IL-1β mRNA levels were determined after 4 h stimulation of HD11 cells with pre-incubated mixtures of S. minnesota LPS (50 ng/ml) and CATH-2 analogs (20 μM). LPS-induced IL-1β expression in HD11 cells was blocked by CATH-2 peptide (90.8%) and truncated analogs C(1–21) (93.5%) and C(4–21) (80.5%) (Fig 4A). Further N-terminal truncation completely abrogated the LPS neutralizing potency of peptides. Interestingly, the most truncated peptide, C(11–21), also partially inhibited LPS.
Fig 4. Neutralization of LPS-induced pro-inflammatory cytokine production by CATH-2 analogs.
IL-1β transcription in HD11 cells stimulated with S. minnesota LPS (50 ng/ml) and CATH-2 derived peptides (20 μM). Transcription was measured by real time QPCR analysis after 4 h of stimulation. Amino acid substitutions: W3, [F2W, F5W, F12W]; W2, [F5W, F12W]; W, [F12W]; Y3, [F2Y, F5Y, F12Y]; Y2, [F5Y, F12Y]; Y, [F12Y]. a LPS-induced IL-1β transcription in HD11 cells was blocked by full-length peptide and N-terminally truncated C(1–21) analogs up to 18 amino acid residues. b Phe/Trp substitution improved the capacity to neutralize LPS-induced IL-1β transcription of peptides C(1–21) and C(4–21) and introduced LPS-neutralizing capacity in formerly inactive peptides C(7–21) and C(10–21). c Phe/Tyr substitution of active peptides C(1–21) and C(4–21) abrogated neutralization of LPS-induced IL-1β expression. Data from 3 to 4 independent experiments; means ± SEM. * p<0.05, ** p<0.01, *** p<0.001.
Aromatic amino acid substitution in CATH-2 analogs modulates neutralization of LPS-induced pro-inflammatory cytokine production
Substitution of Phe by Trp residues in truncated analogs significantly enhanced their capacity to block LPS-induced IL-1β expression in HD11 cells (Fig 4B). Phe/Trp substitution of peptide C(4–21) improved inhibition of LPS-induced IL-1β expression from 80.5% to 95.1%.
Moreover, whereas peptides C(7–21) and C(10–21) lacked LPS-neutralizing activity, their Phe/Trp substituted analogs strongly inhibited LPS-induced pro-inflammatory cytokine production by HD11 cells, i.e. 86.3% and 71.9%, respectively, compared to the control. In contrast, Phe/Tyr substitution of CATH-2 analogs abrogated their capacity to block LPS-induced IL-1β transcription (Fig 4C). Phe/Trp residues did not consistently alter peptide-induced chemokine expression of HD11 cells (Table 3). Substitution by tyrosine increased overall cytokine transcriptional levels during short exposure, and this effect disappeared when exposure was prolonged.
Table 3. Effect of aromatic amino acid substitution on HD11 cytokine expression levels.
| 4h stimulation | 24 h stimulation | |||||||
|---|---|---|---|---|---|---|---|---|
| IL-1β | CXCLi2 | MCP-3 | CCLi4 | IL-1β | CXCLi2 | MCP-3 | CCLi4 | |
| Medium | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 1.0±0.3 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.0 |
| C(1–21) | 3.4±0.2 | 4.8±1.8 | 6.7±3.4 | 2.7±1.8 | 1.9±0.5 | 4.0±1.4 | 11.0±1.6 | 4.9±3.7 |
| C(1–21)W3 | 3.7±0.3 | 4.4±1.4 | 6.2±3.5 | 1.8±0.2 | 3.0±0.2 | 3.5±0.4 | 15.7±3.4 | 2.1±0.6 |
| C(1–21)Y3 | 9.1±6.6 | 12.6±2.4* | 13.7±2.0 | 6.4±1.2 | 2.5±0.5 | 3.3±0.3 | 8.8±2.1 | 3.8±0.7 |
| C(4–21) | 3.5±0.7 | 7.8±0.6 | 10.2±3.0 | 3.0±0.3 | 2.1±0.5 | 3.8±1.0 | 10.8±1.1 | 8.1±2.2 |
| C(4–21)W2 | 5.6±1.4 | 7.3±1.3 | 10.1±1.3 | 3.1±1.0 | 1.7±0.6 | 2.5±0.2 | 7.7±1.1 | 3.9±0.5 |
| C(4–21)Y2 | 7.4±4.1 | 11.6±0.7 | 12.4±3.0 | 6.7±1.0 | 2.1±0.3 | 4.2±0.5 | 6.5±0.8 | 4.6±0.8 |
| C(7–21) | 4.5±1.1 | 7.3±0.8 | 8.3±0.9 | 3.1±1.1 | 1.5±0.7 | 4.7±0.1 | 6.5±0.2 | 4.7±1.0 |
| C(7–21)W | 4.1±0.5 | 4.8±1.0 | 6.6±2.7 | 5.5±0.9 | 0.8±0.1 | 2.7±0.3 | 3.4±0.6 | 1.6±0.5 |
| C(7–21)Y | 7.7±4.1 | 9.4±3.9 | 11.6±4.5 | 5.1±1.5 | 1.8±0.1 | 4.3±0.3 | 6.3±0.2 | 5.4±0.2 |
| C(10–21) | 2.5±0.9 | 0.9±0.1 | 1.2±0.4 | 2.0±1.1 | 0.8±0.2 | 1.0±0.1 | 1.4±0.2 | 1.8±0.6 |
| C(10–21)W | 2.6±0.7 | 2.5±0.3 | 3.9±1.4 | 2.1±1.4 | 0.8±0.2 | 1.2±0.0 | 1.5±0.1 | 1.0±0.2 |
| C(10–21)Y | 1.8±0.1 | 0.9±0.1 | 1.4±0.5 | 1.3±2.0 | 0.7±0.5 | 1.1±0.1 | 1.4±0.2 | 0.7±0.2 |
Comparison of peptide-induced gene expression levels relative to that of basal expression levels in the absence of peptide. Amino acid substitutions: W3, [F2W, F5W, F12W]; W2, [F5W, F12W]; W, [F12W]; Y3, [F2Y, F5Y, F12Y]; Y2, [F5Y, F12Y]; Y, [F12Y].
Significance level: *, p<0.05.
CATH-2 analogs neutralize LPS-induced nitric oxide production by HD11 cells
Membrane-perturbing agents may induce expression of iNOS in macrophages leading to nitric oxide production. Therefore, we examined whether CATH-2 analogs induced NO production in HD11 cells. Exposure (24 h) of HD11 cells to 20 μM LL-37 or CATH-2 analogs slightly increased (2-fold; data not shown) NO levels, with the exception of peptide C(11–21). This effect was abolished by Phe/Trp substitution, but not by Phe/Trp substitution.
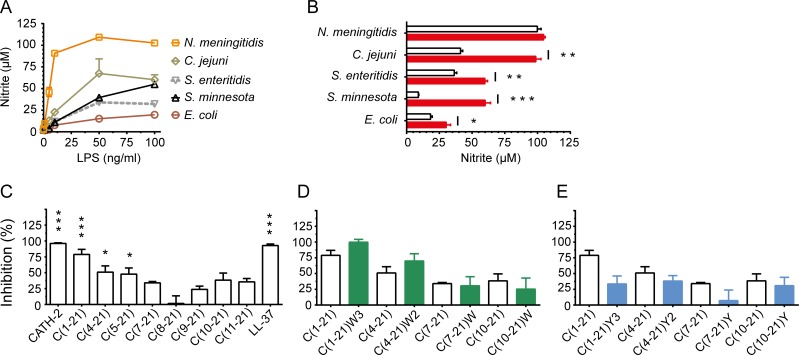
Nitric oxide (NO) production by macrophages plays a dual role; NO can rapidly react with superoxide to produce peroxynitrate, a reactive oxygen species with potent antimicrobial activity, whereas sustained high levels may lead to tissue damage and cell death [21]. To determine the nitric oxide levels produced by HD11 cells in the presence of LPS, HD11 cells were stimulated for 24 h with different concentrations of LPS derived from different bacterial species. HD11 cells were most susceptible to stimulation by N. meningitidis H44/76 LPS, i.e. at 10 ng/ml LPS already induced 83.1% of the maximal NO production (Fig 5A). A moderate response was observed for LPS derived from C. jejuni, S. minnesota and S. enteritidis LPS, e.g. 50 ng/ml of LPS was needed to produce 61.7%, 36.2% and 31.2%, respectively of the maximal NO production. HD11 production of NO was least responsive to E. coli O111:B4 LPS.
Fig 5. CATH-2 analog neutralization of LPS-induced macrophage nitric oxide production.
Nitric oxide production by HD11 cells stimulated with LPS (50 to 100 ng/ml) pre-incubated with CATH-2 derived peptides (20 μM). Nitric oxide (NO) production was measured in supernatants after 24 h incubation using the Griess assay. Amino acid substitutions: W3, [F2W, F5W, F12W]; W2, [F5W, F12W]; W, [F12W]; Y3, [F2Y, F5Y, F12Y]; Y2, [F5Y, F12Y]; Y, [F12Y]. a Dose-dependent production of NO by HD11 cells exposed to different sources of LPS. b CATH-2 significantly inhibited NO production induced by all tested LPS (100 ng/ml) sources, except wild-type Neisseria meningitidis LPS; bars indicate LPS-induced NO production in the absence (closed) and presence (open) of CATH-2 peptide. c Inhibition of S. minnesota LPS-induced (50 ng/ml) NO production by truncated CATH-2 analogs. Production of LPS-induced NO by HD11 cells was significantly reduced in the presence of C(1–21) and N-terminally truncated C(1–21) analogs up to 17 amino acid residues. d, e Phe/Trp substitution enhanced inhibition of active peptides C(1–21) and C(4–21), whereas Phe/Tyr substitution abrogated inhibition of LPS-induced NO production by these peptides. Data from 3 to 4 independent experiments; means ± SEM. * p<0.05, ** p<0.01, *** p<0.001.
Next, neutralization of LPS-induced NO production by full-length CATH-2 peptide was examined. For this purpose HD11 cells were stimulated during 24 h with pre-incubated mixtures of LPS (100 ng/ml) and CATH-2 peptide (20 μM). CATH-2 significantly reduced LPS-induced NO production for LPS of all sources except Neisseria LPS (Fig 5B). The lack of Neisseria LPS inhibition can be explained by the fraction of free LPS. Under the conditions used, CATH-2 most potently inhibited S. minnesota LPS (85%) of which the residual NO production (10 μM) corresponded to10 ng/ml unbound LPS. A similar unbound fraction of N. meningitidis LPS, would induce approx. 90 μM NO. Thus, the most likely explanation is that under these conditions the high potency of the unbound fraction of N. meningitidis LPS was sufficient to obtain a maximal NO production.
Peptide C(1–21) blocked 87.9% of LPS-induced (50 ng/ml) NO production while C(5–21) neutralized NO production by 47.8%. Further truncation diminished the NO neutralization (Fig 5C). Phe/Trp substitution of truncated CATH-2 analogs appeared to increase the inhibition capacity of C(1–21) and C(4–21) (Fig 5D), while Phe/Tyr substitution abrogated inhibition of LPS-induced NO production (Fig 5E).
Structure of LPS-neutralizing peptides
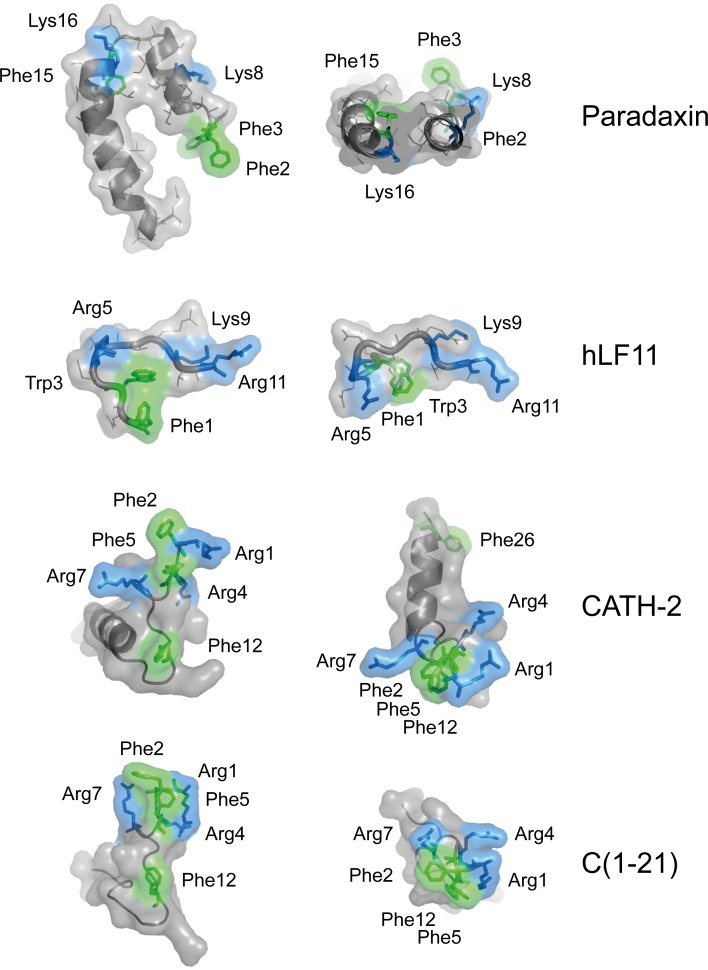
To visualize the possible mode of CATH-2 analog binding to lipid A, the 3D structures of CATH-2 and C(1–21) were predicted using iTASSER [22] and compared with the configuration of known LPS-binding peptides paradaxin and human lactoferrin (hLF11) when in complex with LPS (Fig 6). Lactoferrin-derived peptide LF11 possesses three basic residues (Arg5, Lys9, Arg11) that match the distance between both glucosamine phosphate groups in lipid A and is known to adopt a T-shaped configuration when binding to LPS [23]. Similarly, the Lys8, Lys16 residues and Phe2, Phe3 residues of paradaxin were shown to be in close proximity of lipid A [24]. The generated models of CATH-2 and C(1–21) indicate that in a hydrophobic environment, e.g. a biological membrane, all Phe residues in these peptides align in the same plane where they may interact with lipid A acyl chains and suggest that N-terminal basic residues Arg1, and Arg4 Arg7 are the most likely residues to interact with lipid head phosphate groups.
Fig 6. Structural representation of LPS-neutralizing CATH-2 and C1-21 peptide.
Representations of the known 3-dimensional structure of paradaxin and human lactoferrin-derived peptide hLF11, cationic peptides with known LPS-binding affinity and the deduced structures of CATH-2 and C(1–21). Side chains reported or deduced to be in closest proximity to lipid A are depicted in green (aromatic side chains) and blue (basic side chains). Representations were adapted from the published structures (RSCB Protein Data bank; http://www.rcsb.org/pdb/home/home.do). The structure of CATH-2 analog C(1–21) was predicted using iTasser.
LPS neutralization by CATH-2 analogs does not correlate with LPS binding affinity
Dansyl-polymyxin B (DPmB) experiments were performed to get insight in the relationship between the LPS binding affinity of peptides and their capacity to neutralize LPS. Compared to unlabeled polymyxin B (I50 = 4.19 μM), full-length CATH-2, C(1–21) and C(4–21) displaced DPmB from S. minnesota LPS at a 20-fold lower concentration (Table 4). However, similar high binding affinities were found for C(7–21) (I50 = 0.33 μM) and C(1–15) (I50 = 0.12 μM) that lacked LPS-neutralization capacity. Further peptide truncation gradually decreased LPS binding capacity and was not affected by aromatic amino acid substitution.
Table 4. LPS binding affinity of CATH-2 analogs.
| Peptide | I50 (μM) | ||
|---|---|---|---|
| Phe | Phe/Trp | Phe/Tyr | |
| Polymyxin B | 3.46 ± 0.17 | n.d. | n.d. |
| LL37 | 0.28 ± 0.04 | n.d. | n.d. |
| CATH-2 | 0.18 ± 0.01 | n.d. | n.d. |
| C(1–21) | 0.15 ± 0.02 | 0.16 ± 0.02 | 0.18 ± 0.01 |
| C(4–21) | 0.26 ± 0.05 | 0.20 ± 0.02 | 0.22 ± 0.03 |
| C(5–21) | 0.67 ± 0.16 | n.d. | n.d. |
| C(7–21) | 0.28 ± 0.02 | 0.25 ± 0.01 | 0.35 ± 0.06 |
| C(8–21) | 1.50 ± 0.09 | n.d. | n.d. |
| C(9–21) | 4.89 ± 0.80 | n.d. | n.d. |
| C(10–21) | 5.01 ± 1.27 | 3.09 ± 0.18 | 5.21 ± 0.99 |
| C(11–21) | 42.49 ± 1.83 | n.d. | n.d. |
| C(1–15) | 0.13 ± 0.01 | n.d. | n.d. |
Binding affinity is expressed as the concentration of peptide (μM) needed to displace 50% of Dansyl-labeled polymyxin from S. minnesota LPS micelles.
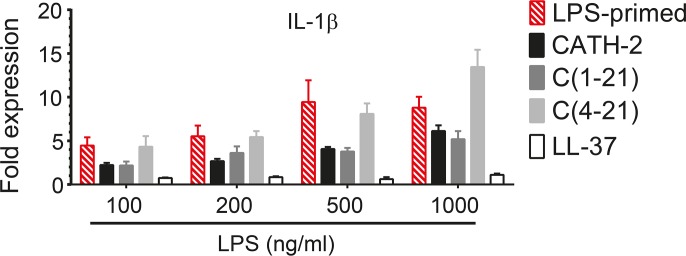
To determine if LPS-neutralization occurred via other mechanisms than direct binding, HD11 cells were primed during 30 minutes with different doses of LPS, washed once to remove exogenous LPS and incubated for 4 h in the absence or presence of peptide. IL-1β transcription induced by LPS priming proved to be partially inhibited by CATH-2 and C(1–21) (p<0.01) and strongly inhibited by LL-37 (p<0.001), whereas no inhibition was observed with C(4–21) (Fig 7).
Fig 7. CATH-2 analogs partially neutralize IL-1β transcription in LPS-primed cells.
HD11 cells were primed for 30 min with S. minnesota LPS (100–1000 ng/ml), washed once and incubated during 4h in the presence or absence of 20 μM peptide. IL-1β transcription was measured by real time QPCR analysis. Data from 3 to 4 independent experiments (means ± SEM).
Discussion
Cathelicidins [25,26] and cathelicidin-derived peptides, such as IDR-1 [27] and IDR1002 [28], have demonstrated in vivo protection against infections by Gram-positive and Gram-negative bacteria. In the case of cathelicidin-derived peptides that lacked direct antimicrobial activity this was found to be associated with the selective induction of chemokines, subsequent recruitment of monocytes and neutrophils, and to be monocyte/macrophage dependent [27]. In this work the immunomodulatory and anti-inflammatory activities of chicken CATH-2 analogs were examined using HD11 cells, a chicken macrophage-like cell line. Full-length CATH-2 peptide and truncated analogs were found to selectively induce CXCLi2/IL-8 and MCP-3 transcription in HD11 cells. These findings corroborate with observations by others for LL-37; induction of MCP-1 expression in murine RAW264.7 cells [8] and induction of MCP-1, MCP-3 and IL-8 via the ERK1/2 and p38 pathways, but not of pro-inflammatory cytokines IL-6, TNF-α or IL-1β, in human monocytes [29]. HD11 cells responded similarly to LL-37 by primarily inducing the transcription of CXCLi2 and MCP-3, suggesting that they may share common signaling pathways.
Experiments with C-terminal and N-terminal truncated analogs of CATH-2 indicated peptide C(7–21) to be the crucial part for peptide-induced chemokine production in HD11 cells. This proved not to be the case for the anti-inflammatory activity of CATH-2 derived peptides. Whereas CATH-2 and truncated analogs C(1–21) and C(4–21) exhibited anti-inflammatory activity, efficiently blocking the LPS-induced IL-1β transcription and NO production by HD11 cells, further truncation abrogated LPS neutralization.
Next we endeavored to enhance the anti-inflammatory properties of smaller CATH-2 analogs while maintaining other immunomodulatory properties. The prominent presence of aromatic moieties in small immunomodulatory peptides [30] and their frequently observed proximity to lipid A acyl chains in peptide-LPS complexes [23,27,31] led us to believe that the position and type of aromatic amino acid may be key factors in enhancing or altering immunomodulatory properties. Indeed, aromatic amino acid residue substitution was found to greatly affect anti-inflammatory activities of truncated CATH-2 analogs, without substantially changing their potency to induce chemokine transcription in HD11 cells. LPS neutralizing capacity could be enhanced in peptide C(4–21) and introduced in inactive peptides C(7–21) and C(10–21) by substitution of the single Phe residue by Trp. Surprisingly, peptide C(1–15) failed to induce chemokine transcription. In addition, C(1–15), previously shown to moderately [13] or weakly [32] inhibit LPS-induced cytokine expression, was under the conditions used not able to block LPS-induced cytokine transcription in HD11 cells (data not shown). Thus, in non-substituted CATH-2 analogs the C-terminal segment (VTITIQ) is pivotal for both immunomodulation and LPS neutralization. However, Phe/Trp substitution of C(1–15) has been shown to introduce significant LPS neutralizing capacity [32]. This can be explained by the behavior of tryptophan in lipid bilayers. Tryptophan side chains and indol groups prefer to localize at the interface of water-associated lipid head groups and acyl chains, probably stabilized by dipole interactions [33,34], suggesting that Phe/Trp-substituted CATH-2 analogs are prone to adopt a different orientation when binding to lipid A. Among aromatic residues Trp is the most polarizable residue and able to form a hydrogen bond via its indol N-H moiety with acyl carbonyl as well as phosphate oxygen groups [35]. Hence, most likely the Phe/Trp substitution in peptide C(10–21) improved LPS neutralization capacity by stabilization of the peptides hydrophobic core to the lipid acyl region. In contrast, substitution of Phe residues by Tyr residues was found to abrogate the LPS neutralizing capacity of ‘active’ CATH-2 analogs. This is comparable to the reported effects of Phe/Trp and Phe/Tyr substitution of Polymyxin B nonapeptide; i.e. LPS-binding and–neutralization were maintained when Phe was substituted by Trp, but strongly abrogated upon substitution with Tyr [36].
It has been shown that cationic peptides in complex with LPS adopt a configuration in which basic residues interact with lipid A glucosaminoglycan phosphate groups while hydrophobic residues interact with its acyl chains. In the case of the polymyxin B-LPS complex, two positively charged α,γ-diaminobutyric acid (DAB) residues and one Phe residue are necessary for LPS-binding [37]. Structure overlay of the LPS-LF11 complex and E. coli iron uptake receptor FhuA-LPS complex revealed a conserved structural LPS-binding motif consisting of three basic residues (Arg or Lys) and a single Phe residue [38]. We reasoned that CATH-2 analogs, possessing multiple basic and hydrophobic residues may adopt a similar configuration. Moreover, arginine residues are flanking each aromatic residue, which may aid to stabilize the conformation through formation of intramolecular cation-π interactions between aromatic and arginine residues [39]. Based on the homology found between the deduced C(1–21) peptide configuration and known lipid A binding peptides we consider it likely that this CATH-2 analog interacts with lipid A via its Arg1, Arg4 and Arg7 residues and multiple Phe residues.
However, our results also indicate that LPS neutralization by CATH-2 analogs cannot be explained by LPS binding affinity alone. CATH-2, C(1–21) and LL-37 could partially or completely neutralize IL-1β transcription in LPS-primed HD11 cells, indicating that LPS neutralization by LL-37 and some CATH-2 analogs is in part independent of binding to LPS. Despite its capacity to neutralize LPS when co-incubated, peptide C(4–21) was not able to neutralize LPS-priming induced IL-1β transcription, suggesting a direct binding mode of action for this peptide. Although LPS-binding has been found to be correlated to inhibition of LPS-induced pro-inflammatory cytokine production for several structurally different cationic peptides [6], LPS signaling may be inhibited via alternative mechanisms. For instance, LL-37 and LL-37-derived peptides were shown to partially inhibit LPS signaling through binding to cell surface CD14 [40]. Furthermore, LPS-induced gene expression profiles were found to be differentially altered in the presence of LL-37 and BMAP-27: strongly inhibiting one set of pro-inflammatory genes while the expression levels of other genes, including negative regulators of NF-κB and certain chemokines, were not substantially affected [41]. For LL-37, BMAP-27 and polymyxin B it has also been described that they can inhibit the nuclear translocation of NF-κB subunits p50 and p65, which is pivotal to LPS-induced pro-inflammatory cytokine production [41]. Additionally, LL-37 is known to selectively and directly affect chemokine production via activation of MAPK pathways [29] and it has been suggested that MAP kinase ERK1/2-mediated CREB phosphorylation could inhibit NF-κB activation by competing with CBP/p300 binding to NF-κB subunit p65 [42].
Overall, the immunomodulatory and anti-inflammatory properties of CATH-2 derived peptides demonstrated towards avian macrophages suggest that treatment of poultry with CATH-2 derived peptides may lead to a selective recruitment and activation of avian immune cells while dampening excessive immune responses. We believe that CATH-2 derived peptides, due to their unique properties, could serve as leads for the design of veterinary animal-specific therapeutics.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was financially supported by the Immuno Valley ALTANT ASIA2 program of the Dutch Ministry of Economic Affairs.
References
- 1.Landers TF, Cohen B, Wittum TE, Larson EL. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127:4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shryock TR, Richwine A. The interface between veterinary and human antibiotic use. Ann N Y Acad Sci. 2010;1213:92–105. 10.1111/j.1749-6632.2010.05788.x [DOI] [PubMed] [Google Scholar]
- 3.Li X, Mehrotra M, Ghimire S, Adewoye L. β-Lactam resistance and β-lactamases in bacteria of animal origin. Vet Mic. 2007;121:197–214. [DOI] [PubMed] [Google Scholar]
- 4.Hancock REW. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis. 2005;5:209–218. [DOI] [PubMed] [Google Scholar]
- 5.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. [DOI] [PubMed] [Google Scholar]
- 6.Scott MG, Vreugdenhil AC, Buurman WA, Hancock RE, Gold MR. Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol. 2000;164:549–553. [DOI] [PubMed] [Google Scholar]
- 7.Bowdish DM, Davidson DJ, Scott MG, Hancock RE. Immunomodulatory activities of small host defense peptides. Antimicrob Agents Chemother. 2005;49:1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock REW. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. [DOI] [PubMed] [Google Scholar]
- 9.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson DJ, Currie AJ, Reid GSD, Bowdish DME, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–1156. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan B, Wu H, Ross CR, Blecha F. PR-39, a porcine antimicrobial peptide, inhibits apoptosis: involvement of caspase-3. Dev Comp Immunol. 2004;28:163–169. [DOI] [PubMed] [Google Scholar]
- 12.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–3052. [DOI] [PubMed] [Google Scholar]
- 13.van Dijk A, Molhoek ME, Veldhuizen EJA, Tjeerdsma-van Bokhoven JLM, Wagendorp E, Bikker F, et al. Identification of chicken cathelicidin-2 core elements involved in antibacterial and immunomodulatory activities. Mol Immunol. 2009;46:2465–2473. 10.1016/j.molimm.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 14.van Dijk A, Tersteeg-Zijderveld MHG, Tjeerdsma-van Bokhoven JLM, Jansman AJM, Veldhuizen EJA, Haagsman HP. Chicken heterophils are recruited to the site of Salmonella infection and release antibacterial mature Cathelicidin-2 upon stimulation with LPS. Mol Immunol. 2009;46:1517–1526. 10.1016/j.molimm.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 15.Westphal O, Jann JK. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 16.Karkhanis YD, Zeltner JY, Jackson JJ, Carlo DJ. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978;85:595–601. [DOI] [PubMed] [Google Scholar]
- 17.Schindler PR, Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975;8:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore RA, Bates NC, Hancock RE. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother. 1986;29:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beug H, von Kirchbach A, Doderlein G, Conscience JF, Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser P, Poh TY, Rothwell L, Avery S, Balu S, Pathania US, et al. A genomic analysis of chicken cytokines and chemokines. J. Interferon Cytokine Res. 2005;25:467–484. [DOI] [PubMed] [Google Scholar]
- 21.Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. [DOI] [PubMed] [Google Scholar]
- 22.J. Yang, R. Yan, A. Roy, D. Xu, J. Poisson and Y. Zhang.The I-TASSER Suite: protein structure and function predictionNat.Methods 127–810.1038/nmeth.3213. [DOI] [PMC free article] [PubMed]
- 23.Japelj B, Pristovsek P, Majerle A, Jerala R. Structural origin of endotoxin neutralization and antimicrobial activity of a lactoferrin-based peptide. J Biol Chem. 2005;280:16955–16961. [DOI] [PubMed] [Google Scholar]
- 24.Bhunia A, Domadia PN, Torres J, Hallock KJ, Ramamoorthy A, Bhattacharjya S. NMR structure of pardaxin, a pore-forming antimicrobial peptide, in lipopolysaccharide micelles: mechanism of outer membrane permeabilization. J. Biol. Chem. 2010;285:3883–3895. 10.1074/jbc.M109.065672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bommineni YR, Achanta M, Alexander J, Sunkara LT, Ritchey JW, Zhang G. A fowlicidin-1 analog protects mice from lethal infections induced by methicillin-resistant Staphylococcus aureus. Peptides. 2010;31:1225–1230. 10.1016/j.peptides.2010.03.037 [DOI] [PubMed] [Google Scholar]
- 26.Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25:465–472. [DOI] [PubMed] [Google Scholar]
- 28.Nijnik A, Madera L, Ma S, Waldbrook M, Elliott MR, Easton DM, et al. Synthetic cationic peptide IDR-1002 provides protection against bacterial infections through chemokine induction and enhanced leukocyte recruitment. J Immunol. 2010;184:2539–2550. 10.4049/jimmunol.0901813 [DOI] [PubMed] [Google Scholar]
- 29.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–3765. [DOI] [PubMed] [Google Scholar]
- 30.Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–564. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–20. [DOI] [PubMed] [Google Scholar]
- 32.Molhoek EM, van Dijk A, Veldhuizen EJA, Dijk-Knijnenburg H, Mars-Groenendijk RH, Boele LCL, et al. Chicken cathelicidin-2-derived peptides with enhanced immunomodulatory and antibacterial activities against biological warfare agents. Int J Antimicrob Agents. 2010;36:271–274. 10.1016/j.ijantimicag.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 33.Wimley WC, White SH. Membrane partitioning: distinguishing bilayer effects from the hydrophobic effect. Biochemistry. 1993;32:6307–12. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs RE, White SH. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989;28:3421–3437. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez KM, Kang G, Wu B, Kim JE. Tryptophan-Lipid Interactions in Membrane Protein Folding Probed by Ultraviolet Resonance Raman and Fluorescence Spectroscopy. Biophys J. 2011;100:2121–2130. 10.1016/j.bpj.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsubery H, Ofek I, Cohen S, Eisenstein M, Fridkin M. Modulation of the hydrophobic domain of polymyxin B nonapeptide: effect on outer-membrane permeabilization and lipopolysaccharide neutralization. Mol Pharmacol. 2002;62:1036–1042. [DOI] [PubMed] [Google Scholar]
- 37.Pristovsek P, Kidric J. Solution structure of polymyxins B and E and effect of binding to lipopolysaccharide: an NMR and molecular modeling study. J Med Chem. 1999;42:4604–4613. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson AD, Welte W, Hofmann E, Lindner B, Holst O, Coulton JW, et al. A conserved structural motif for lipopolysaccharide recognition by procaryotic and eucaryotic proteins. Structure. 2000;8:585–92. [DOI] [PubMed] [Google Scholar]
- 39.Scrutton NS, Raine AR. Cation-π bonding and amino-aromatic interactions in the biomolecular recognition of substituted ammonium ligands. Biochem J. 1996;319 (Pt 1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, et al. Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol. 2002;9:972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mookherjee N, Wilson HL, Doria S, Popowych Y, Falsafi R, Yu JJ, et al. Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J Leukoc Biol. 2006;80:1563–1574. [DOI] [PubMed] [Google Scholar]
- 42.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–6419. 10.4049/jimmunol.1001829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.