Abstract
Background
In the nonclassical form (NC), good correlation has been observed between genotypes and 17OH-progesterone (17-OHP) levels. However, this correlation was not identified with regard to the severity of hyperandrogenic manifestations, which could depend on interindividual variability in peripheral androgen sensitivity. Androgen action is modulated by the polymorphic CAG tract (nCAG) of the androgen receptor (AR) gene and by polymorphisms in 5α-reductase type 2 (SRD5A2) enzyme, both of which are involved in the severity of hyperandrogenic disorders.
Objectives
To analyze whether nCAG-AR and SRD5A2 polymorphisms influence the severity of the nonclassical phenotype.
Patients
NC patients (n = 114) diagnosed by stimulated-17OHP ≥10 ng/mL were divided into groups according to the beginning of hyperandrogenic manifestations (pediatric and adolescent/adult) and CYP21A2 genotypes (C/C: homozygosis for mild mutations; A/C: compound heterozygosis for severe/mild mutations).
Methods
CYP21A2 mutations were screened by allelic-specific PCR, MLPA and/or sequencing. HpaII-digested and HpaII-undigested DNA samples underwent GeneScan analysis to study nCAG, and the SRD5A2 polymorphisms were screened by RLFP.
Results
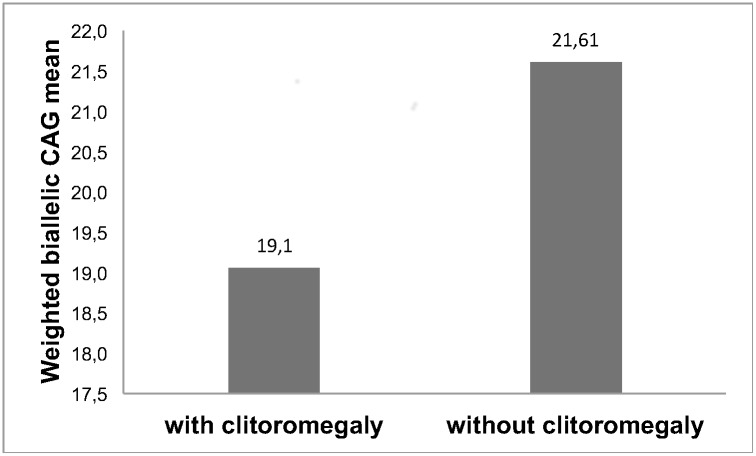
Mean nCAG did not differ among pediatric, adolescent/adult and asymptomatic subjects. In the C/C genotype, we observed a significantly lower frequency of longer CAG alleles in pediatric patients than in adolescent/adults (p = 0.01). In patients carrying the A/C genotype, the frequencies of shorter and longer CAG alleles did not differ between pediatric patients and adolescent/adults (p>0.05). Patients with clitoromegaly had significantly lower weighted CAG biallelic mean than those without it: 19.1±2.7 and 21.6±2.5, respectively (p = 0.007), independent of the CYP21A2 genotype's severity. The SRD5A2 polymorphisms were not associated with the variability of hyperandrogenic NC phenotypes.
Conclusions
In this series, we observed a modulatory effect of the CAG-AR tract on clinical manifestations of the NC form. Although the NC form is a monogenic disorder, our preliminary data suggested that the interindividual variability of the hyperandrogenic phenotype could arise from polygenic interactions.
Introduction
Steroid 21-hydroxylase deficiency is the most frequent cause of congenital adrenal hyperplasia (CAH), accounting for more than 90 to 95% of CAH cases [1, 2]. Due to a lack of negative feedback from cortisol, ACTH stimulation increases, shifting the precursors of steroidogenesis toward androgen synthesis. There is a spectrum of clinical forms, traditionally divided into classical and nonclassical (NC) forms. In the classical form, in addition to the manifestations of cortisol insufficiency, female patients usually present with prenatal external genital virilization, and both sexes present with postnatal virilization. Additionally, approximately 70–80% of patients also present with severe impairment of aldosterone production, resulting in hyponatremic dehydration during the first weeks of life. In the NC form, the hyperandrogenic signs begin later in life: children typically present with precocious pubarche, while adolescents and adults present with hirsutism, menstrual abnormalities and/or infertility. Clitoromegaly has also been observed in both children and adults, occurring in up to 7–10% of NC cases [3]. In fact, these classical and NC forms reflect different impairments of enzymatic activity caused by CYP21A2 mutations.
In CAH, there is a good correlation between genotypes and phenotypes; that is, homozygous patients carrying mutations resulting in total/severe (<7%) and moderate (20–50%) enzymatic activity impairments generally present with the classical and NC forms, respectively [4–9].
Genotypes predicting the NC form carry mild mutations in homozygosis or in compound heterozygosis with severe mutations. Despite the presence of the mild allele, some studies have reported a modulatory effect of the severe allele on the NC phenotype: these patients presented higher ACTH-stimulated 17OH-progesterone (17OHP) levels, higher basal androgen levels and/or earlier onset of hyperandrogenic manifestations compared to those who are homozygous for mild mutations. [10–13] However, this hypothesis has been debated in the literature because other reports have not found these correlations [14–15]. Previously, in our cohort consisting of 114 NC patients, we did not identify correlation among the severity of NC genotypes, age at the beginning of hyperandrogenic manifestations, basal serum androgen levels and the presence of virilizing signs. Interestingly, similar frequencies of both NC genotypes (mild and severe) were observed in asymptomatic female patients and in those with slight clitoromegaly [16]. These data suggested that individual differences in the peripheral androgen sensitivity, modulated by the androgen receptor and 5-α-reductase type 2 genes, could account for this phenotypic variability.
Androgens act via the androgen receptor (AR), the gene for which carries a polymorphic CAG tract at exon 1, varying from 8 to 35 repeats in the normal population [17, 18]. Variations in the CAG repeat numbers (nCAG) have been inversely correlated with AR transactivation activity and consequently with androgen phenotypic variability. Shorter tracts have been associated with idiopathic precocious pubarche, increased severity and earlier age onset of prostate cancer, whereas longer CAG tracts have been associated with oligospermic infertility [19–22].
Dihydrotestosterone (DHT), the main active androgen binding to the androgen receptor (AR), is converted from testosterone by the action of 5α-reductase type 2 enzyme. The 5α-reductase type 2 gene can carry two frequent polymorphisms, V89L and A49T, which modify the enzymatic activity and influence the phenotypic variability of androgen-dependent disorders. The A49T variant might play a role in the etiology and progression of prostate cancer, while the V89L variant has been strongly associated with hypospadias risk in children and also with protection against PCOS [23–26].
Based on these findings, in this study of a noteworthy NC cohort, we evaluated the modulatory effects of androgen receptor and 5α-reductase type 2 gene variants on the hyperandrogenic phenotypic variability of NC patients.
Materials and Methods
This study protocol was approved by the Ethics Committee of the Hospital das Clínicas, Universidade de São Paulo, and written informed consent was obtained from all of the patients and/or their caretakers.
Most patients were from São Paulo state and presented with late onset hyperandrogenic manifestations and hormonal diagnosis of NC-CAH, which was defined by basal 17OHP levels ≥ 10 ng/mL or by ACTH-stimulated 17OHP ≥ 10 ng/mL at 60 min after i.v. injection of synthetic 1–24 ACTH (0.25 mg) [27, 28]. All of the patients had a defined molecular NC genotype, i.e., mutations identified in both CYP21A2 alleles, and according to these criteria, 114 patients were selected [16].
Fifty-three patients (45 females) presented at early or middle childhood with precocious pubarche (6 ± 1.9 years old), and 50 patients (all females) presented at adolescence or adulthood with hirsutism, menstrual abnormalities and/or infertility as their chief complaint (23 ± 11.3 years old). The other 11 cases (5 females) were asymptomatic and were diagnosed during familial molecular studies. Slight clitoromegaly was defined by a clitoris length >9 mm in children and >16 mm in adult females, evaluated by a single examiner [29, 30]. Clitoromegaly was observed in 10 of 114 patients (3 children and 7 adults), but separate urethral and vaginal openings were identified in all of these patients. The mean duration of patient follow-up was 11.0 ± 7.5 years.
Clinical and hormonal data from patients were retrospectively obtained from medical records. These data were correlated with nCAG of the AR gene and with 5α-reductase type 2 allelic variants, and the following manifestations were analyzed: precocious pubarche, amenorrhea, oligomenorrhea, infertility, hirsutism and clitoromegaly. Precocious pubarche was defined by the appearance of pubic hair before 8 years old in girls and before 9 years old in boys. Amenorrhea was defined by the absence of menstrual periods for at least 3 consecutive months and oligomenorrhea by fewer than 6 menstrual periods in the previous year. Infertility was defined by the inability to conceive within 18 months of unprotected intercourse. Hirsutism was defined by male pattern of body hair distribution and a Ferriman and Galley score (FG) ≥ 8. The severity of hirsutism was classified into 2 groups: mild (≤ 14 FG) and severe (≥ 15 FG) [31–33]. Symptomatic patients were grouped according to the beginning of clinical manifestations into pediatric and adolescent/adult groups. None of the subjects had taken any medication for at least 3 months.
Hormone Assays
Serum 17OHP levels were measured by radioimmunoassay (Diagnostic System Laboratories INC/USA, Webster, TX, USA). Cortisol, progesterone and testosterone levels were measured by immunofluorometric assays (AutoDELFIA, Wallac, Finland). Androstenedione levels were determined by chemiluminescence assay (Immulite 2000, Siemens Health Care, UK). The intra- and inter-assay coefficients of variation varied from 5% to 10% [16].
Molecular Studies
CYP21A2 (Gene ID 201910) point mutations were screened using allelic-specific PCR and/or CYP21A2 sequencing, including of the promoter and intronic regions [10, 34, 35]. Large gene rearrangements (CYP21A2 deletions and large gene conversions) were screened by Southern blotting and/or MLPA techniques (SALSA P50B CAH MLPA Mix, MRC-Holland BV, Amsterdam, the Netherlands) [33].
Patient groups
Patient genotypes were classified according to the predicted impairment in enzymatic activity observed in the in vitro studies of 3 groups: A/C (severe), B/C (moderate) and C/C (mild) nonclassical genotypes. Basically, A alleles carry mutations predicting total or almost total impairment of enzymatic activity: CYP21A2 deletion, large gene conversion, IV2-2A>G, p.G110Efs, exon 6 cluster (p.I236N, p.V237E, p.M239K), p.Leu307fs, p.R356W, p.Q318X, p.G424S, p.Arg483fs or the IVS2-13 A/C>G (I2 splice) mutations. B alleles carry the p.I172N mutation, resulting in 3 ± 7% residual enzymatic activity, and C alleles carry the p.P30L, p.V281L or p.P453S mutations, resulting in 20 ± 60% residual enzymatic activity [7, 8, 36, 37].
X-chromosome inactivation analysis
Methylation of the HpaII site, close to the CAG repeats, is correlated with X-inactivation. This site is methylated in the inactive X chromosome, and it resists to cleavage by the HpaII enzyme; therefore, a PCR product is obtained only from the inactive X-chromosome. For each DNA sample, two reactions were performed; in one, 2μg of DNA was digested with 20 U of HpaII (CCGG) at 37°C overnight. A second reaction was similar to the previous one, except for the absence of the enzyme. The reactions were stopped by incubation at 96°C for 5 min. Both digested and undigested DNA (100 ng) were used for PCR amplifications of the CAG polymorphic tract of the AR gene, as previously described [38, 39]. PCR products were submitted to capillary electrophoresis on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA USA) and were analyzed by GeneScan software to determine the sizes of the amplified fragments, which were established from their comparisons with size markers submitted to electrophoresis in the same run. These sizes were correlated with the CAG repeat numbers [18]. The relative inactivation pattern of the AR alleles was calculated using the correction described by Pegoraro et al. [40]. The area of the allele with a smaller repeat number (which is preferentially amplified) was divided by the area of the allele with the greatest number of repeats to determine how many times the first was preferentially amplified. After digestion with HpaII, this factor was applied to the area of the larger allele to normalize the level of amplification between the two alleles. We considered the amplification of one allele ≥ 75% as skewed X-chromosome inactivation [38, 41, 42]. The results of X-inactivation analysis were used to generate a mean value that represented differences in the expression of constituent alleles. It was achieved by multiplying each allele in a genotypic pair by its percentage of total expression (100 minus % inactivity) and by totaling the two adjusted repeat values to achieve a new mean value, which we called the X-weighted biallelic mean [43].
SRD5A2 polymorphisms
Screening for the V89L and A49T SRD5A2 allelic variants was performed using 3 UI of the restriction enzymes RsaI (GT//CA) and MwoI (GCNNNNN//NNGC) (New England Biolabs, Beverly, MA, USA), respectively, and 10 μL of the PCR product of exon 1 at 37°C overnight in a final reaction volume of 20 μL [44]. The restriction products were analyzed by electrophoresis on 3% agarose gel. To monitor the size of the fragments generated by restriction enzymes, the molecular weight marker ΦX174/ HaeIII was used (Invitrogen, Life Technology, Gaithersburg, MD, USA). Each reaction was performed with a known control sample, without polymorphisms.
Statistical Analysis
Qualitative variables were compared using the chi-squared test. Quantitative data, after normality testing, were compared using Wilcoxon’s test or Student’s t-test.
For the multivariate analysis, modification of the linear regression, which considered different patterns of genetic inheritance (recessive, dominant or codominant), was used [45]. Traditional linear regression, adjusted for covariates of interest, was performed and three results were provided based on recessive inheritance (recessive, dominant or codominant). For the linear regression, we included hormonal data, clinical data, CYP21A2 genotype, mean CAG repeat number and SRD5A2 alleles. For univariate statistical analysis, p<0.05 was considered significant, and 95% confidence intervals were generated that did not include the unit. For multivariate analyzes, we planned Bonferroni’s correction for multiple comparisons; however, because no associations attained statistical significance, this correction was not necessary. All of the statistical analyses were performed using Small Stata software, version 11.1 (2010 StataCorp., College Station, TX, USA).
Results
The patients were divided into three groups according to their ages at the beginning of manifestations: pediatric (n = 53), adolescent/adult ≥ 12 years old (n = 50), and asymptomatic (n = 11).
The CAG repeat numbers presented a normal distribution, and 88% of women were heterozygous for this number. The mean CAG repeat numbers in pediatric, adolescent/adult and asymptomatic groups were 22.2 ± 3.1 (12–30), 22.6 ± 3.5 (16–31) and 22.4 ± 3.6 (14–28), respectively (p>0.05). The allelic frequency of CAG repeats was also evaluated among these three groups. The frequencies of shorter CAG alleles (≤ 18 repeats) in the pediatric, adolescent/adult and asymptomatic groups were 15%, 14% and 9%, respectively (p>0.05). The frequencies of longer CAG alleles (≥ 26 repeats) were 6.6% in the pediatric group, 16% in the adolescent/adult group and 13% in the asymptomatic group (p> 0.05).
Nonclassical genotypes from the A/C (severe) and C/C (mild) groups were identified in 52% and 48% of patients, respectively. Only one patient carried the B/C genotype group, and this patient was excluded from the analysis. Initially, frequencies of CAG alleles were compared among the patient groups carrying the same CYP21A2 genotype group. Among the patients carrying the A/C CYP21A2 genotype, frequencies of shorter and longer CAG alleles did not differ between the pediatric and adolescent/adult groups (p>0.05). Among patients carrying the C/C CYP21A2 genotype, the frequency of longer CAG alleles was significantly greater in adolescent/adult than in the pediatric group (p = 0.01), whereas the frequencies of shorter alleles did not differ between these groups (p>0.05) (Table 1). The CAG distribution and period of manifestations according to most frequent CYP21A2 genotypes were described in Table 2.
Table 1. Frequencies of shorter (≤ 18 repeats) and longer (≥ 26 repeats) CAG alleles between pediatric and adolescent/ adult groups according to CYP21A2 genotypes.
| A/C genotype group | C/C genotype group | |||||
|---|---|---|---|---|---|---|
| Age group | CYP21A2 genotype | Allelic frequency (%) | Age group | CYP21A2 genotype | Allelic frequency (%) | |
| Longer alleles | Pediatric | A/C | 13 | Pediatric | C/C | 16 |
| Adolescent/ adult | A/C | 18 p = 0.5 | Adolescent/ adult | C/C | 9 p = 0.28 | |
| Shorter alleles | Pediatric | A/C | 10 | Pediatric | C/C | 2 |
| Adolescent/ adult | A/C | 15 p = 0.37 | Adolescent/ adult | C/C | 17 p = 0.01 | |
Table 2. Most frequent CYP21A2 genotypes, CAG allelic distribution and period of onset of hyperandrogenic manifestations.
| CYP21A2 genotype | Patients n (%) | Mean nCAG | Shorter Alleles n (%) | Period of manifestations | |
|---|---|---|---|---|---|
| childhood (n) | adult (n) | ||||
| V281L/V281L | 42 (36.8) | 21.3 ± 2.8 | 9 (10.7) | 16 | 26 |
| I2Sp/V281L | 15 (13.2) | 21.3 ± 3.2 | 4 (13.3) | 8 | 7 |
| LR/V281L | 12 (10.5) | 22.6 ± 1.2 | 0 | 4 | 8 |
| P453S/V281L | 7 (6.1) | 21 ± 3.6 | 3 (21.4) | 4 | 3 |
| R356W/V281L | 4 (3.5) | 22.7 ± 1.3 | 0 | 3 | 1 |
| Del 8nt/V281L | 3 (2.6) | 19.6 ± 2.1 | 2 (33.3) | 2 | 1 |
LR: large gene rearrangements, included large gene conversions and the CYP21A2 deletions; Del: deletion
Subsequently, the frequencies of shorter and longer CAG alleles were compared between CYP21A2 genotypes in patients from the same group according to the beginning of manifestations. The frequencies of longer CAG alleles between pediatric patients carrying the A/C and C/C CYP21A2 genotypes were 10% and 2%, respectively (p = 0.09); no difference was observed in the frequency of shorter CAG alleles. Regarding adolescent/adult patients carrying the A/C and C/C genotypes, no difference was observed in the frequencies of shorter and longer alleles (Table 1).
Skewed X-chromosome inactivation was observed in 13% of NC females, and its frequency did not differ in female subjects between the pediatric and adolescent/adult groups (23% vs 10%, respectively) (p>0.05).
The weighted biallelic CAG mean was also evaluated according to the number of hyperandrogenic symptoms at diagnosis. In asymptomatic patients, in patients with one symptom and in those with two or more symptoms, the mean CAGs were 21.8 ± 2, 21.2 ± 2.9 and 21.6 ± 2.8, respectively (p>0.05). In the hirsute patients, the weighted biallelic CAG mean was 20.9 ± 2.5, and in those without hirsutism it was 21.7 ± 2.8 (p> 0.05). Ferriman scores were compared across 33 women who were first evaluated before the use of any topical and/or antiandrogen therapies. In patients with severe hirsutism (Ferriman score ≥ 15), the weighted biallelic CAG mean was 21.5 ± 3.0 and, in those with mild hirsutism (Ferriman score ≤ 14), it was 19.9 ± 2.3 (p>0.05).
The influence of CAG repeats on the presence of virilization was also evaluated and the weighted biallelic CAG mean was compared between patients´ groups with and without clitoromegaly. The weighted biallelic CAG mean was significantly lower in patients with clitoromegaly than in those without it: 19.1 ± 2.7 vs 21.6 ± 2.5, respectively (p = 0.007) (Fig 1). Eight of ten (80%) patients with clitoromegaly and 14 of 93 (15%) without clitoromegaly carried shorter CAG alleles (≤ 18 repeats) (p<0.001). There were no differences in serum testosterone levels between adult women with and without clitoromegaly: 138 ± 73 ng/dL and 94 ± 66 ng/dL, respectively (p>0.05).
Fig 1. Weighted biallelic CAG mean of the AR gene in NC-CAH patients with and without clitoromegaly.
The V89L-SRD5A2 variant was identified in 31% of alleles in Hardy-Weinberg equilibrium; 45% of the patients were heterozygous, and 9% were homozygous carriers. Unlike the V89L, the A49T variant was rare in this series (1% of alleles) and was excluded from association analysis. No difference in the frequency of the V89L variant was observed regarding the onset of symptoms, severity of hirsutism and presence of clitoromegaly in patients carrying both CYP21A2 genotypes.
Discussion
The NC form presents great phenotypic variability, even among subjects carrying similar genotypes. Additionally, in the literature, it has not been well defined whether the severe allele, in a compound heterozygous patient, modulates the severity of hyperandrogenic manifestations. Previous studies have reported conflicting results regarding the association between the severity of the NC genotype and the onset of symptoms [12–15]. In this context, we emphasized two studies with large NC cohorts that found no influence of CYP21A2 genotypes on the onset and/or severity of hyperandrogenic manifestations [11, 16].
The phenotypic variability of hyperandrogenism in the NC form could result from interindividual differences in peripheral androgen sensitivity, which might arise from genetic variants related to androgen action and/or metabolism.
The influence of these variants has been widely evaluated in hyperandrogenic disorders. Some studies have identified that shorter CAG alleles of the AR gene present with greater frequency in women with PCOS in relation to the normal population [46, 47]. However, other studies did not find this association [48, 49], and it is likely that these discordant findings result from methodological differences, such as those for defining the criteria for X-inactivation skewing and for defining shorter CAG alleles. An influence of the CAG tract was observed on the phenotypic severity of prostate cancer, and a significant correlation between shorter alleles and an earlier age at diagnosis was identified [50, 51].
In this present study, besides the great miscegenation of Brazilian population, we found a normal distribution of CAG repeat numbers in the NC cohort, and no differences were observed compared to our normal population or with Caucasians [18, 52]. It seems more important to evaluate the influence of the CAG tract at the beginning of manifestations in the NC form; we hypothesized that pediatric group would carry a higher frequency of shorter CAG alleles, since they present at younger age. However, the weighted biallelic CAG mean, which reflects both the number of repeats and the percentage of activity of each allele, did not differ between NC patients from the pediatric and adolescent/adult groups. This weighted biallelic mean did not differ, even when we considered the severity of the 21-hydroxylase genotype. We cannot exclude that these negative results could be related to a sample size effect. It is worth emphasizing the higher frequency of longer CAG tracts in the adult group bearing the mild NC genotype compared with the pediatric group bearing the same genotype; consequently, we speculated that this higher frequency of alleles with lower AR activity contributed to the later onset of symptoms in patients carrying similar genotypes.
In the same manner, the influence of CAG repeat numbers was previously analyzed at the beginning and with regard to the severity of hyperandrogenic manifestations in the NC form. Ben-Shachar et al. [53], analyzing a group of 119 NC female patients, identified that patients carrying shorter CAG alleles (< 25 repeats) had a higher frequency of precocious pubarche and precocious puberty. Ibáñez et al. [54], in a cohort of 181 women, found that shorter CAG alleles (≤ 20 repeats) were associated with increased risk for premature pubarche relative to the normal population and also with subsequent development of ovarian hyperandrogenism. Interestingly, Hickey et al. [43] found that shorter CAG alleles (≤ 22 repeats) were associated with infertility in a subset of 122 PCOS patients. In contrast, our study revealed no influence of nCAG on the prevalence of premature pubarche, hirsutism, menstrual abnormality or infertility or in the number of manifestations at diagnosis, such as isolated hirsutism and/or associated with menstrual irregularity. Similarly to our results, it was observed in a large PCOS cohort that CAG repeats and serum testosterone levels were not predict factors of hirsutism and acne [49]. However, we would like to emphasize that our criteria for classifying shorter alleles (≤ 18 repeats) might have been stricter than those used in the studies cited above.
To assess the severity of hyperandrogenism, we used the Ferriman score for hirsutism and the presence of an enlarged clitoris, which indicates a stronger phenotype of hyperandrogenism. Association analyses disclosed no influence of the weighted biallelic CAG mean on the severity of hirsutism, similar to the findings of Dasgupta et al. [48], who evaluated PCOS patients. However, the number of hirsute NC patients, who started follow-up in our service without ever having received treatment before, was small. Furthermore, we also speculated that small variations in the CAG repeats within the normal range might not be significant in the modulation of hirsutism. Although this is a large cohort considering nonclassical patients, probably to definitively exclude the influence of CAG repeats in the less severe hyperandrogenic manifestations, besides clitoromegaly, it should be necessary higher number of patients.
Interestingly, in our patients with clitoromegaly, basal androgen concentrations were not different from those in subjects without clitoromegaly [49], but we noted a positive association between shorter CAG alleles and the presence of clitoromegaly, as well as with a lower weighted biallelic CAG mean. This finding reinforced those observed in a previous study conducted in our laboratory in patients with the classical form of CAH, in whom we found an influence of the CAG tract in the phenotypic variability of external genitalia virilization [18]. In men, the influence of the CAG tract on the phenotype of the external genitalia was also reported. Ogata et al. [55] described a patient with 46,XY and with genital ambiguity, carrying a rare allele with 44 CAG repeats, without any other allelic variant in exonic/intronic regions of the AR gene and no changes in testosterone synthesis.
Interestingly, no differences in CAG allelic distributions were observed between our asymptomatic and symptomatic NC female subjects. Asymptomatic NC females despite the 21-hydroxylase deficiency, presented with normal basal androgen levels through consecutive evaluations. It is likely that this cryptic phenotype is related to genetic variations in androgen synthesis. However, in this study few asymptomatic cases were evaluated, since they were diagnosed during familial study of an index case.
We also call attention to the process of gene regulation of the androgen receptor, which is extremely complex and differs in various tissues according to the activity of co-regulatory proteins. These proteins might also play a modulatory role in peripheral sensitivity to androgens and corroborate the phenotypic variability of the NC form [56].
Another protein of importance in the peripheral action of androgens is the 5α-reductase enzyme. The peripheral action of testosterone is boosted by the activity of this enzyme converting testosterone into dihydrotestosterone, which is a more potent androgen than testosterone, largely responsible for the growth and stimulation of hair follicles. The increased peripheral activity of 5α-reductase was considered a predisposing factor for the development of idiopathic hirsutism and precocious idiopathic pubarche [57].
Allelic variants in the SRD5A2 gene have also been associated with the development of androgen-dependent disorders, such as prostate cancer and PCOS. In a series enrolling 187 PCOS women, the status carrier for the 89L allele, which is known to reduce the activity of 5α-reductase type 2, was associated with a lower likelihood of developing PCOS [23]. However, this variant was not correlated with a lower intensity of hirsutism. The 89L variant was identified among approximately 31% of the alleles in our patients, similar to the normal Brazilian population [58]. In contrast, our association studies have not identified an influence of the 89L allele on the hyperandrogenic phenotype of the NC form. This allele was not protective against the development of premature pubarche, and its frequency did not differ significantly between hirsute and non-hirsute patients and or between those with and without menstrual irregularity.
Our NC patients carrying the 89L variant had lower median scores for hirsutism and later onset of manifestations relative to wild-type carriers, but these differences were not significant. Although this variant decreased the activity of 5α-reductase to 30% in vitro [59], it is possible that was not significant in vivo for the NC phenotype. It is likely that the 5α-reductase type 1 enzyme is more important to the severity of hirsutism, as previously reported in women in PCOS [23].
In conclusion, we described that the CAG tract of the androgen receptor gene could explain, at least partially, the phenotypic variability in the NC form, but these data must be replicated in other populations. Although the NC form of CAH is a monogenic disorder, our preliminary data suggested that the interindividual variability in the hyperandrogenic phenotype could arise from polygenic interactions.
Supporting Information
(TIF)
(PDF)
LR: large gene rearrangements, included large gene conversions and the CYP21A2 deletions; Del: deletion.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by grants from the Sao Paulo Research Foundation (FAPESP) #2014/07878-4, to Moura-Massari V by FAPESP #08/51624-6 and FAPESP #05/04726-0, and to Bachega TASS and Mendonca BB by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) #308318/2012-9 and 305743/2011-2, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133–60. 10.1210/jc.2009-2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White PC, Bachega TA. Congenital adrenal hyperplasia due to 21 hydroxylase deficiency: from birth to adulthood. Semin Reprod Med. 2012;30(5):400–9. 10.1055/s-0032-1324724 . [DOI] [PubMed] [Google Scholar]
- 3.Moran C, Azziz R, Carmina E, Dewailly D, Fruzzetti F, Ibañez L, et al. 21-Hydroxylase-deficient nonclassic adrenal hyperplasia is a progressive disorder: a multicenter study. Am J Obstet Gynecol. 2000;183(6):1468–74. 10.1067/mob.2000.108020 . [DOI] [PubMed] [Google Scholar]
- 4.Bachega TA, Billerbeck AE, Madureira G, Marcondes JA, Longui CA, Leite MV, et al. Molecular genotyping in Brazilian patients with the classical and nonclassical forms of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1998;83(12):4416–9. 10.1210/jcem.83.12.5350 . [DOI] [PubMed] [Google Scholar]
- 5.Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96(1):E161–72. 10.1210/jc.2010-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85(3):1059–65. 10.1210/jcem.85.3.6441 . [DOI] [PubMed] [Google Scholar]
- 7.Speiser PW, New MI, Tannin GM, Pickering D, Yang SY, White PC. Genotype of Yupik Eskimos with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Genet. 1992;88(6):647–8. . [DOI] [PubMed] [Google Scholar]
- 8.Wedell A, Thilén A, Ritzén EM, Stengler B, Luthman H. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab. 1994;78(5):1145–52. 10.1210/jcem.78.5.8175971 . [DOI] [PubMed] [Google Scholar]
- 9.Wilson RC, Nimkarn S, Dumic M, Obeid J, Azar MR, Azar M, et al. Ethnic-specific distribution of mutations in 716 patients with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Mol Genet Metab. 2007;90(4):414–21. 10.1016/j.ymgme.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachega TA, Billerbeck AE, Marcondes JA, Madureira G, Arnhold IJ, Mendonca BB. Influence of different genotypes on 17-hydroxyprogesterone levels in patients with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2000;52(5):601–7. . [DOI] [PubMed] [Google Scholar]
- 11.Bidet M, Bellanné-Chantelot C, Galand-Portier MB, Tardy V, Billaud L, Laborde K, et al. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab. 2009;94(5):1570–8. 10.1210/jc.2008-1582 . [DOI] [PubMed] [Google Scholar]
- 12.Weintrob N, Brautbar C, Pertzelan A, Josefsberg Z, Dickerman Z, Kauschansky A, et al. Genotype-phenotype associations in non-classical steroid 21-hydroxylase deficiency. Eur J Endocrinol. 2000;143(3):397–403. . [DOI] [PubMed] [Google Scholar]
- 13.Speiser PW, Knochenhauer ES, Dewailly D, Fruzzetti F, Marcondes JA, Azziz R. A multicenter study of women with nonclassical congenital adrenal hyperplasia: relationship between genotype and phenotype. Mol Genet Metab. 2000;71(3):527–34. 10.1006/mgme.2000.3036 . [DOI] [PubMed] [Google Scholar]
- 14.Deneux C, Tardy V, Dib A, Mornet E, Billaud L, Charron D, et al. Phenotype-genotype correlation in 56 women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2001;86(1):207–13. 10.1210/jcem.86.1.7131 . [DOI] [PubMed] [Google Scholar]
- 15.Ezquieta B, Cueva E, Varela J, Oliver A, Fernández J, Jariego C. Non-classical 21-hydroxylase deficiency in children: association of adrenocorticotropic hormone-stimulated 17-hydroxyprogesterone with the risk of compound heterozygosity with severe mutations. Acta Paediatr. 2002;91(8):892–8. . [DOI] [PubMed] [Google Scholar]
- 16.Moura-Massari VO, Bugano DD, Marcondes JA, Gomes LG, Mendonca BB, Bachega TA. CYP21A2 genotypes do not predict the severity of hyperandrogenic manifestations in the nonclassical form of congenital adrenal hyperplasia. Horm Metab Res. 2013;45(4):301–7. 10.1055/s-0032-1330007 . [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22(15):3181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha RO, Billerbeck AE, Pinto EM, Melo KF, Lin CJ, Longui CA, et al. The degree of external genitalia virilization in girls with 21-hydroxylase deficiency appears to be influenced by the CAG repeats in the androgen receptor gene. Clin Endocrinol (Oxf). 2008;68(2):226–32. 10.1111/j.1365-2265.2007.03023.x . [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A. 1997;94(7):3320–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsing AW, Gao YT, Wu G, Wang X, Deng J, Chen YL, et al. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2000;60(18):5111–6. . [PubMed] [Google Scholar]
- 21.Lappalainen S, Utriainen P, Kuulasmaa T, Voutilainen R, Jääskeläinen J. Androgen receptor gene CAG repeat polymorphism and X-chromosome inactivation in children with premature adrenarche. J Clin Endocrinol Metab. 2008;93(4):1304–9. 10.1210/jc.2007-2707 . [DOI] [PubMed] [Google Scholar]
- 22.Mifsud A, Sim CK, Boettger-Tong H, Moreira S, Lamb DJ, Lipshultz LI, et al. Trinucleotide (CAG) repeat polymorphisms in the androgen receptor gene: molecular markers of risk for male infertility. Fertil Steril. 2001;75(2):275–81. . [DOI] [PubMed] [Google Scholar]
- 23.Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R. Variants in the 5alpha-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab. 2006;91(10):4085–91. 10.1210/jc.2006-0227 . [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Zhu Y, He J, Wang M, Zhu M, Shi T, et al. Steroid 5-alpha-reductase type 2 (SRD5A2) V89L and A49T polymorphisms and sporadic prostate cancer risk: a meta-analysis. Mol Biol Rep. 2013;40(5):3597–608. 10.1007/s11033-012-2434-x . [DOI] [PubMed] [Google Scholar]
- 25.Makridakis NM, Ross RK, Pike MC, Crocitto LE, Kolonel LN, Pearce CL, et al. Association of mis-sense substitution in SRD5A2 gene with prostate cancer in African-American and Hispanic men in Los Angeles, USA. Lancet. 1999;354(9183):975–8. 10.1016/S0140-6736(98)11282-5 . [DOI] [PubMed] [Google Scholar]
- 26.Samtani R, Bajpai M, Vashisht K, Ghosh PK, Saraswathy KN. Hypospadias risk and polymorphism in SRD5A2 and CYP17 genes: case-control study among Indian children. J Urol. 2011;185(6):2334–9. 10.1016/j.juro.2011.02.043 . [DOI] [PubMed] [Google Scholar]
- 27.New MI. An update of congenital adrenal hyperplasia. Ann N Y Acad Sci. 2004;1038:14–43. 10.1196/annals.1315.009 . [DOI] [PubMed] [Google Scholar]
- 28.Speiser PW, New MI, White PC. Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14,DR1. N Engl J Med. 1988;319(1):19–23. 10.1056/NEJM198807073190104 . [DOI] [PubMed] [Google Scholar]
- 29.Brown J, Warne G. Practical management of the intersex infant. J Pediatr Endocrinol Metab. 2005;18(1):3–23. . [DOI] [PubMed] [Google Scholar]
- 30.Verkauf BS, Von Thron J, O'Brien WF. Clitoral size in normal women. Obstet Gynecol. 1992;80(1):41–4. . [PubMed] [Google Scholar]
- 31.FERRIMAN D, GALLWEY JD . Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. 10.1210/jcem-21-11-1440 . [DOI] [PubMed] [Google Scholar]
- 32.Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(4):1105–20. 10.1210/jc.2007-2437 . [DOI] [PubMed] [Google Scholar]
- 33.Costa-Barbosa FA, Tonetto-Fernandes VF, Carvalho VM, Nakamura OH, Moura V, Bachega TA, et al. Superior discriminating value of ACTH-stimulated serum 21-deoxycortisol in identifying heterozygote carriers for 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2010;73(6):700–6. 10.1111/j.1365-2265.2010.03871.x . [DOI] [PubMed] [Google Scholar]
- 34.Billerbeck AE, Bachega TA, Frazatto ET, Nishi MY, Goldberg AC, Marin ML, et al. A novel missense mutation, GLY424SER, in Brazilian patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1999;84(8):2870–2. 10.1210/jcem.84.8.5937 . [DOI] [PubMed] [Google Scholar]
- 35.Araújo RS, Mendonca BB, Barbosa AS, Lin CJ, Marcondes JA, Billerbeck AE, et al. Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(10):4028–34. 10.1210/jc.2006-2163 . [DOI] [PubMed] [Google Scholar]
- 36.Higashi Y, Hiromasa T, Tanae A, Miki T, Nakura J, Kondo T, et al. Effects of individual mutations in the P-450(C21) pseudogene on the P-450(C21) activity and their distribution in the patient genomes of congenital steroid 21-hydroxylase deficiency. J Biochem. 1991;109(4):638–44. . [DOI] [PubMed] [Google Scholar]
- 37.Tusie-Luna MT, Traktman P, White PC. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990;265(34):20916–22. . [PubMed] [Google Scholar]
- 38.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51(6):1229–39. [PMC free article] [PubMed] [Google Scholar]
- 39.Kaupert LC, Billerbeck AE, Brito VN, Mendonca BB, Bachega TA. Could the leukocyte x chromosome inactivation pattern be extrapolated to hair bulbs? Horm Res Paediatr. 2010;73(4):238–43. 10.1159/000284387 . [DOI] [PubMed] [Google Scholar]
- 40.Pegoraro E, Schimke RN, Arahata K, Hayashi Y, Stern H, Marks H, et al. Detection of new paternal dystrophin gene mutations in isolated cases of dystrophinopathy in females. Am J Hum Genet. 1994;54(6):989–1003. [PMC free article] [PubMed] [Google Scholar]
- 41.Gale RE, Wheadon H, Boulos P, Linch DC. Tissue specificity of X-chromosome inactivation patterns. Blood. 1994;83(10):2899–905. . [PubMed] [Google Scholar]
- 42.Sharp A, Robinson D, Jacobs P. Age- and tissue-specific variation of X chromosome inactivation ratios in normal women. Hum Genet. 2000;107(4):343–9. . [DOI] [PubMed] [Google Scholar]
- 43.Hickey T, Chandy A, Norman RJ. The androgen receptor CAG repeat polymorphism and X-chromosome inactivation in Australian Caucasian women with infertility related to polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(1):161–5. 10.1210/jcem.87.1.8137 . [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Habuchi T, Mitsumori K, Kamoto T, Kinoshitu H, Segawa T, et al. Association of V89L SRD5A2 polymorphism with prostate cancer development in a Japanese population. J Urol. 2003;169(6):2378–81. 10.1097/01.ju.0000056152.57018.31 . [DOI] [PubMed] [Google Scholar]
- 45.Cleves MA. Exploratory analysis of single nucleotide polymorphism (SNP) for quantitative traits. Stata Journal. 2005;5(2):141–53. WOS:000231710800001. [Google Scholar]
- 46.Shah NA, Antoine HJ, Pall M, Taylor KD, Azziz R, Goodarzi MO. Association of androgen receptor CAG repeat polymorphism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(5):1939–45. 10.1210/jc.2008-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xita N, Georgiou I, Lazaros L, Psofaki V, Kolios G, Tsatsoulis A. The role of sex hormone-binding globulin and androgen receptor gene variants in the development of polycystic ovary syndrome. Hum Reprod. 2008;23(3):693–8. 10.1093/humrep/dem382 . [DOI] [PubMed] [Google Scholar]
- 48.Dasgupta S, Sirisha PV, Neelaveni K, Anuradha K, Reddy AG, Thangaraj K, et al. Androgen receptor CAG repeat polymorphism and epigenetic influence among the south Indian women with Polycystic Ovary Syndrome. PLoS One. 2010;5(8):e12401 10.1371/journal.pone.0012401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skrgatic L, Baldani DP, Cerne JZ, Ferk P, Gersak K. CAG repeat polymorphism in androgen receptor gene is not directly associated with polycystic ovary syndrome but influences serum testosterone levels. J Steroid Biochem Mol Biol. 2012;128(3–5):107–12. . [DOI] [PubMed] [Google Scholar]
- 50.Mishra D, Thangaraj K, Mandhani A, Kumar A, Mittal R. Is reduced CAG repeat length in androgen receptor gene associated with risk of prostate cancer in Indian population? Clin Genet. 2005;68(1):55–60. 10.1111/j.1399-0004.2005.00450.x . [DOI] [PubMed] [Google Scholar]
- 51.Shimbo M, Suzuki H, Kamiya N, Imamoto T, Komiya A, Ueda T, et al. CAG polymorphic repeat length in androgen receptor gene combined with pretreatment serum testosterone level as prognostic factor in patients with metastatic prostate cancer. Eur Urol. 2005;47(4):557–63. 10.1016/j.eururo.2004.10.016 . [DOI] [PubMed] [Google Scholar]
- 52.Adamovic T, Nordenskjöld A. The CAG repeat polymorphism in the androgen receptor gene modifies the risk for hypospadias in Caucasians. BMC Med Genet. 2012;13:109 10.1186/1471-2350-13-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ben-Shachar S, Ayalon I, Reznik-Wolf H, Tenenbaum-Rakover Y, Zuckerman-Levin N, Cohen O, et al. Androgen Receptor CAG Repeat Length in Relation to Phenotype Among Females with Nonclassical 21-Hydroxylase Deficiency. Horm Metab Res. 2015;47(7):491–6. 10.1055/s-0034-1389901 . [DOI] [PubMed] [Google Scholar]
- 54.Ibáñez L, Ong KK, Mongan N, Jääskeläinen J, Marcos MV, Hughes IA, et al. Androgen receptor gene CAG repeat polymorphism in the development of ovarian hyperandrogenism. J Clin Endocrinol Metab. 2003;88(7):3333–8. 10.1210/jc.2002-021791 . [DOI] [PubMed] [Google Scholar]
- 55.Ogata T, Muroya K, Ishii T, Suzuki Y, Nakada T, Sasagawa I. Undermasculinized genitalia in a boy with an abnormally expanded CAG repeat length in the androgen receptor gene. Clin Endocrinol (Oxf). 2001;54(6):835–8. . [DOI] [PubMed] [Google Scholar]
- 56.Bebermeier JH, Brooks JD, DePrimo SE, Werner R, Deppe U, Demeter J, et al. Cell-line and tissue-specific signatures of androgen receptor-coregulator transcription. J Mol Med (Berl). 2006;84(11):919–31. 10.1007/s00109-006-0081-1 . [DOI] [PubMed] [Google Scholar]
- 57.Zouboulis CC, Degitz K. Androgen action on human skin—from basic research to clinical significance. Exp Dermatol. 2004;13 Suppl 4:5–10. 10.1111/j.1600-0625.2004.00255.x . [DOI] [PubMed] [Google Scholar]
- 58.Ribeiro ML, Santos A, Carvalho-Salles AB, Hackel C. Allelic frequencies of six polymorphic markers for risk of prostate cancer. Braz J Med Biol Res. 2002;35(2):205–13. . [DOI] [PubMed] [Google Scholar]
- 59.Nam RK, Toi A, Vesprini D, Ho M, Chu W, Harvie S, et al. V89L polymorphism of type-2, 5-alpha reductase enzyme gene predicts prostate cancer presence and progression. Urology. 2001;57(1):199–204. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(PDF)
LR: large gene rearrangements, included large gene conversions and the CYP21A2 deletions; Del: deletion.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.