Abstract
Following bone fracture, the repair process starts with an inflammatory reaction at the fracture site. Fracture healing is disturbed when the initial inflammation is increased or prolonged, whereby, a balanced inflammatory response is anticipated to be crucial for fracture healing, because it may induce down-stream responses leading to tissue repair. However, the impact of the immune response on fracture healing remains poorly understood. Here, we investigated bone healing in NOD/scid-IL2Rγcnull mice, which exhibit severe defects in innate and adaptive immunity, by biomechanical testing, histomorphometry and micro-computed tomography. We demonstrated that NOD/scid-IL2Rγcnull mice exhibited normal skeletal anatomy and a mild bone phenotype with a slightly reduced bone mass in the trabecular compartment in comparison to immunocompetent Balb/c mice. Fracture healing was impaired in immunodeficient NOD/scid-IL2Rγcnull mice. Callus bone content was unaffected during the early healing stage, whereas it was significantly reduced during the later healing period. Concomitantly, the amount of cartilage was significantly increased, indicating delayed endochondral ossification, most likely due to the decreased osteoclast activity observed in cells isolated from NOD/scid-IL2Rγcnull mice. Our results suggest that—under aseptic, uncomplicated conditions—the immediate immune response after fracture is non-essential for the initiation of bone formation. However, an intact immune system in general is important for successful bone healing, because endochondral ossification is delayed in immunodeficient NOD/scid-IL2Rγcnull mice.
Introduction
A close relationship exists between the bone and immune systems. Both systems share a large number of regulatory molecules, macrophages and osteoclasts develop from the same progenitor and inflammatory disorders can be associated with bone loss [1–4]. The immune system also appears to play an important role in bone healing, because the fracture repair process starts with an inflammatory response [5, 6]. The fracture leads to tissue damage and blood vessel rupture, initiating acute inflammation and the development of a haematoma, which is characterized by low pH, hypoxia and a high concentration of inflammatory mediators and chemokines being released from resident immune cells after sensing injury associated danger signals [7, 8]. Polymorphonuclear neutrophils (PMNs), which are rapidly recruited at the early stage of inflammation, act against endogenous and exogenous pathogens by secreting reactive oxygen species, proteases and cytokines, and phagocytize debris and dead cell remnants. By releasing chemokines, PMNs attract macrophages, which further remove pathogens and initiate tissue repair by producing pro-angiogenic and trophic factors [9]. Later, the immune response shifts towards adaptive immunity, reflected by the invasion of lymphocytes into the fracture zone [10]. The inflammatory phase is orchestrated by many pro- and anti-inflammatory mediators (e.g. interleukin (IL)-1, IL-6, tumour necrosis factor-α), pro-angiogenic mediators and growth factors (e.g. of the bone morphogenetic protein superfamily) [11]. With the resolution of acute inflammation, mesenchymal progenitor cells are attracted and new bone is formed by intramembranous and endochondral ossification [5, 6].
A balanced inflammation at the fracture site restricts tissue damage and initiates tissue repair by providing pro-angiogenic mediators and attracting mesenchymal progenitors cells, and is, therefore, anticipated to be crucial for fracture healing [6, 12]. In contrast, fracture healing is disturbed when the inflammatory response is increased or prolonged. For example, excessive inflammation associated with complex local tissue injury results in delayed healing [13, 14]. Systemic inflammatory conditions, including polytrauma, which induce an acute systemic immune response, significantly increase the risk for non-union [13, 15]. There is also clinical evidence that fracture healing is disturbed in patients with chronic immune disorders, including rheumatoid arthritis, and diabetes [16, 17].
Currently, it is poorly understood how much inflammation may be too much, and whether an immune response is actually crucial for bone repair. Here, we investigated bone healing in NOD/scid-IL2Rγcnull mice, which exhibit severe defects in innate and adaptive immunity, and hypothesized that fracture healing would be considerably disrupted when a balanced immune response, which is proposed to have a positive effect on regeneration, is disturbed.
Materials and Methods
Mouse model
All experiments were performed according to national and international regulations for the care and use of laboratory animals and were approved by the Local Ethics Committee (No. 1000,Regierungspräsidium Tübingen, Germany). NOD/scid-Il2Rγcnull (NOD.Cg-Prkdcscid Il2Rgtm1Wjl/SzJ) and Balb/cByJ (referred to as Balb/c) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The immunedeficient NOD/scid-Il2Rγcnull mouse is characterized by the lack of functional monocytes, dendritic cells, natural-killer cells and mature lymphocytes [18]. Furthermore, they display no detectable activity of haemolytic complement. The immunogenic organs are degenerated and consist mainly of stroma. Splenic follicles are absent and lymph nodes are completely atrophic. All other organs are normally developed. The mice exhibit severe deficiencies in cytokine signalling due to the defect in the so-called common gamma-chain of the IL2 receptor (γc). Therefore, the signalling of IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 is defective [19].
BALB/c mice were chosen as the immunocompetent control, because the spontaneous mutation for scid (severe combined immunodeficiency) in the Prkdc-locus (protein kinase, DNA-activated, catalytic polypeptide) originally occurred in this strain.
Animal studies
To investigate whether the immune defect of NOD/scid-Il2Rγcnull mice influences bone formation, the skeleton of Balb/c and NSG (male, 12 weeks old, n = 10 for both genotypes) mice was comparatively analysed as described below. Fracture healing was investigated in NOD/scid-Il2Rγcnull and BALB/c mice of the same age (n = 24 per genotype). Surgery was performed as previously described [20]. Briefly, an osteotomy was created at the mid-shaft of the right femur and stabilized using an external fixator that was fitted to the bone using four mini-Schanz screws (axial stiffness 18.1 N/mm, RISystem, Davos, Switzerland). To reduce pain, an analgesic (tramadol hydrochloride, 15 mg/kg) was administered subcutaneously before the operation and via the drinking water (25 mg/L) for the first 3 postoperative days. The mice received daily subcutaneous injections of clindamycin-2-dihydrogenphosphate (45 mg/kg) until the third postoperative day. During the first three post-operative days, the animals were monitored daily, afterwards twice a week until sacrification. Specifically, the use of the operated limb was assessed as well general health condition (bodyweight, grooming, overall behaviour). Eight animals per group were sacrificed by CO2 asphyxiation after 21, 28 and 35 days and the osteotomized bone was analysed as described below.
Biomechanical testing
To determine the mechanical quality of the intact and osteotomized femurs, the flexural rigidity was assessed by a non-destructive three-point bending test using a material testing machine as described previously [20, 21]. Briefly, the proximal end of the femur was fixed to an aluminium cylinder using SelfCem (Heraeus Kulzer, Hanau, Germany). The cylinder was fixed in a hinge joint, serving as the proximal support for the bending test. The femoral condyles rested on the bending support, the distance between both supports being 20 mm (l). The bending load F was applied on the mid-shaft and continuously recorded vs. sample deflection (d) up to a maximum force of 5 N. Because the callus was not always located exactly in the middle of the supports (l/2), the distances between the load vector and the proximal (a) and distal (b) supports were considered when calculating the flexural rigidity EI = k((a2b2)/3l) [20].
Micro-computed tomography (μCT)
The intact and osteotomized femurs were scanned using a μCT-device (Skyscan 1172, Bruker, Belgium) at a resolution of 8 μm using a peak voltage of 50 kV and 200 μA. In the cortical bone of the non-osteotomized femurs, a volume of interest (VOI) of 168 μm height was defined in the mid-diaphysis. The trabecular bone was evaluated in the distal part of the femur in a 280 μm high VOI with its lower end 200 μm above the growth plate. To assess the fracture-healing outcome, the former osteotomy gap was defined as the VOI. A global threshold of 37% of the maximal grey value of each specimen was used to distinguish between mineralized and non-mineralized tissue [22]. Common standard parameters of the American Society of Bone and Mineral Research (ASBMR) were evaluated [23, 24].
Histology and histomorphometry
The osteotomized and intact femora were processed for undecalcified histology. The specimens were fixed in 4% formaldehyde, dehydrated, and embedded in methyl methacrylate. Sections of 10 μm of fractured femurs were stained using Giemsa. To determine the osteoblast numbers, 10-μm thick sections of intact femurs were stained using toluidine blue for better visualization of osteoblasts. To visualize osteoclasts, tartrate resistant acid phosphatase (TRAP) staining was performed using naphthol AS-MX phosphate (Sigma-Aldrich, Taufkirchen, Germany) and Fast Red TR-Salt (Sigma-Aldrich) in 0.2 M acetate buffer pH 5.0. For histomorphometric analysis of the fracture callus, the sections were examined using light microscopy (Leica DMI6000B, Leica, Switzerland) at 50-fold magnification. In the fracture callus, the relative cartilage fraction was analysed using the software Leica MMAF 1.4.0 (MetaMorph®, Leica, Switzerland). The osteoblasts and osteoclasts were counted using the OsteoMeasure histomorphometry system (OsteoMetrics, Decatur, USA). The analyses were performed according the recommendations of the ASBMR [23].
Ex vivo analyses of osteoblast- and osteoclast-like cells
To investigate whether osteoblasts and osteoclasts exhibit cell-autonomous defects in the NOD/scid-Il2Rγcnull mouse, cell function was analysed ex vivo. Osteoblasts were isolated from cortical bone of both Balb/c and NSG mice. Briefly, the bones were minced and digested for 2 h using 300 U/ml collagenase type IV (Sigma-Aldrich) in α-minimum essential eagle medium (α-MEM, Biochrom AG, Berlin, Germany). The bone chips were cultivated in α-MEM supplemented with 15% foetal calf serum (FCS), 4 nM L-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin (all Biochrom AG) and 0.25 mg/mL amphotericin-B (Fungizone®, Gibco, Darmstadt, Germany) at 37°C under 5% CO2, until colonies started to form. The colonies were sub-cultivated and cells in passages 3 to 5 were seeded for osteogenic differentiation at a density of 10,000 cells/cm2 in medium further supplemented with 10 mM disodium β-glycerophosphate and 0.2 mM ascorbate-2-phosphate (all Sigma-Aldrich). After 21 days, matrix mineralization was analysed by von-Kossa-staining. Briefly, incubation with silver nitrate leads to the replacement of calcium with silver ions. Reduction of the silver ions results in a dark staining of mineralized areas. Alkaline phosphatase activity was detected by staining using a commercially available kit (Sigma, Germany).
Osteoclast-like cells (OCL) were generated from bone marrow that was flushed from humeri and tibiae. The cells were plated at 3x105 cells/cm2 and cultivated in α-MEM supplemented with 10% FCS (Gibco), 4 nM L-glutamine, 100 U/mL penicillin and 0.1 mg/mL streptomycin (all Biochrom AG). To stimulate osteoclast fusion, 10 ng/mL recombinant human macrophage-colony stimulating factor (rh-MCSF, Chemicon, Limburg, Germany) were added for 3 days. The non-adherent cell fraction was plated at a density of 5x105 cells/cm2 on normal tissue culture plastic and in plates with a synthetic calcium phosphate coating (BD BioCoat™ Osteologic™ Bone Cell Culture System plates; Becton Dickinson GmbH, Germany) and cultivated for 7 days. To assess OCL formation, cells were stained for TRAP using a commercial kit (Sigma, Germany). TRAP-positive cells with ≥3 nuclei were counted as OCL. To analyse the resorption activity of the OCL, BD BioCoat™ Osteologic Bone Cell Culture System™ slides were treated using 6% sodium hypochloride and von-Kossa stained to visualise the resorbed areas in the calcium phosphate coating.
Statistics
Data is presented as the mean ± standard error of the mean. Comparisons between two groups of normally distributed variables, analysed using Shapiro-Wilk normality test, were performed using student’s T-test. (GraphPad Prism6, GraphPad Software, Inc., La Jolla, CA, USA). The level of significance was p<0.05.
Results
Immunodeficiency in NOD/scid-IL2Rγcnull mice induces a moderate bone phenotype
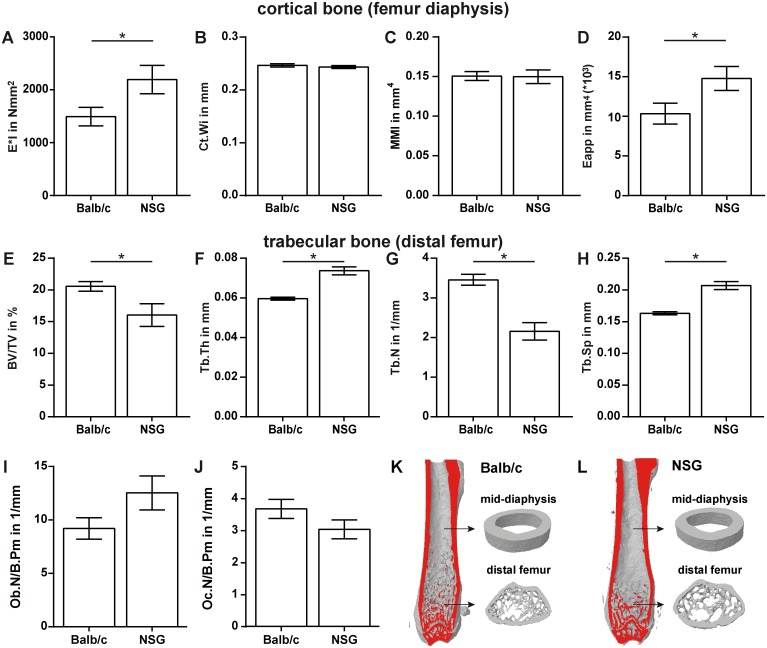
To assess the skeletal phenotype of NOD/scid-IL2Rγcnull mice, whole body X-rays were evaluated in comparison to Balb/c mice. We observed no gross abnormalities when comparing the skeleton of both strains (data not shown). The flexural rigidity of the femurs assessed by three-point bending was significantly higher (+47%, p = 0.0495) in NOD/scid-IL2Rγcnull compared to Balb/c mice (Fig 1A). Micro-computed tomography analysis of the femur diaphysis revealed no significant alterations in cortical width (Ct.Wi), moment of inertia (MMI) or mineralization represented by the mean grey value (115.76±1.43 for Balb/c vs. 112.67±1.40 for NOD/scid-IL2Rγcnull). The apparent Young’s Modulus (Eapp), representing the mechanical properties of the bone matrix, was significantly increased by 43% in NOD/scid-IL2Rγcnull (p = 0.0448) (Fig 1B–1D).
Fig 1. Skeletal phenotype of Balb/c and NOD/scid-IL2Rγcnull (NSG) mice.
Cortical bone was analysed by a three-point bending test and micro-computed tomography (μCT) (A-D); trabecular bone in the distal femur (E–H) was analysed by μCT. The osteoblast number per bone perimeter (Ob.N/B.Pm) was assessed in toluidine-blue-stained sections (I). The number of osteoclasts per bone perimeter (Oc.N/B.Pm) was evaluated in sections stained for tartrate resistant acid phosphatase (TRAP) (J). K and L depict representative 3-dimensional reconstructions of femurs from Balb/c (K) and NSG mice (L). Data is presented as the mean ± standard error of the mean. n = 9–10. Asterisks denote significant differences; p<0.05.
Analysis of trabecular bone at the distal femur revealed a moderately reduced bone mass in NOD/scid-IL2Rγcnull compared to Balb/c mice (Fig 1E–1H). The bone per tissue volume was significantly decreased due to a reduced trabecular number and increased trabecular spacing, whereas trabecular thickness was increased (all parameters p<0.0001) (Fig 1). Representative 3D-reconstructions of cortical and trabecular bone are depicted in Fig 1K and 1L. Osteoblast and osteoclast numbers were not significantly different (Fig 1I and 1J).
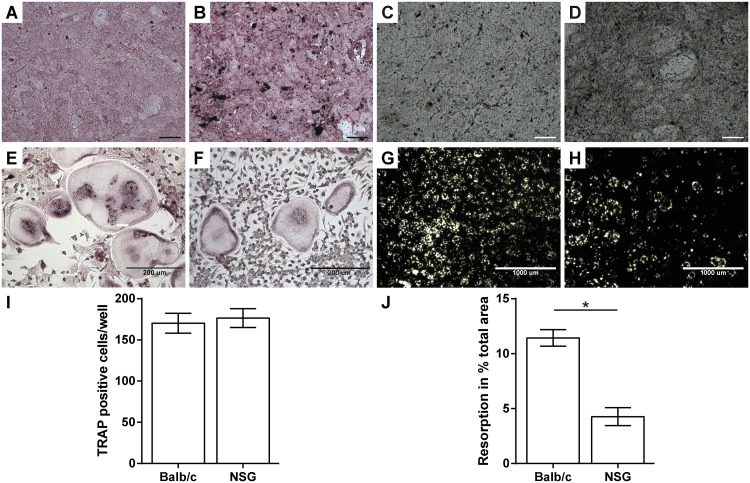
Primary osteoblasts isolated from NOD/scid-IL2Rγcnull mice exhibited a stronger in vitro differentiation capacity compared to Balb/c mice, as demonstrated by increased alkaline phosphatase activity and mineral deposition (Fig 2A–2D). The in vitro formation of osteoclast-like cells was unaffected in NOD/scid-IL2Rγcnull mice (Fig 2E, 2F and 2I); however, the resorption activity of the cells was significantly reduced (Fig 2G, 2H and 2J).
Fig 2. Ex vivo analysis of osteoblast and osteoclast function.
Osteogenic differentiated primary osteoblasts from Balb/c (A, C) and NOD/scid-IL2Rγcnull mice (NSG; B, D) were stained for alkaline phosphatase activity (A, B) and mineral deposition (C, D) using von-Kossa-stain, respectively. Osteoclast-like (OCL) cell fusion from mononuclear cells was analysed using tartrate resistant acid phosphatase (TRAP)-staining (E, F, I). The resorption activity of the OCL was analysed on calcium-phosphate-coated discs. (G, H, J). Data is depicted as the mean ± standard error of the mean. I: n = 4; J: n = 7. Scale bar in A–D = 100 μm.
Immunodeficiency in NOD/scid-IL2Rγcnull mice delays fracture healing
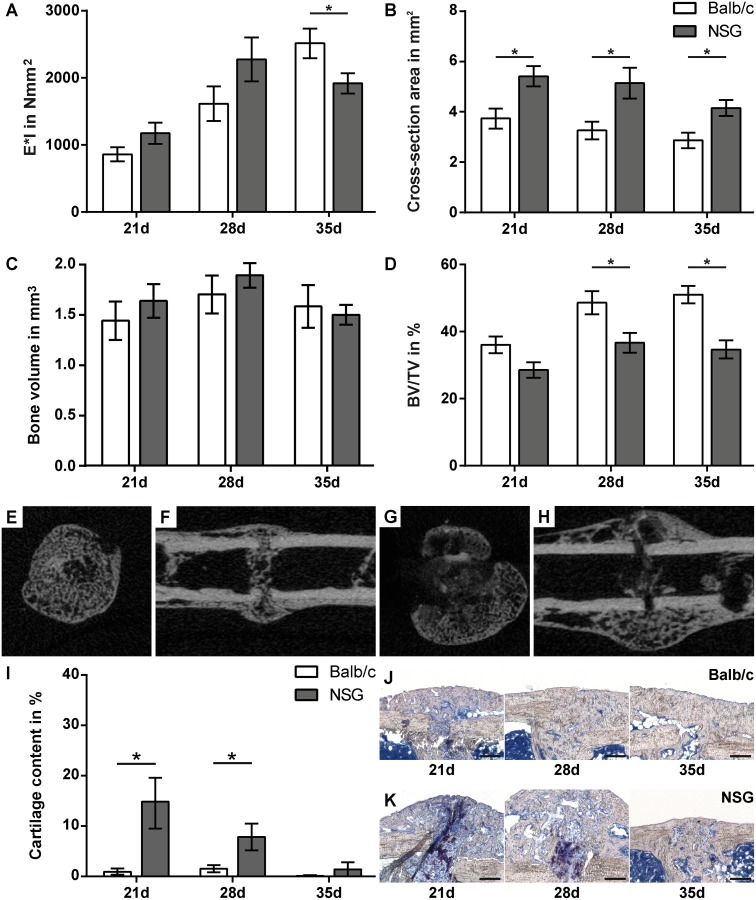
Biomechanical testing of healed femurs in Balb/c and NOD/scid-IL2Rγcnull mice revealed no statistically significant differences after a healing period of both, 21 and 28 days. On day 35, flexural rigidity was significantly lower in NOD/scid-IL2Rγcnull mice (p = 0.0430) (Fig 3A).
Fig 3. Fracture healing is moderately altered in NOD/scid-IL2Rγcnull (NSG) mice.
Assessment of the fracture-healing outcome by three-point bending test (A), micro-computed tomography (B-D) and histomorphometry (I). Representative cross sections (E, G) and longitudinal sections (F, H) on day 21 of calli from Balb/c (E, F) and NSG mice (F, G). Representative micrographs of fracture calli of Balb/c (J) and NSG (K) mice on day 21, 28 and 35. Data is presented as the mean ± standard error of the mean; n = 6–8. Asterisks indicate significant differences between Balb/c and NSG mice at the indicated time-point; p<0.05. Scale bars in J and K = 200 μm.
At all time points, μCT analysis demonstrated a significant increase in callus cross-section area in NOD/scid-IL2Rγcnull mice compared to Balb/c mice (p = 0.0115–0.015) (Fig 3B and 3E–3H). Although the absolute bone volume was not significantly different (Fig 3C), the relative bone content of the fracture callus was significantly lower in NOD/scid-IL2Rγcnull mice on days 28 and 35 (p = 0.0197 and p<0.0006, respectively) due to the increased callus size (Fig 3D).
Histomorphometric analysis revealed a significantly increased cartilage content in the calli of NOD/scid-IL2Rγcnull mice on days 21 and 28 (p = 0.048 and 0.0297, respectively) (Fig 3I, 3J and 3K). Therefore, the longer persistence of cartilage and the reduced bone fraction in the facture callus of the immunodeficient mice suggest delayed endochondral bone formation. The reduced callus tissue quality in the NOD/scid-IL2Rγcnull mice did not affect the flexural rigidity on days 21 and 28, because the callus was larger. However, on day 35, the greater callus size no longer compensated for the poor quality.
Discussion
To investigate the importance of the immune response in fracture healing, we studied bone healing in NOD/scid-IL2Rγcnull mice, which exhibit severe immune defects. Fracture healing was impaired in the immunodeficient mice compared to immunocompetent Balb/c mice, as demonstrated by a significantly reduced bone content of the fracture callus in the late healing phase. Concomitantly, the amount of cartilage was significantly increased, indicating delayed endochondral ossification.
We chose NOD/scid-IL2Rγcnull mice for this study, because they have severe defects in innate and adaptive immunity, but do not exhibit obvious dysfunctions of other organs and have a normal life expectancy. Therefore, these mice are an established model, for example, for xenogeneic transplantation [25]. NOD/scid-IL2Rγcnull mice lack functional leucocytes, macrophages, dendritic cells, natural killer cells and lymphocytes. They also display no detectable activity of haemolytic complement. The mice exhibit deficiencies in cytokine signalling due to the lack of the common gamma chain (γc) of the IL-2 receptor [18]. This receptor subunit is described as important, if not essential, for the binding and signalling of various interleukins, including IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 [26, 27], which also partially play a role in bone homeostasis [28, 29].
Because—to our best knowledge—the bone phenotype was not previously described in detail, we first analysed the skeleton of 12-week-old NOD/scid-IL2Rγcnull mice. Gross inspection of the skeleton revealed no obvious abnormalities, indicating that bone development was not disturbed. Furthermore, immunodeficient mice developed only a mild bone phenotype. Structural parameters of the cortical bone were not significantly affected. In contrast, mechanical properties were increased, indicating alterations to the bone matrix that we could not detect by the applied methods. In the trabecular compartment, bone mass was slightly reduced in NOD/scid-IL2Rγcnull mice due to a diminished trabecular number; however, the existing bone trabeculae were thicker. Bone cell numbers were unaltered in vivo; however, osteoblasts isolated from immunodeficient mice and cultivated ex vivo displayed an increased differentiation capacity, whereas osteoclast resorption activity was reduced, indicating cell-autonomous defects. These results explain the increased trabecular thickness in immunodeficient mice. As stated above, cells of the hematopoietic lineage are affected by the defects of the NOD/scid-IL2Rγcnull mouse. As osteoclasts derive from the myeloid lineage, the disturbed osteoclast function observed ex vivo might be caused by the deletion of the γc-subunit of the IL-2 receptor, which affects the signalling of various interleukins [26, 27]. Cytokine signalling plays a central role in osteoclastogenesis and defects in this signalling pathway could cause osteoclast dysfunctions [30]. The increased differentiation capacity of osteoblasts is difficult to account for by a single underlying factor. Some of the cytokines affected by the γcnull mutation also play a role in bone homeostasis [28, 29, 31]; however, the impact on isolated osteoblasts is unclear. In conclusion, 12-weeks-old NOD/scid-IL2Rγcnull mice exhibit only a mild bone phenotype in comparison to Balb/c mice.
A possible limitation of our study is that we could not use immunocompetent wild-type mice with the same genetic background as the NOD/scid-IL2Rγcnull mice, because these mice were generated by intercrossing several mouse strains with different immune deficiencies [18]. Also, using the founder strains as controls was not possible, as they display abnormalities that NOD/scid-IL2Rγcnull mice do not display like wound healing disorders in the NOD/ShiLt mouse. So, as a compromise, we used Balb/c mice as an immunocompetent control because the spontaneous mutation for scid (severe combined immune deficiency) in the Prkdc-locus (protein kinase, DNA-activated, catalytic polypeptide) originally occurred in this strain and thus the genetic background may have a high degree of similarity. However, because bone phenotype and healing characteristics in mice depend on the genetic background [32, 33], an influence on bone cell function cannot be completely excluded. To overcome this problem, one could think about a rescue-approach, where bone marrow of immunocompetent mice is transplanted into NOD/scid-IL2Rγcnull mice in order to generate a functional immune system. However, irradiation of the mice is necessary for such an approach, thus, other problems arise.
Our results demonstrated that fracture healing was impaired in immunodeficient NOD/scid-IL2Rγcnull mice. The callus bone content was unaffected on day 21, but it was significantly reduced in the later healing phase. The longer persistence of cartilage indicates delayed cartilage-to-bone transformation during endochondral ossification, most likely through reduced osteoclast activity. Impaired osteoclast activity is likely to arise from cell-autonomous defects, as suggested by our ex vivo experiments, or by the absence of functional immune cells, including T-lymphocytes, which support osteoclast activity by producing receptor activator of nuclear factor κ-B ligand [34]. Reduced osteoclast activity could also account for the increased callus size in NOD/scid-IL2Rγcnull mice, which indicates delayed callus remodelling. Osteoclasts are crucial for the degradation of hypertrophic cartilage and for callus remodelling in the later healing period, whereas periosteal primary bone formation during the earlier healing phase is osteoclast independent [35, 36]. Therefore, delayed fracture healing in NOD/scid-IL2Rγcnull mice was rather caused by impaired osteoclast function than disturbed osteoblast precursor cell recruitment and differentiation during the early healing period. Therefore, our results suggest that—under sterile, uncomplicated conditions—the immediate immune response after fracture is nonessential for the initiation of bone formation.
Because we aimed to study the general impact of the immune system on fracture healing, we used a mouse model of severe immune deficiency. Other authors investigated the impact of specific immune cell subsets on fracture healing using transgenic mouse models or antibody induced cell depletion [37, 38]. Thereby, the impact of immune cells, which are classically attributed to innate immunity and act primarily as first line of defence, is intensely discussed. Increased recruitment of neutrophils induced by granulocyte-colony stimulating factor was shown to accelerate bone formation [39]. In contrast, neutrophil depletion impaired fracture healing [40]. Therefore, it appears that a balanced activation of PMNs is necessary for regular bone regeneration [41]. Macrophages are generally considered to contribute to the resolution of inflammation and initiation of tissue repair through the clearance of apoptotic neutrophils and secretion of anti-inflammatory factors, including IL-10 and transforming growth factor-β [42]. In support of this, recent data suggest that bone-resident macrophages are necessary for the initiation of fracture repair and promote anabolic mechanisms during intramembranous and endochondral callus formation, as macrophage-depleted mice display delayed healing of bone injuries [9, 43, 44]. We, in contrast, found only minor changes in fracture healing in the macrophage-deficient NOD/scid-IL2Rγcnull mouse, possibly because the deficiencies in this model are much more complex.
The role of other innate immune cells, including natural killer cells and mast cells in bone healing remains unclear. Cells attributed to adaptive immunity are believed to play an important role mainly in later stages of fracture healing [10]. Bone healing was improved in lymphocyte-deficient, recombination activating gene-1 (RAG-1)-knockout mice, indicating a negative effect of these cells [37]. In agreement with this, depletion of CD8-positive cells improved fracture healing, whereas transplantation of CD8-positive cells resulted in delayed healing [38]. Furthermore, mice lacking functional B-lymphocytes displayed enhanced bone formation [45]. These studies suggest that lymphocytes may provoke negative effects on bone regeneration. However, the studies mentioned above investigated the effect of single cell types and did not consider the delicate interplay of cells and factors of the immune system.
In conclusion, this study described, for the first time, fracture healing in a mouse model with severe immune deficiency. Fracture healing was delayed due to impaired endochondral ossification. However, primary bone formation in the early healing stage was unaffected in the model used for uncomplicated fracture healing. Further studies are necessary to unravel the multifaceted interactions between immune cells and bone cells in fracture healing.
Acknowledgments
We thank Ursula Maile, Marion Tomo, Sevil Essig and Helga Bach for their excellent technical assistance. This study was supported by the 7th Framework Programme (FP7) of the European Commission through the REBORNE Project, grant no. 241879.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the 7th Framework Programme (FP7) of the European Commission through the REBORNE Project, grant no. 241879 and the German Research Foundation (DFG) in the framework of the Collaborative Research Center 1149 (CRC1149). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol. 2009;29(5):555–67. 10.1007/s10875-009-9316-6 [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi H. New immune connections in osteoclast formation. Annals of the New York Academy of Sciences. 2010;1192(1):117–23. 10.1111/j.1749-6632.2009.05303.x [DOI] [PubMed] [Google Scholar]
- 3.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nature Reviews Rheumatology. 2009;5(12):667–76. 10.1038/Nrrheum.2009.217 [DOI] [PubMed] [Google Scholar]
- 4.Danks L, Takayanagi H. Immunology and bone. Journal of biochemistry. 2013;154(1):29–39. 10.1093/jb/mvt049 [DOI] [PubMed] [Google Scholar]
- 5.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nature reviews Rheumatology. 2012;8(3):133–43. 10.1038/nrrheum.2012.1 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue engineering Part B, Reviews. 2015;21(4):354–64. 10.1089/ten.TEB.2014.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clinical orthopaedics and related research. 2011;469(11):3118–26. 10.1007/s11999-011-1865-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt-Bleek K, Schell H, Kolar P, Pfaff M, Perka C, Buttgereit F, et al. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: a study in sheep. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27(9):1147–51. 10.1002/jor.20901 [DOI] [PubMed] [Google Scholar]
- 9.Wu AC, Raggatt LJ, Alexander KA, Pettit AR. Unraveling macrophage contributions to bone repair. BoneKEy reports. 2013;2 10.1038/bonekey.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konnecke I, Serra A, El Khassawna T, Schlundt C, Schell H, Hauser A, et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone. 2014;64:155–65. 10.1016/j.bone.2014.03.052 [DOI] [PubMed] [Google Scholar]
- 11.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. Journal of cellular biochemistry. 2003;88(5):873–84. 10.1002/jcb.10435 [DOI] [PubMed] [Google Scholar]
- 12.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, et al. The early fracture hematoma and its potential role in fracture healing. Tissue engineering Part B, Reviews. 2010;16(4):427–34. 10.1089/ten.TEB.2009.0687 [DOI] [PubMed] [Google Scholar]
- 13.Bhandari M, Tornetta P 3rd, Sprague S, Najibi S, Petrisor B, Griffith L, et al. Predictors of reoperation following operative management of fractures of the tibial shaft. Journal of orthopaedic trauma. 2003;17(5):353–61. [DOI] [PubMed] [Google Scholar]
- 14.Bunn RJ, Burke G, Connelly C, Li G, Marsh D. Inflammation—A double edged sword in high-energy fractures? The Journal of bone and joint surgery British volume. 2005;87-B(SUPP_III):265-c-6. [Google Scholar]
- 15.Karladani AH, Granhed H, Karrholm J, Styf J. The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch Orthop Trauma Surg. 2001;121(6):325–8. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez RK, Do TP, Critchlow CW, Dent RE, Jick SS. Patient-related risk factors for fracture-healing complications in the United Kingdom General Practice Research Database. Acta Orthop. 2012;83(6):653–60. 10.3109/17453674.2012.747054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niikura T, Lee SY, Sakai Y, Nishida K, Kuroda R, Kurosaka M. Causative factors of fracture nonunion: the proportions of mechanical, biological, patient-dependent, and patient-independent factors. J Orthop Sci. 2014;19(1):120–4. 10.1007/s00776-013-0472-4 [DOI] [PubMed] [Google Scholar]
- 18.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human Lymphoid and Myeloid Cell Development in NOD/LtSz-scid IL2R{gamma}null Mice Engrafted with Mobilized Human Hemopoietic Stem Cells. Journal of immunology. 2005;174(10):6477–89. [DOI] [PubMed] [Google Scholar]
- 19.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. IMR203 [pii] 10.1111/j.0105-2896.2004.00203.x [DOI] [PubMed] [Google Scholar]
- 20.Röntgen V, Blakytny R, Matthys R, Landauer M, Wehner T, Gockelmann M, et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2010;28(11):1456–62. 10.1002/jor.21148 [DOI] [PubMed] [Google Scholar]
- 21.Heilmann A, Schinke T, Bindl R, Wehner T, Rapp A, Haffner-Luntzer M, et al. Systemic treatment with the sphingosine-1-phosphate analog FTY720 does not improve fracture healing in mice. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31(11):1845–50. 10.1002/jor.22426 [DOI] [PubMed] [Google Scholar]
- 22.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(7):1468–86. 10.1002/jbmr.141 [DOI] [PubMed] [Google Scholar]
- 23.Parfitt. Bone Histomorphometry. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1987;2(6):595–610. [DOI] [PubMed] [Google Scholar]
- 24.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(1):2–17. 10.1002/jbmr.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma cnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–82. 10.1182/blood-2001-12-0207 [DOI] [PubMed] [Google Scholar]
- 26.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. 10.1146/annurev.immunol.14.1.179 [DOI] [PubMed] [Google Scholar]
- 27.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. Journal of immunology. 2001;167(1):1–5. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: cytokines and the skeletal system. BMB Rep. 2008;41(7):495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djaafar S, Pierroz DD, Chicheportiche R, Zheng XX, Ferrari SL, Ferrari-Lacraz S. Inhibition of T cell-dependent and RANKL-dependent osteoclastogenic processes associated with high levels of bone mass in interleukin-15 receptor-deficient mice. Arthritis Rheum. 2010;62(11):3300–10. 10.1002/art.27645 [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Kim HH. The Role of Jak/STAT Pathways in Osteoclast Differentiation. Biomol Ther. 2011;19(2):141–8. 10.4062/Biomolther.2011.19.2.141 [DOI] [Google Scholar]
- 31.Mori G, D'Amelio P, Faccio R, Brunetti G. The Interplay between the bone and the immune system. Clinical & developmental immunology. 2013;2013:720504 10.1155/2013/720504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Gu W, Masinde G, Hamilton-Ulland M, Rundle CH, Mohan S, et al. Genetic variation in bone-regenerative capacity among inbred strains of mice. Bone. 2001;29(2):134–40. [DOI] [PubMed] [Google Scholar]
- 33.Manigrasso MB, O'Connor JP. Comparison of fracture healing among different inbred mouse strains. Calcified tissue international. 2008;82(6):465–74. 10.1007/s00223-008-9144-3 [DOI] [PubMed] [Google Scholar]
- 34.Pacifici R. T cells: critical bone regulators in health and disease. Bone. 2010;47(3):461–71. 10.1016/j.bone.2010.04.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19(5):459–66. 10.1016/j.semcdb.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 36.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5. 10.1016/j.injury.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, et al. Fracture healing is accelerated in the absence of the adaptive immune system. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(1):113–24. 10.1002/jbmr.185 [DOI] [PubMed] [Google Scholar]
- 38.Reinke S, Geissler S, Taylor WR, Schmidt-Bleek K, Juelke K, Schwachmeyer V, et al. Terminally Differentiated CD8(+) T Cells Negatively Affect Bone Regeneration in Humans. Science translational medicine. 2013;5(177):177ra36 10.1126/scitranslmed.3004754 [DOI] [PubMed] [Google Scholar]
- 39.Bozlar M, Aslan B, Kalaci A, Baktiroglu L, Yanat AN, Tasci A. Effects of human granulocyte-colony stimulating factor on fracture healing in rats. Saudi Med J. 2005;26(8):1250–4. [PubMed] [Google Scholar]
- 40.Chan JK, Glass GE, Ersek A, Freidin A, Williams GA, Gowers K, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO molecular medicine. 2015;7(5):547–61. 10.15252/emmm.201404487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41(4):457–65. [PMC free article] [PubMed] [Google Scholar]
- 42.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO molecular medicine. 2013;5(5):661–74. 10.1002/emmm.201202382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(7):1517–32. 10.1002/jbmr.354 [DOI] [PubMed] [Google Scholar]
- 44.Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. The American journal of pathology. 2014;184(12):3192–204. 10.1016/j.ajpath.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 45.Marusic A, Grcevic D, Katavic V, Kovacic N, Lukic IK, Kalajzic I, et al. Role of B lymphocytes in new bone formation. Lab Invest. 2000;80(11):1761–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.