SUMMARY
Objective
To assess long-term direct medical costs, health care utilization, and mortality following resective surgery in persons with uncontrolled epilepsy.
Methods
Retrospective longitudinal cohort study of Medicaid beneficiaries with epilepsy from 2000 - 2008. The study population included 7,835 persons with uncontrolled focal epilepsy age 18 to 64 years, with an average follow-up time of 5 years. Of these, 135 received surgery during the study period. To account for selection bias, we used risk-set optimal pairwise matching on a time-varying propensity score, and inverse probability of treatment weighting. Repeated measures generalized linear models were used to model utilization and cost outcomes. Cox proportional hazard was used to model survival.
Results
The mean direct medical cost difference between the surgical group and control group was $6,806 after risk-set matching. The incidence rate ratio of inpatient, emergency room, and outpatient utilization was lower among the surgical group in both unadjusted and adjusted analyses. There was no significant difference in mortality after adjustment. Among surgical cases, mean annual costs per subject were on average $6,484 lower, and all utilization measures were lower after surgery compared to before.
Significance
Subjects that underwent epilepsy surgery had lower direct medical care costs and health care utilization. These findings support that epilepsy surgery yield substantial health care cost savings.
Keywords: Economic, Medicaid, Comparative Effectiveness, Lobectomy, Refractory
INTRODUCTION
Direct health care costs decline after successful epilepsy surgery in the short-term, but the long-term impact on costs and utilization is unknown.1 Studies that have found epilepsy surgery to be cost-effective have mostly relied on extrapolating short-term outcomes and cost data.2,3 A more recent study with a long follow-up period after surgery found that about half of patients undergoing surgery remain seizure free after five years, and a review of long-term outcome studies has shown similar results.4,5 Since late seizure recurrence is not uncommon, post-surgical health care costs that are based on short-term data might be underestimated.
Our objective was to compare long-term health care expenditures, utilization, and mortality in those receiving resective surgical treatment for uncontrolled epilepsy versus non-surgical controls. We used a longitudinal observational cohort of Medicaid enrollees with an average follow-up period of up to 5 years. We hypothesized that epilepsy surgery leads to both short-term and long-term declines in health care expenditures, utilization, and mortality compared to continued medical treatment.
METHODS
Study Design and Data Source
This study is a retrospective longitudinal cohort study using administrative claims data. The Case Western Reserve University Institutional Review Board and the Ohio Department of Medicaid approved the study protocol. We used Ohio Medicaid claims and eligibility files from 2000 – 2008. The Medicaid data were linked to a database of Ohio death certificates covering the same period using a deterministic algorithm described in previous studies, which uses a combination of social security number, first and last name, date of birth, and gender.6
Study Population
The study population consists of adults age 18 - 64 enrolled in Ohio Medicaid. The eligibility criteria for Medicaid are complex and can change over time. Briefly, the most common way that an adult with epilepsy was Medicaid-eligible in Ohio during the study period (2000 – 2008) was to have a qualifying low household income and either: (1) be a parent or caretaker of children under age 19 and/or be pregnant or (2) have a disability according to the Social Security Administration criteria (epilepsy is one of the qualifying disabilities depending on severity).7 Most of our study population (64.5%) qualified under the second criteria at baseline.
People with epilepsy were identified through ICD-9-CM diagnosis codes and prescription drug claims. The data from 2000 to 2001 were used strictly for “look back” to obtain baseline measures. Inclusion criteria were (1) one epilepsy code (345.xx) or two seizure codes (780.39) greater than 30 days apart, and (2) at least one antiepileptic drug (AED) prescription claim after meeting diagnosis criteria or within 30 days prior.8 The date both criteria were first met was considered the epilepsy index date. Subjects with <12 months of enrollment prior to the epilepsy index date were not included. The follow-up period was the number of months enrolled in Medicaid from epilepsy index date. Additionally we required subjects to have at least one ICD-9-CM code indicating focal epilepsy (345.4x or 345.5x).
Subjects were then classified into 3 groups: (1) uncontrolled epilepsy, (2) active epilepsy, and (3) stable epilepsy using similar algorithm published in previous study.9 Briefly, those with at least one emergency room (ER) or inpatient visit with a primary diagnosis code of epilepsy or seizure, and at least two AED switches in one year were categorized as uncontrolled.9 Those without an ER or inpatient visit and no AED switches were defined as stable.9 All others were assigned to active epilepsy. Because very few subjects in the stable group ever had surgery (<10) in the study period we decided to only include those with uncontrolled or active epilepsy. Finally, we excluded subjects that had a surgery claim before the study period (n=34) and people that were living in a nursing home (n=969). Our final cohort included 7,835 persons, of whom 135 received surgical treatment. A flow diagram depicting the inclusion and exclusion criteria has been published previously.10
Surgical Treatment
The main exposure was resective epilepsy surgery, which includes temporal lobectomy, extratemporal lobectomy, and amygdalohippocampectomy. Surgical treatment was defined by the presence of an ICD-9-CM procedure code for brain lobectomy (01.53) or other excision of the brain including partial lobectomy (01.59) in institutional claims11, or by CPT codes for temporal lobectomy (61537, 61538), extratemporal lobectomy (61539, 61540), or amygdalohippocampectomy (61566) in professional claims, with a simultaneous presence of a primary diagnosis code of epilepsy on the same record.
Outcomes
The primary outcomes were direct health care costs, health care utilization, and mortality, each measured quarterly. Costs were defined as Medicaid expenditures for all health services except prescription drugs in US dollars. These costs were also sub-classified into inpatient, ER, and outpatient costs. Indirect costs (e.g. lost work productivity) were not evaluated in this study. Utilization measures are counts of inpatient stays, ER visits, and outpatient visits. Costs and utilization related to the surgery itself and procedures performed in the three months prior to surgery such as video-EEG monitoring were excluded from comparative analysis. As a sensitivity analysis we also tried excluding costs six months and one year prior to surgery, but this did not change the overall results in any meaningful way. Cost and utilization was measured at the per-member per-quarter level for the time each subject was enrolled in Medicaid. Mortality was measured using Ohio death certificate claims linked to Medicaid enrollment.
Baseline Covariates
All demographic and insurance characteristics were measured at baseline. These included age, gender, race, marital status, distance to the nearest epilepsy centers, dual-Medicare eligibility, enrollment in an HMO or fee-for service plan, and Medicaid aid category. Medicaid aid category was grouped into aged, blind, and disabled (ABD), aid to income-eligible families and children, and other. Distance to nearest epilepsy center was the minimum value obtained by calculating the straight-line distance between each subject's home zip code and each Ohio epilepsy center.
Time-varying covariates
Comorbidities, AEDs, and diagnostic procedures were measured as time-varying covariates in each quarter through claims data. We also measured specific AEDs based on whether or not a prescription was filled in each quarter. We measured 34 comorbidities using algorithms based on ICD-9-CM codes published on epilepsy-specific risk adjustment.12 A scoring algorithm was used to also combine comorbidities into a single composite score.12 We used CPT codes and ICD-9-CM procedure codes to flag whether a subject received an EEG, video-EEG monitoring, or brain MRI. Finally, we measured total health care costs in the previous 4 quarters of enrollment to form a log-transformed moving average per quarter. A complete list of AEDs, comorbidities, and all other covariates, and their operational definitions are included in the appendix (Supplemental table e-1).
Propensity Score Matching and Weighting
The people that undergo epilepsy surgery may be different than those who do not receive surgery on a number of characteristics that could confound the results. To control for selection bias, we used two types of propensity score adjustment on known variables. The first was risk-set optimal matching,13,14 which permits us to make use of our longitudinal data on time-varying covariates. In risk-set matching, a patient receiving surgery at time t is matched to a “control” who has not yet received surgery as of time t (but may in the future), based only on the (potentially time-varying) characteristics of these up to time t. All information occurring after time t is ignored in identifying matched sets. To obtain time-varying propensity scores, a Cox proportional hazards model was fit using surgical treatment as the dependent variable14 and including both covariates measured only at baseline (demographic, insurance and epilepsy characteristics) and time-varying covariates (comorbidities, AED utilization, EEG history, and health care costs in previous quarters). The Cox model yields a predicted hazard for each patient at each time point. This hazard becomes the time-varying propensity score and is used to match a treated subject to a control in the same risk set using optimal pairwise matching without replacement with a caliper for acceptable matches of one-tenth of the standard deviation of the linear propensity score to ensure close balance on the propensity scores. An advantage of this method is that it accounts for the possibility that controls are just patients that have not had surgery yet, but may have surgery at some later time point. In other words, subjects chosen as a control at time period t could also become a case at a later time period. We used the %VMATCH macro for SAS to calculate the distance matrix and perform the matching algorithm.15
Risk-set matching, using time-varying covariates, is a novel approach that is suitable for epilepsy because seizure control, type of AEDs, and multimorbidity in persons with epilepsy often change over time. The risk-set matching approach adjusts for these changes and allows the matching of a surgery case to a control at the same time point adjusting for all confounders up that time point.14
Because risk-set matching is an innovative method, we also used a more conventional propensity score adjustment approach, inverse probability of treatment weighting (IPTW). The average treatment effect among the treated (ATT) was used so that a surgical patient was assigned a weight of 1, and a control was assigned a weight equal to PS/(1-PS), where PS is the propensity score.16 The propensity score was calculated using a logistic regression model with the same covariates as described in risk-set matching.
After matching and IPTW, we assessed the covariate balance using plots of standardized differences,17 and a set of rules proposed by Rubin which assess the appropriateness of regression-style models for the adjusted population comparisons.18 Slightly different specifications of the propensity score models and calipers for acceptable matches were tried until all three of Rubin's rules were satisfied, before moving on to adjusted outcome analyses.
Statistical Analysis
We tested differences between the surgical group and controls for each categorical (chi-square) and continuous (t-tests) variable. The differences in means or proportions between surgical and non-surgical groups for all outcomes were calculated. We also compared the difference in mean costs and utilization before and after surgery within the surgical cases only. To model the effect of surgery on outcomes, we used repeated measures generalized linear regression models with the identity link and normal distribution to model log-transformed costs and negative binomial distribution and log link to model utilization. The generalized estimating equations (GEE) approach was used in all models for parameter estimation to account for within-subject correlation due to repeated measures. Unadjusted and IPTW models included all data points, whereas risk-set matching models used only the data points that occurred after the point of matching among cases and matched controls – a feature of this method. We also fit GEE models with group-time interactions to compare estimated mean cost over time between the surgical group and matched controls. We used Cox proportional hazards models to estimate the effect of surgery on the risk of mortality. SAS version 9.3 for Unix was used for statistical analysis.
RESULTS
Among 7,835 subjects included in our study, 135 had epilepsy surgery during the observed study period. The average follow-up time was 5 years, with a minimum of 0.25 years, and a maximum of 7 years. There were significant differences between the surgical and non-surgical group on most variables (Table 1). The surgical group was younger (mean age 31.5 years old vs. 37.5) and a lower percentage of the surgical group was female (46% vs 59%) compared to the non-surgical group. Racial minorities were underrepresented in the surgical group (14% vs 23%) compared to the non-surgical group. The surgical group had fewer comorbidities than the non-surgical group overall (mean of 0.5 vs 1.4), and in each measured individual comorbidity. The average count of AED medications at baseline was similar in both groups, but some of the newer generation drugs - lamatrigine, levetiracetam, and oxcarbazepine, and zonisamide - were more commonly prescribed in the surgical group. The percentage of persons classified as having uncontrolled seizures was higher in the surgery group (67% vs 45%).
Table 1.
Baseline characteristics of study population
| Non-surgical group | Surgical group | p-value | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Number of Subjects | 7700 | 100 | 135 | 100 | |
| Male | 3,124 | 40.6 | 72 | 53.3 | 0.003 |
| Race | 0.03 | ||||
| White | 5,898 | 76.6 | 116 | 85.9 | |
| Black or other race | 1,802 | 23.4 | 19 | 14.1 | |
| Seizure Control | <0.001 | ||||
| Uncontrolled | 3,444 | 44.7 | 90 | 66.7 | |
| Active | 4,256 | 55.3 | 45 | 33.3 | |
| Marital status | 0.22 | ||||
| Single / Other | 4,685 | 60.8 | 92 | 68.1 | |
| Married | 1,159 | 15.1 | 17 | 12.6 | |
| Divorced/Separated | 1,856 | 24.1 | 26 | 19.3 | |
| Medicare eligible | 1,763 | 22.9 | 17 | 12.6 | 0.005 |
| Fee-for-service | 7,322 | 95.1 | 133 | 98.5 | 0.07 |
| Aid Category | <0.001 | ||||
| Aged, Blind, Disabled | 4,991 | 64.8 | 65 | 48.1 | |
| Aid to Families | 1,607 | 20.9 | 30 | 22.2 | |
| Other | 1,102 | 14.3 | 40 | 29.6 | |
| Antiepileptic drug use | |||||
| Barbiturates | 617 | 8.0 | <10 | *** | 0.37 |
| Benzodiazepines | 1445 | 18.8 | 14 | 10.4 | 0.01 |
| Carbamazepine | 1698 | 22.1 | 35 | 25.9 | 0.28 |
| Gabapentin | 1058 | 13.7 | 17 | 12.6 | 0.70 |
| Lamatrigine | 784 | 10.2 | 32 | 23.7 | <0.001 |
| Levetiracetam | 891 | 11.6 | 36 | 26.7 | <0.001 |
| Oxcarbazepine | 501 | 6.5 | 16 | 11.9 | 0.01 |
| Phenytoin | 2045 | 26.6 | 30 | 22.2 | 0.26 |
| Topiramate | 745 | 9.7 | 19 | 14.1 | 0.09 |
| Valproic Acid | 1423 | 18.5 | 17 | 12.6 | 0.08 |
| Zonisamide | 239 | 3.1 | 10 | 7.4 | 0.005 |
| Other AEDs | 244 | 3.2 | <10 | *** | 0.19 |
| Selected Comorbidities | |||||
| Alcoholism | 647 | 8.4 | <10 | *** | 0.10 |
| Brain/Head Injury | 921 | 12.0 | <10 | *** | 0.02 |
| Cardiac Arrhythmia | 945 | 12.3 | 10 | 7.4 | 0.09 |
| Cerebrovascular Disease | 1393 | 18.1 | 13 | 9.6 | 0.01 |
| COPD | 2011 | 26.1 | 12 | 8.9 | <0.001 |
| Depression | 2719 | 35.3 | 38 | 28.1 | 0.08 |
| Diabetes, uncomplicated | 1016 | 13.2 | <10 | *** | 0.003 |
| Drug Abuse | 703 | 9.1 | <10 | *** | 0.20 |
| Encephalopathy | 333 | 4.3 | <10 | *** | 0.73 |
| Fracture | 934 | 12.1 | <10 | *** | 0.003 |
| Hypertension | 2054 | 26.7 | 10 | 7.4 | <0.001 |
| Paraplegic | 561 | 7.3 | <10 | *** | 0.11 |
| Psychotic Disorders | 1024 | 13.3 | <10 | *** | 0.006 |
| Continuous variables | Mean | SD | Mean | SD | p-value |
| Age | 37.5 | 12.2 | 31.5 | 9.6 | <0.001 |
| Distance to Epilepsy Centers | 31.2 | 26.9 | 38.9 | 27.8 | 0.001 |
| Epilepsy specific comorbidity Index | 1.4 | 2.3 | 0.5 | 1.5 | <0.001 |
| Previous year costs (log) | 6.1 | 3.2 | 3.9 | 3.5 | <0.001 |
| Follow up time (qtrs) | 20.0 | 7.8 | 19.9 | 7.0 | 0.88 |
| Count of concurrent AED medications | 1.3 | 1.1 | 1.5 | 1.4 | 0.180 |
Statistical tests were Fisher's exact test for categorical variables and t-test for continuous variables. Only comorbidities with at least five cases in both groups are shown.
The exact frequency of cells with less than 10 subjects are masked for patient confidentiality.
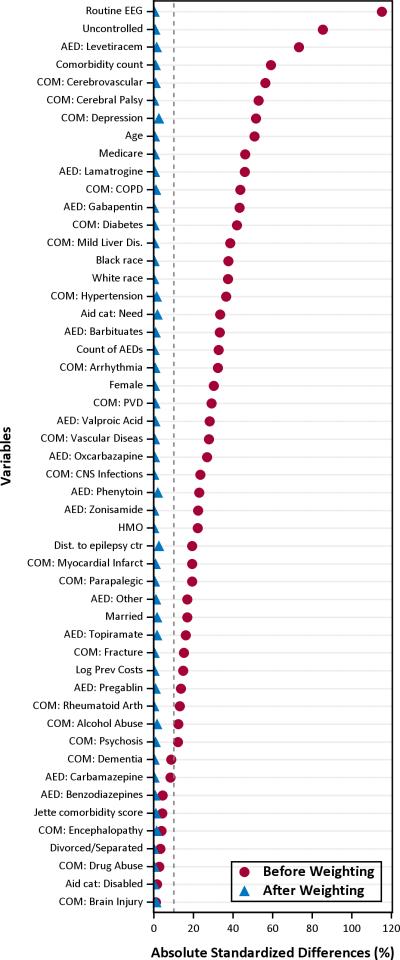
After inverse probability of treatment weighting, all variables had standardized mean differences less than 10% (Figure 1). The risk-set optimal pairwise matching approach using a time-varying propensity score yielded 131 matched pairs. Seven subjects that were chosen as controls later became surgical cases. After matching, the absolute standardized mean differences for all variables used in the propensity score model were less than 20% difference in all cases. There was no statistically significant difference for any measured variable in both weighted and risk-set matching analyses.
Figure 1. Love plot of absolute standardized differences before and after inverse probability of treatment weighting.
The red dots show the absolute standardize differences of each covariate between the surgery and control groups before weighting (i.e. unadjusted), and the blue triangles are the differences after propensity score inverse probability of treatment weighting. Values within the dotted grey line and the vertical axis represent less a 10% standardized difference between groups and are negligible.
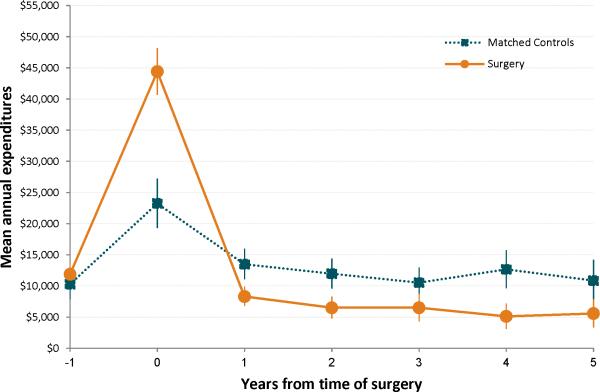
The mean differences in annual health care costs and utilization between cases and controls are shown in Table 2. Total costs and category-specific (inpatient, outpatient, and ER) costs and utilization were lower in the unadjusted and all adjusted analyses. The risk-set matching yielded the most conservative differences, but these were still significant for all but the inpatient costs and utilization. The more conservative differences are possibly due to loss of power as most of the controls and some of the cases are dropped from the analysis after matching. The adjusted analysis suggests that persons who receive surgery incur an average of $6,806 - $13,454 less per year after the surgery compared to control. Using a repeated measures GEE model with group time interaction, the estimated average costs per year was lower in the surgical group compared to the propensity score matched control group in each of the first five years following surgery (Figure 2). Surgery patients also had 0.58 – 1.19 fewer ER visits and 0.92 – 2.64 fewer outpatient visits per year (Table 2). In weighted analyses, mortality is higher among the control group (10% vs. 4%, p=0.018), but there is no significant difference in the matched analysis (5% vs 4%, p=0.734).
Table 2.
Mean difference in outcomes between surgical group and non-surgical group
| Unadjusted Mean/year (±95% CI) | Risk-set propensity score optimal matching Mean/year (±95% CI) | Inverse probability of treatment weighting Mean/year (±95% CI) | |
|---|---|---|---|
| Utilization (# of stays/visits) | |||
| Inpatient stays | 0.37 ±0.12 | 0.06 ±0.10 | 0.29 ±0.02 |
| Outpatient visits | 2.87 ±0.69 | 0.92 ±0.61 | 2.18 ±0.10 |
| Emergency room visits | 1.06 ±0.33 | 0.58 ±0.35 | 0.86 ±0.06 |
| Costs (US Dollars) | |||
| All direct medical costs | $19,096 ±3,800 | $6,806 ±1,925 | $13,454 ±519 |
| Inpatient costs | $4,748 ±3,075 | $343 ±966 | $5,502 ±441 |
| Outpatient costs | $1,461 ±560 | $862 ±557 | $1,449 ±78 |
| Emergency room costs | $133 ±35 | $77 ±33 | $111 ±4 |
Each cell shows the mean difference (X̄non–surgery – X̄surgery) and 95% confidence interval between controls and the surgical group. All bolded differences were significant at the p<0.05 level using t-test for unadjusted and weighted analyses, and paired t-test for matched pairs.
Figure 2. Comparison of Mean Annual Expenditures over Time between Surgery and Propensity Score Matched Controls.
The estimated mean annual expenditures and standard error bars are shown at each time point from the time of surgery (or at the time of matching for controls). Estimates derived from a repeated measures generalized estimating equation model with group x time interaction effects modeled. This analysis includes the surgery and pre-surgical costs at year 0.
Comparisons of before and after surgery (among surgical group only) also show significant cost and utilization reductions (Table 3). After surgery, subjects had $6,481 (95%CI: ±2,241) reduction in annual costs, 0.58 (±0.21) fewer inpatient visits, 2.93 (±0.84) fewer outpatient visits, and 1.03 (±0.42) fewer ER visits per year, compared to before surgery.
Table 3.
Costs and utilization before and after surgery among the cases only
| Before Surgery | After Surgery | Difference | p-value | |
|---|---|---|---|---|
| Mean/year (±95% CI) | Mean/year (±95% CI) | |||
| Utilization (# of stays/visits) | ||||
| Inpatient stays | 0.92 ±0.20 | 0.34 ±0.08 | 0.58 | <0.001 |
| Outpatient visits | 8.03 ±0.80 | 5.10 ±0.39 | 2.93 | <0.001 |
| Emergency room visits | 2.84 ±0.35 | 1.81 ±0.22 | 1.03 | <0.001 |
| Costs (US Dollars) | ||||
| All direct medical costs | 13,989 ±1,913 | 7,508 ±1,166 | $6,481 | <0.001 |
| Inpatient costs | 7,212 ±1,554 | 1,695 ±702 | $5,517 | <0.001 |
| Outpatient costs | 3,066 ±504 | 1,070 ±259 | $1,995 | <0.001 |
| Emergency room costs | 226 ±37 | 61 ±14 | $164 | <0.001 |
Differences in utilization and costs within the surgical group (n=135) before and after the procedure. Utilization is measured as a count of number of visits, and costs are measured as the mean expenditures in dollars by Medicaid per subject. Statistical testing was done using paired t-tests.
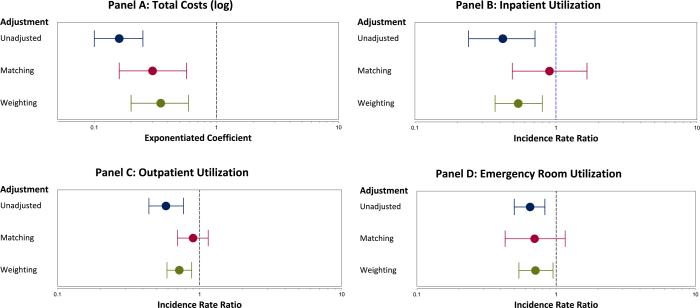
The effect of surgery on costs in the repeated measures linear GEE model is shown in Figure 3. Surgery is associated with nearly 3 times lower costs compared to the non-surgical group in both weighted and matched analysis (Figure 3: Panel A). Surgery was also associated with a significantly lower incidence rate ratio of inpatient, outpatient, and ER visit in the unadjusted and weighted analysis, but this was not significant in the risk-set matching analysis (Figure 3: Panels B,C,D). There were no significant differences in the hazard ratio (HR) of surgery vs. control for mortality outcomes after matching (HR: 0.54, 95%CI: 0.23, 1.31) or weighting (HR: 1.54, 95%CI: 0.37, 6.45), likely due to a small number of deaths in our cohort.
Figure 3. Effectiveness of Surgery on Cost and Utilization Outcomes after Propensity Score Adjustment.
Each panel shows the unadjusted, propensity score matched, and inverse probability of treatment weighted comparison of surgery vs. control on selected outcomes. Panel A shows the effect of surgery on total costs from a repeated measures generalized linear model. Panels B, C, and D show the effect of surgery on the number of inpatient visits, outpatient visits, and emergency room visits, respectively, from a repeated measures negative binomial regression model.
DISCUSSION
We found strong evidence that health care expenditures decrease after epilepsy surgery. Although surgery and the pre-surgical evaluation are expensive, our results suggest that Medicaid incurs $6,800 - $13,500 less per patient per year, which is cost saving in the long run. Previous cost-effectiveness analyses of epilepsy surgery were based on extrapolating short-term results, or were based on following a specific group of patients from a clinical trial, cohort study, or a particular health system.2,3,19 Our study uses actual cost data from a payer's claims, which also accounts for costs incurred due to other acute or chronic conditions besides epilepsy. A recent systematic review of direct epilepsy costs in the United States showed that epilepsy-specific costs are just a fraction of the total health expenditures for people with epilepsy, indicating the importance of using all-cause total costs.20 This same systematic review demonstrated that those with uncontrolled or refractory epilepsy have markedly higher direct health care costs compared to those with controlled or stable epilepsy, further highlighting the importance of long-term cost-savings in this population.
Our study also showed that inpatient, outpatient, and ER utilization decreases following epilepsy surgery. Lower utilization post-surgery is likely due to reduced seizures or seizure freedom. It is possible that other comorbid conditions may also improve after surgery, which can also contribute to lower health care utilization, but additional studies are needed to investigate this. In other studies, increased seizure frequency is associated with increased utilization of health services, particularly ER visits and hospitalizations.9,21 Epilepsy patients who utilize health services have also been shown to have poorer overall quality of life.22
Epilepsy surgery has been shown to be a cost-effective procedure from a societal perspective,2,3 but another interesting question is whether the surgery is actually cost-saving (the savings exceed the initial cost of treatment) to an insurer. A previous cost analysis estimated that the direct costs of medical management would exceed surgery after 14.4 years.23 When indirect costs were also considered, surgery became cost-saving at 7.3 years. Their model was based on 70% seizure freedom, which is higher than the 50% shown in recent long-term follow up studies. Two separate cost-effectiveness studies from 1997 estimated the cost of surgery at $16,000 (King) and $28,000 (Langfitt), and currently the Epilepsy Foundation estimates the total cost of surgery to be $50,000-$200,000 depending on the tests required.3,19,24 The mean total costs on the day of surgery in our study population was $24,039 and an additional $2,934 in the 15 day period before and after surgery. Using our most conservative estimate of $6800 lower cost per year after surgery, this would be cost-saving to Medicaid after just 4 years. Because we did not include prescription drugs in our cost calculations, the actual cost difference could be greater if there are long-term reductions in AED use and dosage after surgery.
Since cost and utilization of health care services can be influenced by many things, it is possible that some important confounder is missing from our model, even after adjustment for certain comorbidities and prior health expenditures. In addition, the primary purpose of surgery is to control seizures, and costs and utilization are really a secondary outcome influenced by the success of the primary outcome. Because of this, there are more possibilities for confounders to influence costs and utilization, compared to a study looking at the effect of treatment on a more direct clinical outcome.
One of the purposes of propensity score methods is to move beyond measures of association and to make causal inferences. Some of the Bradford Hill criteria can be used to evaluate the evidence that epilepsy surgery leads to reduced health care costs and utilization.25 The criteria for temporality and plausibility seem to be clearly met, as we know exactly when a surgery took place, and it is conceivable that persons whose seizures are cured will use less health services. Our results are consistent with previous experimental and observational studies, although this is the first study to look at the impact of surgery on costs from a payer perspective using a longer follow-up period.1,23 The strength of association for reduced costs was high in all adjustment methods. The effect of surgery on reducing utilization was high in all cases except risk-set matching which showed no significant effect.
The differences in terms of demographics, comorbidity, and seizure control between the surgical group and nonsurgical controls indicated a need to control for selection bias. We demonstrated two different approaches – risk-set matching and weighting. Risk-set matching using time-varying covariates is a novel approach, but one that may work well for studies involving epilepsy because seizure control, type of anticonvulsant medication, and multimorbidity can change often in epilepsy patients over time. The direction of the effect of surgery was consistent for all outcomes with both matching and weighting. However, the magnitudes varied, with the risk-set matching producing more conservative estimates. A possible explanation is that in weighting and unadjusted analysis all data points are used for all subjects, whereas in risk-set matching only the data points for the surgery cases and the matched controls are used, and only after the time period where the two are matched. Therefore the weighted and unadjusted analyses may have more noise in the data from costs occurring before surgery and from controls that are too dissimilar from surgery patients. It is also for this reason that we feel the results from risk-set matching provide the best head-to-head comparison between surgical cases and controls. When we included the before-match data points in the regression models for risk-set matching as a sensitivity analysis, the magnitude of effect was more similar to the weighted analysis. It is also important to note that matching necessarily leads to a reduced sample size that could also partially explain the more conservative differences.
There are several limitations that are common in studies using administrative databases including the accuracy of billing codes. Validation studies have shown a high degree of specificity and sensitivity in defining epilepsy cases.26–28 However, there are few, if any studies, that have developed and validated algorithms in administrative data to sub-classify epilepsy patients by seizure control, seizure localization, or specific syndrome. Likewise, several studies have used billing codes to identify cases of epilepsy surgery, but the accuracy of these codes has not been validated.11,29,30 It is also important to note that the aforementioned validation studies were not conducted using Medicaid data, and the accuracy of using ICD-9-CM diagnosis codes to define cases of epilepsy may differ between Medicaid and other sources. Filling these knowledge gaps is important as the use of administrative data in epilepsy research will likely continue to grow in this “big data” era.
More detailed clinical information would have been useful in the calculation of propensity scores, as we were limited to data available in claims data. Also, test results from MRI and EEG may have been useful to exclude people shown to not be candidates for surgery. Although we used an epilepsy-specific comorbidity index, this index was developed as a risk-adjustment for mortality, and may not be comprehensive when the outcomes of interest are utilization and cost. We attempt to control for this by incorporating a moving average of previous health care costs. Additional research is needed to determine which conditions should be adjusted for in cost and utilization analyses of epilepsy. The generalizability of the findings could be limited since the Medicaid population is different from the general population, and Medicaid programs vary across states in terms of services covered, reimbursement rates, and eligibility criteria. Because Medicaid reimburses health care services at a substantially lower rate than most private insurers in the U.S., the direct cost differences between the surgical and non-surgical group might be even greater in people with private insurance. We however show that for the sickest group of epilepsy patients in a low socioeconomic status population, surgery helps lower the subsequent direct cost of health care.
Supplementary Material
KEY POINT BOX.
Health care costs decline in the long-term after epilepsy surgery
Subjects that underwent epilepsy surgery had lower direct medical care costs and health care utilization in subsequent years compared to matched controls.
From a payer perspective, the initial cost of epilepsy surgery may be offset by future savings after just four years.
ACKNOWLEDGEMENTS
This publication was made possible, in part, by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 and KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Dr. Schiltz was also supported by the T32 Institutional Training Grant, #5T32HS000059-18 (PI: Alfred Rimm) from the Agency for Healthcare Research and Quality (AHRQ). The authors would like to thank Paul Bakaki, MBChB, PhD (Research Scientist; Case Western Reserve University), for his contributions in preparing the data for analysis, including the SAS code to identify the study cohort in the Medicaid claims data and defining some of the study variables.
Footnotes
DISCLOSURES AND CONFLICTS OF INTEREST:
None of the authors have any conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology. 2007;68:1290–1298. doi: 10.1212/01.wnl.0000259550.87773.3d. [DOI] [PubMed] [Google Scholar]
- 2.Choi H, Sell RL, Lenert L, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300:2497–2505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 3.King JT, Jr, Sperling MR, Justice AC, et al. A cost-effectiveness analysis of anterior temporal lobectomy for intractable temporal lobe epilepsy. J Neurosurg. 1997;87:20–28. doi: 10.3171/jns.1997.87.1.0020. [DOI] [PubMed] [Google Scholar]
- 4.De Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. The Lancet. 2011;378:1388–1395. doi: 10.1016/S0140-6736(11)60890-8. [DOI] [PubMed] [Google Scholar]
- 5.Téllez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 6.Koroukian SM, Beaird H, Duldner JE, et al. Analysis of injury- and violence-related fatalities in the Ohio Medicaid population: identifying opportunities for prevention. J Trauma. 2007;62:989–995. doi: 10.1097/01.ta.0000210359.98816.45. [DOI] [PubMed] [Google Scholar]
- 7.Ohio Department of Jobs and Family Services [September 22, 2015];Ohio Medicaid Fact Sheet. Undated. http://www.pickawayjfs.org/files/McaidPgm0306.pdf.
- 8.Kaiboriboon K, Bakaki PM, Lhatoo SD, et al. Incidence and prevalence of treated epilepsy among poor health and low-income Americans. Neurology. 2013;80:1942–9. doi: 10.1212/WNL.0b013e318293e1b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manjunath R, Paradis PE, Parisé H, et al. Burden of uncontrolled epilepsy in patients requiring an emergency room visit or hospitalization. Neurology. 2012;79:1908–1916. doi: 10.1212/WNL.0b013e318271f77e. [DOI] [PubMed] [Google Scholar]
- 10.Schiltz NK. Access to Care and Surgery Outcomes Among People with Epilepsy on Medicaid. 2013 http://rave.ohiolink.edu/etdc/view?acc_num=case1372678525.
- 11.Schiltz NK, Koroukian SM, Lhatoo SD, et al. Temporal trends in pre-surgical evaluations and epilepsy surgery in the U.S. from 1998 to 2009. Epilepsy Res. 2012;103:270–8. doi: 10.1016/j.eplepsyres.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Germaine-Smith C, Liu M, Quan H, et al. Development of an epilepsy-specific risk adjustment comorbidity index. Epilepsia. 2011;52:2161–2167. doi: 10.1111/j.1528-1167.2011.03292.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR. Design of Observational Studies. 2010th ed. Springer; 2009. [Google Scholar]
- 14.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61:721–728. doi: 10.1111/j.1541-0420.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 15.Bergstralh EJ, Kosanke JL, Jacobsen SJ. Software for optimal matching in observational studies. Epidemiology. 1996;7:331–332. [PubMed] [Google Scholar]
- 16.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love TE. Displaying Covariate Balance After Adjustment for Selection Bias. Presentation at Joint Statistical Meetings. 2002 http://www.chrp.org/love/JSM_Aug11_TLove.pdf.
- 18.Rubin DB. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Services & Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 19.Langfitt JT. Cost-effectiveness of anterotemporal lobectomy in medically intractable complex partial epilepsy. Epilepsia. 1997;38:154–163. doi: 10.1111/j.1528-1157.1997.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 20.Begley CE, Durgin TL. The direct cost of epilepsy in the United States: A systematic review of estimates. Epilepsia. 2015;56:1376–1387. doi: 10.1111/epi.13084. [DOI] [PubMed] [Google Scholar]
- 21.Peña P, Sancho J, Rufo M, et al. Driving cost factors in adult outpatients with refractory epilepsy: a daily clinical practice in clinics of neurology in Spain. Epilepsy Res. 2009;83:133–143. doi: 10.1016/j.eplepsyres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Taylor RS, Sander JW, Taylor RJ, et al. Predictors of health-related quality of life and costs in adults with epilepsy: A systematic review. Epilepsia. 2011;52:2168–2180. doi: 10.1111/j.1528-1167.2011.03213.x. [DOI] [PubMed] [Google Scholar]
- 23.Platt M, Sperling MR. A comparison of surgical and medical costs for refractory epilepsy. Epilepsia. 2002;43(Suppl 4):25–31. doi: 10.1046/j.1528-1157.43.s.4.5.x. [DOI] [PubMed] [Google Scholar]
- 24.Epilepsy Foundation [May 17, 2013];Pre-Surgical Evaluation. http://www.epilepsyfoundation.org/aboutepilepsy/treatment/surgery/assessment.cfm.
- 25.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 26.Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005;8:1–14. doi: 10.1089/dis.2005.8.1. [DOI] [PubMed] [Google Scholar]
- 27.Jetté N, Reid AY, Quan H, et al. How accurate is ICD coding for epilepsy? Epilepsia. 2010;51:62–69. doi: 10.1111/j.1528-1167.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 28.Kee VR, Gilchrist B, Granner MA, et al. A systematic review of validated methods for identifying seizures, convulsions, or epilepsy using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):183–193. doi: 10.1002/pds.2329. [DOI] [PubMed] [Google Scholar]
- 29.Pestana Knight EM, Schiltz NK, Bakaki PM, et al. Increasing utilization of pediatric epilepsy surgery in the United States between 1997 and 2009. Epilepsia. 2015;56:375–381. doi: 10.1111/epi.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClelland S, 3rd, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Arch Neurol. 2011;68:725–729. doi: 10.1001/archneurol.2011.7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.