Abstract
Genistein is a naturally occurring isoflavone phytoestrogen commonly found in plant products such as soybeans, lentils, and chickpeas. Genistein, like other phytoestrogens, has the potential to mimic, enhance, or impair the estradiol biosynthesis pathway, thereby potentially altering ovarian follicle growth. Previous studies have inconsistently indicated that genistein exposure may alter granulosa cell proliferation and hormone production, but no studies have examined the effects of genistein on intact antral follicles. Thus, this study was designed to test the hypothesis that genistein exposure inhibits follicle growth and steroidogenesis in intact antral follicles. To test this hypothesis, antral follicles isolated from CD-1 mice were cultured with vehicle (dimethyl sulfoxide; DMSO) or genistein (6.0 and 36 μM) for 18 – 96 hours (h). Every 24 h, follicle diameters were measured to assess growth. At the end of each culture period, the media were pooled to measure hormone levels, and the cultured follicles were collected to measure expression of cell cycle regulators and steroidogenic enzymes. The results indicate that genistein (36 μM) inhibits growth of mouse antral follicles. Additionally, genistein (6.0 and 36 μM) increases progesterone, testosterone, and dehydroepiandrosterone (DHEA) levels, but decreases estrone and estradiol levels. The results also indicate that genistein alters the expression of steroidogenic enzymes at 24, 72 and 96 h, and the expression of cell cycle regulators at 18 h. These data indicate that genistein exposure inhibits antral follicle growth by inhibiting the cell cycle, alters sex steroid hormone levels, and dysregulates steroidogenic enzymes in cultured mouse antral follicles.
Keywords: genistein, ovary, follicle, steroidogenesis
Introduction
Recent studies indicate that numerous people consume botanical compounds to prevent or treat a variety of medical conditions, including cancer, kidney disease, cardiovascular disease, neuronal injury, sexual dysfunction, inflammation, depression, and menopausal symptoms (Shibata, 2000; Ho and Jie, 2007; Andres et al., 2011; Chen et al., 2011; Khan et al., 2011). Many botanical compounds are known phytoestrogens, plant-derived chemicals that can bind to and signal through estrogen receptors (Kuiper et al., 1998; Khan et al., 2011; Yoon et al., 2014). Genistein is the predominant phytoestrogen in soy (Glycine max) and soy-derived products. It accounts for two thirds of soy isoflavone content and has been shown to bind both estrogen receptor alpha (ESR1) and estrogen receptor beta (ESR2), though it is thought to have a greater affinity for ESR2 than ESR1 (Kuiper et al., 1998; Khan et al., 2011; Yoon et al., 2014). Genistein is also a nutraceutical compound used as a chemo-preventive agent in women undergoing chemotherapy and as a treatment for menopausal symptoms (Khan et al., 2011; Yoon et al., 2014). Genistein is, therefore, considered to be one of the most relevant environmental estrogens in the human diet (Reinli and Block, 1996; Khan et al., 2011).
Although studies indicate that botanical compounds such as genistein may be useful in treating some adverse medical conditions (Khan et al., 2011; Yoon et al., 2014), the broader physiological impacts are less well understood. In particular, genistein exposure is a concern because it binds to estrogen receptors and can impact estrogen signaling pathways, such as steroidogenesis, which may have long-lasting adverse female health effects. Very little is known about the impact of botanical compounds on the ovary, the major site of estrogen receptors, steroidogenesis, and estradiol biosynthesis. Proper functioning of ovarian follicles is absolutely required for normal female fertility and estradiol biosynthesis. Further, low estradiol levels have been associated with reduced fertility, cardiovascular disease, mood disorders, adverse menopausal symptoms, and osteoporosis (Bush et al., 1987; Armamento-Villareal et al., 1992; Bagur and Mautalen, 1992; Christiansen, 1993; Everson et al., 1995; Cooper and Sandler, 1998; Mosca, 1998; Dennerstein et al., 1999; Hu et al., 1999). Thus, botanical compounds that target the ovary have the potential to adversely impact overall female health.
Unfortunately, studies on the effects of genistein on the ovary are limited and mainly focus on the impact of embryonic and neonatal exposures to genistein on the developing ovary (Chen et al., 2007; Jefferson et al., 2007; Jefferson et al., 2009). Such studies have shown that embryonic or neonatal exposures to genistein cause the development of multi-oocyte follicles, increase atresia (Medigovic et al., 2012), and reduce fertility in rodents (Britt et al., 2005; Jefferson et al., 2005; Chen et al., 2007; Jefferson et al., 2007; Jefferson et al., 2009; Cimafranca et al., 2010; Zhuang et al., 2010). In addition, one study indicates that exposure to genistein after weaning alters circulating estradiol levels in rats, with the lower dose (10 mg/kg for three weeks) increasing estradiol levels and the higher dose (100 mg/kg for three weeks) inhibiting estradiol levels (Zin et al., 2013).
Although the impacts of genistein on the developing and pre-pubertal ovary are important, it is also important to evaluate the effects of botanical compounds on the adult ovary. Women are often exposed to botanical compounds in their diet and as potential preventive agents and treatments for a variety of conditions, including cancer, neuronal loss, kidney disease, and menopausal symptoms (Khan et al., 2011). Unfortunately, epidemiological studies investigating the effects of botanicals, including genistein, on women's health are scarce. One study suggests that consumption of a soy-based diet is associated with decreased circulating estradiol levels in premenopausal women (Lu et al., 2001). Another study indicates that genistein inhibits steroidogenesis and steroidogenic enzymes in cultured human luteinized granulosa cells (Whitehead et al., 2002; Lacey et al., 2005; Rice et al., 2006). Interestingly, some studies using animal models suggest similar effects on steroidogenesis in isolated and cultured ovarian follicular cells. Genistein inhibits steroidogenesis or steroidogenic enzymes in cultured rat pre-antral follicles (Myllymaki et al., 2005), rat granulosa-luteal cells (Whitehead and Lacey, 2000), and porcine granulosa, theca, or luteal cells (Gregoraszczuk et al., 1999; Nynca and Ciereszko, 2006; Tiemann et al., 2007; Basini et al., 2010). Exposure to genistein also alters follicle growth (Zhuang et al., 2010), induces follicular atresia (Zin et al., 2013), and inhibits oocyte maturation (Chan, 2009). However, these studies have not examined the effects of genistein on the intact, adult antral follicle, the functional unit of the ovary. Further, they have not fully determined the mechanism by which genistein alters steroidogenesis in the adult ovary. Thus, the goal of these studies was to test the hypotheses that genistein inhibits 1) antral follicle growth and steroidogenesis in the adult mouse ovary and 2) steroidogenesis by inhibiting the levels of precursor hormones and the necessary steroidogenic enzymes.
Materials and Methods
Chemicals
Genistein (98% purified via HPLC, Botanical Research Center, University of Illinois) was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) then diluted in DMSO to achieve final treatment concentrations of 1.6 and 9.6 μg of genistein per mL of culture media (6.0 and 36 μM). These concentrations were selected based on previous studies using a range of similar doses that show that genistein affects female reproductive function (Gregoraszczuk et al., 1999; Whitehead and Lacey, 2000; Jefferson et al., 2002). The concentrations are higher than observed in vivo after dietary consumption in rodents (Santell et al., 1997; Holder et al., 1999; Chang et al., 2000; Doerge et al., 2002), but serve as models for the mechanistic effects of genistein on the ovary.
Animals
Adult, cycling, female CD-1 mice were purchased from Charles River (Wilmington, MA). The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility and were provided food (Harlan Teklad 8626) and water for ad libitum consumption. Temperature was maintained at 22 ± 1°C and animals were exposed to 12 hour light-dark cycles. The Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign approved all procedures concerning animal care, euthanasia, and tissue collection.
In vitro time-course follicle culture
Female CD-1 mice were euthanized on postnatal days (PND) 32–35 and their ovaries removed using aseptic technique. Antral follicles were mechanically isolated from the ovary based on relative size (250–400 μm), cleaned of interstitial tissue using fine watchmaker forceps, individually placed in wells of a 96-well culture plate, and covered with unsupplemented α-minimum essential media (α-MEM) prior to treatment. Follicles from 2–3 mice were isolated per experiment, providing approximately 20–40 antral follicles from each mouse. Each experiment contained a minimum of 8–12 follicles per treatment group.
Concentrations of vehicle control (DMSO) and genistein (6.0 and 36 μM) were individually prepared in supplemented α-MEM. Supplemented α-MEM was prepared with the following: 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (FSH; Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, Torrance, CA), 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA) (Cortvrindt and Smitz, 2002; Miller et al., 2005; Gupta et al., 2006). Various stock concentrations of genistein were prepared (2.2 and 12.8 mg/mL) so that an equal volume of chemical could be added to each well to control for solvent concentration (0.75 μL of genistein or DMSO per mL of media).
Antral follicles were cultured from 18 to 96 h in an incubator supplying 5% CO2 at 37°C. After each culture, follicles were pooled by treatment group and snap-frozen in liquid nitrogen and then subjected quantitative real time PCR (qPCR) as described below. Media were collected and stored at −80°C until subjected to hormone assays as described below.
Analysis of follicle growth
To evaluate follicle growth over time, follicles were measured along perpendicular axes every 24 h for 96 h. The diameters were recorded in microns, averaged among treatments groups per 24 h interval, and then converted to percent change at each time-point. Percent change was determined by dividing the average diameters of the follicles at each 24 h interval per treatment group by the initial average measurement (0 h) of each respective treatment group.
Analysis of hormone levels
Media were collected from the in vitro culture system at 24 h intervals, from at least three separate experiments, and subjected to enzyme-linked immunosorbent assays (ELISA) for measurement of progesterone, dehydroepiandrosterone (DHEA), androstenedione, testosterone, estrone, and estradiol levels. ELISA kits were purchased from DRG International Inc. (Springfield, NJ). Assays were run according to the manufacturer's instructions. Some samples were diluted to match the dynamic range of each ELISA kit. The samples were run in triplicate and had intra- and inter-assay coefficients of variability below 10%.
Analysis of gene expression by qPCR
Cultured antral follicles were collected at 24 h intervals and total RNA was extracted using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's protocol. Reverse transcriptase generation of cDNA was performed with 0.3–0.5 μg of total RNA using an iScript RT kit (Bio-Rad Laboratories, Hercules CA). qPCR was conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) and accompanying software (CFX Manager Software) according to the manufacturer's instruction. qPCR was performed using 1 μL cDNA, 1 μL of gene specific primers (Integrated DNA Technologies, Inc., Coralville, IA; Table 1), 3 μL of molecular water, and 5 μL of a SsoFast EvaGreen Supermix qPCR kit (Bio-Rad laboratories, Hercules, CA) per sample. The qPCR program protocol was similar to that used in previous studies (Hannon et al., 2015). A standard curve was generated from five serial dilutions of a combination of samples to calculate the amplification efficiency of each primer and determine relative expression of each target gene to the reference gene, glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Gapdh was used as a reference gene for each sample because its expression did not differ between treatment groups.
Table 1.
Primers used in Quantitative Real-Time Polymerase Chain Reactions (qPCR)
| Gene | Gene Symbol | Primer Sequence | |
|---|---|---|---|
| Forward | Reverse | ||
|
| |||
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | TGTGTCCGTCGTGGATCTGA | CCTGCTTCACCACCTTCTTGAT |
|
| |||
| Steroidogenic acute regulatory protein | StAR | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
|
| |||
| Cytochrome P450 cholestrol side-chain cleavage | Cyp11a1 | AGATCCCTTCCCCTGGCGACAATG | CGCATGAGAAGAGTATCGACGCATC |
|
| |||
| 3b-Hydroxysteroid dehydrogenase 1 | Hsd3b1 | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC |
|
| |||
| Cytochrome P450 steroid 17-a-hydroxylase 1 | Cyp17a1 | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG |
|
| |||
| 17b-Hydroxysteroid dehydrogenase 1 | Hsd17b1 | ACTGTGCCAGCAAGTTTGCG | AAGCGGTTCGTGGAGAAGTAG |
|
| |||
| Cytochrome P450 aromatase | Cyp 19a1 | CATGGTCCCGCAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
|
| |||
| Estrogen receptor 1 (alpha) | Esr1 | CCGTGTGCAATGACTATGCC | GTGCTTCAACATTCTCCCTCCTC |
|
| |||
| Estrogen receptor 2 (beta) | Esr2 | GGAATCTCTTCCCAGCAGCA | GGGACCACATTTTTGCACTT |
|
| |||
| Cyclin A2 | Ccna2 | GCTCTACTGCCCGGAGGCTGA | TGGCCTACATGTCCTCTGGGGAA |
|
| |||
| Cyclin B1 | Ccnb1 | TGCATTCTCTCAGTGCCCTCCACA | AGACAGGAGTGGCGCCTTGGT |
|
| |||
| Cyclin D2 | Ccnd2 | CCTTTGACGCAGGCTCCCTTCT | ACCCTGGTGCACGCATGCAAA |
|
| |||
| Cyclin E1 | Ccne1 | GGTGTCCTCGCTGCTTCTGCTT | CCGGCTAACCATGGCGAACGGA |
|
| |||
| Cyclin-dependent kinase 4 | Cdk4 | AGAAACCCTCGCTGAAGCGGCA | TGGGGGTGAACCTCGTAAGGAGA |
|
| |||
| Cyclin-dependent kinase inhibitor 1A (p21) | Cdkn1a | TTAGGCAGCTCCAGTGGCAACC | ACCCCCACCACCACACACCATA |
|
| |||
| B cell lymphoma 2 | Bcl2 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
|
| |||
| Bcl2-associated X protein | Bax | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG |
Statistical Analyses
All data were analyzed using SPSS software (SPSS Chicago, IL) and expressed as mean ± the standard error of the mean (SEM). All normally distributed data were analyzed using a one-way analysis of variance test (ANOVA). A Dunnett's t post-hoc test was used for the follicle growth and gene expression analyses, and a Tukey's post-hoc test was used for hormone level data analysis. Any non-normally distributed data were analyzed using a Kruskal-Wallis test. Statistical significance was assigned at p ≤ 0.05, n = 3 - 5 separate experiments. At 48 h, DHEA levels for the DMSO and genistein 1.6 μM groups were below the level of detection of our ELISA kits. Thus, to complete statistical analysis, we used the lowest detectable amount of DHEA provided by the kit.
Results
Effect of Genistein on Follicle Growth
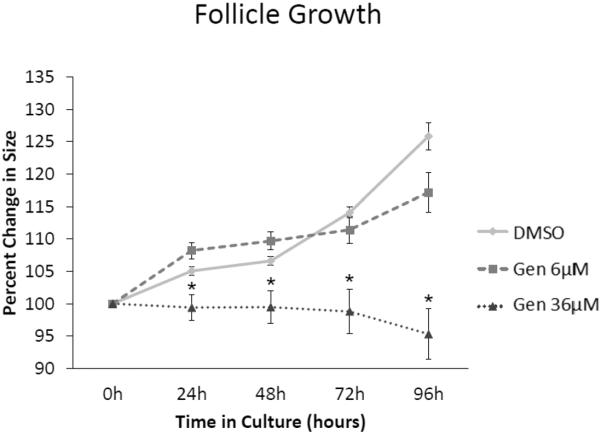
Follicles treated with vehicle control (DMSO) grew significantly over time (Figure 1). However, exposure to genistein (36 μM) significantly inhibited antral follicle growth compared to DMSO, beginning at 24 h and continuing throughout the 96 h culture (Figure 1; n=3-5, p≤0.05). Exposure to a lower dose of genistein (6 μM) did not significantly inhibit follicle growth compared to DMSO at any time point (Figure 1).
Figure 1. Effect of genistein on follicle growth over time.
After isolation, antral follicle growth was measured daily along perpendicular axes and percent change in growth was determined from 24 to 96 h. The graph represents the means ± SEM of percent change in follicle growth from 3 separate experiments. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3; p≤ 0.05).
Effect of Genistein on Apoptotic Factors
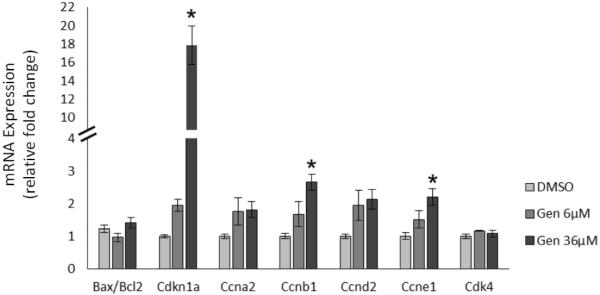
Because we observed inhibited growth with genistein exposure at 24 h of culture, we examined gene expression of factors that regulate atresia at an earlier time point (18 h) to determine if genistein was inhibiting follicle growth by causing atresia. We selected the anti-apoptotic factor B-cell lymphoma 2 (Bcl2) and the pro-apoptotic factor Bcl-2-associated X protein (Bax) because they are important factors in regulating apoptosis in the ovary (Kaipia and Hsueh, 1997). Our data indicate that genistein does not affect the expression of the ratio of Bax to Bcl2 (Figure 2), suggesting that genistein exposure at this early time point does not inhibit antral follicle growth by inducing atresia.
Figure 2. Effects of genistein on apoptotic factors and cell cycle regulators at 18 h.
After culture of antral follicles for 18 h, follicles were collected and subjected to RNA extraction. The RNA was reverse transcribed to cDNA and used to measure gene expression of Bax, Bcl2, Cdkn1a, Ccna2, Ccnb1, Ccnd2, Ccne1, and Cdk4 by quantitative polymerase chain reaction (qPCR). Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=4; p≤ 0.05).
Effect of Genistein on Cell Cycle Regulators
Given that genistein does not affect growth by inducing atresia, we examined whether it inhibits growth by altering cell cycle regulation. Our data show that genistein significantly increases expression of the cell cycle inhibitor cyclin-dependent kinase inhibitor 1a (Cdkn1a) about 17-fold compared to control (Figure 2; n=4, p ≤ 0.05). Genistein did not affect the expression of the cell cycle activators cyclin A2 (Ccna2), cyclin D2 (Ccdn2), and cyclin-dependent kinase 4 (Cdk4) (Figure 2), but it did slightly increase the expression of the cell cycle activators cyclin B1 (Ccnb1) and cyclin E1 (Ccne1) compared to control (Figure 2; n=4, p ≤ 0.05).
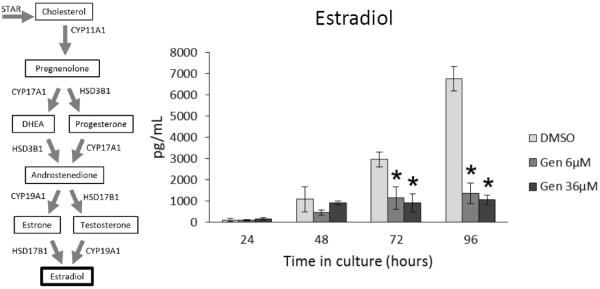
Effect of Genistein on Estradiol Production
Given that growing antral follicles are major producers of estradiol (Hirshfield, 1991; Findlay et al., 2001) and that our data indicate that genistein inhibits follicle growth, we next examined whether genistein affects the ability of antral follicles to produce estradiol. At 24 and 48 h, genistein exposure did not significantly affect estradiol levels produced by antral follicles compared to controls (Figure 3). However, at 72 and 96 h both doses of genistein (6 and 36 μM) significantly decreased the levels of estradiol produced by antral follicles compared to controls (Figure 3; n= 3, p ≤ 0.05).
Figure 3. Effects of genistein on estradiol production over time.
After culture of antral follicles for 24-96 h, media were collected and subjected to enzyme-linked immunosorbent assays for estradiol. The graph represents the means ± SEM from 3 separate experiments. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3; p≤ 0.05).
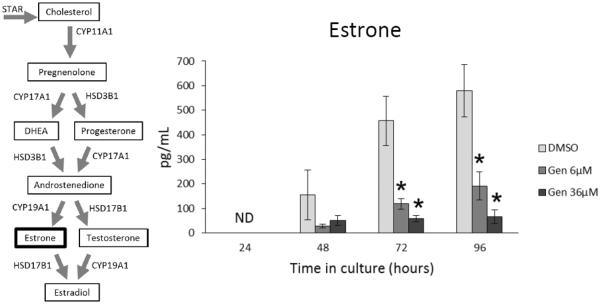
Effect of Genistein on Metabolic Pre-Cursors to Estradiol
Production of estradiol is dependent partly on the availability of its metabolic precursors, estrone, testosterone, androstenedione, DHEA, and progesterone. Thus, inhibition of these sex steroid hormones would indirectly inhibit production and levels of estradiol. To test this possibility, we examined whether exposure to genistein alters the production of estrone, testosterone, androstenedione, DHEA, or progesterone by adult antral follicles. At 24 h, estrone levels in all treatment groups were below the level of detection of the ELISA kits (Figure 4). At 48 h, genistein exposure did not affect estrone levels compared to controls. However, at 72 and 96 h, both doses of genistein (6.0 and 36 μM) significantly decreased estrone levels compared to controls (Figure 4; n=3-5; p ≤ 0.05).
Figure 4. Effects of genistein on estrone production over time.
After culture of antral follicles for 24-96 h, media were collected and subjected to enzyme-linked immunosorbent assays for estrone. The graph represents the means ± SEM from 3-5 separate experiments. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3-5; p≤ 0.05).
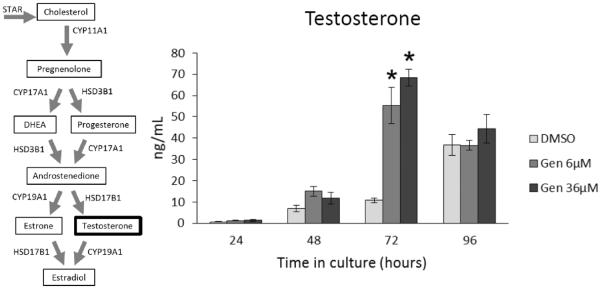
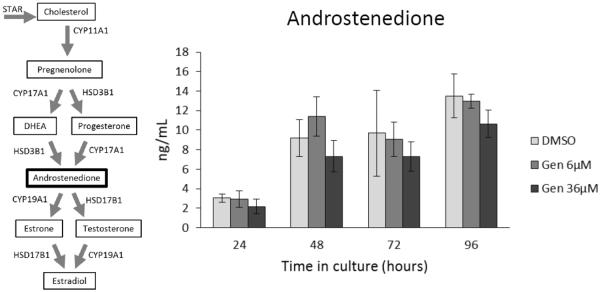
At 24, 48, and 96 h, genistein exposure did not significantly affect testosterone levels compared to controls (Figure 5). At 72 h, both doses of genistein (6 and 36 μM) significantly increased the levels of testosterone produced by antral follicles compared to controls (Figure 5; n=3; p ≤ 0.05). In contrast, genistein exposure did not significantly affect androstenedione levels compared to controls at any time-point (Figure 6).
Figure 5. Effects of genistein on testosterone production over time.
After culture of antral follicles for 24-96 h, media were collected and subjected to enzyme-linked immunosorbent assays for testosterone. The graph represents the means ± SEM from 3 separate experiments. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3; p≤ 0.05).
Figure 6. Effects of genistein on androstenedione production over time.
After culture of antral follicles for 24-96 h, media were collected and subjected to enzyme-linked immunosorbent assays for androstenedione. The graph represents the means ± SEM from 3 separate experiments.
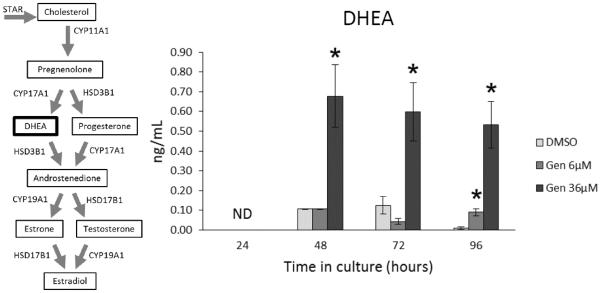
At 24 h, DHEA levels in all treatment groups were below the level of detection of the ELISA kits (Figure 7). However, at 48 and 72 h, genistein exposure (36 μM) significantly increased DHEA levels compared to control (Figure 7; n=3-5; p ≤ 0.05). At 96 h, both concentrations of genistein (6 and 36 μM) significantly increased DHEA levels compared to control (Figure 7; n=3-5; p ≤ 0.05).
Figure 7. Effects of genistein exposure on DHEA production over time.
After culture of antral follicles for 24-96 h, media were collected and subjected to enzyme-linked immunosorbent assays for DHEA. The graph represents the means ± SEM from 3-5 separate experiments. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3-5; p≤ 0.05).
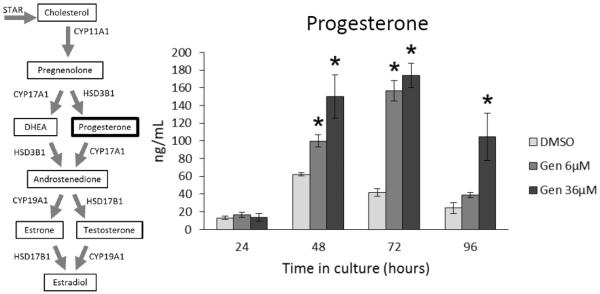
At 24 h, genistein exposure did not significantly affect progesterone levels produced by antral follicles compared to controls (Figure 8). At 48 and 72 h, however, both doses of genistein (6 and 36 μM) significantly increased the levels of progesterone compared to controls (Figure 8; n=3; p ≤ 0.05). At 96 h, only the high dose of genistein (36 μM) significantly increased the levels of progesterone compared to controls (Figure 8; n=3; p ≤ 0.05).
Figure 8. Effects of genistein on progesterone production over time.
After culture of antral follicles for 24-96 h, media were collected and subjected to enzyme-linked immunosorbent assays for progesterone. The graph represents the means ± SEM from 3 separate experiments. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3; p≤ 0.05).
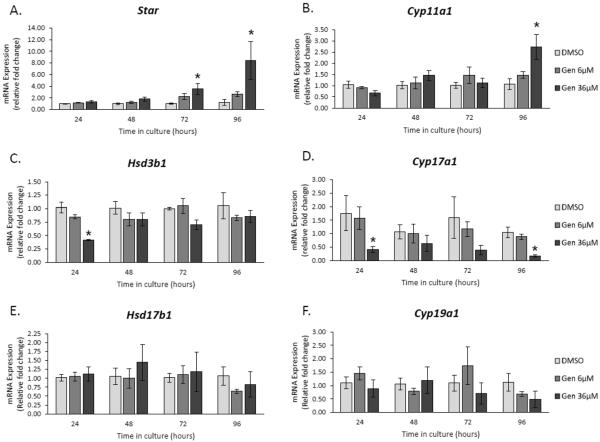
Effect of Genistein on the Expression of Steroidogenic Enzymes
Our data indicate that genistein increased testosterone, DHEA, and progesterone levels (Figures 5, 7, and 8), but decreased estradiol and estrone levels compared to control (Figures 3 and 4), suggesting that genistein may alter the ability of antral follicles to synthesize sex steroid hormones. Therefore, we investigated if genistein alters the expression of the following steroidogenic enzymes at each time-point in the culture.
Steroidogenic acute regulatory protein (Star)
STAR is a transport protein responsible for regulating cholesterol transfer into the mitochondria and is a rate-limiting step in steroidogenesis (Miller and Strauss, 1999; Strauss et al., 1999). At 24 and 48 h, genistein did not affect Star expression compared to control (Figure 9A). However, at 72 and 96 h, genistein (36 μM) significantly increased Star expression compared to controls (Figure 9A; n=3-5; p ≤ 0.05).
Figure 9. Effects of genistein on the gene expression of steroidogenic enzymes over time.
After culture of antral follicles for 24-96 h, follicles were collected and subjected to RNA extraction. The RNA was reverse transcribed to cDNA and used to measure gene expression of key steroidogenic enzymes by qPCR. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3-5; p≤ 0.05).
Cytochrome P450 side chain cleavage (Cyp11a1)
CYP11A1 is another rate-limiting step in steroidogenesis and it is responsible for metabolizing cholesterol into pregnenolone, the required pre-cursor for progesterone (Miller and Strauss, 1999; Payne and Hales, 2004). At 24–72 h, genistein did not significantly alter the expression of Cyp11a1 compared to controls (Figure 8B). However, at 96 h, it increased the expression of Cyp11a1 compared to control (Figure 9B; n=3-5; p ≤ 0.05).
3β-hydroxysteroid dehydrogenase 1 (Hsd3b1)
HSD3B1 is integral in metabolizing and converting Δ5-steroids (pregnenolone and DHEA) into Δ4-steroids (progesterone and androstenedione) (Readhead et al., 1983; Payne and Hales, 2004). At 24 h, genistein (36 μM) decreased the expression of Hsd3b1 compared to controls (Figure 9C; n=3-5; p ≤ 0.05), but it did not affect expression at any other time-point (Figure 9C).
Cytochrome P450 17a1 (Cyp17a1)
CYP17A1 is important for the synthesis of DHEA and androstenedione from pregnenolone and progesterone, respectively (Conley and Bird, 1997; Payne and Hales, 2004). At 24 and 96 h genistein (36 μM) significantly decreased Cyp17a1 expression compared to controls. (Figure 9D; n=3-5; p ≤ 0.05), but not at 48 and 72 h. At 72 h, there was a trend towards decreased expression with genistein exposure (36 μM) when compared to controls (Figure 9D).
17β-hydroxysteroid dehydrogenase 1 (Hsd17b1)
HSD17B1 is responsible for conversion of androstenedione to testosterone as well as estrone to estradiol (Armstrong, 1968; Payne and Hales, 2004). Genistein did not affect the expression of Hsd17b1 compared to controls at any time-point (Figure 9E; n=3-5).
Cytochrome P450 19 (Cyp19a1)
CYP19A1 is responsible for the conversion of androstenedione to estrone as well as testosterone to estradiol (Armstrong, 1968; Payne and Hales, 2004). Genistein did not affect the expression of Cyp19a1 compared to controls at any time-point (Figure 9F; n=3-5).
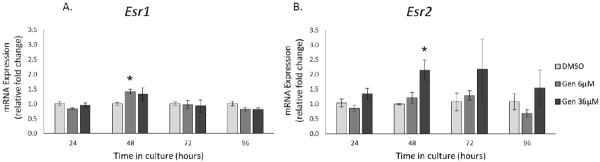
Effect of Genistein on the Expression of Estrogen Receptors
Genistein is known as an estrogenic compound and has the ability to bind and act through ESR1 and ESR2 (Kuiper et al., 1998). Thus, we examined the effect of genistein exposure on Esr1 and Esr2 expression in antral follicles. At 24, 72, and 96 h, genistein did not affect the expression of Esr1 or Esr2. However, at 48h, genistein exposure increased the expression of Esr1 (6 μM) and Esr2 (36 μM) compared to controls (Figure 10; n=3, p ≤ 0.05).
Figure 10. Effects of genistein on the gene expression of Esr1 and Esr2.
After culture of antral follicles for 24-96 h, follicles were collected and subjected to RNA extraction. The RNA was reverse transcribed to cDNA and used to measure gene expression of Esr1 and Esr2 by qPCR. Asterisks (*) indicate a significant difference between control groups and genistein at each time point (n=3-5; p≤ 0.05).
Discussion
Using an in vitro follicle culture system, we have shown that genistein inhibits growth of antral follicles, alters the expression of cell cycles regulators, disrupts the production of sex steroid hormones, and alters the expression of steroidogenic enzymes. Specifically, we observed that genistein exposure inhibits antral follicle growth as early as 24 h in culture (Figure 1). Our data are consistent with a previous in vivo study indicating that exposure to genistein (160 mg/kg/day) significantly inhibits follicle growth because it increases the percentage of primordial follicles, but decreases the number of total antral follicles in 4 month and 15 month old rats (Zhuang et al., 2010).
To determine the cause of genistein-induced antral follicle growth inhibition, we examined the expression of apoptotic factors Bax and Bcl2 at 18 h of culture. We found that genistein did not affect apoptotic factors in antral follicles (Figure 2), suggesting that atresia is not the cause of genistein-induced inhibition of antral follicle growth. Further, when observed the genistein-treated follicles under a light microscope, we noticed that the follicles appeared to be healthy, but not growing (data not shown). These results differ from previous studies that indicate that genistein causes atresia of rat follicles. Medigovic et al. dosed immature female rats with 50 mg/kg/day genistein daily from postnatal days (PND) 18 to 20 and found decreased numbers of primordial, primary, and secondary follicles and increased atresia (Medigovic et al., 2012). Zin et al. also found that genistein treatment (10 or 100 mg/kg/day) from PND 22 to 42 increases atresia in Sprague-Dawley rats (Zin et al., 2013). Our results likely differ from these studies due differences in age, species, and doses of genistein.
The observation that genistein inhibits follicle growth, but does not induce atresia led us to examine the effects of genistein on cell cycle regulators at 18 h. Previous studies on other endocrine disruptors such as bisphenol A and phthalates show that they can affect follicle growth by altering expression of cell cycle regulators (Peretz et al., 2012; Craig et al., 2013). Our data indicate that genistein significantly increases the expression of the cell cycle inhibitor Cdkn1a about 17-fold compared to control (Figure 2). Further, our data indicate that although genistein also significantly increases the expression of the cell cycle activators Ccnb1 and Ccne1 (Figure 2), the change is minor in comparison to that of Cdkn1a, and is likely a compensatory effect. Collectively, these data suggest that genistein inhibits follicle growth by inhibiting the cell cycle.
Previous studies indicate that estradiol is necessary for normal follicle growth (Channinget al., 1980; Findlay et al., 2001; Drummond, 2006; Chaffin and Vandevoort, 2013). Therefore, we initially hypothesized that genistein would inhibit antral follicle growth due to altered steroidogenesis. Instead, we found that genistein (36 μM) inhibits growth as early as 24 h (Figure 1), whereas genistein does not affect estradiol levels until 72 h (Figure 3). Additionally, the lower dose of genistein (6 μM) does not affect follicle growth at any time point (Figure 1), but still decreases estradiol levels beginning at 72 h (Figure 3). Thereby, it is unlikely that the genistein-induced inhibition of growth is due to decreased levels of estradiol. Instead, it is more likely that inhibition of follicle growth is the cause of decreased estradiol production.
Our data indicate that genistein exposure decreases estradiol and estrone levels in the media of cultured antral follicles. Estradiol and estrone are produced by converting precursor hormones in the theca and granulosa cells of the follicles (Armstrong, 1968). Thus, it is possible that the inhibition of antral follicle growth by genistein (36 μM) results in fewer granulosa cells being present in the follicle to produce estradiol. This is supported by our data indicating that genistein exposure (36 μM) inhibits antral follicle growth first at 24 h (Figure 1), whereas it inhibits estradiol levels beginning at 72 h (6 and 36 μM) (Figure 3). Another likely scenario is that genistein is affecting the metabolism of estradiol and estrone. It is possible that genistein increases estradiol and estrone metabolism by inducing cytochrome P450 1A1 (CYP1A1) and cytochrome P450 1B1 (CYP1B1). Thus, future studies should determine whether genistein affects estradiol metabolism by inducing CYP1A1 and CYP1B1. Additionally, our data indicate that genistein exposure increases the expression of Esr1 (6 μM) and Esr2 (36 μM) (Figure 10). It is possible that this genistein-induced increase could lead to greater responsiveness of antral follicles to genistein and further result in decreased production of estradiol, but this needs to be examined in future studies.
Our data on the effects of genistein on estradiol levels are consistent with several, but not all previous studies. Specifically, we showed that genistein exposure reduced estradiol production from cultured antral follicles. Similarly, dietary genistein exposure decreased estradiol levels in male mice (8 mg/kg; (Ryokkynen et al., 2006)), cultured swine granulosa cells (185 μM; (Basini et al., 2010)), and Sprague Dawley rats (10 and 100 mg/kg/day; (Zin et al., 2013)). Additionally, daily consumption of soy products containing genistein reduced circulating levels of estradiol over the menstrual cycle in nine healthy regularly cycling women (Lu et al., 2001). Further, acute (4 h) and chronic (24 h) genistein exposure inhibited estradiol production in cultured, luteinized human granulosa cells (Whitehead et al., 2002). In contrast, neonatal genistein exposure (50 mg/kg/day) did not affect serum levels of estradiol when measured before puberty or during pregnancy in female mice (Jefferson et al., 2005). Further, genistein did not affect FSH-stimulated estradiol production in cultured porcine (0.5-50 μM; (Nynca and Ciereszko, 2006)) or immature rat granulosa cells (1–10 μM; (Myllymaki et al., 2005)). The reasons for these differences in results pertaining to the effects of genistein on estradiol levels are unclear, but likely stem from differences in species (mice versus rats, humans, and pigs), and study design (doses of genistein, timing of treatment, age of animals, and in vivo versus in vitro methods). It is also possible that our in vitro results differ from other in vitro studies because we used an antral follicle culture system, which consists of the intact functional units of the ovary. Other studies used isolated granulosa cells (Myllymaki et al., 2005; Nynca and Ciereszko, 2006), which normally produce sex steroid hormones in conjunction with theca cells and, thus, may behave differently alone compared to intact antral follicles.
Our data indicate that genistein exposure also alters the levels of other precursor hormones (Figures 4–8). Specifically, we observed that genistein exposure increases testosterone, DHEA, and progesterone levels compared to control (Figures 5, 7, and 8). Similar to our results, one study indicates that genistein exposure (10–10,000 ng/mL) increases progesterone release by bovine granulosa cells and rabbit granulosa cells (100–10,000 ng/mL) (Makarevich et al., 1997). Additionally, genistein exposure (0.1–3 μM) increases FSH-induced progesterone levels in rat granulosa cells (Haynes-Johnsonet al., 1999). This same study, however, shows that higher concentrations of genistein (30–100 μM) decrease FSH-induced progesterone levels (Haynes-Johnson et al., 1999), thereby indicating dose related differences in the effects of genistein on steroidogenesis.
Our findings on the effects of genistein on progesterone and testosterone differ from other previously conducted studies. One study indicates that neonatal genistein exposure (50 mg/kg/day) does not affect serum levels of progesterone or testosterone when measured before puberty and during pregnancy in mice (Jefferson et al., 2005). Additionally, genistein exposure (1, 18.5, and 185 μM) inhibits FSH-stimulated and basal progesterone levels in cultured porcine granulosa cells (Nynca and Ciereszko, 2006; Basini et al., 2010)), LH-stimulated progesterone secretion in porcine luteinized granulosa cells (0.5-50 μM; (Nynca et al., 2015)), and prolactin-stimulated progesterone secretion in porcine thecal cells (45 μM; (Gregoraszczuk et al., 1999)). Furthermore, genistein exposure (50 μM) inhibits the ability of primary porcine granulosa cells to produce progesterone (Tiemann et al., 2007). Genistein exposure (1–100 μM) also decreases progesterone levels in human granulosa cells (Whitehead et al., 2002; Lacey et al., 2005). It is likely that our results differ from previous studies largely due to species differences in the effects of genistein on sex steroid hormone levels. Most previous studies were conducted with pigs (Gregoraszczuk et al., 1999; Nynca and Ciereszko, 2006; Tiemann et al., 2007; Basini et al., 2010; Nynca et al., 2015) or human cells (Whitehead et al., 2002; Lacey et al., 2005), whereas our study was conducted in mice. Further, our results likely differ from the other study conducted in mice (Jefferson et al., 2005) because of the differences in study design, such as in vitro vs in vivo experiments and pre-pubertal vs adult, non-pregnant exposure timing, as well as the differences in genistein concentrations. Traditional toxicology dogma indicates that endocrine disrupting chemicals can have differing effects across a range of concentrations and that low dose effects may be different than those observed at higher doses (Vandenberg et al., 2012).
Adequate estradiol biosynthesis is dependent on the availability of upstream precursor hormones and steroidogenic enzymes. In the estradiol biosynthesis pathway, StAR transports cholesterol from the outer membrane to the inner membrane of the mitochondria (Miller and Strauss, 1999; Strauss et al., 1999). Cholesterol then is converted to pregnenolone by CYP11A1 (Payne and Hales, 2004). Pregnenolone is then converted to estradiol via two pathways. In the first pathway, pregnenolone is converted to DHEA by CYP17A1 (Conley and Bird, 1997). DHEA is then converted to androstenedione by HSD3B1 (Armstrong, 1968; Readhead et al., 1983). Androstenedione is converted to either estrone by CYP19A1 or testosterone by HSD17B1 (Armstrong, 1968; Payne and Hales, 2004). Finally, testosterone is converted to estradiol via CYP19A1 (Armstrong, 1968; Payne and Hales, 2004). In the second pathway, pregnenolone is converted to progesterone by HSD3B1 (Readhead et al., 1983). The progesterone is converted to androstenedione by CYP17A1 (Conley and Bird, 1997), then testosterone, and finally estradiol (Armstrong, 1968; Payne and Hales, 2004).
Although the mechanism by which genistein alters testosterone and DHEA, remains unknown, our steroidogenic enzyme findings shed some light on why we observe a genistein-induced increase in progesterone levels. We observed a genistein-induced decrease in Cyp17a1 as early as 24 h and it continued until 96 h (Figure 9D). Because CYPA17A1 is the enzyme responsible for progesterone metabolism, a decrease in its expression would decrease progesterone metabolism. Further, beginning at 72 h, we observed an increase in Star and Cyp11a1 expression (Figures 9A and 9B), two enzymes that increase progesterone production. Collectively, the decrease in progesterone metabolism and increase in progesterone production led to unusually high levels of progesterone in response to genistein.
In conclusion, our results indicate that genistein exposure inhibits the growth of mouse antral follicles in vitro, likely by inhibiting the cell cycle. Our results also indicate that estradiol, a key factor involved in the growth of antral follicles, is significantly decreased in genistein-treated follicles compared to control. Additionally, our results show that genistein alters the levels of the estradiol precursor hormones estrone, testosterone, DHEA, and progesterone. Finally, our data indicate that genistein induces dysregulation of steroidogenic enzyme expression and this may be the cause of genistein-induced altered hormone levels in cultured mouse antral follicles. This study, as well as future ones that further elucidate how genistein alters steroidogenic enzymes and steroid hormone production, are important because genistein-induced altered hormone levels and inhibition of antral follicle growth could lead to numerous health risks and female infertility. Many people, both men and women, are exposed to genistein and other phytoestrogen-based botanicals regularly as part of their diet or as therapeutics for reproductive and non-reproductive diseases. Therefore, it is imperative to understand how genistein and other botanicals may impact overall public health.
Highlights.
Genistein exposure inhibits antral follicle growth
Genistein exposure alters expression of cell cycle regulators
Genistein exposure alters sex steroid hormones
Genistein exposure alters expression of steroidogenic enzymes
Genistein exposure alters Esr1 and Esr2 expression
Acknowledgements
The authors would like to acknowledge the members of the Flaws' lab for their support and input throughout the project.
Funding This work was supported by NIHR03ES023972 (JAF) and the Botanical Research Center at the University of Illinois (WGH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Critical reviews in toxicology. 2011;41:463–506. doi: 10.3109/10408444.2010.541900. [DOI] [PubMed] [Google Scholar]
- Armamento-Villareal R, Villareal DT, Avioli LV, Civitelli R. Estrogen status and heredity are major determinants of premenopausal bone mass. The Journal of clinical investigation. 1992;90:2464–2471. doi: 10.1172/JCI116138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DT. Gonadotropins, ovarian metabolism, and steroid biosynthesis. Recent progress in hormone research. 1968;24:255–319. doi: 10.1016/b978-1-4831-9827-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Bagur AC, Mautalen CA. Risk for developing osteoporosis in untreated premature menopause. Calcified tissue international. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- Basini G, Bussolati S, Santini SE, Grasselli F. The impact of the phyto-oestrogen genistein on swine granulosa cell function. Journal of animal physiology and animal nutrition. 2010;94:e374–382. doi: 10.1111/j.1439-0396.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- Britt KL, Simpson ER, Findlay JK. Effects of phytoestrogens on the ovarian and pituitary phenotypes of estrogen-deficient female aromatase knockout mice. Menopause (New York, N.Y.) 2005;12:174–185. doi: 10.1097/00042192-200512020-00012. [DOI] [PubMed] [Google Scholar]
- Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Vandevoort CA. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Experimental biology and medicine (Maywood, N.J.) 2013;238:539–548. doi: 10.1177/1535370213489437. [DOI] [PubMed] [Google Scholar]
- Chan WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reproductive toxicology (Elmsford, N.Y.) 2009;28:52–58. doi: 10.1016/j.reprotox.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Chang HC, Churchwell MI, Delclos KB, Newbold RR, Doerge DR. Mass spectrometric determination of Genistein tissue distribution in diet-exposed Sprague-Dawley rats. The Journal of nutrition. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- Channing CP, Schaerf FW, Anderson LD, Tsafriri A. Ovarian follicular and luteal physiology. International review of physiology. 1980;22:117–201. [PubMed] [Google Scholar]
- Chen Y, Huang JH, Ning Y, Shen ZY. Icariin and its pharmaceutical efficacy: research progress of molecular mechanism. Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine. 2011;9:1179–1184. doi: 10.3736/jcim20111104. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Christiansen C. Prevention and treatment of osteoporosis with hormone replacement therapy. International journal of fertility and menopausal studies. 1993;38(Suppl 1):45–54. [PubMed] [Google Scholar]
- Cimafranca MA, Davila J, Ekman GC, Andrews RN, Neese SL, Peretz J, Woodling KA, Helferich WG, Sarkar J, Flaws JA, Schantz SL, Doerge DR, Cooke PS. Acute and chronic effects of oral genistein administration in neonatal mice. Biology of reproduction. 2010;83:114–121. doi: 10.1095/biolreprod.109.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biology of reproduction. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Sandler DP. Age at natural menopause and mortality. Annals of epidemiology. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Human reproduction update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Lehert P, Burger H, Dudley E. Mood and the menopausal transition. The Journal of nervous and mental disease. 1999;187:685–691. doi: 10.1097/00005053-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Banks EP, Jefferson WN, Newbold RR. Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer letters. 2002;184:21–27. doi: 10.1016/s0304-3835(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Drummond AE. The role of steroids in follicular growth. Reproductive biology and endocrinology : RB&E. 2006;4:16. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson SA, Matthews KA, Guzick DS, Wing RR, Kuller LH. Effects of surgical menopause on psychological characteristics and lipid levels: the Healthy Women Study. Health psychology : official journal of the Division of Health Psychology, AmericanPsychological Association. 1995;14:435–443. doi: 10.1037//0278-6133.14.5.435. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Britt K, Kerr JB, O'Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation: the role of oestrogens. Reproduction, fertility, and development. 2001;13:543–547. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk E, Slomczynska M, Stoklosowa S. Effect of genistein, tyrphostin and herbimycin on prolactin-stimulated progesterone production by porcine theca and luteal cells. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 1999;50:477–484. [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicology and applied pharmacology. 2006;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hannon PR, Brannick KE, Wang W, Flaws JA. Mono(2-Ethylhexyl) Phthalate Accelerates Early Folliculogenesis and Inhibits Steroidogenesis in Cultured Mouse Whole Ovaries and Antral Follicles. Biology of reproduction. 2015 doi: 10.1095/biolreprod.115.129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes-Johnson D, Lai MT, Campen C, Palmer S. Diverse effects of tyrosine kinase inhibitors on follicle-stimulating hormone-stimulated estradiol and progesterone production from rat granulosa cells in serum-containing medium and serum-free medium containing epidermal growth factor. Biology of reproduction. 1999;61:147–153. doi: 10.1095/biolreprod61.1.147. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. International review of cytology. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Ho JW, Jie M. Pharmacological activity of cardiovascular agents from herbal medicine. Cardiovascular & hematological agents in medicinal chemistry. 2007;5:273–277. doi: 10.2174/187152507782109854. [DOI] [PubMed] [Google Scholar]
- Holder CL, Churchwell MI, Doerge DR. Quantification of soy isoflavones, genistein and daidzein, and conjugates in rat blood using LC/ES-MS. Journal of agricultural and food chemistry. 1999;47:3764–3770. doi: 10.1021/jf9902651. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Archives of internal medicine. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biology of reproduction. 2002;67:1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environmental health perspectives. 2009;117:1883–1889. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biology of reproduction. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the developing female reproductive system by phytoestrogens: genistein as an example. Molecular nutrition & food research. 2007;51:832–844. doi: 10.1002/mnfr.200600258. [DOI] [PubMed] [Google Scholar]
- Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annual review of physiology. 1997;59:349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- Khan SI, Zhao J, Khan IA, Walker LA, Dasmahapatra AK. Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reproductive biology and endocrinology : RB&E. 2011;9:91. doi: 10.1186/1477-7827-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lacey M, Bohday J, Fonseka SM, Ullah AI, Whitehead SA. Dose-response effects of phytoestrogens on the activity and expression of 3beta-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. The Journal of steroid biochemistry and molecular biology. 2005;96:279–286. doi: 10.1016/j.jsbmb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of an isoflavone-free soy diet on ovarian hormones in premenopausal women. The Journal of clinical endocrinology and metabolism. 2001;86:3045–3052. doi: 10.1210/jcem.86.7.7684. [DOI] [PubMed] [Google Scholar]
- Makarevich A, Sirotkin A, Taradajnik T, Chrenek P. Effects of genistein and lavendustin on reproductive processes in domestic animals in vitro. The Journal of steroid biochemistry and molecular biology. 1997;63:329–337. doi: 10.1016/s0960-0760(97)00092-7. [DOI] [PubMed] [Google Scholar]
- Medigovic I, Ristic N, Trifunovic S, Manojlovic-Stojanoski M, Milosevic V, Zikic D, Nestorovic N. Genistein affects ovarian folliculogenesis: a stereological study. Microscopy research and technique. 2012;75:1691–1699. doi: 10.1002/jemt.22117. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicological sciences : an official journal of the Society of Toxicology. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Miller WL, Strauss JF., 3rd Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. The Journal of steroid biochemistry and molecular biology. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- Mosca L. Estrogen and atherosclerosis. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 1998;46:381–386. [PubMed] [Google Scholar]
- Myllymaki S, Haavisto T, Vainio M, Toppari J, Paranko J. In vitro effects of diethylstilbestrol, genistein, 4-tert-butylphenol, and 4-tert-octylphenol on steroidogenic activity of isolated immature rat ovarian follicles. Toxicology and applied pharmacology. 2005;204:69–80. doi: 10.1016/j.taap.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Nynca A, Ciereszko RE. Effect of genistein on steroidogenic response of granulosa cell populations from porcine preovulatory follicles. Reproductive biology. 2006;6:31–50. [PubMed] [Google Scholar]
- Nynca A, Sadowska A, Orlowska K, Jablonska M, Ciereszko RE. The Effects of Phytoestrogen Genistein on Steroidogenesis and Estrogen Receptor Expression in Porcine Granulosa Cells of Large Follicles. Folia biologica. 2015;63:119–128. doi: 10.3409/fb63_2.119. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine reviews. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Readhead C, Lobo RA, Kletzky OA. The activity of 3 beta-hydroxysteroid dehydrogenase and delta 4–5 isomerase in human follicular tissue. American journal of obstetrics and gynecology. 1983;145:491–495. doi: 10.1016/0002-9378(83)90323-x. [DOI] [PubMed] [Google Scholar]
- Reinli K, Block G. Phytoestrogen content of foods--a compendium of literature values. Nutrition and cancer. 1996;26:123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. The Journal of steroid biochemistry and molecular biology. 2006;101:216–225. doi: 10.1016/j.jsbmb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Ryokkynen A, Kukkonen JV, Nieminen P. Effects of dietary genistein on mouse reproduction, postnatal development and weight-regulation. Animal reproduction science. 2006;93:337–348. doi: 10.1016/j.anireprosci.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. The Journal of nutrition. 1997;127:263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- Strauss JF, 3rd, Kallen CB, Christenson LK, Watari H, Devoto L, Arakane F, Kiriakidou M, Sugawara T. The steroidogenic acute regulatory protein (StAR): a window into the complexities of intracellular cholesterol trafficking. Recent progress in hormone research. 1999;54:369–394. discussion 394–365. [PubMed] [Google Scholar]
- Tiemann U, Schneider F, Vanselow J, Tomek W. In vitro exposure of porcine granulosa cells to the phytoestrogens genistein and daidzein: effects on the biosynthesis of reproductive steroid hormones. Reproductive toxicology (Elmsford, N.Y.) 2007;24:317–325. doi: 10.1016/j.reprotox.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SA, Cross JE, Burden C, Lacey M. Acute and chronic effects of genistein, tyrphostin and lavendustin A on steroid synthesis in luteinized human granulosa cells. Human reproduction (Oxford, England) 2002;17:589–594. doi: 10.1093/humrep/17.3.589. [DOI] [PubMed] [Google Scholar]
- Whitehead SA, Lacey M. Protein tyrosine kinase activity of lavendustin A and the phytoestrogen genistein on progesterone synthesis in cultured rat ovarian cells. Fertility and sterility. 2000;73:613–619. doi: 10.1016/s0015-0282(99)00580-4. [DOI] [PubMed] [Google Scholar]
- Yoon K, Kwack SJ, Kim HS, Lee BM. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. Journal of toxicology and environmental health. Part B, Critical reviews. 2014;17:127–174. doi: 10.1080/10937404.2014.882194. [DOI] [PubMed] [Google Scholar]
- Zhuang XL, Fu YC, Xu JJ, Kong XX, Chen ZG, Luo LL. Effects of genistein on ovarian follicular development and ovarian life span in rats. Fitoterapia. 2010;81:998–1002. doi: 10.1016/j.fitote.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Zin SR, Omar SZ, Khan NL, Musameh NI, Das S, Kassim NM. Effects of the phytoestrogen genistein on the development of the reproductive system of Sprague Dawley rats. Clinics (Sao Paulo, Brazil) 2013;68:253–262. doi: 10.6061/clinics/2013(02)OA21. [DOI] [PMC free article] [PubMed] [Google Scholar]