Abstract
Objectives
We sought to a) use a novel method of late gadolinium enhancement (LGE) quantification that utilizes normalized intensity measures to confirm the association between LGE extent and atrial fibrillation (AF) recurrence following ablation, and b) examine the presence of interaction and effect modification between LGE and AF persistence.
Background
Recurrent AF after catheter ablation has been reported to associate with the baseline extent of left atrial (LA) LGE on cardiac magnetic resonance (CMR). Traditional methods for measurement of intensity lack an objective threshold for quantification and interpatient comparisons of LGE.
Methods
The cohort included 165 participants (60.0±10.2 years, 77% men, 57% persistent AF) that underwent initial AF ablation. The association of baseline LGE extent with AF recurrence was examined using multivariable Cox proportional hazard models. Multiplicative and additive interaction between AF type and LGE extent were examined.
Results
During 10.2±5.7 months of follow-up, 63 (38.2%) patients experienced AF recurrence. Baseline LGE extent was independently associated with AF recurrence after adjusting for confounders [hazard ratio (HR) 1.5 per 10% increased LGE, P<0.001]. The HR for AF recurrence progressively increased as a function of LGE. The magnitude of association between LGE >35% and AF recurrence was greater among patients with persistent AF (HR 6.5, P=0.001 versus HR 3.6, P=0.001); however, there was no evidence for statistical interaction.
Conclusions
Regardless of AF persistence at baseline, participants with LGE ≤ 35% have a favorable outcome, whereas those with LGE > 35% have a higher rate of AF recurrence in the first year after ablation. These findings suggest a role for a) patient selection for AF ablation using LGE extent, and b) substrate modification in addition to pulmonary vein isolation in patients with LGE extent exceeding 35% of LA myocardium.
Keywords: magnetic resonance imaging, late gadolinium enhancement, atrial fibrillation, catheter ablation
Introduction
Atrial fibrillation (AF) is associated with increased risk for mortality, heart failure, and thromboembolic events, and has a worldwide prevalence of >33.5 million (1-3). Catheter ablation of AF is evolving as an effective therapy for symptomatic AF (4). Recurrent AF after ablation, however, remains a problem and has been reported to associate with the baseline extent of left atrial (LA) late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR)(5). Mechanistically, persistent AF appears to be more reliant upon fibroblast proliferation and myocyte-fibroblast coupling than paroxysmal AF, which is primarily dependent upon pulmonary vein triggers (6-8). Therefore, we sought to a) confirm the association of LA LGE with recurrent AF following ablation, and b) examine the presence of interaction and/or effect modification between LGE and AF persistence prior to ablation.
Methods
Patient Characteristics
The Johns Hopkins Institutional Review Board approved the study and all patients provided written informed consent. Between November 2011 and December 2013, 171 consecutive patients with drug refractory AF who were referred for an initial catheter ablation procedure, were scheduled for a pre-procedure CMR, and consented to participate, were prospectively enrolled.
Cardiac Magnetic Resonance
All subjects underwent pre-procedural CMR as previously described (9-11). Images were acquired using a 1.5 Tesla CMR scanner (Avanto, Siemens, Erlangen, Germany) with a phased array cardiac coil. Pulse oximetry, blood pressure, and ECG monitoring were maintained during the CMR examination. Contrast enhanced 3D fast low angle shot magnetic resonance angiography images were used to define LA and PV anatomy. LGE-CMR scans were acquired approximately 20 minutes following 0.2 mmol/kg gadolinium injection (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Montville, NJ). The LGE sequence was a 3D inversion recovery prepared respiratory triggered and navigated, ECG gated, and fat suppressed fast spoiled gradient echo sequence (repetition time of 2.5-5.5 ms, echo time of 1.52 ms, field of view at 340 mm, flip angle at 10 degrees, inversion time 240-300 ms, 1.3 × 1.3 mm in-plane spatial resolution, 2 mm slice thickness).
LA LGE Extent Quantification
The LGE-CMR images were processed using QMass MR software (Version 7.2, Leiden University Medical Center, Leiden, The Netherlands). The LA myocardium was defined by manual placement of epicardial and endocardial contours by observers that were masked to clinical data (approximately 30 minutes of analysis time per image set). The image intensity ratio (IIR), a previously described (10,11) LGE-CMR analysis technique that normalizes the myocardial image intensity by blood pool intensity, was utilized. The extent of LA LGE was quantified using the 0.97 image intensity threshold previously validated against LA bipolar voltage <0.5 mV (11). For this study, we utilized a threshold of <0.5 mV given prior use of this threshold to demarcate abnormal LA myocardium (12). LA volume measurements included the LA appendage as well as pulmonary vein antra and were limited anteriorly to the mitral valve plane.
Catheter Ablation
All patients underwent wide area circumferential pulmonary vein isolation (PVI) as previously described (10,11). Briefly, a double trans-atrial septal puncture was performed under fluoroscopic guidance. An endocardial map of the left atrium was created with an electroanatomic mapping system (CARTO, Biosense-Webster) and superimposed upon the pre-existing CMR image of the chamber. With routine hemodynamic and electrocardiographic monitoring, a four-millimeter-tipped irrigated ablation catheter (Thermocool, Biosense-Webster, Diamond Bar, CA) was advanced under fluoroscopic guidance to the left atrium. Circumferential lesions were applied surrounding the pulmonary veins. Additional ostial lesions were targeted to remaining pulmonary vein potentials using a circular multipolar electrode-mapping catheter (Lasso, Biosense, Diamond Bar, CA, USA). Entrance block into the pulmonary veins was confirmed in all patients as the primary procedural endpoint. Additionally, when possible by demonstration of PV capture, exit block was documented. To prevent short-term recurrences of AF, previously ineffective antiarrhythmic medications were continued for at least 3 months.
Clinical Follow-up
Recurrent AF was defined based on the 2012 HRS Consensus Document as symptomatic or asymptomatic AF, atrial tachycardia, or atrial flutter of > 30 seconds duration after a 3-month blanking period (4). Close communication via clinic visits and phone was maintained with all patients following the ablation. If symptoms suggestive of an arrhythmia occurred, patients were asked to undergo 24-hour Holter monitoring or 30-day event monitoring depending upon symptom frequency. In the absence of reported symptoms, patients were evaluated for recurrence at 6 and 12 months.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as frequencies and percentages. Differences between group means were evaluated with Student's t-tests (continuous variables) or chi-square analysis (categorical variables) as appropriate. The association of baseline LA LGE extent with recurrent AF was examined using multivariable Cox proportional hazards models adjusting for potential confounders including age, LA volume, AF type, and history of congestive heart failure, coronary or peripheral vascular disease. To explore a potential non-linear association between LA LGE extent and recurrent AF, we used a restricted cubic spline model with incorporated knots at the 5th, 50th, and 95th percentiles (13). Results were also visualized using Kaplan Meier Survival Curves and Log Rank tests. The possibility of multiplicative and additive interaction between our main effect variable (LA LGE extent) and AF type, as well as effect modification by AF type were explored (14). Additive interaction was explored using the relative excess risk due to interaction, the attributable proportion due to interaction, and the synergy index (15,16). Statistical analyses were performed using STATA software (Version 12, STATA Corp, Texas).
Results
Patients Characteristics
Of 171 patients, 6 (3.5%) were excluded due to poor CMR image quality that precluded analysis by consensus of 2 observers. Detailed demographics of the remaining 165 patients are summarized in Table 1. The mean age was 60.0±10.2 years, and 71 (43.0%) had persistent AF. The mean CHA2DS2-VASc score (17) was 1.6±1.5. Of all patients, 76 (46.1%) had prior history of hypertension and 19 (11.5%) had prior history of congestive heart failure. The mean baseline LA LGE extent (corresponding to bipolar voltage <0.5 mV) (11) was 35.9±14.8% (median 35%, range 7-72%).
Table 1.
Patient Demographics
| Variables | Total (n=165) | Paroxysmal (n=94) | Persistent (n=71) | P |
|---|---|---|---|---|
| Age, years | 60.0±10.2 | 60.9±10.6 | 58.8±9.5 | 0.189 |
| Male gender, n (%) | 127 (77.0) | 65 (69.2) | 62 (87.3) | 0.006 |
| Caucasian Ethnicity, n (%) | 144 (87.8) | 84 (89.4) | 60 (85.7) | 0.644 |
| Hypertension, n (%) | 76 (46.1) | 40 (42.6) | 36 (50.7) | 0.298 |
| Diabetes, n (%) | 11 (6.7) | 5 (5.3) | 6 (8.5) | 0.425 |
| Congestive Heart Failure, n (%) | 19 (11.5) | 7 (7.5) | 12 (16.9) | 0.060 |
| Prior Stroke/TIA, n (%) | 10 (6.1) | 7 (7.5) | 3 (4.2) | 0.265 |
| Coronary/Peripheral Vascular Disease, n (%) | 20 (12.1) | 9 (9.6) | 11 (15.5) | 0.249 |
| Obstructive Sleep Apnea, n (%) | 39 (23.6) | 20 (21.3) | 19 (26.8) | 0.412 |
| Body Mass Index, kg/m2 | 28.9±5.9 | 27.7±5.1 | 30.5±6.4 | 0.003 |
| CHA2DS2-Vasc Score | 1.6±1.5 | 1.6±1.5 | 1.5±1.3 | 0.517 |
| AF Duration, years | 5.6±6.4 | 5.3±5.5 | 6.0±7.4 | 0.546 |
| Left Ventricular Ejection Fraction (%) | 57.3±6.8 | 58.2±6.2 | 56.1±7.3 | 0.044 |
| LA Volume (mL) | 153.8±50.4 | 145.8±45.4 | 164.2±54.8 | 0.021 |
| LA LGE Extent, (% of LA Myocardium) | 35.9±14.8 | 31.9±13.1 | 41.1±15.3 | <0.001 |
| Number of Image Planes per Patient, n | 21.9±2.7 | 21.8±2.7 | 22.1±2.4 | 0.536 |
Patients with persistent AF were more likely to be male (87.3% versus 69.2%, P=0.006), to have higher body mass index (30.5±6.4 versus 27.7±5.1 kg/m2, P=0.003), to have lower left ventricular ejection fraction (56.1±7.3 versus 58.2±6.2 %, P=0.044), to have higher LA volume (164.2±54.8 versus 145.8±45.4, P=0.021) and to have greater LA LGE extent than those with paroxysmal AF (41.1±15.3 versus 31.9±13.1%, P<0.001). The mean LGE sequence duration was 5.5±1.6 minutes. Eighteen (10.9%) patients were in AF during CMR image acquisition. Image quality was adequate in the subset of patients with AF; however, CMR scan duration was longer for those in AF (7.4±2.2 vs. 5.2±1.3 minutes; p <0.001).
AF Recurrence
Of 165 patients, 63 (38.2%) had AF recurrence during the follow-up duration of 307±170 days. Of 71 patients with persistent AF, 29 (40.9%) had AF recurrence; whereas of 94 patients with paroxysmal AF 34 (36.2%) had AF recurrence. In univariate analyses, age, history of congestive heart failure, CHA2DS2-VASc Score, and the extent of LA LGE were associated with AF recurrence (Table 2).
Table 2.
Characteristics of patients with versus those without AF recurrence
| Variables | Total (n=165) | No AF Recurrence (n=102) | AF Recurrence (n=63) | P |
|---|---|---|---|---|
| Persistent AF, n (%) | 71 (43.0) | 42 (41.2) | 29 (46.0) | 0.541 |
| Age, years | 60.0±10.2 | 58.6±10.4 | 62.4±9.3 | 0.018 |
| Male gender, n (%) | 127 (77.0) | 81 (79.4) | 46 (73.0) | 0.343 |
| Caucasian Ethnicity, n (%) | 144 (87.8) | 87 (86.1) | 57 (90.5) | 0.771 |
| Hypertension, n (%) | 76 (46.1) | 46 (45.1) | 30 (47.6) | 0.752 |
| Diabetes, n (%) | 11 (6.7) | 5 (4.9) | 6 (9.5) | 0.248 |
| Congestive Heart Failure, n (%) | 19 (11.5) | 7 (6.9) | 12 (19.1) | 0.017 |
| Prior Stroke/TIA, n (%) | 10 (6.1) | 6 (5.9) | 4 (6.4) | 0.903 |
| Coronary/Peripheral Vascular Disease, n (%) | 20 (12.1) | 9 (8.8) | 11 (17.5) | 0.099 |
| Obstructive Sleep Apnea, n (%) | 39 (23.6) | 21 (20.6) | 18 (28.6) | 0.241 |
| Body Mass Index, kg/m2 | 28.9±5.9 | 28.3±5.7 | 30.0±6.0 | 0.064 |
| CHA2DS2-Vasc Score | 1.6±1.5 | 1.4±1.3 | 1.9±1.6 | 0.041 |
| AF Duration, years | 5.6±6.4 | 5.6±6.1 | 5.7±6.8 | 0.922 |
| Left Ventricular Ejection Fraction (%) | 57.3±6.8 | 57.3±6.9 | 57.3±6.7 | 0.940 |
| LA Volume (mL) | 153.8±50.4 | 151.3±49.2 | 157.9±52.5 | 0.423 |
| LA LGE Extent, (% of LA Myocardium) | 35.9±14.8 | 32.4±13.3 | 41.5±15.4 | <0.001 |
| Number of Image Planes per Patient, n | 21.9±2.7 | 21.6±2.9 | 22.3±2.1 | 0.148 |
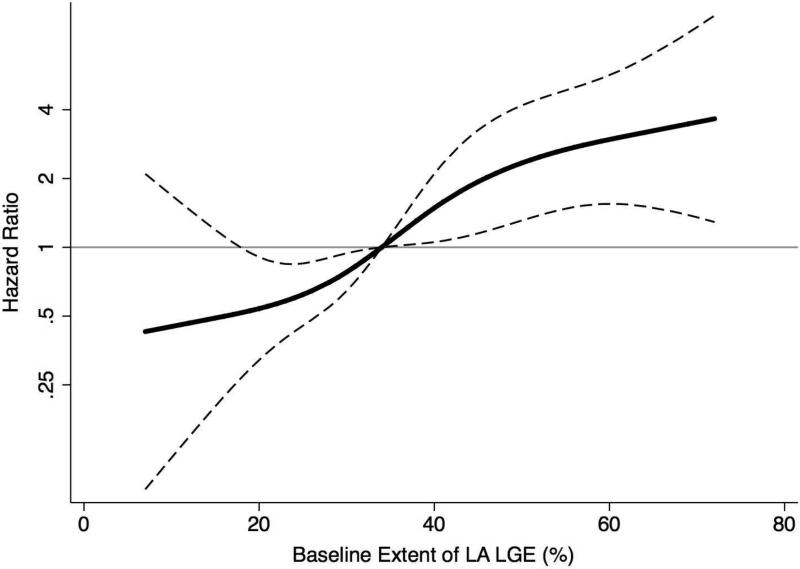
In unadjusted analysis, each 10% increase in the extent of baseline LA LGE was associated with 1.5 fold-increased hazard of AF recurrence [hazard ratio (HR) 1.5, 95% confidence interval (CI): 1.3-1.8, P<0.001]. In a multivariable Cox proportional hazard model adjusting for age, LA volume, AF type, and history of congestive heart failure, coronary or peripheral vascular disease, each 10% increase in the extent of baseline LA LGE remained independently associated with AF recurrence (HR 1.5, 95% CI: 1.3-1.8, P<0.001). The multivariable adjusted spline regression model revealed no evidence for non-linear effects (Figure 1). The HR for AF recurrence progressively increased with increasing extent of baseline LA LGE extent.
Figure 1. Multivariable Adjusted Hazard Ratios for AF Recurrence as a Function of the LA LGE Extent.
The solid black indicates multivariable-adjusted hazard ratios (HRs) for AF recurrence as a function of the baseline extent of LA LGE using restricted quadratic splines. The dashed lines delineate the upper and lower 95% CI. The horizontal gray line indicates an HR of 1.0. The model was adjusted for age, LA volume, AF type, and history of congestive heart failure, coronary or peripheral vascular disease.
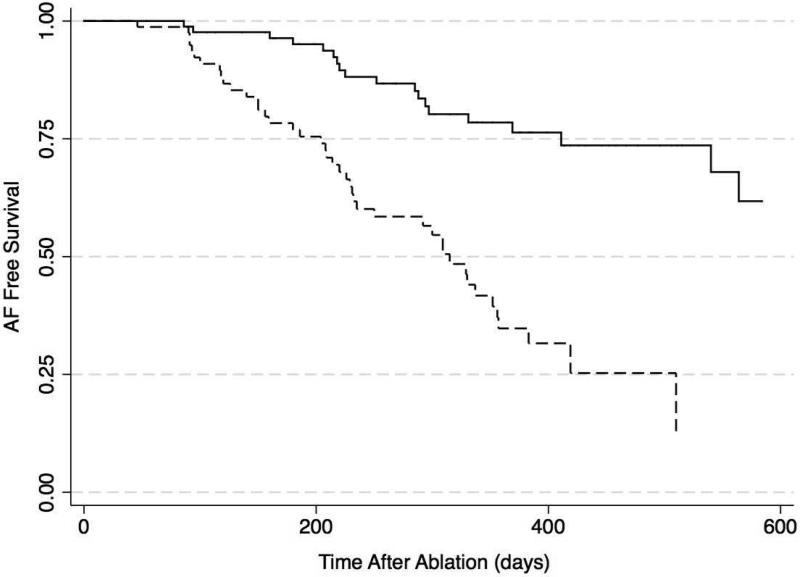
The extent of LA LGE extent was stratified at the median. In the multivariable Cox proportional hazard model adjusting for age, LA volume, AF type, and history of congestive heart failure, coronary or peripheral vascular disease, baseline LGE extent >35% was associated with AF recurrence (HR 3.8, 95% CI: 2.1-6.8, P<0.001). Figure 2 demonstrates Kaplan Meier plots for time to AF recurrence in patients stratified by median LGE extent. Patients with LGE ≤ 35% of LA myocardium had prolonged freedom from AF (15% with AF recurrence at 288±40 days) compared to those with LGE extent >35% (15% with AF recurrence at 140±18 days).
Figure 2. Kaplan–Meier AF Free Survival Curves After PVI in Participants Stratified by Median LA LGE Extent.
The figure illustrates time to first AF recurrence in 86 (52%) participants with LGE extent ≤ 35% (solid line) and 79 (48%) participants with LGE extent > 35% of LA myocardium (dashed line) (P<0.001).
Effect Modification and Statistical Interaction with AF Type
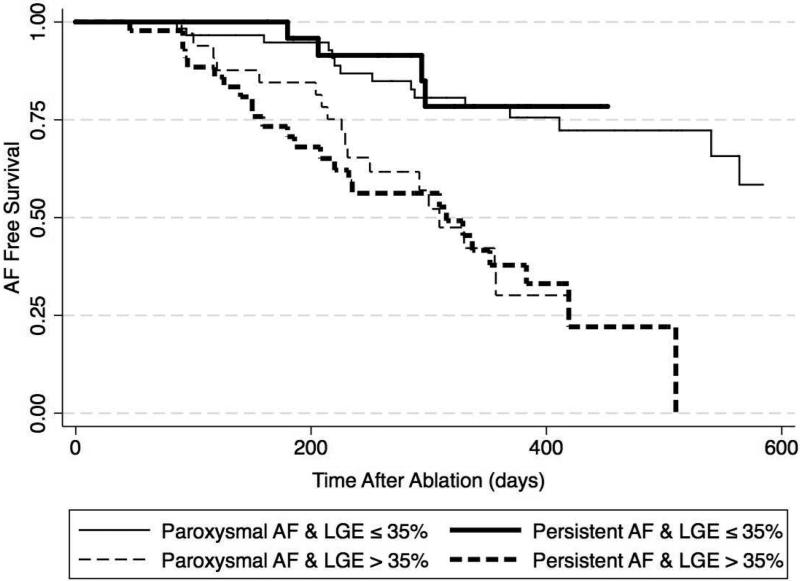
There was no evidence of multiplicative interaction between AF type and baseline LA LGE extent in the multivariable Cox proportional hazards model of the association between LGE extent and AF recurrence (P=0.511). Similarly, the measures relative excess risk due to interaction (1.29, 95% CI −1.32-3.90), attributable proportion due to interaction (0.31, 95% CI −0.23-0.84), and synergy index (1.68, 95% CI 0.53-5.35) did not suggest additive interaction between AF type and baseline LA LGE extent. However, as demonstrated in Figure 3, the magnitude of association between baseline LGE extent >35% and AF recurrence was attenuated when the cohort was stratified based upon AF type. In patients with paroxysmal AF the HR for AF recurrence with baseline LGE extent >35% was 3.6 (95% CI: 1.7-7.7, P=0.001) after adjusting for age, LA volume, and history of congestive heart failure, coronary or peripheral vascular disease. In contrast, in patients with persistent AF, the HR for AF recurrence with LGE extent >35% was 6.5 (95% CI: 2.1-19.6, P=0.001) after adjusting for the same covariates. Figure 4 demonstrates Kaplan Meier plots for time to AF recurrence in patients stratified by median LGE extent as well as AF persistence. Patients with LGE ≤ 35% of LA myocardium had prolonged freedom from AF regardless of AF persistence prior to ablation (15% with AF recurrence at 252±45 days and 294±7.6 days for patients with paroxysmal and persistent AF, respectively). In contrast, those with LGE extent >35% had early recurrence regardless of AF persistence prior to ablation (15% with AF recurrence at 156±47 days and 126±22 days for patients with paroxysmal and persistent AF, respectively).
Figure 3. Forest Plot of Hazard Ratios for AF Recurrence if LGE Extent > 35% After Stratification by AF Type.
The forest plot summarizes multivariable-adjusted HRs for AF Recurrence. Models were adjusted for age, LA volume, AF type, and history of congestive heart failure, coronary or peripheral vascular disease. The magnitude of association between AF recurrence and LGE extent was higher among patients with persistent AF; however, there was no evidence for statistical interaction.
Figure 4. Kaplan–Meier AF Free Survival Curves After PVI in Participants Stratified by Median LA LGE Extent and AF Type.
The figure illustrates time to first AF recurrence in 60 (36%) participants with paroxysmal AF and LGE extent ≤ 35% (thin solid line), 26 (16%) participants with persistent AF and LGE extent ≤ 35% (thick solid line), 34 (21%) participants with paroxysmal AF and LGE extent > 35% (thin dashed line), and 45 (27%) participants with persistent AF and LGE extent > 35% of LA myocardium (thick dashed line) (P<0.001).
Discussion
The main finding of the present study is that regardless of AF persistence at baseline, LGE ≤ 35% of LA myocardium, measured using the IIR, is associated with a favorable outcome, whereas LGE > 35% is associated with early AF recurrence post ablation.
Prior Studies of the Association of LA LGE with AF Recurrence Post PVI
Our study is in agreement with prior reports of an association between AF recurrence and LA LGE extent. Peters and colleagues’ were the first to utilize LGE-MRI for detection of post ablation PVI lesions within LA myocardium (18). Several follow on studies reported that AF recurrence was negatively associated with the extent of post-ablation LGE (19,20). Marrouche and colleagues recently completed a multicenter, observational cohort study of MRI prior to PVI in 272 patients with AF and reported 1.06 fold increase in AF hazard with each 1% increase in LGE extent (5). In the study by Marrouche and colleagues, as well as all other prior studies of the association between LGE extent and AF recurrence, LGE extent has been estimated by the relative intensity of contrast enhancement. CMR signal intensity is measured in “arbitrary units” with variable magnitude and scale across examinations. Although LA wall image intensity on LGE-CMR primarily varies as a function of gadolinium retention in fibrotic regions, it is also affected by parameters such as surface coil proximity, contrast dose, delay time of image acquisition after contrast injection, patient hematocrit, glomerular filtration rate, and body mass index. In order to overcome the limitations of the “arbitrary unit scale” as well as improve objectivity and generalizability, we utilized the IIR for quantification of LGE. In a prior study, we showed that the IIR is closely associated with local intracardiac bipolar LA voltage measures, and exhibits favorable intraclass correlations (> 0.9) for inter- and intra-observer variability and reliability of measurement (11). It should be noted, however, that given the variable methodologies for acquisition and analysis of LGE images, LGE prevalence and extent should not be compared among the different studies. It is our hope that utilization of a standardized technique as described here, will improve the future ability to compare results across cohorts. Nevertheless, the overall results are consistent and increasing LGE appears to be associated with AF recurrence in all studies to date.
Interaction and Effect Modification with AF Type
To our knowledge, this is the first study to examine the presence of interaction or effect modification between LGE and AF type. Paroxysmal AF appears to be related to triggered activity primarily from pulmonary vein foci (6). Therefore, it was expected that AF ablation with PVI would be more efficacious in the subgroup of patients with paroxysmal AF (21). In contrast, persistent AF appears to be more reliant upon myocyte-fibroblast coupling resulting in non pulmonary vein triggered activity, as well as enhanced AF perpetuation due to fibroblast proliferation (7,8). We hypothesized that the association of AF recurrence with baseline LGE extent would be dependent upon AF type prior to ablation. In this study, we found no evidence of statistical interaction or effect modification between AF type and LGE extent. However, the magnitude of association between LGE extent and AF recurrence was stronger in patients with persistent AF. As a result, patients with persistent AF that had ≤35% LGE had equivalent outcomes as those with paroxysmal AF and limited LGE. Similarly, patients with >35% LGE had poor outcomes regardless of AF type. This important result is contrary to current beliefs regarding uniformly lower AF ablation efficacy in persistent AF that result in discouragement of many such patients from undergoing ablation. Additionally, extensive ablations in addition to PVI have been employed with the goal of improving efficacy in persistent AF patients. Recent results suggest that additional ablation does not necessarily reduce AF recurrence (22,23). Our results suggest that patients with persistent AF and LGE ≤ 35% are likely to benefit from PVI without additional extensive ablations.
Limitations
The study was performed in a single tertiary care center, thus the sample size is relatively small, and the results may not be generalizable to centers with less experience with LGE-CMR or lower PVI volumes. The limited sample size may have decreased study power to detect statistical interaction between AF type and LGE extent. However, it is important to note that due to the protocol homogeneity of this single center study, only 3.5% of patients had to be excluded due to poor image quality versus 17% in the comparable multicenter study (5). The LGE-CMR sequence used in this study provided a 1.3 × 1.3 mm in-plane resolution. Atrial wall thickness may be near the limit of image resolution in some cases. Thus the LGE analysis may have included blood-pool or epicardial fat in some cases. Data regarding the duration of continuous AF was unavailable in this cohort. The possibility of statistical interaction between baseline LGE extent and AF duration in their association with AF recurrence warrants further investigation. Finally, although patients were closely monitored via symptom prompted and scheduled (6 and 12 month) electrocardiographic monitoring, continuous monitoring was not performed and some asymptomatic recurrences may have been missed.
Conclusions
Regardless of AF persistence at baseline, and after adjusting for potential confounders, participants with LGE ≤ 35% have a favorable outcome, whereas those with LGE > 35% have early AF recurrence after ablation. These findings suggest that patients with LGE ≤ 35% of LA myocardium should be considered as candidates for simple PVI regardless of AF persistence. Additionally, our results suggest that substrate modification ablation strategies in addition to PVI may have a role when LGE extent exceeds 35% of LA myocardium.
Perspectives.
Competency in Medical Knowledge
Among patients with paroxysmal or persistent AF, a baseline contrast CMR scan of the LA with >35% LGE is associated with high AF recurrence following ablation. In contrast, regardless of AF persistence, LGE burden ≤35% is associated with a favorable response to ablation.
Translational Outlook
Future studies are warranted to examine a) the utility of CMR-based patient selection for AF ablation and b) modification of current ablation methodologies in patients with LGE extent exceeding 35% of LA myocardium.
Acknowledgments
Funding: The study was funded by NIH grants K23HL089333 and R01HL116280 as well as a Biosense-Webster grant to Dr. Nazarian, the Dr. Francis P. Chiaramonte Foundation, The Norbert and Louise Grunwald Cardiac Arrhythmia Fund, the Marv Weiner Cardiac Arrhythmia Fund, and the Marilyn and Christian Poindexter Research Fund.
Abbreviations
- AF
atrial fibrillation
- LA
left atrial
- LGE
late gadolinium enhancement
- CMR
cardiac magnetic resonance
- IIR
image intensity ratio
- PVI
pulmonary vein isolation
- SD
standard deviation
- HR
hazard ratio
- CI
confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Nazarian is a scientific advisor to Medtronic, CardioSolv, and Biosense Webster Inc and principal investigator for research funding to Johns Hopkins University from Biosense-Webster Inc.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. Journal of the American College of Cardiology. 2001;37:371–8. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:632–696 e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. Jama. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 6.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England journal of medicine. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Inoue K, Kurotobi T, Kimura R, et al. Trigger-based mechanism of the persistence of atrial fibrillation and its impact on the efficacy of catheter ablation. Circulation Arrhythmia and electrophysiology. 2012;5:295–301. doi: 10.1161/CIRCEP.111.964080. [DOI] [PubMed] [Google Scholar]
- 8.Wilber DJ. Fibroblasts, focal triggers, and persistent atrial fibrillation: is there a connection? Circulation Arrhythmia and electrophysiology. 2012;5:249–51. doi: 10.1161/CIRCEP.111.968750. [DOI] [PubMed] [Google Scholar]
- 9.Beinart R, Khurram IM, Liu S, et al. Cardiac magnetic resonance T1 mapping of left atrial myocardium. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:1325–31. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumoto K, Habibi M, Gucuk Ipek E, et al. Comparison of preexisting and ablation-induced late gadolinium enhancement on left atrial magnetic resonance imaging. Heart rhythm : the official journal of the Heart Rhythm Society. 2014 doi: 10.1016/j.hrthm.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurram IM, Beinart R, Zipunnikov V, et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11:85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Wazni OM, Marrouche NF, et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation - An independent predictor of procedural failure. Journal of the American College of Cardiology. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–517. doi: 10.1016/j.jclinepi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 14.VanderWeele TJ. On the Distinction between Interaction and Effect Modification. Am J Epidemiol. 2009;169:S34–S34. doi: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 15.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 16.Hallqvist T, Ahlbom A, Diderichsen F, Reuterwall C. How to evaluate interaction between causes: A review of practices in cardiovascular epidemiology. J Intern Med. 1996;239:377–382. doi: 10.1046/j.1365-2796.1996.431782000.x. [DOI] [PubMed] [Google Scholar]
- 17.Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Brit Med J. 2011:342. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–5. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 19.McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. Journal of the American College of Cardiology. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 20.Peters DC, Wylie JV, Hauser TH, et al. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovascular imaging. 2009;2:308–16. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Journal of the American Heart Association. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma A, Sanders P, Macle L, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial-Part II (STAR AF II): Design and Rationale. Am Heart J. 2012;164:1–+. doi: 10.1016/j.ahj.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. The New England journal of medicine. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]