Abstract
The continuing horrors of military conflicts and terrorism often involve the use of chemical warfare agents (CWAs) and toxic industrial chemicals (TICs). Many CWA and TIC exposures are difficult to treat due to the danger they pose to first responders and their rapid onset that can produce death shortly after exposure. While the specific mechanism(s) of toxicity of these agents are diverse, many are associated either directly or indirectly with increased oxidative stress in affected tissues. This has led to the exploration of various antioxidants as potential medical countermeasures for CWA/TIC exposures. Studies have been performed across a wide array of agents, model organisms, exposure systems, and antioxidants, looking at an almost equally diverse set of endpoints. Attempts at treating CWAs/TICs with antioxidants have met with mixed results, ranging from no effect to nearly complete protection. The aim of this commentary is to summarize the literature in each category for evidence of oxidative stress and antioxidant efficacy against CWAs and TICs. While there is great disparity in the data concerning methods, models, and remedies, the outlook on antioxidants as medical countermeasures for CWA/TIC management appears promising.
Graphical abstract
1.0 Background on CWAs and TICs
Since the dawn of warfare, the threat of chemical weapons use has been one of the most serious and alarming concerns to ground troops, in part because of their devastating ability to incapacitate soldiers and the challenges associated with treating this type of injury. Concerns over the exposure to toxic chemicals are not limited to the battlefield however; civilian exposures to toxic chemicals in the workplace are far more regular and can also have lethal consequences. Chemical warfare agents (CWAs) and toxic industrial chemicals (TICs) are an exceptionally toxic group of chemicals, exposure to which may result in death or injury. The most common sites of exposure include the skin, eyes and lung. Lung exposures by inhalation are the most difficult to manage due to rapid absorption and difficulties in decontamination.
1.1 History and Relevance of CWA and TIC exposures to contemporary military conflicts and civilian Threats
Large scale chemical warfare began in World War I and the threat of CWA deployment against troops has forever changed the way wars are fought. While CWAs have seen extensive use in many conflicts around the globe, attempts have been made to outlaw their use. In 1925 the Geneva Convention prohibited the use of chemical weapons in warfare, but many nations continued to stockpile CWAs[1]. The chemical weapons convention of 1993 further prohibited the stockpiling of these agents which led to the destruction of many CWA stockpiles[2]. However, despite the ban on the use and storage of CWAs, many have not yet been destroyed[1]. Since some of the banned CWAs are relatively easy to synthesize, there may be undeclared stockpiles of these agents around the world. The UN estimates that there have been more than one million casualties globally since World War I and large scale use of CWAs have been recently reported in Syria[3]. For these reasons, they remain an ever present threat to the world population from potential (illegal) military use, terrorist attacks, or unintentional exposures from CWA storage depots.
Another group of chemicals, classified as TICs, also present a significant threat to human life. These are highly toxic chemicals which have many industrial uses and are produced in large amounts for manufacturing. The widespread use of these chemicals has led to accidental occupational exposures as well as exposures to residents of surrounding communities. Additional threats come from the potential for dangerous spills during transportation (such as was seen in the 2005 Graniteville, SC accident) and easy access opportunities for terrorists planning on misusing TICs for nefarious purposes[4]. The potential for CWAs/TICs to cause harm to civilian or military personnel is very real, which makes it vital to discover effective medical countermeasures against these agents.
2.0 Overview of the evidence for oxidative stress in CWAs and TICs induced injury
While the specific mechanisms of action vary by agent, a common thread linking many of the CWAs and TICs is the observation that they produce oxidative stress in target tissues. Whether the agents are directly generating reactive oxygen species (ROS)/reactive nitrogen species (RNS) or whether it is a consequence of other damaging interactions within cells is not always clear. The damaging potential of these agents is often attributed to redox imbalances within the cell. ROS/RNS may cause irreversible damage to DNA, cellular proteins and other macromolecules once the antioxidant defenses of the cell and/or tissue have been compromised. ROS/RNS can also readily disrupt cellular signaling pathways leading to apoptosis or necrosis. The high reactivity and low specificity of these chemicals often means they are not limited to a single cytotoxic pathway within cells, but instead may initiate damage to multiple cellular targets. The role oxidative stress plays in the propagation of damage may vary by toxin, due to the multiple mechanisms of action. This commentary will focus on identifying the presence of oxidative imbalances in models of toxicity for each agent. Chemicals discussed are divided into categories by their toxic effects; these include vesicating agents, choking agents, blood agents, and TICs.
2.1 Vesicating agents
Vesicating agents such as sulfur mustard (SM) and nitrogen mustard (NM) are named for the characteristic chemical burns they produce, which results in the formation of blisters (vesicles). These CWAs are particularly destructive to the skin, eyes, and lungs where they may cause painful blisters, blindness, edema, and respiratory distress[5]. Vesicating agents are highly toxic, and while exposures to these agents can be lethal, their primary value lies in incapacitating their victims and causing fear [6].
2.1.1 Sulfur Mustard and analogs
SM (bis-2-chloroethyl sulfide, Figure 1A) has a long history of military use dating back to 1917, and is considered to pose a threat to this day from both military and terrorist groups[7]. There are currently no FDA approved effective medical countermeasures for SM, and even non-lethal exposures may lead to skin, eye, and pulmonary dysfunction for many years. SM is a CWA that alkylates a wide variety of cellular macromolecules through the formation of a cyclic sulfonium ion intermediates[8]. While the exact molecular mechanism of sulfur mustard-mediated injury has not been elucidated, there are three commonly accepted cellular macromolecule targets which are believed to be responsible for the majority of SM induced damage. The first mechanism involves direct damage to DNA by SM and can occur either by alkylation at a single base or by cross-linking of base pairs utilizing both of the terminal chloroethyl groups. DNA alkylation produces strand breaks, genotoxicity, and triggers DNA repair which depletes cellular NAD stores[9]. Another mechanism of SM damage is through interactions with proteins, leading to their alkylation and subsequent inactivation[7]. A third possible mechanism of action is through the depletion of cellular GSH reserves, disrupting the redox equilibrium, causing the initiation of lipid peroxidation cascades and indirectly leading to apoptosis[5,10]. SM sulfonium ions may also be directly reduced by cytochrome P450 reductase to form carbon-based free radicals capable of forming oxygen free radicals[11]. Additionally, inducible nitric oxide synthase (iNOS) is upregulated following SM exposures, which contributes to the nitrosative stress through the formation of peroxynitrite[12]. Evidence supporting one or all of these mechanisms of action include findings of increased lipid peroxidation, depletion of GSH, and increases in 8-hydroxy-2-deoxyguanosine (8-OHdG)[7,10]. Lethal SM exposures produce bronchial airway casts that are associated with restrictive airflow and obstruction and can be lethal within 48 hours[13,14]. Animals that survive the airway obstruction go on to die with lung fibrosis and bronchiolitis obliterans at 4 to 6 weeks post exposure[15,16].
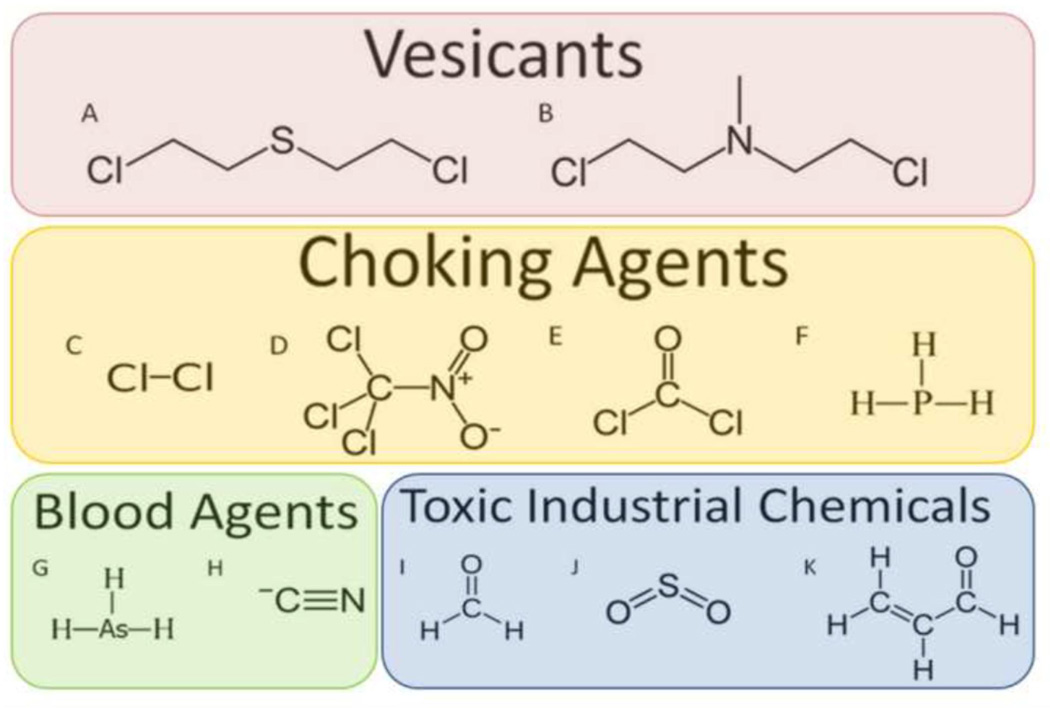
Figure 1. Selected Chemical Structures of Chemical Warfare Agents and Toxic Industrial Chemicals.
A) Sulfur Mustard (Bis(2-chloroethyl)sulfide); B) Nitrogen Mustard (Bis(2-chloroethyl)methylamine); C) Chlorine; D) Chloropicrin; E) Phosgene; F) Phosphine; G) Arsine; H) Hydrogen Cyanide; I) Formaldehyde; J) Sulfur Dioxide; and K) Acrolein.
Additional mechanistic studies have been performed with 2-chloroethyl ethyl sulfide (CEES), a monofunctional SM analog. CEES has been used as a model to evaluate antioxidant effects on SM induced acute lung injury [17,18]. Half mustards can only alkylate cellular macromolecules and lack the ability to crosslink. Many of the same types of injury to the skin, eyes, and lungs have been documented with CEES as have been seen in SM[18,19]. CEES has been reported to increase cellular ROS and RNS, produce oxidative DNA damage, increase mitochondrial ROS formation, and mitochondrial dysfunction [20]. CEES exposures also result in the formation of obstructive airway casts that has been observed with SM[13,14,21].
2.1.2 Nitrogen Mustard
NM ((Bis(2-chloroethyl)methylamine), Figure 1B) is a vesicating agent which was stockpiled for use as a chemical weapon. NM is particularly toxic to the skin, and able to cause systemic toxicity from topical absorption[22]. However unlike SM, NM has never been used in combat and fell out of favor for military use due to difficulties with its storage[23]. NM may have a similar mechanism of action as SM due to its high reactivity, ability to alkylate DNA, and cause oxidative stress. NM mechanism of action produces DNA alkylation that is mediated with an immonium ion instead of a sulphonium ion[22]. NM is believed to directly cause oxidative stress in affected tissues, which then mediates activation of signaling pathways leading to cell microvesication and death[24].
2.2 Choking agents
A choking agent, also known as a pulmonary agent, is one whose main toxic effects are characterized by lung irritation, damage, and pulmonary edema[6].
2.2.1 Chlorine gas
Chlorine (Cl2, Figure 1C) is a choking agent and a commonly utilized TIC with strong oxidizing potential. Massive amounts of chlorine are produced and transported each year for industrial purposes leading to the potential for accidental civilian exposures. Chlorine has been used many times as a CWA by military forces in the early 1900’s, and may have been used by insurgents in the Middle East as recently as 2015, making it both a CWA and a TIC. Chlorine inhalation can result in fatal acute lung injury due to its rapid hydrolysis to hydrochloric acid (HCl) and hypochlorous acid (HOCl) in the lung epithelial lining fluid[25]. HOCl is a ROS that can directly oxidize and chlorinate cellular macromolecules[26]. Upon inhalation of Cl2, direct oxidative injury can occur causing extensive epithelial damage[27]. Inhalation of high concentrations of chlorine gas can result in death within minutes. Chlorine gas exposures at lower doses results in acute lung injury, leading to pneumonitis, pulmonary edema, decreased lung function, and reactive airway disease that resembles asthma[27]. The oxidation of macromolecules in the lung is believed to be a primary contributor to chlorine toxicity, a hypothesis supported by lowered GSH levels in lung tissues[25]. Chlorine gas inhalation can disrupt nitric oxide signaling pathways, which may further enhance its toxicity[28].
2.2.2 Chloropicrin
Chloropicrin (trichloronitromethane, Figure 1D) was used as a CWA during WW1 where its function as a strong lacrimator and lung irritant made it a useful harassing agent. Currently, chloropicrin is primarily used as a fungicide for soil fumigation[29]. Accidental exposures to this chemical causes irritation to the mucous membranes, nausea, vomiting, and difficulties breathing[30]. In severe cases, exposure resulted in pulmonary edema and death[31]. Although little is understood about the mechanism of toxicity, it is known that chloropicrin forms protein adducts with biological thiols and may act through inhibition of thiol-containing enzymes[32]. Chloropicrin is a potent inhibitor of mitochondrial enzymes including the pyruvate dehydrogenase complex[29]. Cell culture experiments have demonstrated that chloropicrin produces a dose dependent increase in ROS[30,33] .
2.2.3 Phosg ene
Phosgene (COCl2, Figure 1E) is a highly potent pulmonary agent, which was used during WW1 as a CWA. Inhalation of phosgene causes acute lung injury (ALI) which may result in death. Phosgene is also a TIC, commonly used in modern times for industrial chemosynthesis reactions. Phosgene rapidly hydrolyzes to HCl and CO2 in solution, and also quickly reacts with amino, hydroxyl, hydrazino, and sulfhydryl groups[34]. Inhalation of phosgene has been shown to alter non-protein sulfhydryl concentrations and lead to the up-regulation of antioxidant enzymes[35]. The exact means of phosgene induced injury remains unclear though studies indicate that oxidative stress is likely a primary mechanism of toxicity[36]. Increases in markers of lipid oxidation and decreases in GSH are observable in rat lung tissue after inhalation exposures to phosgene gas[37] .
2.2.4 Phosphine
Phosphine (PH3, Figure 1F) was used as a CWA used during WW1 and is classified as a warfare and terrorism agent by the Agency for Toxic Substances and Disease Registry (ATSDR). Currently, it is used by several manufacturing industries as well as being widely used as a pesticide [38]. Phosphine increased ROS formation in cultured liver cells along with several markers of oxidative stress[39]. Research shows that phosphine disrupts metabolic processes though interactions with mitochondrial complex IV [32]. As a byproduct of this, several markers of oxidative stress have been confirmed in several models of phosphine toxicity [38,40].
2.3 Blood agents
Blood agents are agents that exhibit their damaging effects by being absorbed into the blood, usually targeting blood cells, oxygen transport, or oxygen utilization[41]. Two commonly studies blood agents are arsine and cyanide.
2.3.1 Arsine
Arsine(AsH3, Figure 1G) is a highly toxic form of arsenic which was proposed for use as a CWA due to its lethality at concentrations lower than its odor threshold. Arsine was never used as a CWA however, due to its disadvantages compared to phosgene[42,43]. Occupational exposures are also a concern since arsine is regularly used in the semiconductor industry. Accidental exposures have occurred in workplace environments that refine metals when arsine is formed as a byproduct of industrial processes[44]. Arsine acts primarily as a hemolytic agent, which can produce toxicities ranging from delayed GI effects/chest pain to death[45]. Once absorbed, arsine impairs oxygen transport in the blood[42]. This effect may be related to the production of ROS and formation of hemoglobin adducts from thiol binding[42]. It has been hypothesized that depletion of intracellular GSH may play a role in hemolysis[46].
2.3.2 Cyanide
Hydrogen Cyanide (CN, Figure 1H) has a history of use as a CWA during WW1, but chemical stability issues limited its effectiveness. Exposures to cyanide are not uncommon due to the widespread use of cyanide for industrial chemical production. Cyanide is one of the most potent inhibitors of mitochondrial respiration[47]. Cyanide tightly binds and inhibits cytochrome C oxidase in the mitochondrial electron transport chain leading to an inability utilize oxygen to replenish cellular ATP[48]. Cyanide has long been known to stimulate ROS/RNS that contribute to its neurological and cardiovascular toxicity[49–52]. Additional in-vitro and in-vivo studies have also revealed that GSH depletion and elevated lipid peroxidation may contribute to the toxic effects of cyanide poisoning[50,53].
2.4 Other CWAs
The above list is not comprehensive of all CWAs. Agents excluded in this commentary include those lacking the need for a therapeutic such as less-lethal agents (e.g. tear agents/vomiting agents/malodorants), outdated/obsolete agents (e.g. most arsenicals), and agents against which antioxidants have not been evaluated. Chemical Warfare Nerve Agents (CWNAs) are another type of CWAs which are not addressed in this commentary. CWNAs primarily exert their effects through excessive stimulation of the parasympathetic nervous system[45]. Potent inhibition of acetylcholine esterase AChE (the enzyme responsible for breaking down acetylcholine) causes accumulation of acetylcholine in the in the synaptic cleft producing hyper-stimulation of the nerves[45]. Lethality is swift and often occurs due to seizures and suffocation from loss of diaphragm contraction[45]. A combination of atropine sulfate (a muscarinic antagonist) and 2-pralidoxime (2-PAM, an oxime that displaces the AChE inhibitor and reactivates the enzyme) are able to rescue from the lethal effects if they are administered quickly after exposure[41,45,54]. Although CWNAs are an important category of CWAs, the extreme rapidity of their lethal effects in combination with the existence of effective therapeutics undermines the exploration of antioxidants as a primary CWNA therapeutics. However, survivors of CWNA exposures often have delayed neurologic symptoms of memory loss and cognitive dysfunction that may be amendable to antioxidant therapy[55].
2.5 Toxic Industrial Chemicals
TICs are highly toxic chemicals which are produced in massive quantities by numerous industries. Due to the large volume of production and storage of these chemicals, there is a strong concern for unintentional exposures such as accidental leaks/spills during storage, transport, or use. These chemicals are also noted for the ease with which terrorists might obtain these chemicals and their potential for misuse. TICs are characterized as possessing a high hazard index, meaning that they are widely produced, stored, transported, have high toxicity and are easily vaporized[56]. It should be noted that several agents are considered to be both TICs as well as CWAs and have been described earlier[56].
2.5.1 Formaldehyde
Formaldehyde (CH2O, Figure 1I) is a chemical produced in large quantities for use in many industries, as well as a common environmental pollutant. The International Agency for Research on Cancer (IARC) has categorized formaldehyde as a leukemogenic agent[57]. Formaldehyde is capable of cross-linking DNA as well as proteins on their amino groups[57]. Formaldehyde inhalation has been associated with increasing epithelial permeability and edema in the lung[58]. Research has shown that animals inhaling formaldehyde exhibit increases in ROS, MDA, DNA-protein cross-linking and decreased levels of GSH in the lung and systemically[57,59]. Formaldehyde mediated elevation in ROS and associated genotoxicity are implicated in the development of leukemia[59]. Other studies have shown formaldehyde exposures are associated with elevated ROS/RNS levels in the lung as well as alterations in antioxidant enzyme concentrations[60].
2.5.2 Sulfur Dioxide
Sulfur dioxide (SO2, Figure 1J) is a common industrial chemical used in the manufacture and refinement of many products and also used as a food preservative. Sulfur dioxide is present in the environment as a pollutant formed from the burning of sulfur-containing fossil fuels[61]. Inhalation of sulfur dioxide can cause bronchoconstrition and airway obstructions which can be life-threatening[62]. In-vitro data suggests that sulfur dioxide may also have mutagenic potential in human lymphocytes[61]. Elevated oxidative stress and lipid peroxidation levels following occupational sulfur dioxide exposures have been recognized as a potential mechanism leading to the observed bronchoconstriction[63]. Other experiments have confirmed that sulfur dioxide inhalation causes oxidative damage systemically, with lipid peroxidation and alterations in many non-pulmonary tissue antioxidant enzyme levels [64].
2.5.3 Acrolein
Acrolein (C3H4O, Figure 1K) is an industrial chemical intermediate used for synthesizing numerous organic substances. The general population is also exposed to acrolein which is present as an environmental pollutant generated from fires. Acrolein is a major source of toxicity from smoke inhalation and cigarette smoke[65]. Acrolein exposures produce ocular, GI, and respiratory irritation, the severity of which is dose dependent[66]. The mechanism through which acrolein exerts its toxic effects is through adduction of nucleophilic sites on biological macromolecules [66]. GSH depletion, followed by the binding of acrolein to sulphydryl groups on proteins and peptides is believed to be a principal contributor to its toxic effects. DNA damage and mitochondrial dysfunction have also been found following acrolein exposures[67–69].
3.0 Molecular consequences and hypothesized pathways of oxidative/nitrosative stress mediated toxicity
The role played by oxidative stress in CWA/TIC mediated damage can be difficult to separate from other mechanisms of toxicity. Oxidative stress is characterized as a disruption of the balance between the generation of reactive oxidizing species and their detoxification by antioxidant and repair defenses. Studying the intricacies that oxidative stress plays in disease models is complicated due to the many indirect sources of ROS/RNS and tissue damage mediated by the influx of inflammatory cells as additional sources of ROS/RNS.
3.1 Major Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) implicated in CWA/TIC toxicity
The major ROS which play a role in CWA/TIC induced oxidative stress include superoxide(O2•−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH•). Superoxide is formed by the one electron reduction of molecular oxygen and can be generated enzymatically from NADPH oxidases or from electron leak from electron transport systems such as those found in the mitochondria[70]. Superoxide rapidly undergoes non-enzymatic dismutation or enzymatic dismutation by superoxide dismutase (SOD) which results in the formation of O2 and H2O2[71]. If nitric oxide (NO•) is present, it rapidly reacts with superoxide to form peroxynitrite (ONOO−) that can result in hydroxyl-radical-like damage to cellular macromolecules[72]. Peroxynitrite is capable of covalently modifying membrane lipids, thiols, proteins, and DNA[12]. The antioxidant enzymes catalase (CAT) and glutathione peroxidase (GPx) can convert hydrogen peroxide to oxygen and water[73,74]. If the hydrogen peroxide is not detoxified, it may be free to participate in the generation of the hydroxyl radical (through reactions with transition state metals), which is the most reactive and damaging of the biologically relevant ROS[75]. Hydroxyl radicals are reactive enough to oxidize any cellular macromolecule or begin a lipid peroxidation cascade[76].
Under normal conditions, the cell is able to maintain low levels of ROS/RNS with its endogenous antioxidant defenses. These defenses include small molecule antioxidants such as GSH, vitamin E, thioredoxin, and peroxiredoxin, as well as antioxidant enzymes such as SOD, GPx, glutathione S-transferase, CAT, glutathione reductase and thioredoxin reductase[25]. Examination of changes in these endogenous antioxidant defenses can be used in models to quantify oxidative stress conditions. Restoration of these markers to normal levels can also be used to judge the efficacy of potential therapies against oxidative toxicity.
3.2 The evolution of cellular damage from excessive cellular macromolecule oxidation
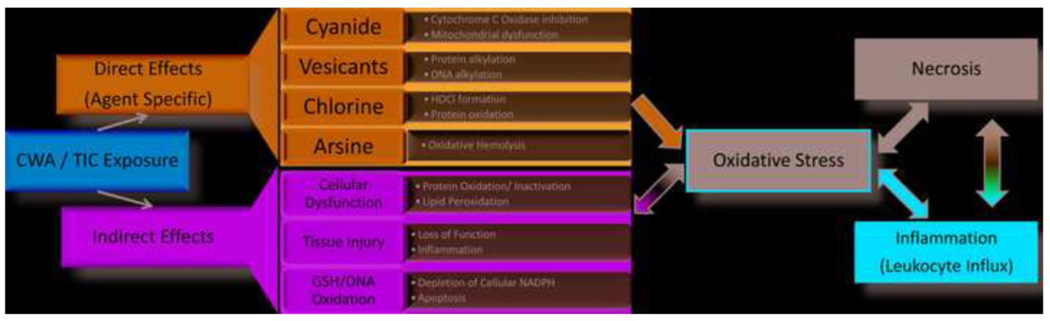
There are a copious number of possible mechanisms of oxidative stress mediated cellular damage from CWAs and TICs (Figure 2). Some of these include genotoxicity from oxidative DNA damage, protein oxidation leading to enzyme inactivation, and cellular energy depletion. While agents like sulfur mustard are capable of alkylating single base pairs or cross-linking DNA purine bases leading to strand breaks, most oxidative DNA damage is believed to occur through interaction with the hydroxyl radical (OH•)[77]. Sulfur and nitrogen mustards can undergo enzymatic one-electron reductions resulting in their conversion to carbon-based free radicals. One target of these radical molecules is molecular oxygen, resulting in the formation of superoxide radicals[11]. Many agents can redox cycle with oxygen to propagate their damaging effects[11]. Damage to the mitochondria can lead mitochondrial dysfunction and increased leak of ROS/RNS along with loss of energy production. Another potential mechanism for energy depletion occurs from over-activation of cellular repair mechanisms that can deplete cellular energy reserves and induce apoptosis. One example of this involves oxidative DNA damage causing over-activation of poly (ADP-ribose) polymerase (PARP). Excessive activation of PARP leads to depletion of cellular nicotinamide adenine dinucleotide (NAD+) and adenosine triphosphate (ATP) reserves, a known trigger of cell death[9].
Figure 2. Proposed mechanisms of oxidative stress from CWA/TIC exposure.
These agents produce both direct and indirect effects that lead to cellular events known to trigger oxidative stress. In addition to these effects, the agents produce tissue injuries that promote leukocyte influx. Activation of leukocytes is yet another source of ROS/RNS production that further promotes oxidative stress. All of these points in CWA/TIC pathways to tissue injury and damage are potential targets for intervention with antioxidant therapy.
4.0 Potential antioxidant therapies: mechanisms, efficacy, and practicality of use
Many compounds are labeled as antioxidants, however many of these compounds produce antioxidant effects indirectly or even paradoxically[70]. Direct acting antioxidants that directly scavenge ROS/RNS may often produce a reactive product. This happens with SOD that dismutates superoxide and produces hydrogen peroxide. Some compounds are precursor molecules that can be cofactors or substrates for other endogenous antioxidants, such as N-acetyl cysteine that is a direct scavenger but can be utilized as a source of cysteine for GSH synthesis. Thiol containing antioxidants may also exert effects through interactions with targets other than ROS, such as carbon-centered radicals. Other compounds may be electrophiles that produce a mild oxidative stress response, which in turn, evokes an endogenous antioxidant response resulting in decreased oxidative stress. This is usually the case for many polyphenolic compounds sold as dietary products. The following is a summary of some of the findings for antioxidants being tested as potential therapeutics for CWA/TIC induced damage (Table 1).
Table 1.
Summary of CWA/TIC literature on Oxidative Stress and Antioxidant Interventions*
| Agent | Category | Abbreviations | Target Organ | Role of Oxidative Stress | Antioxidant Countermeasure Efficacy* |
|---|---|---|---|---|---|
| Sulfur Mustard (Bis(2- chloroethyl)sulfide) / CEES (2-Chloroethyl ethyl sulfide) |
Vesicant | SM, HD | Lung [2,4,9– 12,14–17,68,70,77,92], Skin[6,13,69,77,82,90] |
[4,6,7,10,25,40,68] | NAC[70,71], GSH[77], MEL[2,8,40], MP[12,82], TRO[2,77,92,93], QUE[2,77,88,93], SIL[2,90,91] |
| Nitrogen Mustard |
Vesicant | NM, HN1, HN2, HN3 |
Lung [79,94],Skin[18,19,89] |
[18,19,40,94] | NAC[95], GSH[96], MEL[40,79], SIL[89] |
| Chlorine | Choking | Cl | Lung [20,22,23,83,97–105] | [22,23,100–107] | NAC[20,97], GSH[98,99], MEL[40], MP[83] |
| Chloropicrin | Choking | PS | Lung [28],Eye[24] | [24–26,28] | NAC[28] |
| Phosgene | Choking | CG | Lung [29– 31,72,73,108,109] |
[29–31,40,108,109] | NAC[72,73], MEL[40] |
| Phosphine | Choking | PH3 | Lung [110] | [32] | NAC[111], GSH[33], MEL[34,110] |
| Arsine | Blood | SA | [36,37,39,74] | NAC[74], TRO[74,86], QUE[74], SIL[112] |
|
| Cyanide | Blood | CN, HCN, AC | Lung [85] | [42,113] | NAC[45], MEL[40], TRO[85] |
| Formaldehyde | TICs | FA | Lung [51,52] | [50–52,114] | NAC[75], MEL[115,116], TRO[117] |
| Sulfur Dioxide | TICs | SO2 | Lung [53,55,56] | [53–53] | TRO[86] |
| Acrolein | TICs | ACR | Lung[58,118,119], Eye [58,59], Skin [58] |
[58,59] | NAC[76], GSH[60], MEL[118], QUE[119] |
Antioxidant abbreviations: N-acetylcysteine (NAC), Glutathione (GSH), Melatonin (MEL), Metalloporphyrin (MP), Trolox (TRO), Quercetin (QUE), Silibinin (SIL).
4.1 N-acetyl cysteine
NAC (N-acetyl cysteine, Figure 3A) is a thiol containing precursor to the endogenous antioxidant GSH, which is used pharmacologically to replenish GSH levels as an antidote for acetaminophen overdose as well as being used as a mucolytic agent. NAC may also interact directly with oxidants and electrophiles such as mustard agents. In models of in-vivo sulfur mustard inhalation, NAC has been shown to protect against markers of lung injury and edema [78]. The effectiveness of NAC and its relatively low toxicity profile has led to its recommendation for consideration for use as a SM chemoprophylactic to military personnel despite its adverse effects that have limited clinical usage that include severe nausea[79]. Chlorine inhalation toxicity also appears to be reduced with the administration of NAC[25]. In a rat model of phosgene induced acute lung injury, NAC administration reduced markers of lung oxidative stress. Additionally, it appeared that NAC administration was able to increase Nrf2 activation, thereby strengthening the endogenous antioxidant defense systems[80]. NAC treatments may attenuate pulmonary edema following phosgene exposure by maintaining protective GSH levels and decreasing lipid peroxidation[81]. In models of chloropicrin-induced epithelial cell toxicity, NAC has been shown to prevent cell vacuolization and preserve cell viability[33]. For blood agents, NAC has been shown in cell culture models to protect cells exposed to cyanide from GSH depletion and lipid peroxidation[53]. It has also been used in several studies which have reported positive outcomes associated with NAC administration after arsenic poisoning[82]. NAC has also been used in models of TIC toxicity, in which NAC has been shown to have a protective effect against the oxidative stress produced from formaldehyde exposures [83] and acrolein exposures[84].
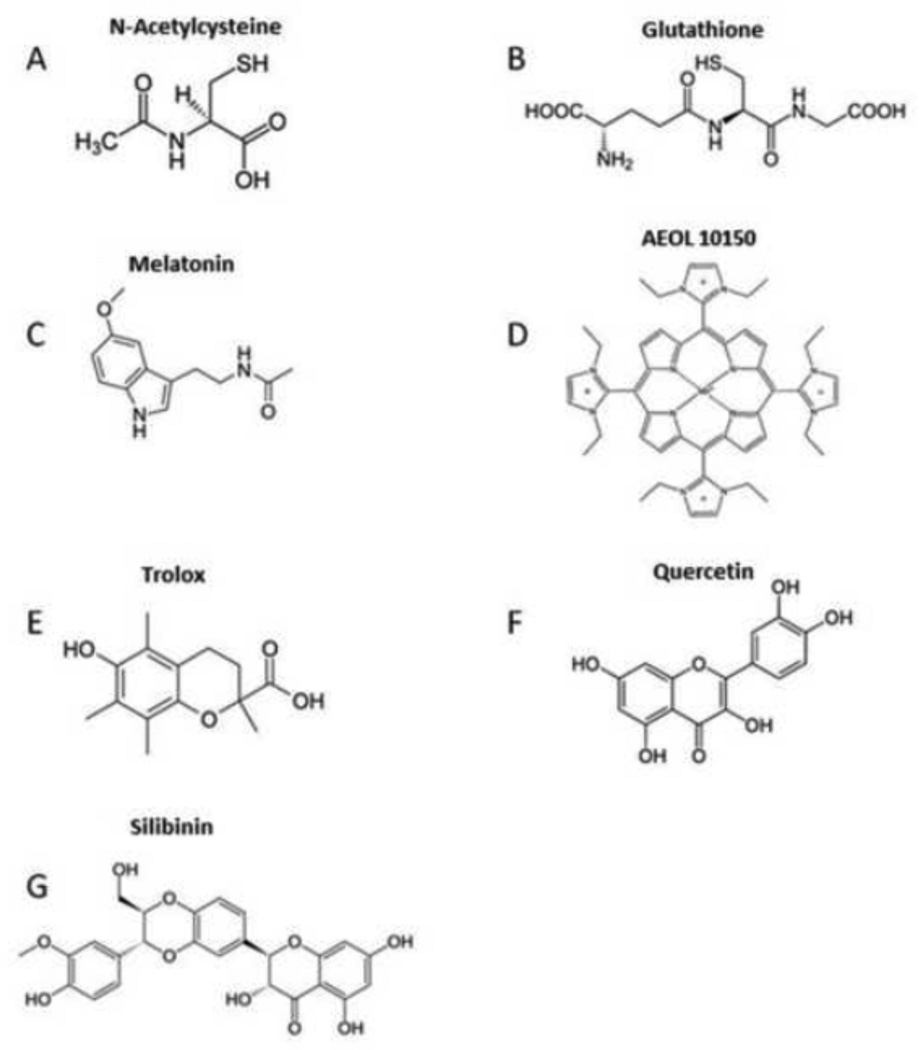
Figure 3. Chemical Structures of Relevant Antioxidants.
A) N-Acetylcysteine; B) Glutathione; C) Melatonin; D) Metal-Containing Catalytic Antioxidants; E) Trolox; F) Quercetin; and G) Silibinin.
4.2 Glutathione (GSH)
GSH (γ-L-Glutamyl-L-cysteinylglycine, Figure3B) is an endogenous tripeptide consisting of glutamate, cysteine, and glycine and the most abundant non-protein thiol in cells. GSH is a vital cellular antioxidant for maintenance of redox equilibrium. GSH may not be as effective as other antioxidant therapeutics administered orally due to its limited absorption and degradation in the stomach[5,85]. Despite complications with absorption and cell permeability, GSH treatments have been shown to exhibit protection against in-vitro and in-vivo models of SM toxicity[85]. GSH was able to greatly reduce the oxidative damage produced by phosphine toxicity, in -vitro[39]. Compounds with free sulfhydryl groups such as GSH have also been shown to protect from the DNA damaging abilities of acrolein[68].
4.3 Melatonin
Melatonin (N-acetyl-5-methoxytryptamine, Figure 3C) is an endogenous hormone involved in regulation of circadian rhythms and used as an alternative medicine for sleep disorders. Melatonin can act as both an indirect and direct acting antioxidant[86]. It is a highly lipophilic compound and easily cross lipid barriers to participate in detoxification reactions[48]. Melatonin has been tested as a potential therapeutic against several CWA models of toxicity including sulfur mustard, nitrogen mustard, phosgene, chlorine, cyanide and others. Against the vesicant class agents, melatonin has been shown to reduce oxidative stress and inflammation in rodent models of mustard gas exposure[48]. It was hypothesized that melatonin’s protection from nitrogen mustard might be linked to its effects as an iNOS inhibitor[87]. In a study following nitrogen mustard injection, melatonin was able to restore all oxidative and nitrosative stress markers in rat lungs to control levels[87]. Melatonin has also shown protective effects against cell culture models of chlorine and phosgene exposures[88]. In a rat model of phosphine-induced toxicity, melatonin was able to protect animals from lipid peroxidation, GSH depletion and oxidative DNA damage[40]. A comparison study of multiple antioxidants found melatonin to be the most effective at protecting rats from phosphine injury[40]. In-vitro and in-vivo studies have also shown that melatonin treatments reduce cyanide induced brain damage[50]. Melatonin shows a great deal of promise as an antioxidant therapeutic producing favorable outcomes across a wide span of injury models. Melatonin, with its low toxicity profile, appears to be a promising antioxidant therapeutic for CWA/TIC exposures[48].
4.4 Metal-Containing Ca ta lytic Antioxidants
Metal-containing catalytic antioxidants are a novel class of potential therapeutic agents for the treatment of CWA/TIC exposures. These synthetic complexes are designed to mimic the function of endogenous antioxidant enzymes such as SOD and CAT. Metalloporphyrin antioxidants have been shown to be capable of scavenging superoxide, hydrogen peroxide, peroxynitrite, and lipid peroxides[89]. A series of metalloporphyrins were screened in vitro against the sulfur mustard analog 2-chloroethyl ethyl sulfide (CEES) and found to be cytoprotective with AEOL10150 (Figure 3D) having the best effect[20]. These studies also demonstrated CEES-induced formation of mitochondrial ROS and dysfunction that was rescued by AEOL 10150. The catalytic antioxidant AEOL 10150 has been shown to be effective in vivo to lessen markers of oxidative stress, markers of acute lung injury, and airway cast formation in a model of CEES inhalation[18]. AEOL10150 was also tested in a skin model of topical CEES-induced injury both in vitro and in vivo[90]. These studies found 10150 treatment improved skin cell viability, decreased CEES-induced ROS, and DNA damage. In vivo CEES studies in mice revealed AEOL10150 mediated decrease skin bi-fold thickness, microvesication, and epidermal thickness. Topical CEES produced increases in myeloperoxidase activity and oxidative DNA damage that were all rescued with AEOL10150 treatment. AEOL 10150 has also been shown to have a protective effect on chlorine inhalation toxicity by reducing airway hyperresponsiveness, inflammation, oxidative stress and acute lung injury in mice[91].
4.5 Trolox
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Figure 3E) is a water soluble analog of vitamin E[92]. Trolox appears to improve survival times and protect from oxidative damage in models of SM toxicity[5,85]. Oral supplementation of antioxidant vitamins (including vitamin E) appears to reduce the severity of the pathological changes produced from In-vivo cyanide exposures[93]. Vitamin E has also been shown to protect against arsenic intoxication[82]. In another study, the combined use of antioxidant vitamins C and E administered to animals following sulfur dioxide inhalation, protected from sulfur dioxide mediated lipid peroxidation [94].
4.6 Quercetin
Quercetin (Figure 3F) is a bioflavanoid compound which is abundant in many fruits and vegetables and sold as a dietary supplement. There is evidence that quercetin offers dose dependent protection from topical SM application in mice, however clinical studies are inconclusive about its value in the treatment of any disease conditions in humans[95]. The protective effect of quercetin against SM toxicity is attributed to its ability to detoxify SM intermediates as well as interruption of the lipid peroxidation cascade[96]. Studies also suggest that quercetin can improve survival times following SM exposure [5,85]. Quercetin has also been shown to relieve oxidative stress conditions following arsenic toxicity[82].
4.7 Silib inin
Silibinin (Figure 3G) is a natural product flavanone extract of the milk thistle plant[97]. It is commonly sold as a dietary supplement for treatment of hepatotoxicity but is also taken for a diverse range of ailments. Silibinin has been studied for its utility against oxidative stress, inflammation, and cancer, and has demonstrated potential medical utility in several models of toxicity. Its exact mechanism of action is poorly understood, but it has shown protection in models of vesicant toxicity including nitrogen mustard, CEES and SM[10,24,97–99]. Silibinin might also reduce the nitrosative stress observed following in-vitro SM exposures through its ability to inhibit iNOS[99].
5.0 Models of CWA/TIC exposures for evaluating antioxidant efficacy
The hazardous potential of the CWAs/TICs discussed here make them difficult to handle and study in a laboratory environment. The high reactivity profile of many of these agents also makes it difficult to ascertain which of the biological targets that they are interacting with are most important to their toxidrome. Studying how these agents affect human physiology can be especially challenging. Obtaining samples from exposed humans to analyze is typically difficult or impossible, since exposures are infrequent and usually occur in chaotic environments. Therefore most of our clinical and mechanistic data must be extrapolated from animal and cell culture models.
The exact mechanisms of action of the antioxidants presented here, with a few exceptions, tend to be poorly understood. These agents have proven abilities to reduce the amount of oxidative stress in models systems, but rarely have their mechanisms been shown to be limited exclusively to ROS/RNS scavenging. The myriad of processes these compounds may be interacting with on a biochemical level obfuscates the distinction between antioxidant, anti-inflammatory, and direct detoxification of toxicants. The trend observable across many models of toxicity supports that the potency of the antioxidant is proportional to its ability to protect from injury, further highlighting the role played by oxidative stress in CWA/TIC injury.
6.0 Future Directions and Challenges
The models used to generate the data summarized in this commentary are highly variable. These studies have been performed using many different cell culture lines, treatment doses, experimental times/conditions, and toxicant exposure methods. The animal studies used to achieve these results reflect even more experimental diversity. These issues prevent meaningful side-by-side comparisons of antioxidant agents, therefore each experiment, agent, and antioxidant must be considered independently on its own merit. Although in the broadest sense, there is a clear trend showing the potential of antioxidants to be developed as medical countermeasures for CWAs/TICs.
Establishing meaningful and ethical models for the evaluation of CWA/TIC toxicity is a complex and challenging task. The exact mechanisms of toxicity of the CWAs/TICs discussed are not fully characterized to date. Improving our understanding of how these agents produce their harmful outcomes is essential to finding successful treatments. Human contact with CWAs in a battlefield scenario would be expected to have unpredictable exposure profiles since the dose received would directly depend on the combatants’ proximity to the delivery vehicle, concentration of agent, and access to safety equipment. Factors such as access to decontamination equipment and egression speed from the contaminated areas add further complexity to determining a realistic dose and dose windows for therapy modeling. While there is no “right answer” for the proper model, a worst-case-scenario/lethal dose provides the most utility for final testing of potential antidotes in animal models.
Survival studies provide valuable data about the effectiveness of an antidote, but usually reveal little about mechanistic processes at work. Logical exposure routes must also be selected for these types of studies. Sub-lethal in-vivo studies can be very useful for analyzing biomarkers of toxicity, but require the selection of appropriate biomarkers that reflect the most relevant mechanisms of damage for the agent. Cell culture studies can provide insight into determining drugable targets and viable therapeutics for initial determination of whether a therapeutic is worth looking at in vivo. Cell culture experiments are valuable for determining mechanistic pathways when studying CWAs because they are relatively inexpensive. They allow for some limited work between human and animal systems for comparable cytoprotection data and mechanisms. Unfortunately, results derived from these systems can be deceiving, as many of the in-vivo signaling pathways and tissue-specific effects are not present in in-vitro models. Another consideration to be wary of in vitro cell culture models of CWAs is the reactivity of the CWA with the culture media. The culture media is capable of scrubbing the CWA/TIC before reaching the target cells requiring larger exposures. In addition to the problems faced with studying agents that affect an assortment of intracellular targets, the situation is further convoluted by attempting to define specific mechanisms of antioxidant therapeutics. Characterization of the antioxidant effects of the treatments are limited by technical difficulties of measuring the unstable specific radical species. Many of the antioxidants described have also been shown to reduce inflammation and influence signaling pathways. Since oxidative stress may induce inflammation, and vice-versa, the reduction in one may alleviate the other. Both in vitro and in vivo models must be used together to conclude whether an antioxidant therapeutic is a realistic solution to a specific CWA/TIC injury paradigm.
Exposure to CWAs or TICs can be some of the most serious and potentially lethal toxic experiences faced by humans. The necessity of defining their toxicological properties and the search for effective treatments are of paramount importance. Elevated markers of oxidative stress are observable and implicated in many models of CWA/TIC exposure for contributing to toxicity. Many articles summarized in this commentary show evidence supporting the efficacy of antioxidant treatments as CWAs/TICs rescue agents. A wealth of literature suggests that future research into antioxidant therapeutics for CWA/TIC exposures is warranted and shows potential in developing useful countermeasures. The low toxicity, low cost, high availability, high stability, and apparent effectiveness of many of the antioxidants presented here makes them practical and favorable options to supplement the supportive care currently prescribed. Continued improvements in developing more potent broad spectrum antioxidants are needed. The applications of antioxidant therapeutics are just beginning to be realized and as more antioxidants are developed and studied, the more promising their prospects appear for use as pharmacological countermeasures to CWA/TIC injury.
Acknowledgements
This work was supported in part by a NIH Counteract grant to BJD, U54 ES015678. BJD is a consultant for and holds equity in Aeolus Pharmaceuticals that is developing metalloporphyrins as potential therapeutic agents.
Abbreviations
- CAT
Catalase
- CEES
2-Chloroethyl-ethyl sulfide
- CWA
Chemical Warfare Agent
- CWNAs
Chemical Warfare Nerve Agents
- GP
Glutathione Peroxidase
- GSH
Glutathione
- 8-OH2G
8-hydroxy-2-deoxyguanosine
- iNOS
Inducible Nitric Oxide Synthase
- MDA
Malondialdehyde
- MEL
Melatonin
- MP
Metalloporphyrin
- NAC
N-Acetylcysteine
- NM
Nitrogen Mustard
- Nrf2
Nuclear Factor Erythroid 2 Related Factor 2
- PARP
Poly (ADP-Ribose) Polymerase
- QUE
Quercetin
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SIL
Silibinin
- SM
Sulfur Mustard
- SOD
Superoxide Dismutase
- TIC
Toxic Industrial Chemical
- TRO
Trolox
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillis M. Disarmament: A Basic Guide, United Nations. 2009 [Google Scholar]
- 2.Tucker JB. The Chemical Weapons Convention Implementation Challenges and Solutions. Monterey Instutute Int. Stud. (n.d.) [Google Scholar]
- 3.Davenport K, Horner D. U.S. Says Chemical Weapons Used in Syria. Strateg. Surv. 1966;67:33–36. [Google Scholar]
- 4.New study examines effects of Graniteville, S. C. Chlorine Gas Disaster, Bioterrorism Week. 2009:1–2. [Google Scholar]
- 5.Keyser BM, Andres DK, Holmes WW, Paradiso D, Appell a, Letukas Va, et al. Mustard Gas Inhalation Injury: Therapeutic Strategy. Int. J. Toxicol. 2014;33:271–281. doi: 10.1177/1091581814532959. [DOI] [PubMed] [Google Scholar]
- 6.Jonathan L, Israel B. Chemical Warfare Agents, Part I: Choking Agents. Vesicants, and Halogenated Oximes. 2003:1–25. [Google Scholar]
- 7.Zhu X-J, Xu R, Meng X, Chu H-B, Zhao C, Lian C-J, et al. Mechanistic Insights of Sulfur Mustard-Induced Acute Tracheal Injury in Rats. Int. J. Toxicol. 2014;33:382–392. doi: 10.1177/1091581814548730. [DOI] [PubMed] [Google Scholar]
- 8.Stein WH BM, Moore S. Chemical reactions of mustard gas and related compounds; the transformations of mustard gas in water; formation and properties of sulfonium salts derived from mustard gas. J Org Chem. 1946:664–674. [PubMed] [Google Scholar]
- 9.Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Inturi S, Tewari-Singh N, Gu M, Shrotriya S, Gomez J, Agarwal C, et al. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free Radic. Biol. Med. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brimfield AA, Soni SD, Trimmer Ka, Zottola Ma, Sweeney RE, Graham JS. Metabolic activation of sulfur mustard leads to oxygen free radical formation. Free Radic. Biol. Med. 2012;52:811–817. doi: 10.1016/j.freeradbiomed.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Korkmaz A, Tan D-X, Reiter RJ. Acute and delayed sulfur mustard toxicity; novel mechanisms and future studies. Interdiscip. Toxicol. 2008;1:22–26. doi: 10.2478/v10102-010-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veress LA, O’Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am. J. Respir. Crit. Care Med. 2010;182:1352–1361. doi: 10.1164/rccm.200910-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rancourt RC, Ahmad A, a Veress L, Rioux JS, Garlick RB, White CW. Antifibrinolytic Mechanisms in Acute Airway Injury after Sulfur Mustard Analog Inhalation. 51. 2014:559–567. doi: 10.1165/rcmb.2014-0012OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veress LA, Anderson DR, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. Airway Tissue Plasminogen Activator Prevents Acute Mortality Due to Lethal Sulfur Mustard Inhalation. Toxicol. Sci. 2014;143:178–184. doi: 10.1093/toxsci/kfu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberger B, Laskin JD, Sunil VR, Sinko PJ, Heck DE, Laskin DL. Sulfur mustard-induced pulmonary injury: Therapeutic approaches to mitigating toxicity. Pulm. Pharmacol. Ther. 2011;24:92–99. doi: 10.1016/j.pupt.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClintock SD, Till GO, Smith MG, a Ward P. Protection from half-mustard-gas-induced acute lung injury in the rat. J. Appl. Toxicol. 2002;22:257–262. doi: 10.1002/jat.856. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill HC, White CW, Veress LA, Hendry-Hofer TB, Loader JE, Min E, et al. Treatment with the catalytic metalloporphyrin AEOL 10150 reduces inflammation and oxidative stress due to inhalation of the sulfur mustard analog 2-chloroethyl ethyl sulfide. Free Radic. Biol. Med. 2010;48:1188–1196. doi: 10.1016/j.freeradbiomed.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abel EL, Bubel JD, Simper MS, Powell L, McClellan SA, Andreeff M, et al. Protection against 2-chloroethyl ethyl sulfide (CEES) - induced cytotoxicity in human keratinocytes by an inducer of the glutathione detoxification pathway. Toxicol. Appl. Pharmacol. 2011;255:176–183. doi: 10.1016/j.taap.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. J. Pharmacol. Exp. Ther. 2009;328:732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veress LA, Hendry-Hofer TB, Loader JE, Rioux JS, Garlick RB, White CW. Tissue plasminogen activator prevents mortality from sulfur mustard analog-induced airway obstruction. Am. J. Respir. Cell Mol. Biol. 2013;48:439–447. doi: 10.1165/rcmb.2012-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goswami DG, Kumar D, Tewari-Singh N, Orlicky DJ, Jain AK, Kant R, et al. Topical nitrogen mustard exposure causes systemic toxic effects in mice. Exp. Toxicol. Pathol. 2015;67:161–170. doi: 10.1016/j.etp.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of the Army, POTENTIAL MILITARY CHEMICAL/BIOLOGICAL AGENTS AND COMPOUNDS. Army Field Manual. 2005 [Google Scholar]
- 24.Kumar D, Tewari-Singh N, Agarwal C, Jain AK, Inturi S, Kant R, et al. Nitrogen mustard exposure of murine skin induces DNA damage, oxidative stress and activation of MAPK/Akt-AP1 pathway leading to induction of inflammatory and proteolytic mediators. Toxicol. Lett. 2015;235:161–171. doi: 10.1016/j.toxlet.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, et al. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L733–L743. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim. Biophys. Acta - Gen. Subj. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 27.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc. Am. Thorac. Soc. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honavar J, Bradley E, Bradley K, Oh JY, Vallejo MO, Kelley EE, et al. Chlorine gas exposure disrupts nitric oxide homeostasis in the pulmonary vasculature. Toxicology. 2014;321:96–102. doi: 10.1016/j.tox.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sparks SE, Quistad GB, Li W, Casida JE. Chloropicrin dechlorination in relation to toxic action. J. Biochem. Mol. Toxicol. 2000;14:26–32. doi: 10.1002/(sici)1099-0461(2000)14:1<26::aid-jbt4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Pesonen M, Pasanen M, Loikkanen J, Naukkarinen a, Hemmilä M, Seulanto H, et al. Chloropicrin induces endoplasmic reticulum stress in human retinal pigment epithelial cells. Toxicol. Lett. 2012;211:239–245. doi: 10.1016/j.toxlet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Pesonen M, Vähäkangas K, Halme M, Vanninen P, Seulanto H, Hemmilä M, et al. Capsaicinoids, chloropicrin and sulfur mustard: Possibilities for exposure biomarkers. Front. Pharmacol. DEC. 2010:1–12. doi: 10.3389/fphar.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparks SE, Quistad GB, Casida JE. Chloropicrin: Reactions with biological thiols and metabolism in mice. Chem. Res. Toxicol. 1997;10:1001–1007. doi: 10.1021/tx9700477. [DOI] [PubMed] [Google Scholar]
- 33.Pesonen M, Häkkinen M, Rilla K, Juvonen R, Kuitunen T, Pasanen M, et al. Chloropicrin-induced toxic responses in human lung epithelial cells. Toxicol. Lett. 2014;226:236–244. doi: 10.1016/j.toxlet.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Subcommittee on Acute Exposure Guideline levels. Committee on Toxicology, Nrc, Acute Exposure Guideline Levels for Selected Airborne Chemicals. 2002 [Google Scholar]
- 35.Jaskot RH, Grose EC, Richards JH, Doerfler DL. Effects of inhaled phosgene on rat lung antioxidant systems. Toxicol. Sci. 1991;17:666–674. doi: 10.1016/0272-0590(91)90176-5. [DOI] [PubMed] [Google Scholar]
- 36.Grainge C, Rice P. Management of phosgene-induced acute lung injury. Clin. Toxicol. (Phila) 2010;48:497–508. doi: 10.3109/15563650.2010.506877. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Ye XL, Liu R, Chen HL, Liang X, Li WL, et al. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp. Toxicol. Pathol. 2013;65:311–318. doi: 10.1016/j.etp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Ebert PR, Nath NS, Bhattacharya I, Tuck AG, Schlipalius DI. Mechanisms of phosphine toxicity. J. Toxicol. 2011;2011 doi: 10.1155/2011/494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu CH, Quistad GB, Casida JE. Phosphine-induced oxidative stress in Hepa 1c1c7 cells. Toxicol. Sci. 1998;46:204–210. doi: 10.1006/toxs.1998.2552. [DOI] [PubMed] [Google Scholar]
- 40.Hsu C-H, Chi B-C, Liu M-Y, Li J-H, Chen C-J, Chen R-Y. Phosphine-induced oxidative damage in rats: role of glutathione. Toxicology. 2002;179:1–8. doi: 10.1016/s0300-483x(02)00246-9. [DOI] [PubMed] [Google Scholar]
- 41.Agents CW, Agents B, Gear P, Physician E, Springs C, Services CT, et al. Chemical Warfare Agents : Part II - Nerve Agents , Blood Agents. and Protective. 2003:1–20. [Google Scholar]
- 42.Pakulska D, Czerczak S. Hazardous effects of arsine: a short review. Int. J. Occup. Med. Environ. Health. 2006;19:36–44. doi: 10.2478/v10001-006-0003-z. [DOI] [PubMed] [Google Scholar]
- 43.Facts about Arsine. 2003 http://www.bt.cdc.gov/agent/arsine/pdf/arsinefactsheet.pdf.
- 44.Bannister M. Poisoning By Arsine. Br. Med. J. 1920;2:476. doi: 10.1136/bmj.2.3117.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson PD. Emergency Management of Chemical Weapons Injuries. J. Pharm. Pract. 2012;25:61–68. doi: 10.1177/0897190011420677. [DOI] [PubMed] [Google Scholar]
- 46.Blair PC, Thompson MB, Bechtold M, Wilson RE, Moorman MP, a Fowler B. Evidence for oxidative damage to red blood cells in mice induced by arsine gas. Toxicology. 1990;63:25–34. doi: 10.1016/0300-483x(90)90065-o. [DOI] [PubMed] [Google Scholar]
- 47.Satyanarayana bU. Biochemistry, 4, revised, Elsevier Health Sciences. 2014 [Google Scholar]
- 48.Pita R, Marco-Contelles J, Ramos E, Del Pino J, Romero A. Toxicity induced by chemical warfare agents: Insights on the protective role of melatonin. Chem. Biol. Interact. 2013;206:134–142. doi: 10.1016/j.cbi.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: Implications for acute cyanide toxicity. Toxicol. Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 50.Ardelt BK, Borowitz JL, Isom GE. Brain Lipid Peroxidation and Antioxidant Protectant Mechanisms Following Acute Cyanide Intoxication. 1989;56:147–154. doi: 10.1016/0300-483x(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 51.G Gunasekar P, Borowitz JL, Isom GE. Cyanide-induced generation of oxidative species: involvement of nitric oxide synthase and cyclooxygenase-2. J. Pharmacol. Exp. Ther. 1998;285:236–241. [PubMed] [Google Scholar]
- 52.Kanthasamy AG, Ardelt B, Malave A, Mills EM, Powley TL, Borowitz JL, et al. Reactive oxygen species generated by cyanide mediate toxicity in rat pheochromocytoma cells. Toxicol. Lett. 1997;93:47–54. doi: 10.1016/s0378-4274(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 53.Satpute RM, Hariharakrishnan J, Bhattacharya R. Effect of alpha-ketoglutarate and N-acetyl cysteine on cyanide-induced oxidative stress mediated cell death in PC12 cells. Toxicol. Ind. Health. 2010;26:297–308. doi: 10.1177/0748233710365695. [DOI] [PubMed] [Google Scholar]
- 54.Nambiar MP, Gordon RK, Rezk PE, Katos AM, Wajda Na, Moran TS, et al. Medical countermeasure against respiratory toxicity and acute lung injury following inhalation exposure to chemical warfare nerve agent VX. Toxicol. Appl. Pharmacol. 2007;219:142–150. doi: 10.1016/j.taap.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 55.RamaRao G, Bhattacharya BK. Multiple signal transduction pathways alterations during nerve agent toxicity. Toxicol. Lett. 2012;208:16–22. doi: 10.1016/j.toxlet.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 56.National Institute of Justice. Guide for the Selection of Chemical and Biological Decontamination Equipment for Emergency First Responders. 1998 JR000235. [Google Scholar]
- 57.Ye X, Ji Z, Chenxi W, McHale CM, Ding S, Thomas R, et al. Inhaled Formaldehyde Induces DNA-Protein Crosslinks andOxidative Stress in Bone Marrow and Other Distant Organs of Exposed Mice. Environ. Mol. Mutagen. 2010;51:229–235. doi: 10.1002/em.21821. [DOI] [PubMed] [Google Scholar]
- 58.Kastner PE, Casset A, Pons F. Formaldehyde interferes with airway epithelium integrity and functions in a dose- and time-dependent manner. Toxicol. Lett. 2011;200:109–116. doi: 10.1016/j.toxlet.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Liu X, McHale C, Li R, Zhang L, Wu Y, et al. Bone Marrow Injury Induced via Oxidative Stress in Mice by Inhalation Exposure to Formaldehyde. PLoS One. 2013;8:e74974. doi: 10.1371/journal.pone.0074974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lino-dos-Santos-Franco A, Correa-Costa M, Dos Santos Durão ACC, Ligeiro de Oliveira AP, Breithaupt-Faloppa AC, Bertoni JDA, et al. Formaldehyde induces lung inflammation by an oxidant and antioxidant enzymes mediated mechanism in the lung tissue. Toxicol. Lett. 2011;207:278–285. doi: 10.1016/j.toxlet.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 61.Uren N, Yuksel S, Onal Y. Genotoxic effects of sulfur dioxide in human lymphocytes. Toxicol. Ind. Health. 2012;30:311–315. doi: 10.1177/0748233712457441. [DOI] [PubMed] [Google Scholar]
- 62.Atsdr, Public Health Statement - Sulfur Dioxide. Public Health. 1998:7. www.atsdr.cdc.gov/
- 63.Gokirmak M, Yildirim Z, Hasanoglu HC, Koksal N, Mehmet N. The role of oxidative stress in bronchoconstriction due to occupational sulfur dioxide exposure. Clin. Chim. Acta. 2003;331:119–126. doi: 10.1016/s0009-8981(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 64.Meng Z. Oxidative Damage of Sulfur Dioxide on Various Organs of Mice : Sulfur Dioxide. Inhal. Toxicol. 2003;15:181–195. doi: 10.1080/08958370304476. [DOI] [PubMed] [Google Scholar]
- 65.Bein K, Leikauf GD. Acrolein - a pulmonary hazard. Mol. Nutr. Food Res. 2011;55:1342–1360. doi: 10.1002/mnfr.201100279. [DOI] [PubMed] [Google Scholar]
- 66.Faroon O, Roney N, Taylor a Ashizawa J, Lumpkin MH, Plewak DJ. Acrolein health effects. Toxicol. Ind. Health. 2008;24:447–490. doi: 10.1177/0748233708094188. [DOI] [PubMed] [Google Scholar]
- 67.Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, et al. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: Protection by (R)-??-lipoic acid. Investig. Ophthalmol. Vis. Sci. 2007;48:339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott-Minty C, Howe P. Concise International Chemical Assessment Document 43: Acrolein. IPCS Concise Int. Chem. Assess. Doc. 2002 [Google Scholar]
- 69.Li L, Jiang L, Geng C, Cao J, Zhong L. The role of oxidative stress in acrolein-induced DNA damage in HepG2 cells. Free Radic. Res. 2008;42:354–361. doi: 10.1080/10715760802008114. [DOI] [PubMed] [Google Scholar]
- 70.Day BJ. Antioxidant therapeutics: Pandoras box. Free Radic. Biol. Med. 2014;66:58–64. doi: 10.1016/j.freeradbiomed.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCord JM FI. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 72.Beckman JS, Beckman TW, Chen J, a Marshall P, a Freeman B. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fridovich I. The biology of oxygen radicals - The superoxide radical is an agent of oxygen toxicity; superoxide dismutases provide an important defense. Science (80-. ) 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 74.Day BJ. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett. 1978;96:238–242. doi: 10.1016/0014-5793(78)80409-8. [DOI] [PubMed] [Google Scholar]
- 76.Gutteridge JM, Richmond R, Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferoxamine. Biochem. J. 1979;184:469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsayed NM, Omaye ST, Klain GJ, Korte DW. Free radical-mediated lung response to the monofunctional sulfur mustard butyl 2-chloroethyl sulfide after subcutaneous injection. Toxicology. 1992;72:153–165. doi: 10.1016/0300-483x(92)90109-r. [DOI] [PubMed] [Google Scholar]
- 78.Anderson DR, Byers SL, Vesely KR. Treatment of sulfur mustard (HD)-induced lung injury. J. Appl. Toxicol. 2000;(20 Suppl. 1):S129–S132. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat670>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 79.Bobb AJ, Arfsten DP, Jederberg WW. N-acetyl-L-Cysteine as prophylaxis against sulfur mustard. Mil. Med. 2005;170:52–56. doi: 10.7205/milmed.170.1.52. [DOI] [PubMed] [Google Scholar]
- 80.Ji L, Liu R, Di Zhang X, Chen HL, Bai H, Wang X, et al. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal. Toxicol. 2010;22:535–542. doi: 10.3109/08958370903525183. [DOI] [PubMed] [Google Scholar]
- 81.Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhal. Toxicol. 2004;16:565–580. doi: 10.1080/08958370490442584. [DOI] [PubMed] [Google Scholar]
- 82.Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Ayaki H, Lee MJ, Sumino K, Nishio H. Different cytoprotective effect of antioxidants and change in the iron regulatory system in rodent cells exposed to paraquat or formaldehyde. Toxicology. 2005;208:73–79. doi: 10.1016/j.tox.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 84.Yoshida M, Tomitori H, Machi Y, Hagihara M, Higashi K, Goda H, et al. Acrolein toxicity: Comparison with reactive oxygen species. Biochem. Biophys. Res. Commun. 2009;378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 85.Kumar O, Sugendran K, Vijayaraghavan R. Protective effect of various antioxidants on the toxicity of sulphur mustard administered to mice by inhalation or percutaneous routes. Chem. Biol. Interact. 2001;134:1–12. doi: 10.1016/s0009-2797(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 86.Mayo JC, Tan DX, Sainz RM, Natarajan M, Lopez-Burillo S, Reiter RJ. Protection against oxidative protein damage induced by metal-catalyzed reaction or alkylperoxyl radicals: Comparative effects of melatonin and other antioxidants. Biochim. Biophys. Acta - Gen. Subj. 2003;1620:139–150. doi: 10.1016/s0304-4165(02)00527-5. [DOI] [PubMed] [Google Scholar]
- 87.Ucar M, Korkmaz A, Reiter RJ, Yaren H, Öter S, Kurt B, et al. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol. Lett. 2007;173:124–131. doi: 10.1016/j.toxlet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Pita R, Marco-Contelles J, Ramos E, Del Pino J, Romero A. Melatonin as potential candidate to prevent the toxicity induced by chemical warfare agents. Arch. Toxicol. 2014;88:3–4. doi: 10.1007/s00204-013-1111-8. [DOI] [PubMed] [Google Scholar]
- 89.Day BJ. Catalytic antioxidants: A radical approach to new therapeutics. Drug Discov. Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 90.Tewari-Singh N, Inturi S, Jain AK, Agarwal C, Orlicky DJ, White CW, et al. Catalytic antioxidant AEOL 10150 treatment ameliorates sulfur mustard analog 2-chloroethyl ethyl sulfide-associated cutaneous toxic effects. Free Radic. Biol. Med. 2014;72:285–295. doi: 10.1016/j.freeradbiomed.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: A novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic. Biol. Med. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sagach VF, Scrosati M, Fielding J, Rossoni G, Galli C, Visioli F. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol. Res. 2002;45:435–439. doi: 10.1006/phrs.2002.0993. [DOI] [PubMed] [Google Scholar]
- 93.Okolie NP, Iroanya CU. Some histologic and biochemical evidence for mitigation of cyanide-induced tissue lesions by antioxidant vitamin administration in rabbits. Food Chem. Toxicol. 2003;41:463–469. doi: 10.1016/s0278-6915(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 94.Etlik ODO, Tomur A, Kutman MN, Yorukan S. The Effects of Sulfur Dioxide Inhalation and Antioxidant Vitamins on Red Blood Cell Lipoperoxidation. J. Appl. Toxicol. 16:365–371. doi: 10.1006/enrs.1995.1063. (n.d.) [DOI] [PubMed] [Google Scholar]
- 95.Miles SL, McFarland M, Niles RM. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014;72:720–734. doi: 10.1111/nure.12152. [DOI] [PubMed] [Google Scholar]
- 96.Gautam A, Vijayaraghavan R, Pant SC, Kumar O, Singh S, Kumar HTS. Protective effect of quercetin against sulphur mustard-induced oxidative stress in mice. Def. Sci. J. 2007;57:707–720. http://www.scopus.com/inward/record.url?eid=2-s2.0-34548596862&partnerID=40&md5=99763f60510c5a724eac1d973a38c439. [Google Scholar]
- 97.Jain AK, Tewari-Singh N, Inturi S, Kumar D, Orlicky DJ, Agarwal C, et al. Flavanone silibinin treatment attenuates nitrogen mustard-induced toxic effects in mouse skin. Toxicol. Appl. Pharmacol. 2015;285:71–78. doi: 10.1016/j.taap.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tewari-Singh N, Jain AK, Inturi S, Agarwal C, White CW, Agarwal R. Silibinin Attenuates Sulfur Mustard Analog-Induced Skin Injury by Targeting Multiple Pathways Connecting Oxidative Stress and Inflammation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balszuweit F, John H, Schmidt A, Kehe K, Thiermann H, Steinritz D. Silibinin as a potential therapeutic for sulfur mustard injuries. Chem. Biol. Interact. 2013;206:496–504. doi: 10.1016/j.cbi.2013.06.010. [DOI] [PubMed] [Google Scholar]