Abstract
Objective
Retinal degenerations are a class of devastating blinding diseases that are characterized by photoreceptor dysfunction and death. In this study, we tested whether grape consumption, in the form of freeze-dried grape powder (FDGP), improves photoreceptor survival in a mouse model of retinal degeneration.
Methods
Retinal degeneration was induced in mice by acute oxidative stress using subretinal injection of paraquat and comparisons were to equivalent volumes of injected saline. The grape-supplemented diet was made by formulating base mouse chow with FDGP, corresponding to 3 daily human servings of grapes, and a control diet was formulated with equivalent sugar composition as FDGP (0.68% glucose/0.68% fructose mixture). Mice were placed on the diets at weaning for 5 weeks prior to oxidative stress injury until analysis at 2 weeks post-injection. Retinal function was measured using electroretinography, thickness of the photoreceptor layer was measured using optical coherence tomography, and rows of photoreceptor nuclei were counted on histological sections.
Results
In mice fed the control diet, oxidative stress significantly reduced photoreceptor layer thickness and photoreceptor numbers. In contrast, retina thickness and photoreceptor numbers were not reduced by oxidative stress in mice on the grape-supplemented diet, indicating significantly higher photoreceptor survival after injury than mice on the control diet. Furthermore, mice on the grape diet showed preservation of retina function after oxidative stress injury compared with mice on the control diet.
Conclusions
A diet supplemented with grapes rescued retinal structure and function in an oxidative stress-induced mouse model of retinal degeneration, which demonstrates the beneficial effect of grapes on photoreceptors.
Keywords: Retinal degeneration, freeze-dried grape powder, oxidative stress, paraquat, neuroprotection, nutrition, retina, grapes
Introduction
Retinal degenerations are a class of diseases that cause progressive photoreceptor death and irreversibly impair vision. These diseases include age-related macular degeneration (AMD) and retinitis pigmentosa (RP), and have high prevalence throughout the world. In the United States, AMD affects over 1.75 million people [1] and RP affects every 1 in 4000 individuals [2]. A major focus in the field is identifying new therapeutic strategies that promote photoreceptor survival. Nutrition and diet-based therapies are of particular interest because they have the potential to ameliorate progression of the disease with potentially fewer side effects than pharmacotherapy.
Numerous studies have explored the benefits to the retina of specific dietary supplements, ingested either as isolated compounds or as whole foods. A large randomized controlled trial showed that in patients with AMD, progression to advanced disease was delayed by ingesting certain nutrients, including anti-oxidant vitamins and specific carotenoids [3]. This finding led to the development of the AREDS dietary supplement formulation that is recommended to patients with AMD and is widely available commercially, and includes a combination of vitamins C, E, zinc, and beta-carotene or lutein and zeaxanthin. Various other nutritional supplements, such as minerals, dietary fatty acids and resveratrol, have also been associated with improved retinal health in small sample observational studies, although those tested in subsequent larger studies often show lack of statistical significant benefits [4]. However, an overall healthy diet, which includes low fat and high fruit and vegetable intake, appears to be important for reducing progression of retinal degeneration [5].
Grapes are an ideal candidate for dietary supplementation because polyphenols and other molecules isolated from grapes have anti-oxidant and anti-inflammatory effects in numerous tissues [6]. Oxidative damage and inflammation contribute to retinal degenerations [7, 8], leading to the hypothesis that the bioactive components found in grapes could protect photoreceptors. Grape consumption and grape-derived compounds are reported to be neuroprotective elsewhere in the CNS [9], and in mice with an retinal pigment epithelium (RPE)-specific phagocytosis defect, whole grapes prevented RPE damage and secondary retinal dysfunction [10], indicating that grape-derived compounds are biologically active in the retina. However, the effect of grape consumption on the neural retina, especially on photoreceptors, during retinal degeneration has not previously been examined.
Characterizing basic mechanisms of photoreceptor survival in simple models of retinal degeneration in animals is frequently used to identify novel therapeutic strategies that could be relevant for complex degenerations in humans such as AMD. Elevated oxidative stress is strongly associated with retinal disease and has been widely studied in the development of AMD and other retinal degenerations. An acute oxidative stress-induced retinal degeneration model is commonly used to mimic oxidative stress damage that contributes to retinal degenerations [11]. Oxidative stress injury is induced by subretinal injection of paraquat in mice and has the advantage as a non-genetic model that it does not require complex breeding schemes. Paraquat is processed in the mitochondria into free radicals [12] and induces oxidative damage to the retina when injected directly into the retina [11]. We previously reported that paraquat induced retinal degeneration in mice, and reduced retinal thickness, lower photoreceptor counts, attenuated ERG responses and decreased visual acuity, which could all be prevented by delivery of specific molecules [13].
In this study, we characterized the effect of dietary supplementation of grapes in a mouse model of oxidative stress-induced retinal degeneration. We demonstrated that mice fed a grape enriched diet, in the form of freeze-dried grape powder, showed remarkably improved photoreceptor survival at both the structural and functional levels. Therefore, our data provide new evidence for a beneficial effect of grapes to photoreceptor health. In conclusion, these results suggest that dietary grape intake may be a viable preventative or adjuvant therapy for retinal degenerations.
Materials and Methods
Diets
All procedures involving mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee at the University of Miami. Dietary supplementation with grapes was achieved using a biochemically characterized freeze-dried grape powder (FDGP) that was a prepared from Californian whole grapes and seeds of different varietals, red, green, and black, and was provided by the California Table Grape Commission. FDGP has been used in numerous studies for testing the effect of grapes in human and animal studies [6, 10, 14]. The biochemical composition of FDGP has previously been described [15, 16]. FDGP is composed of fresh red, green and black California grapes (seeded and seedless varieties), which have been frozen, ground with food-quality dry ice, freeze-dried, and re-ground using Good Manufacturing Practices for food products. The FDGP was incorporated into AIN-76A base diet mouse chow (prepared by Purina Mills, Gray Summit, MO) to provide approximately 43 mg of FDGP per day, based on the reported average amount of food ingested per mouse per day, and corresponds to approximately 3 human servings of grapes (USDA serving size is 1 cup of grapes per serving) [10]. This dose is similar to what was used in a recent report to allow direct comparisons [10]. A control diet that was sugar-matched for the sugar content in grapes was made by incorporating 0.68% glucose/0.68% fructose into the base diet mouse chow. The mice were placed on the grape-supplemented or sugar-matched control diets upon weaning at age 21 days and maintained on the diet for five weeks prior to retinal injections until analysis at 2 weeks post-injections (Fig 1). There were a total of 26 mice in the study: there were 13 mice on each diet, and 8 mice per diet received a PQ injection and the remaining 5 mice received a saline injection.
Figure 1.
Schematic of the oxidative-stress induced model. Wildtype mice at age 3 weeks (weaning age) were placed on the grape-supplemented or sugar control diet. After 5 weeks, the mice were placed randomly into two study groups, the paraquat (PQ)-injected group and the saline control group. The mice were maintained on the diet until the retina phenotype was analyzed after an additional 2 weeks.
Oxidative stress injury
Wild type mice (male and female, strain B6;129SF2/J, Jackson Laboratory, Bar Harbor, ME) were used. Oxidative stress injury was induced in the retina at age 8 weeks by subretinal injection of paraquat, which has been used by many groups to simulate the elevated oxidative stress that is observed in retinal degenerations. The mice were first anesthetized by intraperitoneal injection of a xylazine-ketamine mixture and the eyes were locally anesthetized with eye drops containing 0.5% proparacaine hydrochloride (Akorn, Lake Forest, IL). Mice were then subretinally injected with either 2 μl of paraquat (PQ, 1mM) or saline control, as described [13]. Briefly, an incision was made in the conjunctiva and the sclera and injections were performed using a 1.5 cm 33-gauge Hamilton needle (Hamilton Company, Reno, NV) between the RPE and neural retina. The left eye of each mouse was injected, and all injections were localized in the same area of the eye. All mice received the same volume of injection fluid. A successful injection was indicated by a transient retinal detachment that spontaneously resolved, which was confirmed by OCT analysis at 1 week post-injection. We excluded from the study any mice with unresolved retinal detachments, bleeding or infection.
Electroretinography
Retinal function was analyzed two weeks after paraquat injury using electroretinography (ERG), as described [13]. The mice were dark adapted and then anesthetized with a ketamine/xylazine cocktail and the pupils were dilated with topical 10% phenylepherine hydrochloride. To maintain a constant body temperature, the mice were wrapped in a modified heating blanket attached to a heated running water bath. A reference electrode was placed under the skin between the ears of the animal, a ground electrode was placed in the tail, and silver wire recording electrodes were positioned on each cornea. The mice were then placed in a Ganzfeld light emitting chamber, and flash intensity and duration was set using the UTAS system controlled by EM for Windows software (LKC Technologies, Gaithersburg, MD). The eyes were kept moist by the application of artificial tears. To measure scotopic responses, mice were exposed to single flashes of white light stimuli at intensities varying from 1 through 100 cd·s/m2. To measure photopic responses, the mice were exposed to flashes of green light stimuli at intensities varying from 0.01 through 10 cd·s/m2 in the presence of a dim green background light intensity of 1.0 cd/m2. Ten responses to each light stimulus were averaged.
Optical Coherence Tomography
Retina layer thickness was measured in vivo using spectral domain optical coherence tomography (SD-OCT) (Bioptigen, Research Triangle Park, NC) that is optimized for rodents, as described [13]. Mice were anesthetized with ketamine/xylazine cocktail, and then pupils were dilated with topical phenylephrine (Akorn, Lake Forest, IL) and kept moist using artificial tears. A 1.3×1.3×1.56 mm3 volume of the mouse retina centered on the optic disk was imaged, generating 100 b-scans. The average photoreceptor thickness was measured by segmenting the OCT images in 70-80 cross-sectional b-scans across the retina from the outer nuclear layer (ONL) to the inner and outer segments, inclusive, using MATLAB software and analytical programs developed by the Ophthalmic Biophysics Center at the University of Miami (OBC Segmentation).
Histology
Enucleated eyes were embedded and cryosectioned as described in [17]. Eyes were fixed in cold 4% paraformaldehyde, incubated in increasing sucrose concentration solutions, then embedded in optimal cutting temperature compound and frozen. Eight μm thick cross-sections were cut through the entire globe, and the sections were mounted onto Superfrost micro slides (VWR International, Radnor, PA). The retinas were stained with 4',6-diamidino-2-phenylindole (DAPI) to visualize nuclei or with H&E to visualize the retina structure, and then viewed using a fluorescent microscope (Zeiss Axiovert 200). Photoreceptor nuclei counts were performed by counting rows of nuclei in the ONL in the central retina near the optic nerve region. Columns were counted in 9 regions per mouse eye and averaged.
Statistical Analysis
Investigators were masked to the identity of the injected compound and diet for all analyses. Statistical analysis of the data was performed using ANOVA with Tukey test for post-hoc analysis, and p<0.05 was considered statistically significant.
Results
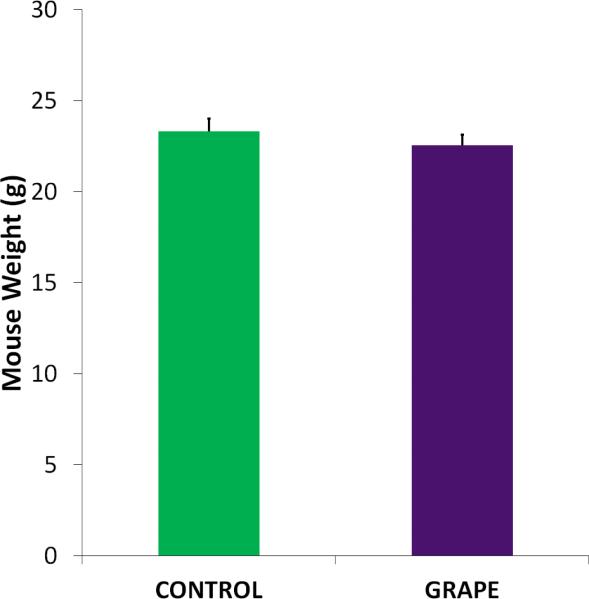
To mimic the oxidative stress damage that contributes to inherited and non-inherited retinal degenerations, we used a mouse model in which photoreceptor death is induced by oxidative stress by subretinal injection of paraquat. The paraquat-induced degeneration model has been used in many studies and leads to significant structural and functional loss of photoreceptors compared with the control injection of saline [13], as well as degeneration of cells in the inner retina (Patel et al., in press). In the present study, mice were randomly divided into the control (sugar equivalent diet) and grape (FDGP-supplemented diet) groups, and then randomly divided again for the paraquat or saline injections (Fig 1). Mice were weighed prior to being placed on the diets and then weekly until the end of the experiment. As shown in Fig 2, there was no significant difference in weight between the mice on the different diets at any time point.
Figure 2.
Body weights during the experiment. Mice were weighed each week after being placed on the diet. There was no significant difference in weights at any time point. The average weights of the mice at the end of the experiment are shown (n=10, mean + SE shown).
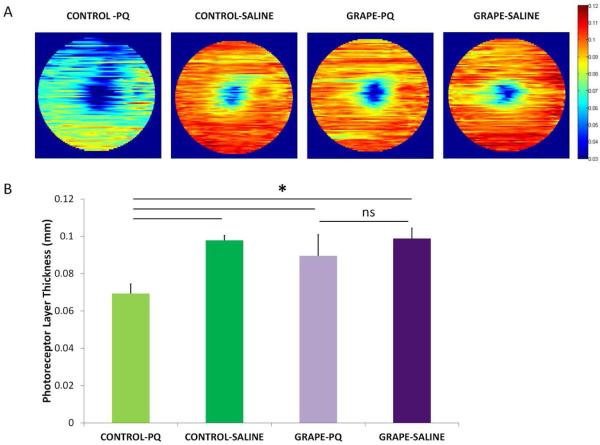
To assess the amount of retinal degeneration after oxidative stress injury, the structural integrity of the retina was examined by quantifying thinning of the outer nuclear layer (ONL), which is the region in which rod and cone nuclei are located. Representative heat maps that depict average thickness across the retina for each treatment group are shown in Fig 3A. In mice on the control diet, paraquat injection resulted in dramatic disruption of the normal lamellae structure of the ONL, and caused holes and lesions that were absent in mice that received the saline injection. The mice on the control diet that were injected with paraquat had an ONL thickness of 0.0694 ± 0.0051 mm (average ± SD), whereas the saline injection thickness was 0.0979 ± 0.00253 mm, which indicates a significant decrease in ONL thickness (Fig 3B, n=8 (PQ), 5 (saline); p<0.05). In contrast, in mice on the grape supplemented diet, the retinas injected with paraquat were not damaged and look remarkably similar to retinas injected with saline. Indeed, mice on the grape diet showed preservation of the ONL: after paraquat injection, the average ONL thickness was 0.08956 ± 0.01128 mm, whereas the ONL thickness after the saline injection was 0.098873 ± 0.005502 mm, which was a non-significant 10% change (Fig 3B, n=8 (PQ), 5 (saline)). Furthermore, the thickness of the ONL was significantly greater, by 20%, in the injured grape diet mice than the injured control diet mice (Fig 3B, n=8 (grape + PQ), 8 (saline + PQ); p<0.05). The paraquat injections in the mice on the grape diet were performed at the same time with the identical paraquat aliquot as the injections in the mice on the control diet, and the mice were analyzed on the same day. Therefore, the difference in degeneration is due to the influence of the diets.
Figure 3.
A. The structural integrity of the retina was imaged using OCT. Representative retina thickness heat maps are shown. Orange-red indicates thicker retinas and blue-green indicates thinner retinas. The grape-supplemented diet preserved the retina in the paraquat injected eyes (Grape-PQ) whereas the retina degenerated and was thinner in mice fed the control diet (Control-PQ). (B) Quantification of photoreceptor layer thickness. OCT measurements demonstrated that the nuclear layer plus inner and outer segments were significantly thinner after paraquat injection in mice fed the control diet (compare Control-PQ and Control-Saline). This decrease was absent in paraquat-injected mice fed the grape diet (compare Grape-PQ and Grape-Saline), and mice on the grape diet had thicker retinas than mice on the control diet (paraquat-injected mice, n=8; saline-injected mice, n=5). There was minimal variation of degeneration within each treatment group. Mean ± SD shown. *p<0.05.
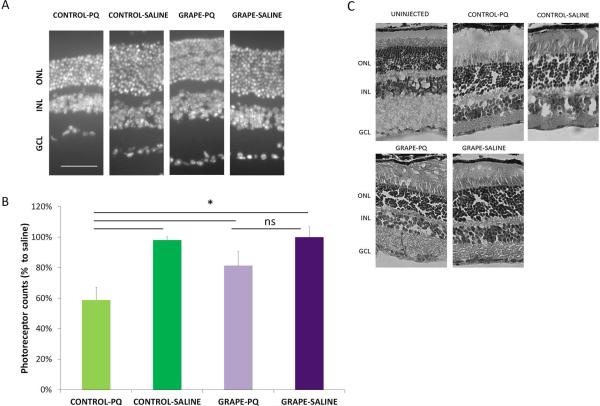
We next used a second method to quantify the extent of retinal degeneration. The number of remaining photoreceptors (rods and cones) were counted in random regions in the retina, which is an established methodology of quantifying photoreceptor survival [18]. In mice on the control diet, the percent of photoreceptor nuclei was reduced by 40% after oxidative stress injury, compared with the saline injection (Fig. 4, p< 0.001). In contrast, in mice on the grape diet, there was no reduction in photoreceptor numbers after the saline and paraquat injections, which is equivalent to our findings in the OCT measurements above. Also, comparisons between the groups injected with paraquat indicated that the mice on the grape diet had 28% more photoreceptors than mice on the control diet (p< 0.05), and corresponding light microscopy images show normal retina structure in grape fed mice (Fig. 4). Therefore, the mice on the grape supplemented diet showed structural preservation of the retina after oxidative stress injury.
Figure 4.
The grape diet significantly protected against photoreceptor loss. (A) Representative retina sections, stained with DAPI to show the photoreceptor nuclei, demonstrates more photoreceptors in injected mice on the grape diet compared with the control diet. GCL= ganglion cell layer, INL= inner nuclear layer, ONL= outer nuclear layer. Scale bar: 50μm. (B) Average photoreceptor row counts to quantify the number of surviving photoreceptors (rods and cones). In mice on the control diet, paraquat induced a significant reduction of photoreceptors compared with saline control. In contrast, mice on the grape diet showed a minimal decrease in photoreceptors, which was not statistically significant from saline control. Mean ± SD is shown. * p<0.05. (C) Cross-sections of the retinas stained with H&E demonstrating normal retinal structure. Gaps in the nuclear layers are artefacts from the staining procedure.
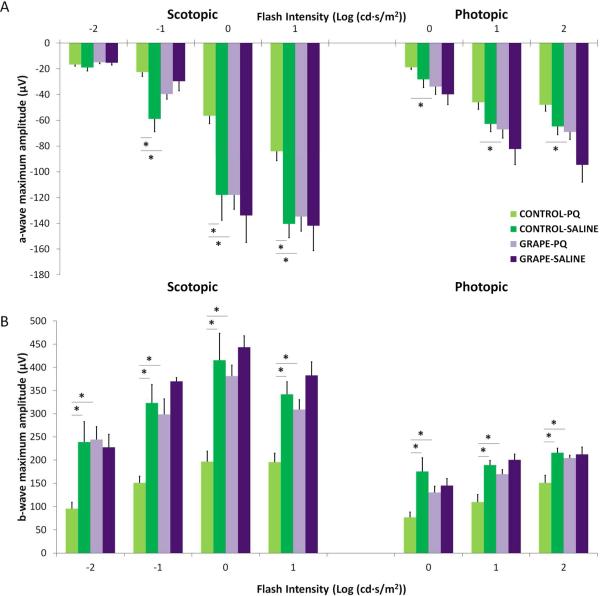
We next assessed whether grape consumption would provide protection from the functional deficits that occur during retinal degenerations. Neuronal cells of the retina often lose their electrical activity, which is characterized by reduced response of the retina to light stimuli, before structural losses are evident. To determine whether the protective effect of the grape supplemented diet was also observed on retina function, retinal responses were quantified using ERGs. As shown in Fig 5, mice on the control diet showed significant reductions in photoreceptor-generated a-wave amplitudes after paraquat injection compared with saline injection (n=8 (PQ), 5 (saline), p<0.05), which is indicative of retinal degeneration. The amount of amplitude reduction observed in Fig. 5 is consistent with our previous studies on paraquat-injected mice [13]. In contrast, the grape diet mice injected with paraquat did not have reduced photoreceptor activity, as indicated by equivalent a-wave amplitudes in the paraquat and saline injected mice (Fig 5).
Fig 5.
The grape diet significantly protected the retina from oxidative stress. ERG was used to quantify retinal function after injury. The a-waves, representing photoreceptor responses, and b-waves, representing inner retina responses, are shown. The intensity of light stimulation in log cd-s/m2 is indicated on the x-axis. Oxidative injury led to reduced a- (A) and b- (B) wave amplitudes in mice on the control diet (light and dark green bars), compared with saline injected eyes. In contrast, ERG responses in mice on the grape diet were equivalent between paraquat-injury and saline control (light and dark purple bars), indicating preserved retina function. Furthermore, the b-wave amplitudes of paraquat-injected mice on the grape diet were significantly higher than paraquat-injected mice on the control diet (n=8 (PQ), n=5 (saline), *p<0.05). Mean ± SE shown.
We next measured b-wave amplitudes to assess the function of the inner retina. There was a dramatic decrease in b-wave amplitudes in mice on the control diet injected with paraquat, as expected. In contrast, in mice on the grape diet, there was no difference in scotopic and photopic b-wave amplitudes between injections with paraquat or saline, which indicates functional preservation of the retina (Fig 5). Furthermore, b-wave amplitudes were significantly higher in the mice on the grape diet: At several light intensities, the b-wave amplitudes in the mice on the grape diet injected with paraquat were double that of the response in the mice on the control diet injected with paraquat (Fig 5). Additionally, the b-wave amplitudes in the mice on the grape diet injected with paraquat were equivalent to the non-degenerating retinas in mice on the control diet injected with saline (Fig 5). Notably, there was no significant difference in retinal function between the mice on the diets in the absence of injury (compare grape+saline vs control+saline, Fig 5B), showing that the protection by the diet was only in the context of injury and not by increasing the baseline health or synaptic responses of the retina.
Discussion
This study demonstrated that a grape-enriched diet significantly preserved retinal structure and function in mice exposed to retinal oxidative stress when compared to animals on the control diet. These results are supported by previous studies that showed that dietary intake of grapes reduced oxidative stress and RPE dysfunction in a mouse model of deficient phagocytosis [10]. Furthermore, molecules present in grapes have been shown to have various beneficial effects in the retina and in cultured retina cells, including anti-oxidant, anti-angiogenic and anti-inflammatory activities. For example, grape-derived compounds were neuroprotective to rat retinal ganglion cells after optic nerve transection [19], protected photoreceptors after light-induced retinal degeneration [20] and grape polyphenols reduced ocular inflammation [21].
The results of this study add to the growing body of literature demonstrating that dietary intake of certain nutrients can affect the pathology and prognosis of degenerative diseases of the retina. In animal models, various fruits, mineral and vitamin supplements are associated with many benefits, including enhanced neuronal survival, decreased oxidative damage, reduced protein misfolding, and decreased pathological angiogenesis in the retina and brain. Also, the effect of diet on human ocular disease has been studied extensively, and there are many review articles describing the roles of different dietary supplements on the pathology of AMD, glaucoma and RP [4]. Indeed, the AREDS formulation is now routinely recommended to delay progression of disease in patients with AMD [22].
The molecular basis of the protective effect of grapes in the retina is not yet established. Several pathways contribute to retinal degeneration, including oxidative stress, excessive inflammation, and cell death signaling, and compounds derived from grapes could inhibit proteins that mediate these pathological processes. Specific polyphenol compounds found in grapes, including quercetin, procyanidin B2, resveratrol and epicatechin are bioactive, and can regulate apoptotic, oxidative stress and inflammatory pathways [23]. Molecular targets of grape-derived compounds include key pro-survival molecules, such as glycogen synthase kinase 3β (GSK3β) [24] and the transcription factor STAT3 [25], among many others. Interestingly, modulating both GSK3β and STAT3 has been shown to improve photoreceptor survival [26]. Several studies have shown that various dietary supplements, such as supplementation with probiotic bacteria given to mice fed a high-fat diet [27, 28], can significantly regulate cytokines and proinflammatory factors, and the grape diet may follow a similar mechanism of action. Therefore, future work will investigate the mechanism of action of the grape diet by assessing levels of oxidative stress, pro-survival molecules and inflammation in the retina. Furthermore, such studies will be helpful for developing additional endpoints for assessing efficacy in mouse and potentially in human studies.
In this study, the approximate equivalent of three human servings of grapes was consumed. Because of the limited quantity of FDGP available, a dose-response relationship for photoreceptor protection could not be determined. However, the data indicate that we have used an ideal dose because almost complete rescue was observed for photoreceptor survival (compare grape+PQ and grape+saline in ERG and OCT). It would be interesting to determine the lowest dose that achieves a protective effect. Although our study suggests the possibility of grape supplementation being used as an adjuvant nutritional therapy for retinal disease, it is important to note that extrapolation from our mouse study to humans must be done with caution. The mice used were genetically inbred and were on identical carefully controlled diets, and the grape diet was initiated prior to the injury. In addition, the disease model used in this study is an acute oxidative stress model. Most neurodegenerative diseases in which oxidative stress plays a role usually occur due to a chronic buildup of oxidative stress damage. It is not yet known how grape consumption regulates oxidative stress over long periods of time and future studies will be necessary to test whether grapes are beneficial in a chronic model of photoreceptor death. Furthermore, some molecules that are efficacious in animal models do not show equivalent protective effects in humans [4]. However, because human retinal degenerative diseases are amendable to nutritional supplementation, it is plausible that a grape-based treatment could benefit patients. Further studies using a randomized controlled experimental paradigm would determine whether grape consumption improves visual outcomes in patients with retinal degenerations, and whether a combination of grapes and the AREDS formulation leads to greater protection of photoreceptors and RPE than each treatment alone.
Highlights.
A grape supplemented diet prevented retinal degeneration after oxidative stress injury
Consumption of grapes preserved retinal function
Grapes may be beneficial as an adjuvant nutritional therapy for retinal degenerative diseases
Acknowledgements
We thank BaoXiang Li and Hany Azcuy for technical support, and William J. Feuer for helpful discussions and statistical analyses. This work was supported by the California Table Grape Commission, and institutional support to BPEI was received from a Research to Prevent Blindness Unrestricted Grant and an NEI Center Core Grant P30 EY014801.
List of Abbreviations
- AMD
age-related macular degeneration
- ONL
outer nuclear layer
- PQ
paraquat
- RPE
retinal pigment epithelium
- FDGP
freeze-dried grape powder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions are as follows: conception and design of the study: AP, AD, AH, collection, analysis and interpretation of data: AP, AD, MER, SA, AH; manuscript and figures: SA, AP, AH; approval of the final version of the manuscript: AH.
Competing Interests:
The authors declare they have no competing interests.
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Eye Diseases Prevalence Research G: Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Evans JR, Lawrenson JG. A review of the evidence for dietary interventions in preventing or slowing the progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2014;34:390–396. doi: 10.1111/opo.12142. [DOI] [PubMed] [Google Scholar]
- 4.Grover AK, Samson SE. Antioxidants and vision health: facts and fiction. Mol Cell Biochem. 2014;388:173–183. doi: 10.1007/s11010-013-1908-z. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CJ, Chang ML, Zhang FF, Li T, Gensler G, Schleicher M, Taylor A. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol. 2014;158:118–127. e111. doi: 10.1016/j.ajo.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Khaoustov VI, Wang H, Yu J, Tabassam F, Yoffe B. Freeze-dried grape powder attenuates mitochondria- and oxidative stress-mediated apoptosis in liver cells. J Agric Food Chem. 2009;57:9324–93. doi: 10.1021/jf900851n. [DOI] [PubMed] [Google Scholar]
- 7.Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histol Histopathol. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 8.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Simonyi A, Li W, Sisk BA, Miller RL, Macdonald RS, Lubahn DE, Sun GY, Sun AY. Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Mol Nutr Food Res. 2005;49:443–451. doi: 10.1002/mnfr.200500019. [DOI] [PubMed] [Google Scholar]
- 10.Yu CC, Nandrot EF, Dun Y, Finnemann SC. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking alphavbeta5 integrin. Free Radic Biol Med. 2011;52:660–670. doi: 10.1016/j.freeradbiomed.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cingolani C, Rogers B, Lu L, Kachi S, Shen J, Campochiaro PA. Retinal degeneration from oxidative damage. Free Radic Biol Med. 2006;40:660–669. doi: 10.1016/j.freeradbiomed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel A, Hackam A. A novel protective role for the innate immunity Toll-Like Receptor 3 (TLR3) in the retina via Stat3. Molecular and Cellular Neuroscience. 2014;68C:38–48. doi: 10.1016/j.mcn.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanausek M, Spears E, Walaszek Z, Kowalczyk MC, Kowalczyk P, Wendel C, Slaga TJ. Inhibition of murine skin carcinogenesis by freeze-dried grape powder and other grape-derived major antioxidants. Nutr Cancer. 2011;63:28–38. doi: 10.1080/01635581.2010.516474. [DOI] [PubMed] [Google Scholar]
- 15.Hu D, Haware RV, Hamad ML, Morris KR. Characterization of critical physical and mechanical properties of freeze-dried grape powder for development of a clinical patient delivery system. Pharm Dev Technol. 2013;18:146–155. doi: 10.3109/10837450.2012.659257. [DOI] [PubMed] [Google Scholar]
- 16.Jing Y, Yumin X, Khaoustov V, Yoffe B. Identification of components of grape powder with anti-apoptotic effects. Toxicol Ind Health. 2011;27:19–28. doi: 10.1177/0748233710380220. [DOI] [PubMed] [Google Scholar]
- 17.Syeda S, Patel AK, Lee T, Hackam AS. Reduced photoreceptor death and improved retinal function during retinal degeneration in mice lacking innate immunity adaptor protein MyD88. Exp Neurol. 2015;267C:1–12. doi: 10.1016/j.expneurol.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michon JJ, Li ZL, Shioura N, Anderson RJ, Tso MO. A comparative study of methods of photoreceptor morphometry. Invest Ophthalmol Vis Sci. 1991;32:280–284. [PubMed] [Google Scholar]
- 19.Kim SH, Park JH, Kim YJ, Park KH. The neuroprotective effect of resveratrol on retinal ganglion cells after optic nerve transection. Mol Vis. 2013;19:1667–1676. [PMC free article] [PubMed] [Google Scholar]
- 20.Kubota S, Kurihara T, Ebinuma M, Kubota M, Yuki K, Sasaki M, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. Am J Pathol. 2010;177:1725–1731. doi: 10.2353/ajpath.2010.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha JH, Shil PK, Zhu P, Gu L, Li Q, Chung S. Ocular inflammation and endoplasmic reticulum stress are attenuated by supplementation with grape polyphenols in human retinal pigmented epithelium cells and in C57BL/6 mice. J Nutr. 2014;144:799–806. doi: 10.3945/jn.113.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall LL, Roach JM. Prevention and treatment of age-related macular degeneration: an update for pharmacists. Consult Pharm. 2013;28:723–737. doi: 10.4140/TCP.n.2013.723. [DOI] [PubMed] [Google Scholar]
- 23.Georgiev V, Ananga A, Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients. 2014;6:391–415. doi: 10.3390/nu6010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XL, Li BY, Cheng M, Yu F, Yin WB, Cai Q, Zhang Z, Zhang JH, Wang JF, Zhou RH, Gao HQ. PIMT prevents the apoptosis of endothelial cells in response to glycated low density lipoproteins and protective effects of grape seed procyanidin B2. PLoS One. 2013;8:e69979. doi: 10.1371/journal.pone.0069979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyagi A, Raina K, Shrestha SP, Miller B, Thompson JA, Wempe MF, Agarwal R, Agarwal C. Procyanidin B2 3,3(”)-di-O-gallate, a biologically active constituent of grape seed extract, induces apoptosis in human prostate cancer cells via targeting NF-kappaB, Stat3, and AP1 transcription factors. Nutr Cancer. 2014;66:736–746. doi: 10.1080/01635581.2013.783602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AK, Surapaneni K, Yi H, Nakamura RE, Karli SZ, Syeda S, Lee T, Hackam AS. Activation of Wnt/beta-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology. 2015;91:1–12. doi: 10.1016/j.neuropharm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novotny Nunez I, Maldonado Galdeano C, de Moreno de LeBlanc A, Perdigon G. Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition. 2015;31:1000–1007. doi: 10.1016/j.nut.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Nunez IN, Galdeano CM, de LeBlanc Ade M, Perdigon G. Evaluation of immune response, microbiota, and blood markers after probiotic bacteria administration in obese mice induced by a high-fat diet. Nutrition. 2014;30:1423–1432. doi: 10.1016/j.nut.2014.03.025. [DOI] [PubMed] [Google Scholar]