Abstract
Objective
To develop a protocol for cryopreservation and recovery of human endometrial epithelial cells (eEC) retaining molecular and functional characteristics of endometrial epithelium in vivo.
Design
This is an in vitro study using human endometrial cells.
Setting
University research laboratory.
Patients
Endometrial biopsies were obtained from premenopausal women undergoing benign gynecological procedures.
Interventions
Primary eEC were cryopreserved in 1% fetal bovine serum (FBS)/10% dimethyl sulfoxide (DMSO) in Defined Keratinocyte Serum Free Medium (KSFM). Recovered cells were observed for endometrial stromal fibroblast (eSF) contamination and subsequently evaluated for morphology, gene expression, and functional characteristics of freshly cultured eECs and in vivo endometrial epithelium.
Main Outcome Measures
Analysis of eEC morphology and the absence of eSF contamination; evaluation of epithelial-specific gene and protein expression; assessment of epithelial polarity.
Results
eEC recovered after cryopreservation (n=5) displayed epithelial morphology and expressed E-cadherin (CDH1), occludin (OCLN), claudin1 (CLDN1), and keratin18 (KRT18). Compared to eSF, recovered eEC displayed increased (P<0.05) expression of epithelial-specific genes AREG, CDH1, DEFB4A, MMP7, and WNT7A, while exhibiting low-to-undetectable (P<0.05) stromal-specific genes COL6A3, HOXA11, MMP2, PDGFRB, and WNT5A. Recovered eEC secrete levels of cytokines and growth factors comparable to freshly cultured eEC. Recovered eEC can formed a polarized monolayer with high transepithelial electrical resistance (TER) and impermeability to small molecules, and expressed apical/basolateral localization of CDH1 and apical localization of OCLN.
Conclusion
We have developed a protocol for cryopreservation of eEC in which recovered cells after thawing demonstrate morphological, transcriptomic, and functional characteristics of human endometrial epithelium in vivo.
Keywords: cryopreservation, freezing medium, endometrium, endometrial epithelium, endometrial epithelial cells
Introduction
The endometrium, the lining of the uterus, undergoes growth and differentiation in response to ovarian hormones in preparation for blastocyst nidation (1, 2) and regenerates cyclically in the absence of pregnancy (2, 3). The tissue is residence to a variety of cell types, including epithelial cells (eEC), stromal fibroblasts (eSF), leukocyte populations, endothelial cells, vascular smooth muscle cells and stem/progenitor cells (1, 2). Because of its importance in the reproductive process and its roles in women’s health (physiology and pathophysiology), the endometrium has been the subject of intense research in a variety of clinical settings. In addition, because it is relatively accessible by biopsy or from surgical specimens, numerous protocols have been developed to obtain, process, and store human endometrial tissue with the goal of preserving in vivo characteristics and also to isolate cell constituents for mostly in vitro and flow cytometry studies for research on cell function and reproductive disease phenotypes (4, 5).
The majority of the endometrial histoarchitecture is comprised of luminal epithelium, the underlying endometrial epithelial glands, and the endometrial stroma. These endometrial cell types exhibit differences with regard to ease of preparation, purification, in vitro stability and functionality. For example, endometrial stromal fibroblasts (eSF), whose programmed response to estradiol (E2) and progesterone (P4) is essential to pregnancy establishment and maintenance, are readily cultured after fresh isolation, have high recovery and viability rates after cryopreservation, are routinely passaged in vitro with fidelity of in vivo functionality, and respond in vitro to E2 and P4 in a predictable manner (1, 6–8). In contrast, the endometrial epithelial cell types present unique challenges in terms of obtaining pure populations, culturing and maintaining in vivo functionality and have limited expansion potential (9–17). Specifically, in vitro the endometrial epithelium needs to be polarized and express specific adherens and tight junction proteins (10) to replicate in vivo apical/basolateral morphology and functionality, and requires paracrine interactions to optimally respond to E2 and P4 both in vivo and in vitro (1, 2, 18). Primary eEC, compared to eSF, have limited expansion potential without immortalization (14, 19, 20), thus restricting the size and versatility of experimental designs using this cell type. Moreover, in the absence of published optimized protocols for cryopreservation of human eEC, further use in experimental models is limited, by dependence on amount and availability of fresh tissues for eEC studies in vitro, underscoring the need for improved methods made available to the research community in this field.
In view of these challenges, the objective of the current study was to develop a cryopreservation protocol that allows successful recovery of cryopreserved eEC of high purity and, most importantly, retaining morphological, molecular, and functional fidelity of the endometrial epithelium in vivo, to enable further research on human endometrial epithelial function and dysfunction. Our results indicate that a cryopreservation medium formulated with Defined Keratinocyte Serum Free Medium (KSFM) supplemented with low (1%) serum and 10% dimethylsulfoxide (DMSO) results in high recovery and viability eEC which express epithelial lineage markers and display endometrial epithelial functionality.
Materials and Methods
Endometrial Tissue Procurement
Endometrial tissues were obtained using standard operating procedures for collecting samples through the NIH National Translational Center for Research in Infertility (NCTRI) Human Endometrial Tissue and DNA Bank at the University of California, San Francisco (UCSF) (5). Briefly, endometrial samples were obtained from women undergoing benign gynecological procedures (n=9) or egg donors (n=5) at the time of oocyte retrieval, after written informed consent in accordance with the guidelines of the Declaration of Helsinki and under approved human subjects protocol by the Committee on Human Research (CHR) at UCSF (CHR Protocol 10-02786). Subjects were premenopausal (ages 28–53) and confirmed not to be pregnant. Samples from patients undergoing oocyte retrieval (n=5) were considered in the early secretory phase (ESE). Patients undergoing benign gynecologic procedures for endometriosis, fibroids, or polycystic ovary syndrome were either in the proliferative phase (n=1, P), secretory phase (n=4, SE), or did not have phase classification available (n=4). Details of each patient’s clinical characteristics at the time of tissue sampling are in the Supplemental Data available at online at www.fertstert.org, Supplemental Table S1.
Endometrial Tissue Processing, Culture, Cryopreservation/Thawing/Recovery
Endometrial tissue samples were processed on the day of collection, and primary cells were isolated for cryopreservation protocol testing was initiated immediately after tissue procurement.
Tissue Processing
The cryopreservation/thawing recovery protocol is shown pictorially in Figure 1. Endometrial tissue was first minced with a scalpel into ~1mm3 pieces in phosphate buffered saline (PBS) and then digested in Hanks Buffered Salt Solution (HBBS) with Ca++ Mg++ (0.1μM each; UCSF Cell Culture Facility, San Francisco, CA) diluted 1:1 with HBSS without Ca++ Mg++ (UCSF) and containing 6.4 mg/mL collagenase type I (Worthington, Lakewood, NJ), 125 U/mL hyaluronidase (Sigma Aldrich, St. Louis, MO) and 0.1nM gentamycin (UCSF) for 2–3h into suspensions containing single cells and luminal epithelial sheets and glandular epithelial fragments. Digests were then size fractionated with a 40-μm cell strainer (BD Biosciences San Jose, CA) to separate single cells (eSF, leukocytes, stem cell populations, vascular cells) from fragments of luminal epithelial sheets and glandular epithelium. The digest >40-μm fraction was backwashed into a petri dish and cultured for 1–2h in selective attachment medium (a 1:10 dilution of stromal fibroblast cell medium [SCM]: 75% Dulbecco’s Modified Eagle’s Medium [DMEM, Gibco, Grand Island, NY]; 25% MCDB 105 with 10% fetal bovine serum [FBS], 500nM sodium pyruvate; 0.1nM gentamycin in PBS) to promote attachment of potentially non-filtered eSF (14, 20–23). Non-attached epithelium was then aspirated, pelleted by centrifugation (300 × g), and washed twice in Defined Keratinocyte Serum Free Medium (KSFM 10744-019, Gibco) with 1% FBS and gentamycin. Undigested, non-epithelial tissue was removed by pipette aspiration. Luminal epithelial sheets and glandular epithelial fragments were resuspended in KSFM with 1% FBS/gentamycin/10% DMSO and aliquoted into cryovials, which were then sequentially frozen at −80°C in Styrofoam insulation for 24h, followed by their transfer into liquid nitrogen for long-term storage.
Figure 1. The workflow diagram of processing eEC for cryopreservation.
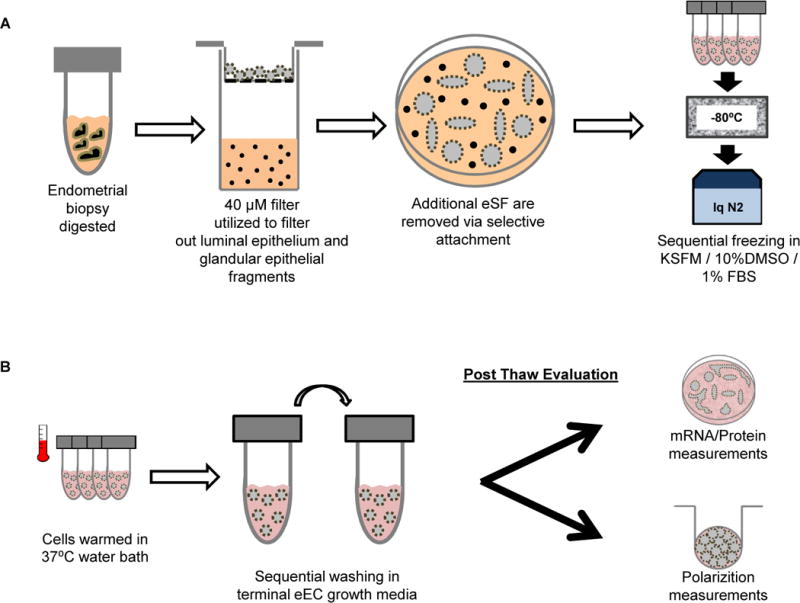
Figure 1A depicts the protocol for processing of the endometrial tissue, including sequential enzyme digestion into single cells and luminal epithelial sheets and glandular epithelial fragments, size fractionation, selective attachment, and cryopreservation. Figure 1B shows the recovery process for subsequent assays. Epithelial fragments were washed in eEC culture medium and then prepared for either protein expression studies on Matrigel-coated dishes or digested into a single cell suspension for plating on transwell inserts for polarization studies.
Recovery of cryopreserved cells
Cryovials were warmed in a 37°C water bath for 1–2 min, and epithelial fragments were washed in KSFM with 1% FBS and gentamycin twice to remove traces of DMSO and resuspended in medium depending on the experimental endpoint (see below). Two post-thaw evaluations of epithelial phenotype were conducted (Figure 1B). First, thawed luminal epithelial sheets and glandular epithelial fragments were plated in KSFM with gentamycin (without FBS) on Matrigel-coated plates (Corning Life Sciences, Corning, NY; 6, 12, or 24 wells) at a density of 5–10 fragments per viewing field at 100× magnification, and cultured for 5–10 days to evaluate epithelial-specific gene and protein expression. A second post-thaw evaluation experiment was to determine if recovered eEC can polarize and form a tight epithelial barrier. To this end, epithelium was digested in 5ml Accutase (EMD Millipore, Billerica, MA) at 37°C for 10–20 min until a single-cell suspension was achieved, washed in KSFM/1%FBS/gentamycin twice to remove Accutase, resuspended in a final concentration of 1×106 cells per ml of KSFM/1% FBS/gentamycin, then plated at 2×105 cells per 24-well size on polyethylene terephthalate membrane, 0.332cm filtration area, 1μm pore Millicell hanging transwell cell culture inserts, (PIRP12R48, EMD Millipore) coated with Matrigel (growth factor reduced, 354230, BD Biosciences) and cultured for 10–15d. Accutase digestion yielded eEC viability of 74 ± 14%, whereas utilizing trypsin-based digestion resulted in poor viability of 14% ± 8%. These and all other cultures were maintained at 37°C in a humidified 5% CO2 incubator.
To compare eEC recovered after cryopreservation versus freshly cultured cells, paired culture were prepared with samples that were large enough reserving an aliquot of epithelium for fresh culture, and another aliquot for cryopreservation. Fresh eEC culture is identical to culturing recovered eEC, and as previously described (21, 22). Briefly, freshly isolated epithelial fragments were plated in KSFM with gentamycin (without FBS) on Matrigel-coated plates. Also, eSF that were sample-matched to eEC obtained and cultured by methods previously described (21, 22) served as cell type controls for gene expression in situ. The filtered <40-μm single-cell fraction was pelleted and washed with PBS twice to remove residual digestion medium. 2.5 ×105 primary cells from this single-cell suspension (comprised mainly of eSF) were plated onto a 10cm petri dish and allowed to reach confluency in 5–10d in SCM. Established cells (eSF) were then passaged (1×105) into 24 well plates and reached confluence in 1–2d.
Light microscopy and immunofluorescence staining of eEC-specific markers
Phase contrast microscopy was used to characterize the morphology of digested epithelium, attached epithelium, and potential eSF contamination. Indirect immunofluorescence was conducted as previously reported (21, 22) to identify eEC-specific markers. Briefly, eEC cultured in Matrigel-coated plates (n=5; 2 oocyte donors in ESE, 3 non-oocyte donors in SE) were fixed in ice cold methanol, permeabilized with 0.1% Triton X-100 (Sigma Aldrich), blocked with 10% normal goat serum (Sigma Aldrich), and incubated overnight at 4°C with the following primary antibodies at 1–200 dilution: mouse anti-human KRT18 (1:200; C-7785, Sigma Aldrich), CDH1 (ab1416, Abcam, Cambridge, MA), rabbit anti-human CLDN1 (ab15098, Abcam), rabbit anti-human OCLN (ab31721, Abcam). Cells were then washed 3 times with phosphate-buffered saline (PBS)/0.1% Tween 20 buffer and incubated for 1h at room temperature with the corresponding Alexafluor 488 conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (1:250; A-11001 and A-11008, respectively; Life Technologies, Carlsbad, CA) and then washed 3 times with buffer. Controls were mouse or rabbit non-immune IgG substituted for the corresponding primary antibodies. Cells were subsequently treated with ProLong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (DAPI; P-36931, Life Technologies) then viewed on a Zeiss Axio Observer Z1 inverted microscope equipped with bright field, phase contrast, and epifluorescence optics, and images captured using ZEN imaging software (Zeiss, San Diego, CA).
Preparation of total RNA and cDNA synthesis
Total RNA from recovered eEC and eSF was obtained using methods previously reported (21, 22). Briefly, non-polarized eEC cultured on Matrigel-coated plates were harvested and purified using the Nucleospin RNA II Purification Kit (740955-250, Machery Nagel, Bethlehem, PA). Isolated mRNA was quantified and evaluated for purity with Nanodrop (Nanodrop, Wilmington, DE). First strand cDNA synthesis was performed using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) to perform a one-cycle first strand synthesis utilizing the Eppendorf Master Cycler (Eppendorf, Hauppauge, NY) using the manufacturer’s protocols. Final cDNAs were diluted to a concentration of 10ng/μl.
Quantitative Real Time PCR (qRT-PCR) analysis of eEC or eSF-specific genes
To assess cellular purity, a selected set of differentially expressed genes previously found to be up or down regulated in comparing eEC with eSF were chosen for qRT-PCR (n=5; 2 oocyte donors in ESE, 3 non-oocyte donors in SE). Total RNA was confirmed for the absence of RNA/protein contaminants by Nanodrop (Nanodrop). cDNA was generated using the Biorad Iscript cDNA synthesis kit (1708891, Biorad, Hercules, CA) using manufacturer’s protocols. Total cDNA (20 ng) was combined with SYBR green and 1 μM custom-made primers (Fluidigm, South San Francisco, CA, USA) directed towards human AREG, CDH1, DEFB4A, MMP7, WNT7A, COL6A3, HOXA11, MMP2, PDGFRB, WNT5A, and the housekeeping gene YWHAZ. Our choice of YWHAZ for reference is based on its stability of expression between eEC and eSF, and based on stability of expression from previous studies (21). Amplification was performed using the Stratagene MX3005P (Agilent, Santa Clara, CA) Thermocycler. Dissociation curves for both target and housekeeping genes were utilized to ensure the absence of primer dimers and other non-specific amplification. Primers were designed by Fluidigm and optimized for SYBR-based qRT-PCR following the Fluidigm Biomark guidelines on mRNA amplification, including primer amplification efficiency, amplicon size, and appropriate dissociation temperatures governing mRNA amplification. These amplification conditions are compliant with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE)(24) and thermo-cycling conditions were similar to those previously reported (25). The comparative (delta-delta) Ct method was used to measure relative gene expression for each cell type (ABI User bulletin 2).
Multiplex protein assays
The secretory activity of eEC recovered after cryopreservation was compared to paired freshly cultured cells (n=5, 3 oocyte donor in ESE, 1 non-oocyte donors in P, 1 non-oocyte donor, hysterectomy, no phase classification available). eEC conditioned (48h) media (500μl from 24-well Matrigel coated culture plates) from sample-paired eECs that were either freshly cultured or cryopreserved/thawed and cultured were centrifuged at 13,000 × g for 5 minutes to remove cellular debris, and supernatants were analyzed for secreted cytokines using a custom multiplex Luminex kit (EMD Millipore), as previously described (21, 22). Select cytokines assayed included fibroblast growth factor (FGF) 2, fractalkine (CX3CL1), granulocyte colony stimulating factor (GCSF), granulocyte macrophage colony stimulating factor (GMCSF), interleukin (IL) 1A, −4, −6, −8, chemokine (C-X-C motif) ligand 1 (GRO α), monocyte chemoattractant protein (MCP)1, 3, macrophage inflammatory protein (MIP)1A, B, regulated on activation, normal T cell expressed and secreted (RANTES), tumor necrosis factor alpha (TNFA), and vascular endothelial growth factor (VEGFA). All protocols were based on the manufacturer’s specifications.
Establishment of eEC polarity
Cryopreserved/thawed eEC (cultured on Matrigel-coated inserts as described above were tested for measurement of transepithelial electrical resistance (TER) (n=5, 1 oocyte donor in ESE, 1 non-oocyte donors in SE, 3 non-oocyte donors no phase classification available) using the Millicell URF-2 voltometer (EMD Millipore) as previously described (21). Briefly, dual electrodes were placed so that one electrode rested in the apical chamber fluid and one in the basolateral chamber and TER recorded. Three measurements were obtained for each sample and averaged for a total of n=5 samples. To test for leakiness of the eEC monolayer, 200 μl of phenol red (32mg/L) in KSFM with 1% FBS was added into the apical chamber, while 1ml of KSFM with 1% FBS without added phenol red was added into the basolateral chamber and allowed to equilibrate for 6h in the 37C humidified 5% CO2 incubator. The optical densities (OD) of both the apical- and basolateral-chamber fluids were read at 559nm using a Beckman Coulter DU 560 specrophotometer (Beckman Coulter, Brea CA). Three readings of the apical and basolateral chambers were taken for each sample and averaged at two time points (2 and 10 days in culture) for a total of n=5 samples. Confocal imaging was carried out using a 20X objective on Leica SP5 TCS microscope (Leica, Buffalo Grove, IL) equipped with a 405, 488, 543, 594 and 633nm lasers. Image stacks were analyzed using Volocity (Improvision, Perkin Elmer, Waltham, MA). Apical and basal compartments of the cells were determined based on the Z focal plane in relation to the Matrigel substrate (basal).
Statistics
To determine significance of differences in the expression levels of eEC or eSF-specific genes, paired T-tests were used for each pair of patient-specific recovered eEC and eSF. Paired T-tests were also used to determine the significance of differences in levels of secreted proteins between eEC freshly cultured vs recovered eEC. TER data were analyzed using ANOVA with Tukey post-hoc testing for comparisons between the blank insert, 48h post plating, and 10d post plating. To analyze differences in phenol red leakage, comparison of OD between the apical and basolateral media was conducted using the paired T-test. To determine if time in culture affected leakage, ANOVA with Tukey post-hoc analysis was conducted in the basolateral chamber media OD readings.
Results
Cryopreserved/thawed eEC express epithelial-specific morphology and proteins
In the process of isolating endometrial cells, separation of epithelial fragments from single cells is commonly performed, with the former serving as a resource for eEC. Herein, we isolated and then cryopreserved epithelial fragments (Figure 1) and then evaluated them morphologically after thawing and for expression of epithelial-specific genes and proteins after culture. Phase contrast microscopy showed no difference in the morphological appearance of epithelium post recovery compared to freshly isolated epithelium, both containing tubular gland fragments and epithelial cell sheets (Figure 2A). Primary cultures of epithelium attached to Matrigel-coated plates, with eECs spreading into island-like masses that then form into a confluent layer, with mound-like structures (Figure 2B). Brightfield microscopy was used to examine the entire area of the plate to assess any cells exhibiting non-epithelial, eSF morphology. Pure epithelial cultures should have distinct borders and the absence of non-epithelioid cells on Matrigel (Figure 2C). Given the published literature of utilizing 10% FBS in cryopreservation media, we attempted to freeze eEC with KSFM with 10% DMSO and 10% FBS, which resulted in eventual contamination by cells that have elongated, spindle-shape morphology characteristic of eSF (n=5, data not shown). The eEC monolayer in culture expressed the tight-junction protein CLDN1 (Figure 2D) and OCLN (Figure 2E). Both eEC-specific factors E-cadherin (the epithelial adherans junction protein) and KRT18 (the epithelial-specific intracellular filament) were also detected (Figure 2F). Negative control IgG slides are also indicated (Figures 2G, H, I).
Figure 2. Phase contrast and immunofluorescence microscopy of recovered eEC.
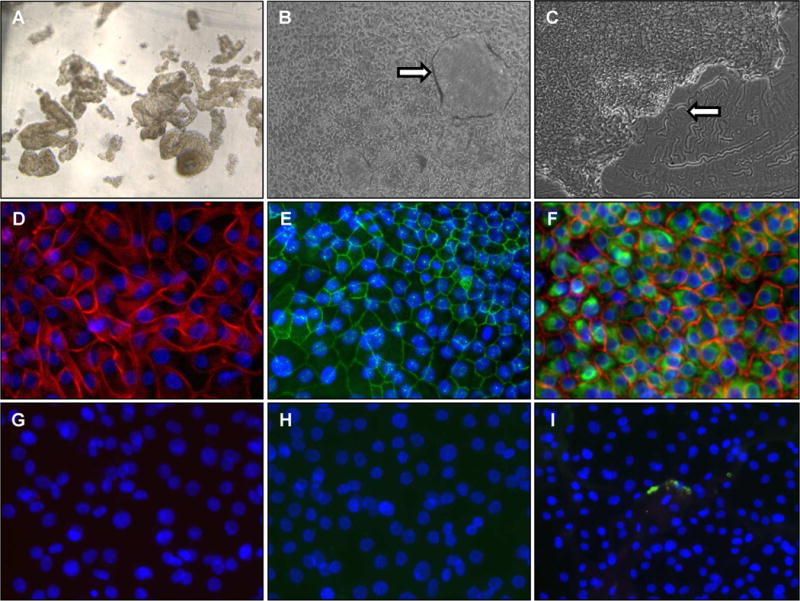
Panel A shows the epithelial fragments immediately after thawing prior to culture (50X). Panel B shows a confluent eEC monolayer culture, with formation of dome-shaped structures indicated by the arrow (50X). Panel C shows the edge of an expanding epithelial colony with defined borders and clean areas of the Matrigel substrate where cells have not attached indicated by the arrow (50X). Panels D and E show immunofluorescence of CLDN1 (red) and OCLN (green) respectively (200X). Panel F shows double staining of KRT18 (green) and CDH1 (red) (200X). Blue indicates DAPI nuclear staining. Panels G, H, I show respective IgG negative controls for Panels D, E, and F.
Cryopreserved and recovered eEC express epithelial-specific genes
To determine if cryopreserved eEC express an epithelial signature at the transcript level, qPCR was used to measure the expression of cell lineage-specific genes Primary-cultured eEC expressed significantly higher levels of the epithelial markers AREG, CDH1, DEFB4A, MMP7, and WNT7A, (21, 22) compared to eSF (P<0.05) (Figure 3A). Conversely, eSF expressed significantly more (P<0.05) COL6A3, HOXA11, MMP2, PDGFRB, WNT5A transcripts compared to eEC (Figure 3B).
Figure 3. Expression of eEC-specific genes and proteins.
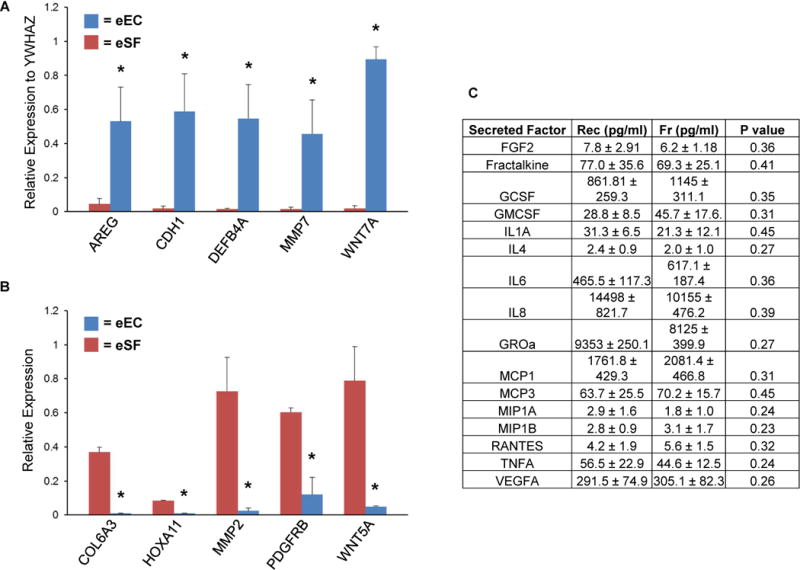
Panel A shows that recovered eEC express eEC-specific genes AREG, CDH1, DEFB4A, MMP7, WNT7A. Panel B shows that in comparison to patient-matched eSF, recovered eEC express significantly lower levels of eSF-specific genes COL6A3, HOXA11, MMP2, PDGFRB, WNT5A. Values in Panels A and B represent relative gene expression for each cell type normalized to the housekeeping gene YWHAZ. Panel C shows comparison of secreted factors from recovered eEC (Rec) versus freshly cultured eEC (Fr) from the same subject and the P value for the corresponding paired T-test. Asterisik (*) indicates P<0.05 between eEC and eSF.
Secretory profile of recovered eEC
The biological activity of recovered eEC was assessed by measuring levels of proteins known to be secreted by freshly cultured eECs, (Figure 3C). Supernatants of sample-paired frozen eEC and freshly cultured eEC experiments revealed that cryopreservation does not significantly affect the patterns or concentrations of secreted factors (P>0.05). Moreover, the concentrations of the secreted factors were comparable to previous studies conducted in our lab using freshly cultured eEC (21, 22), with the highest concentration of secreted proteins being IL8 (10,000–14,000 pg/ml) and the lowest concentration of protein being MIP1A/1B (1–3pg/ml).
Ability of recovered eECs to polarize and form a tight-epithelial barrier
To determine if recovered eECs can polarize and form a tight layer that achieves high trans-epithelial electrical resistance (TER) and is impermeable to small molecules, eEC were plated in single-cell suspension on Matrigel-coated hanging inserts; after monolayers reached confluence, TER and leakage of phenol red from the insert into the basolateral chamber were determined. TER was significantly increased by 48h, and further increased by 10 days (P<0.05; Figure 4A). The presence of phenol red in the apical and basolateral chambers was measured by optical density (OD). In the presence of polarized eEC recovered from cryopreservation, the phenol red signal was significantly higher in the apical medium compared to basolateral media at 10d (P<0.05; Figure 4B). After 48h, increased phenol red signal in the basolateral chamber (P<0.05) compared to basolateral readings at 10d indicates greater leakiness. With an insert in the absence of cells, the apical and basolateral media exchanged evenly by 6h, resulting in comparable phenol red readings, as expected. Brightfield microscopy of recovered eEC polarized on inserts and co-cultured with eSF are shown in Figure 4C. eEC showed only apical expression of the tight junction protein OCLN (Fig. 4D); whereas, the adherens junction protein CDH1 was expressed throughout the apical-basolateral cell border (Fig. 4D).
Figure 4. Polarization of recovered eEC.
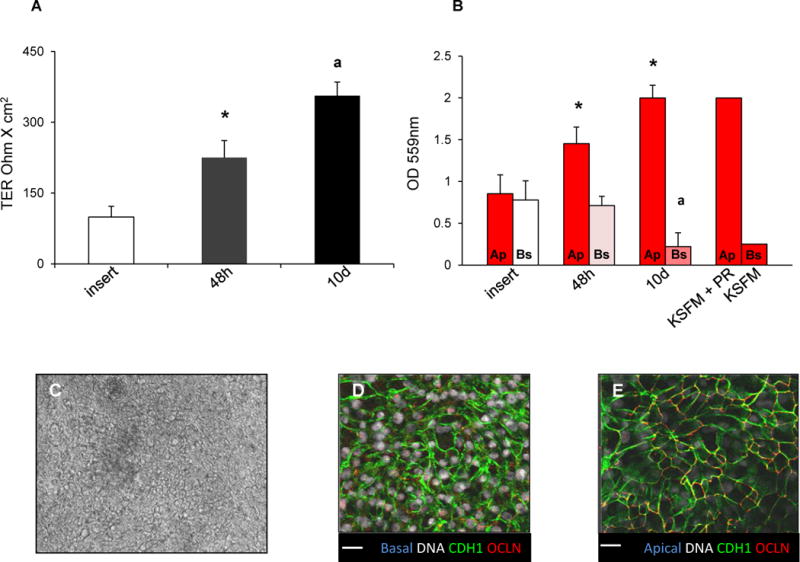
Panel A shows transepithelial electrical resistance (TER) of control inserts without cells, and of insert cultures of recovered eEC 48h and 10d after plating. Panel B shows absorbance readings for measurement of phenol red concentration in reference standards for KSFM with phenol red (KSFM+PR) versus without (KSFM), and in apical (Ap) and basolateral (Bs) chambers of control inserts without cells, and insert cultures of recovered eEC 48h and 10d after plating. Panel C is a representative phase contrast image of polarized eEC on transwell inserts, 50x. Panel D shows basolateral expression of CDH1 (green) in polarized eEC on transwell inserts. Panel E shows apical expression of CDH1 (green) and OCLN (red). Grey indicates Hoechst nuclear staining. Scale bar: 25 microns. Asterisk (*) indicates P<0.05 between groups in the TER study, and between Ap and Bs for the PR study. Letter (a) indicates P<0.05 between groups in the TER study, and between Bs at 10d vs Bs at 48h in the PR study.
Discussion
In the current study, we tested a protocol to cryopreserve and thaw human endometrial epithelial cells and validated that recovered cells were highly pure and demonstrated morphologic, transcriptomic, and functional characteristics of in vivo epithelium. Given the importance of endometrial epithelium in human reproduction and as a mucosal barrier of infection in the upper female reproductive tract, establishing a method to cryopreserve and recover eEC has the promise to enhance reproductive research with this cell type for the greater women’s health research community.
Utilizing KSFM/1%FBS/10%DMSO freezing medium ensures successful recovery of eEC
Traditionally, freezing media contain varying concentrations of DMSO (ranging from 5–10% (26)) and FBS (10% for fibroblasts (15, 27, 28) and 20–90% for leukocytes (29, 30)). Whereas DMSO slows the freezing process and prevents ice crystal formation, FBS contains growth and nutrient factors that improve cell viability during the thaw and recovery process (31). However, we show here that utilizing KSFM with 1% FBS freezing medium efficiently recovers eEC from endometrial biopsies that exhibit morphological, transcriptomic, and secretory properties of epithelium. Interestingly, we found that 10% FBS with KSFM resulted in increased eSF proliferation and overtaking eEC cultures. This finding was surprising, as it was not expected that the composition of the initial freezing medium could affect recovery outcome, especially given that the eEC were thoroughly washed in their eventual culture medium (in this case, KSFM or KSFM with 1% FBS). Culture of epithelial cell lineages has long been conducted under serum free conditions (32), and recent studies have further supported use of serum-free cryopreservation medium for storing amniotic epithelial cells as part of stem cell therapy (33). Thus, serum free conditions are likely conducive for both culturing as well as successfully cryopreserving epithelial cells, more broadly.
Recovered eEC display morphological characteristics similarly to freshly cultured eEC
eEC exhibit distinct patterns of growth and morphology, including attaching to a matrix, such as Matrigel, initiating growth in clusters, and taking from one to two weeks to achieve full confluency. Herein, we observed similar patterns of growth and proliferation in recovered eEC compared to their fresh counterparts. eEC express structural proteins (e.g., adherens and tight junction components) associated with their function as epithelium, which serve as a protective barrier to the external environment (the uterine lumen in the case of the endometrium (1, 21, 34)). We observed that recovered epithelium expressed junctional components CDH1, OCLN, KRT18, and CLDN1, which were previously detected in eEC both at the mRNA and protein level and highly expressed compared to non-epithelial cells, such as eSF (21, 22). Additionally, recovered eEC expressed other eEC-specific genes with minimal expression of eSF-specific genes as previously reported (21, 22). These data demonstrate a high degree of cell specificity and epithelial purity in recovered samples.
Recovered eEC secrete epithelial factors comparable to freshly cultured eEC
Epithelial secreted proteins, such as cytokines, play an important role in uterine mucosal immunity as well as the immune environment during the process of embryonic implantation (35). Several cytokines and growth factors prominently expressed in the eEC, include CCL3, CCL4, IL1A, 4, 6, 8, TNFA, VEGFA, FGF, and fractalkine (21, 22, 36). Cryopreserved and recovered eEC produced secreted factors, equivalent paired freshly cultured eEC or reports from the literature – e.g. IL8 concentrations were 2–3 fold higher than IL6 (21, 22, 36). Furthermore, factors including TNFA, CCL3, CCL4 reportedly secreted at very low concentrations (1–5 pg/ml) by eEC in culture (16, 19), were similarly secreted in very low levels by recovered eEC. The fidelity of the secreted cytokine profiles from recovered eEC gives confidence to the utility of the cryopreservation/thaw protocol for future studies using human endometrial epithelium – whether in response to infectious agents, chemicals, embryo-crosstalk, or communications with other cell types.
Recovered eEC polarize in culture and form a tight epithelial monolayer
Recovered eEC that have been cryopreserved establish functional polarity post recovery and exhibit impermeability to phenol red and develop high TER in a time-dependent manner. Previous work in epithelial cells shows that the tight junction protein OCLN localizes to the apical surface, helping to seal the epithelium and prevent non-specific diffusion of luminal contents to the underlying stroma (37). CDH1 belongs to a family of adherens proteins that stabilize all epithelial junction proteins and interact with intracellular actin throughout the apical-basolateral cell-to-cell contact point (38, 39). The data presented herein support these functions in that polarized recovered eEC express CDH1 on both its apical and basolateral surfaces, while the apical tight junction protein OCLN is restricted to the apical surface.
The demonstrated functional recovery of epithelial polarization from frozen and recovered eEC expands the potential and feasibility of polarized epithelial cell culture models and more complex epithelial/stromal co-culture models. Current limitations on the amount of human epithelial samples for complex experimental designs are further complicated by the difficulty associated with properly polarizing cells in culture. The ability to biobank epithelial samples enables more complex experimental designs with high power and large experimental groups. Examples include measuring the capacity of exogenous factors to compromise integrity of the luminal epithelium; measuring the effect of embryo-secreted factors on the luminal epithelium, and studying how systemic factors delivered through the endometrial stromal affect epithelial responses.
Limitations to the Interpretations of the Current Work
A major limitation to the implementation of this protocol is obtaining samples that are adequate in size to provide yields that are suitable to the seeding density, discussed in the methodology. Yield efficiency will also likely influence the number of treatment conditions for subsequent experimental studies beyond validation of expression of epithelial phenotypic parameters. Our current studies do not evaluate whether recovered, non-polarized eEC grown on regular Matrigel-coated plates display a phenotype consistent with luminal epithelium versus glandular epithelium. Previously (21), we showed that polarized eEC grown on transwell inserts express elevated WNT7A, HBEGF, and KRT13 compared to FACS-sorted eEC, all of which are markers of human luminal, not glandular, epithelium. Further evaluation of the recovered eEC phenotype in a non-polarized environment is warranted. It will be important to ask whether cryopreserved eEC respond to the ovarian hormones E2 and P4, given established hormonal dependence throughout the menstrual cycle and the dependence of eEC on paracrine interactions with eSF for specific hormone responsiveness. Further research is also needed to determine why and how serum concentrations affect eSF viability and predominance in cryopreserved and recovered eEC. Finally, samples were derived from patients with endometrial disorders (e.g. endometriosis) and oocyte donors during a hormonally stimulated cycle, which could confound the results, although samples were distributed as evenly as possible across the experimental methods, and generally behaved similarly in all assays. While these are important considerations, our objective was to recapitulate the epithelial phenotype in frozen samples of high purity and compare secreted cytokines and growth factors by paired fresh vs. frozen samples. With this foundation established, subsequent studies among varying clinical conditions can be successfully undertaken.
Summary
We have developed a cryopreservation protocol that consistently generates viable eEC upon recovery, which will enhance research involving use of eEC in reproductive biology more broadly. While eEC culture and the use of serum-free/low serum commercially available freezing media are not novel per se, the current combination of existing methodologies for eEC culture with altering current freezing media formulations using Defined KSFM have boosted the efficiency of eEC cryopreservation and recovery, and hopefully will enhance research involving eEC in reproductive biology more broadly.
Supplementary Material
Acknowledgments
This project was supported by NIH AI083050-05 (LCG and Warner Greene), the National Institutes of Health (NIH) Eunice Kennedy Shriver NICHD National Centers for Translational Research in Infertility (NCTRI) P50-055764 (LCG), and the F32HD074423-03 National Research Service Award (JCC), UCSF Program for Breakthrough Biomedical Research, which is funded in part by the Sandler Foundation (RA) and NIH DP2OD007420 (DJL). The authors are grateful for the administrative support of Nicole Bloom, Bethanie Brandon, and Shaina Balayan. The authors would like to acknowledge Drs. Warner Greene, Ruth Greenblatt, Barbara Shacklett, Karen Smith-McCune, Nadia Roan, Marielle Cavrois, Terhi Piltonen, David Erikson, Sahar Houshdaran, Trimble Spitzer, Lusine Aghajanova, and Crystal Chan for their invaluable input. The authors also acknowledge the support of the National Institutes of Health Specialized Cooperative Centers Program in Reproduction and Infertility Research, the UCSF Human Endometrial Tissue and DNA Bank, as well as the UCSF/Kaiser Permanente Undergraduate Research Internship (URI) program (JGG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Aplin J, Fazleabas A, Glasser S, Giudice L. The Endometrium: Molecular, Cellular, and Clinical Perspectives. Second. London: Informa Healthcare; 2008. [Google Scholar]
- 2.Giudice LC. Elucidating endometrial function in the post-genomic era. Hum Reprod Update. 2003;9:223–35. doi: 10.1093/humupd/dmg019. [DOI] [PubMed] [Google Scholar]
- 3.Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55:795–810. doi: 10.1507/endocrj.k08e-067. [DOI] [PubMed] [Google Scholar]
- 4.Edgerton ME, Grizzle WE, Washington MK. The deployment of a tissue request tracking system for the CHTN: a case study in managing change in informatics for biobanking operations. BMC Med Inform Decis Mak. 2010;10:32. doi: 10.1186/1472-6947-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheldon E, Vo KC, McIntire RA, Aghajanova L, Zelenko Z, Irwin JC, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril. 2011;95:2120–2. 2 e1–12. doi: 10.1016/j.fertnstert.2011.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giudice LC. Genomics’ role in understanding the pathogenesis of endometriosis. Semin Reprod Med. 2003;21:119–24. doi: 10.1055/s-2003-41318. [DOI] [PubMed] [Google Scholar]
- 7.Irwin J, Giudice L. The Decidua. San Diego: Academic Press; 1998. [Google Scholar]
- 8.Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52:761–8. doi: 10.1016/s0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 9.Bongso A, Gajra B, Lian NP, Wong PC, Soon-Chye N, Ratnam S. Establishment of human endometrial cell cultures. Hum Reprod. 1988;3:705–13. doi: 10.1093/oxfordjournals.humrep.a136770. [DOI] [PubMed] [Google Scholar]
- 10.Classen-Linke I, Kusche M, Knauthe R, Beier HM. Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell Tissue Res. 1997;287:171–85. doi: 10.1007/s004410050743. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Shaw S, Shorter SC, Naish CE, Barlow DH, Starkey PM. Isolation and purification of human endometrial stromal and glandular cells using immunomagnetic microspheres. Hum Reprod. 1992;7:156–61. doi: 10.1093/oxfordjournals.humrep.a137609. [DOI] [PubMed] [Google Scholar]
- 12.Gildea JJ, McGrath HE, Van Sciver RE, Wang DB, Felder RA. Isolation, growth, and characterization of human renal epithelial cells using traditional and 3D methods. Methods Mol Biol. 2013;945:329–45. doi: 10.1007/978-1-62703-125-7_20. [DOI] [PubMed] [Google Scholar]
- 13.Kedinger M, Haffen K, Simon-Assmann P. Intestinal tissue and cell cultures. Differentiation. 1987;36:71–85. doi: 10.1111/j.1432-0436.1987.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirk D, King RJ, Heyes J, Peachey L, Hirsch PJ, Taylor RW. Normal human endometrium in cell culture. I. Separation and characterization of epithelial and stromal components in vitro. In Vitro. 1978;14:651–62. doi: 10.1007/BF02616162. [DOI] [PubMed] [Google Scholar]
- 15.Murakami S, Shibaya M, Takeuchi K, Skarzynski DJ, Okuda K. A passage and storage system for isolated bovine endometrial epithelial and stromal cells. J Reprod Dev. 2003;49:531–8. doi: 10.1262/jrd.49.531. [DOI] [PubMed] [Google Scholar]
- 16.Satyaswaroop PG, Bressler RS, de la Pena MM, Gurpide E. Isolation and culture of human endometrial glands. J Clin Endocrinol Metab. 1979;48:639–41. doi: 10.1210/jcem-48-4-639. [DOI] [PubMed] [Google Scholar]
- 17.Trent JM, Davis JR, Payne CM. The establishment and morphologic characterization of finite cell lines from normal human endometrium. Am J Obstet Gynecol. 1980;136:352–62. doi: 10.1016/0002-9378(80)90862-5. [DOI] [PubMed] [Google Scholar]
- 18.Blauer M, Heinonen PK, Martikainen PM, Tomas E, Ylikomi T. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod. 2005;20:864–71. doi: 10.1093/humrep/deh722. [DOI] [PubMed] [Google Scholar]
- 19.Kyo S, Nakamura M, Kiyono T, Maida Y, Kanaya T, Tanaka M, et al. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am J Pathol. 2003;163:2259–69. doi: 10.1016/S0002-9440(10)63583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Rees MC, Bicknell R. The isolation and long-term culture of normal human endometrial epithelium and stroma. Expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci. 1995;108(Pt 1):323–31. doi: 10.1242/jcs.108.1.323. [DOI] [PubMed] [Google Scholar]
- 21.Chen JC, Erikson DW, Piltonen TT, Meyer MR, Barragan F, McIntire RH, et al. Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril. 2013;100:1132–43. doi: 10.1016/j.fertnstert.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JC, Johnson BA, Erikson DW, Piltonen TT, Barragan F, Chu S, et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Hum Reprod. 2014;29:1255–70. doi: 10.1093/humrep/deu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirk D, Irwin JC. Normal human endometrium in cell culture. Methods Cell Biol. 1980;21B:51–77. doi: 10.1016/s0091-679x(08)60678-0. [DOI] [PubMed] [Google Scholar]
- 24.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 25.Piltonen TT, Chen JC, Khatun M, Kangasniemi M, Liakka A, Spitzer T, et al. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum Reprod. 2015;30:1203–15. doi: 10.1093/humrep/dev055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janz Fde L, Debes Ade A, Cavaglieri Rde C, Duarte SA, Romao CM, Moron AF, et al. Evaluation of distinct freezing methods and cryoprotectants for human amniotic fluid stem cells cryopreservation. J Biomed Biotechnol. 2012;2012:649353. doi: 10.1155/2012/649353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–81. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 28.Mirabet V, Solves P, Minana MD, Encabo A, Carbonell-Uberos F, Blanquer A, et al. Human platelet lysate enhances the proliferative activity of cultured human fibroblast-like cells from different tissues. Cell Tissue Bank. 2008;9:1–10. doi: 10.1007/s10561-007-9048-x. [DOI] [PubMed] [Google Scholar]
- 29.Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldham RK, Dean JH, Cannon GB, Ortaldo JR, Dunston G, Applebaum F, et al. Cryopreservation of human lymphocyte function as measured by in vitro assays. Int J Cancer. 1976;18:145–55. doi: 10.1002/ijc.2910180203. [DOI] [PubMed] [Google Scholar]
- 31.Alipoor FJ, Gilani MA, Eftekhari-Yazdi P, Hampa AD, Hosseinifar H, Alipour H, et al. Achieving high survival rate following cryopreservation after isolation of prepubertal mouse spermatogonial cells. J Assist Reprod Genet. 2009;26:143–9. doi: 10.1007/s10815-009-9298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lechner J, LaVeck M. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. Journal of tissue culture methods. 1985;Volume 9:43–8. [Google Scholar]
- 33.Niknejad H, Deihim T, Peirovi H, Abolghasemi H. Serum-free cryopreservation of human amniotic epithelial cells before and after isolation from their natural scaffold. Cryobiology. 2013;67:56–63. doi: 10.1016/j.cryobiol.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–8. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- 35.Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–67. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 36.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 37.Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, et al. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151:45–54. [PMC free article] [PubMed] [Google Scholar]
- 38.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins PM, Vasavda C, Hostettler J, Davis JQ, Abdi K, Bennett V. E-cadherin polarity is determined by a multifunction motif mediating lateral membrane retention through ankyrin-G and apical-lateral transcytosis through clathrin. J Biol Chem. 2013;288:14018–31. doi: 10.1074/jbc.M113.454439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


