Abstract
The prebiotic concept was introduced twenty years ago, and despite several revisions to the original definition, the scientific community has continued to debate what it means to be a prebiotic. How prebiotics are defined is important not only for the scientific community, but also for regulatory agencies, the food industry, consumers and healthcare professionals. Recent developments in community-wide sequencing and glycomics have revealed that more complex interactions occur between putative prebiotic substrates and the gut microbiota than previously considered. A consensus among scientists on the most appropriate definition of a prebiotic is necessary to enable continued use of the term.
Introduction
The idea that certain nutrients (including carbohydrates) can modify the gut microbiota existed long before definitions for such nutrients had been proposed. Reports on the bifidogenic properties of inulin and oligofructose (produced from inulin), and fructooligosaccharides (FOS) synthetically produced from sucrose, as well as galactose-containing and xylose-containing oligosaccharides appeared in the 1980s and early 1990s [1–4]. Even earlier, in the 1950s, researchers described the presence of a so-called ‘bifidus factor’ in human milk, a component that enriched for bifidobacteria in infants [5]. Later, this factor was identified and shown to consist of complex oligosaccharides and glycans [6,7]. Oligosaccharides were also detected in bovine milk and milk products, but their physiological role was unclear [8]. Remarkably, there were even reports of human subjects consuming massive doses of lactose to enrich for beneficial lactic acid bacteria in the gut [9]. Fructans and other fermentable fibers likely comprised an important part of the diet of humans tens of thousands of years ago [10].
In 1995, Glenn Gibson and Marcel Roberfroid [11•] introduced the prebiotic concept. They defined a prebiotic as ‘a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health’. Although this original definition has been revised multiple times, the main features have mostly been retained [12–15].
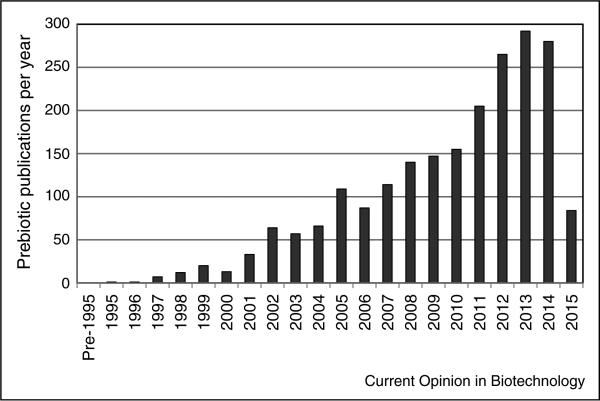
The scientific community quickly adopted the new term. The original paper was cited, according to Web of Science, more than 2500 times. The prebiotic concept was well understood, widely accepted, and extensively used in the science community (Figure 1). It has had a profound effect on gut health research. However, the original and subsequent definitions (see below) have been criticized and misunderstood. First, ‘prebiotic’ had already been defined in the chemistry literature as building block structures that pre-date living organisms. Thus, there is extensive ‘origin of life’ literature on prebiotic chemicals unrelated to prebiotic food ingredients. Secondly, while consumers and health practitioners have a general understanding of probiotics, surveys have shown they are less knowledgeable about prebiotics and may even confuse the two terms [16,17].
Figure 1.
Number of prebiotics publications sited in PubMed (search terms = prebiotic* AND bacteria NOT origins NOT inorganic), data for 2015 are through May.
In addition, while the original term was broadly defined, subsequent papers suggested that the fructans, oligofructose and inulin, FOS, lactulose, as well as the galactan, galactooligosaccharides (GOS) were the only established prebiotics at that time [18]. Early research focused mainly on these few substances. However, even by the early 2000s, development of next generation, rationally selected prebiotics was proposed [19]. Thus, resistant starch (RS), pectin and other fiber components, and milk oligosaccharides are now proposed as having prebiotic potential [20–22].
When first introduced, methods for identifying and quantifying members of the gut microbiota depended mostly on culture and probe-based techniques. The general results of these studies showed a specific enrichment of bifidobacteria (and in some experiments lactobacilli), consistent with the notion that prebiotics selectively stimulated these beneficial bacteria. Indeed, in 2000, Gibson and Fuller suggested that, given the state of scientific knowledge at that time, species of Lactobacillus and Bifidobacterium were the ‘preferred target organisms for prebiotics’ [23]. Physiological mechanisms by which these bacteria metabolize prebiotics have also been established [24].
In the past decade, however, community sequencing has revealed more complex outcomes of prebiotic administration, with quite different and sometimes unexpected members of the microbiota being enriched. In particular, several putatively beneficial autochthonous gut organisms, including Faecalibacterium prausnitzii and Akkermansia muciniphila, have been shown to respond to specific prebiotics [25•, 26•, 27–30]. The consumption of other molecules (e.g. human milk oligosaccharides, resistant starch and various soluble and insoluble fibers) may also promote enrichment of other as yet unidentified members of the gut microbiota, either directly or via cross-feeding [31,32,33•, 34•]. However, there is still a paucity of sequencing studies associated with a clinical intervention for many (potential) prebiotics.
In part to account for these new observations, Bird et al. and Bindels et al. proposed a broader definition for prebiotics. Bird et al. [20] further suggested that prebiotics should be viewed as ‘undigested dietary carbohydrates’ that are fermented by colonic bacteria yielding short chain fatty acids as end products. Importantly, given (i) the potential therapeutic benefits of a diverse gut microbiota, (ii) the broad impact of some well-established prebiotics on gut microbiota composition, and (iii) unresolved questions about the distinctions between beneficial and detrimental members, Bindels et al. proposed that selectivity and specificity were no longer relevant criteria (35). Bird et al. [20] and Martinez et al. [36] suggested that a broader range of food ingredients have prebiotic activity, including resistant starch and various dietary fibers. However, in the absence of evidence that the benefits of such substances are mediated through an impact on the microbiota prebiotics cannot be distinguished from other ingredients that reach the colon intact. Recently a diverse gut microbiota (measured via high microbial gene count) has been associated with health, but it is unknown if any one prebiotic or fiber ingredient could achieve such an effect [37,38]. Comprehensive in vitro studies have characterized the mechanistic strategies by which common gut bacteria degrade more complex fibers and other dietary polysaccharides, including yeast mannan, human milk oligosaccharides, xyloglucan and other complex xylans [39••, 40•, 41,42•, 43,44]. However, many different microbiota species, each with a potentially different utilization strategy, likely compete for these substrates in each individual, making it challenging for researchers to determine specifically which pathways affect the gut microbiota and why certain microbial groups respond.
In this review, our goal is not to advocate for an existing definition nor is it our intent to propose a new definition for prebiotics. Rather, we address the fundamental question of why such a definition is important. The necessity for open discussion about this topic is reflected in that the very basis for defining prebiotics has recently been challenged [45,46]. They argue that these definitions are outdated, too narrow, too limiting, and too simplistic. Given the emerging evidence that many dietary ingredients also influence the microbiota, they suggest that it is better to use ‘pharmabiotics’ to describe substances having microbiota-influencing or therapeutic activities. This broad term may be useful, but it does not obviate the value of the ‘prebiotic’ term that is valued by generally healthy consumers.
Why definitions matter
Definitions for prebiotics and what specific outcomes define a prebiotic effect have been challenged almost since the concept was introduced only 20 years ago. Recently, Shanahan warned researchers to ‘resist becoming captive to restrictive language and outdated terms like prebiotics’ [45]. Whatever the merits or otherwise of this argument, ‘prebiotic’ is now very much ingrained in the lexicon. We contend that scientists, regulators, the food industry, and consumers and healthcare providers must find a way to use this term in a consistent and meaningful way.
For scientists
As noted above, there have been several revisions or clarifications to the original 1995 definition of prebiotics, including one proposed recently by Bindels et al. [35••]. Importantly, once a revised definition is published in the scientific literature, it is usually widely adopted by research scientists, the medical community, and granting agencies. Nonetheless, for scientists, establishing the criteria for what constitutes a prebiotic has proven to be no easy matter and consensus has not yet been achieved. The 2008 FAO definition [14], for example, describes a prebiotic as a ‘nonviable food component that confers a health benefit on the host associated with modulation of the microbiota’. Yet, because this definition does not state that metabolism is necessary, substances such as bacteriocins or dietary compounds that have antimicrobial activity would qualify as prebiotics.
Despite the absence of a consensus definition, a key element of the original and all subsequent definitions is that prebiotics should improve host health or otherwise provide a beneficial physiological effect. Accordingly, Rastall and Gibson [22] suggested that while identification of new prebiotic target organisms are likely to emerge from microbiome analyses, identifying outcomes and health benefits that occur as a result of prebiotic consumption are vital. However, to distinguish prebiotics from other functional foods, it is critical that these benefits be mediated by modulation of the gut microbiota or via measurable changes in the community structure.
Accordingly, this clarification implies that prebiotic-provoked modulation of the microbiota is the actual effector of the health effect or benefit. However, the possibility that a health benefit and shifts in the microbiota could occur independently following prebiotic consumption also exists, but would be, at the present, difficult to establish. Indeed, ‘health benefit’ is, unfortunately, not well defined either, nor is there a clear mechanistic link between microbial activity and putative health benefits.
Although selectivity, the ability of a nutrient to enrich for specific members of the gut microbiota, was a key component of the original definition, this requirement has recently been challenged [35••]. In part, this is because the term, ‘selectivity’, itself is difficult to define or establish in the context of prebiotics. One dictionary definition is ‘the property of affecting some things and not others’. In the context of fermentations in the gut, what then is the ‘limit’ for the number of species or taxa that have to be enriched by a prebiotic to still be considered selective? Would any change in the microbiota composition (that would inherently involve increases in some species and not others) that confers a corresponding health benefit fulfill the ‘selectivity’ criterion?
At the current time there is evidently no consensus on these questions. Moreover, the use of metagenomics techniques to characterize the gut microbiota will inevitably expand the list of species responding to prebiotic consumption. It is also important to note that metabolism of complex dietary polysaccharides (including many prebiotics) involves a consortium of organisms, such that selectivity may not be applicable [35••, 47,48]. Thus, for such polysaccharides, where selfish utilization or cross-feeding of degradation or fermentation products is possible, it may be necessary to obtain a more comprehensive understanding of how these materials influence the microbiota. Indeed, Scott et al. [49] noted that cross-feeding is an important contributor to the prebiotic effect and that the bacteria enriched by prebiotics extend beyond those initially identified. Consistent with these arguments, it has been shown that oligofructose from inulin increases the abundance of A. muciniphila even higher than Bifidobacterium spp. [25•, 26•]. However, A. muciniphila is unable to use oligofructose as an energy source [50]. Altogether, these examples emphasize the importance of cross-feeding as a key mechanism by which prebiotics influence the microbiota, in contrast to a direct impact on specific taxa. Finally, as Frese and Mills suggested recently [51], another way to assess the metabolic fate of prebiotics is by fecal glycomics — simply measuring what is excreted in feces and correlating those products with changes in microbiota composition and health.
Although reaching consensus on the prebiotic definition would not necessarily resolve the open questions noted above, doing so would still provide clarity to gut health researchers, as well as regulators, the food industry, health professionals and consumers.
For regulators
Neither the U.S. Food and Drug Administration (FDA) nor Europe's European Food Safety Authority (EFSA) have established definitions for prebiotics [52,53]. In 2006, the FDA released a draft document that provided guidance to industry on when ‘complementary and alternative medicine (CAM) products’ were subject to FDA regulation [54]. The National Center for Complementary and Alternative Medicine (now known as the National Center for Complementary and Integrative Health), a center within the National Institutes of Health, subsequently included prebiotics as part of a ‘biologically-based’ group of foods that also included botanicals, animal-derived extracts, vitamins, minerals, fatty acids, amino acids, proteins, whole diets, and functional foods. For this document, the 1995 definition of prebiotics was used, which underscores the argument that changes in the definition can take a long time until they find their way into the regulatory process. The FDA also permits food manufacturers to self-affirm GRAS (Generally Recognized as Safe) status for products ultimately labeled as prebiotics [55].
In Europe, EFSA uses the FAO prebiotic definition, which stipulates the need for a health benefit [14,53]. EFSA states that evidence of microbiota changes is not in itself a benefit, and further requires demonstration of a beneficial physiological effect or outcome of human intervention study [56]. Oligofructose and native chicory inulin were recently given positive opinions by EFSA [57,58] for ‘reduction of post-prandial glycaemic responses’ and ‘maintenance of normal defecation’, respectively. With respect to bowel habit as affected by inulin, fermentation leading to high stool bulk, softer stools and SCFA production (which are indirectly due to microbiota activity) were amongst the mechanisms cited.
A similar situation exists in Canada, where the use of the term, ‘prebiotics’ on labels or in advertising is considered as an ‘implied health claim’ (Canada Food Inspection Agency). Accordingly, this would mean that ‘prebiotics’ could be included on a food label only if it satisfied the health claim requirements. Thus, a statement specifying the ‘measurable health benefit conferred by the prebiotic substance, as demonstrated in humans’ would have to be included [59].
The regulatory environment in Japan is much different than in the U.S. and E.U. While prebiotics are not specifically defined, oligosaccharides, dietary fiber, and other polysaccharides are specified as ‘foods to modify gastrointestinal conditions’ and can be approved as ‘foods for specific health uses’ [60].
Collectively, the various ways by which regulatory agencies around the world deal with labeling prebiotic compounds reveals the need for a clear definition, at least in the USA and Europe.
For the food industry
The food industry is interested in prebiotics for their application and promise as functional ingredients in foods targeted toward health-conscious consumers. Benefits, ranging from maintaining and enhancing gut health, modulating the immune system, lowering glycemic response, and reducing insulin resistance are of interest to consumers and therefore attractive targets for new functional foods. Some of these recently received positive opinions from EFSA [57,58]. However, if scientists do not agree on the scope and appropriate use of the term ‘prebiotic’; regulatory agencies may establish their own definition, and this may have a negative impact on how prebiotics are labeled.
The term ‘prebiotic’ is a concept gaining acceptance by consumers, and frequent changes to the definition may be confusing. Therefore, any such change should be well-considered and meaningful. However, without an accepted and clear definition of a prebiotic by academic scientists, it is impossible to know if a substance is accurately labeled as a prebiotic, with implications for misleading consumers. It would be much preferred for scientists to provide the best definition for regulators, rather than have regulators determine this on their own. However, a global consensus effort is key to adopting a definition that is useful and scientifically sound.
For consumers and health professionals
The main source of health information for consumers is now the Internet [61]. In particular, Wikipedia has become a major online reference source for medical and health information, not just among consumers, but also public health professionals and scientists [62]. This site currently refers to prebiotics as ‘chemicals (emphasis added) that induce the growth or activity of microorganisms (e.g. bacteria and fungi) that contribute to the well-being of their host’. On another popular site, WebMD, prebiotics are described as ‘food ingredients that help support growth of probiotic bacteria’. Elsewhere in this website (Vitamins and Supplements Guide), prebiotics are defined as ‘non-digestible ingredients in foods that are used to spur the growth of probiotic bacteria in the body by providing a suitable environment in which the probiotics themselves can flourish’. While the latter definitions convey perhaps a general meaning, they inaccurately imply that probiotic bacteria are already present in the body (by definition, probiotics are an exogenously provided live microbial supplement). It also suggests that prebiotics directly influence the environment.
In contrast, the Mayo Clinic Health Letter (July 2014) provides a more accurate definition, describing prebiotics as ‘nondigestible substances that act as food for the gut microbiota. Essentially, prebiotics stimulate growth or activity of certain healthy bacteria that live in your body.’ This newsletter also notes that ‘prebiotics are found in whole grains, bananas, onions, garlic, honey and artichokes’. However, a typical Western diet is estimated to contain only a few grams of naturally occurring prebiotics, and it is unclear if this is a sufficient dose to have any measurable effects [63,64].
Ultimately, however, what consumers and health care providers actually know about prebiotics is difficult to assess. According to recent surveys, prebiotics appear to be poorly understood by the general public. In one recent survey of 200 U.S. adults (inpatients at an urban hospital), only 11% were familiar with the term, ‘prebiotics’, and only 7% identified the correct definition among four other choices [17]. In a similar survey of 245 health care providers (including 100 physicians), only 22% of respondents were familiar with prebiotics [16]. There was also confusion about differences between probiotics and prebiotics. Interestingly, although most of the health care providers viewed prebiotics as beneficial to health, few actually recommended them to patients. It is likely that this poor understanding of the prebiotic concept by the general population hinders potential applications. It is also possible that this lack of clarity contributes to its low rate of recommendation by health care providers.
Conclusions
A major challenge for gut health scientists is how to best educate and inform other stakeholders about prebiotics. Having a definition that accurately describes prebiotics and is agreed upon by the scientific community is an essential first step. We can hardly expect regulators, consumers and healthcare providers to have a clear understanding of prebiotics if the scientific community does not. The prebiotic definition may evolve as science advances, and it is incumbent on the scientific community to interpret new findings and adjust the prebiotic concept as needed.
Metagenomic, metabolomic, glycomics, and other omic approaches and advances in analytical chemistry, as well as human clinical studies are likely to provide new insights into mechanisms by which prebiotics function in vivo [30]. Even if a consensus definition could be reached, it would need to be sufficiently flexible to accommodate the inevitable new discoveries in gut biology. Otherwise, the science on the effects of prebiotics on the microbiota and host health may simply outpace the definition. Ultimately, for scientists and non-scientists, and for industry and regulators, resolving the definition of prebiotics is necessary to enable continued use of the term.
Acknowledgments
LBB is a Postdoctoral Researcher from the F.R.S.-FNRS (Fond National de la Recherche Scientifique, Belgium). PDC is a research associate at FRS-FNRS (Fonds de la Recherche Scientifique), Belgium and recipient of FNRS, FRFS-WELBIO under grant: WELBIO-CR-2012S-02R and is a recipient of ERC Starting Grant 2013 (European Research Council, Starting grant 336452-ENIGMO). DAM acknowledges NIH awards R01AT008759 and R01AT007079.
Footnotes
This paper was based in part on an expert panel discussion session held at the International Scientific Association for Probiotics and Prebiotics Annual Meeting in Washington D.C in May, 2015.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Tanaka R, Takayama H, Morotomi M, Kuroshima T, Ueyama S, Matsumoto K, Kuroda A, Mutai M. Effects of administration of TOS and Bifidobacterium breve 4006 on the human fecal flora. Bifidobact Microflora. 1983;2:17–24. [Google Scholar]
- 2.Okazaki M, Fujikawa S, Matsumoto N. Effect of xylooligasaccharide on the growth of bifidobacteria. Bifidobact Microflora. 1990;9:77–86. [Google Scholar]
- 3.Ito M, Deuguchi Y, Miyamori A, Matsumote K, Kikuchi H, Matsumoto K, Yajima T, Kan T. Effects of administration of galactooligosaccharides on the human faecal microflora, stool weigh and abdominal sensation. Microb Ecol Health Dis. 1990;3:285–292. [Google Scholar]
- 4.Hidaka H, Tashiro Y, Eida T. Proliferation of bifidobacteria by oligosaccharides and their useful effect on human health. Bifidobact Microflora. 1991;10:65–79. [Google Scholar]
- 5.György P, Norris RF, Rose SR. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48:193–201. doi: 10.1016/0003-9861(54)90323-9. [DOI] [PubMed] [Google Scholar]
- 6.Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol. 2004;38(Suppl. 6):S80–S83. doi: 10.1097/01.mcg.0000128926.14285.25. [DOI] [PubMed] [Google Scholar]
- 7.Kitaoka M, Tian J, Nishimoto M. Novel putative galactose operon involving lacto-N-biose phosphorylase in Bifidobacterium longum. Appl Environ Microbiol. 2005;71:3158–3162. doi: 10.1128/AEM.71.6.3158-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holsinger VH. Lactose. In: Wong NP, Jenness R, Keeney M, Marth EH, editors. Fundamentals of dairy chemistry. 3rd ed. Van Nostrand Reinhold Co.; New York, NY, USA: 1988. pp. 279–342. [Google Scholar]
- 9.Rettger LF, Cheplin HA. A treatise on the transformation of the intestinal flora with special reference to implantation of Bacillus acidophilus. Yale University Press; New Haven: 1921. [Google Scholar]
- 10.Leach JD, Gibson GR, Van Loo J. Human evolution, nutritional ecology and prebiotics in ancient diet. Biosci Microflora. 2006;25:1–8. [Google Scholar]
- 11•.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [This paper introduces the concept of prebiotics for the first time and explains the potential for stimulating potentially beneficial bacteria that are already present in the human colon.] [DOI] [PubMed] [Google Scholar]
- 12.Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, Roberfroid M, Rowland I, Cherbut C, Klaenhammer TR. New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol. 2003;37:105–118. doi: 10.1097/00004836-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 14.Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, Brunser O, Kieran T. FAO technical meeting on prebiotics. J Clin Gastroenterol. 2008;42:S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 15.Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Garau M, Murphy EF, Saulnier D, Loh G, Macfarlane S, Delzenne N, Ringel Y, Kozianowski G, Dickmann R, Lenoir-Wijnkook I, Walker C, Buddington R. Dietary prebiotics: current status and new definition. Food Sci Technol Bull: Funct Foods. 2010;7:1–19. [Google Scholar]
- 16.Oliver L, Rasmussen H, Gregoire MB, Chen Y. Health care provider's knowledge, perceptions, and use of probiotics and prebiotics. Top Clin Nutr. 2014;29:139–149. [Google Scholar]
- 17.Betz M, Azueta A, Rasmussen H, Gregoire M, Vanderwall C, Witowich G. Knowledge, use and perceptions of probiotics and prebiotics in hospitalised patients. Nutr Diet. 2015 In press. [Google Scholar]
- 18.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 19.Rastall RA, Maitin V. Prebiotics and synbiotics: towards the next generation. Curr Opin Biotechnol. 2002;13:490–496. doi: 10.1016/s0958-1669(02)00365-8. [DOI] [PubMed] [Google Scholar]
- 20.Bird AR, Conlon MA, Christophersen CT, Topping DL. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes. 2010;1:423–431. doi: 10.3920/BM2010.0041. [DOI] [PubMed] [Google Scholar]
- 21.Coppa GV, Zampini L, Galeazzi T, Gabrielli O. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(Suppl. 2):S291–S294. doi: 10.1016/S1590-8658(07)60013-9. [DOI] [PubMed] [Google Scholar]
- 22.Rastall RA, Gibson GR. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr Opin Biotechnol. 2015;32:42–46. doi: 10.1016/j.copbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr. 2000;130:391S–395S. doi: 10.1093/jn/130.2.391S. [DOI] [PubMed] [Google Scholar]
- 24.Goh YJ, Klaenhammer TR. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu Rev Food Sci Technol. 2015;6:137–156. doi: 10.1146/annurev-food-022814-015706. [DOI] [PubMed] [Google Scholar]
- 25•.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, Delzenne NM, Schrenzel J, Cani PD. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [This study is the first describing the impact of prebiotics on the gut microbiota using high-throughput sequencing methods, including 454 pyrosequencing, microarrays, and qPCR, to show the impact of prebiotics on Akkermansia muciniphila.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8:2116–2130. doi: 10.1038/ismej.2014.45. [This study provides the first catalog of genes (taxa and function), using metagenomic sequencing, that are affected by a prebiotic treatment under normal or high-fat diet feeding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–550. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 28.Miquel S, Martin R, Bridonneau C, Robert V, Sokol H, Bermudez-Humaran LG, Thomas M, Langella P. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes. 2014;5:146–151. doi: 10.4161/gmic.27651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinken A, Khan MT, Paglia G, Rodionov DA, Harmsen HJM, Thiele I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J Bacteriol. 2014;196:3289–3302. doi: 10.1128/JB.01780-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, Delzenne NM. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;6:1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian S, Blanton LV, Frese SA, Charbonneau M, Mills DA, Gordon JI. Cultivating healthy growth and nutrition through the gut microbiota. Cell. 2015;161:36–48. doi: 10.1016/j.cell.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 33•.Holscher J, Caporaso G, Hooda S, Brulc JM, Fahey GC, Jr, Swanson KS. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr. 2015;101:55–64. doi: 10.3945/ajcn.114.092064. [This study describes the influence of dietary fiber on the composition of the gut microbiota in healthy male adults, showing that polydextrose and SCF cause changes in the community structure of the gut microbiota and in the bacterial metagenome.] [DOI] [PubMed] [Google Scholar]
- 34•.Salonen A, Lahti L, Salojärvi J, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE, Louis P, Flint HJ, de Vos WM. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [Resistant starch increased Ruminococcaceae and decreased diversity among obese adults, but the response was subject to considerable inter-individual variation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [The authors review the evolution of the prebiotic concept and propose a rational framework, based on ecological and functional criteria, for defining this term.] [DOI] [PubMed] [Google Scholar]
- 36.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, MicroObes consortium ANR, Doré J, Zucker JD, Clément K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 38.Kong LC, Holmes BA, Cotillard A, Habi-Rachedi F, Brazeilles R, Gougis S, Gausserès N, Cani PD, Fellahi S, Bastard JP, Kennedy SP, Doré J, Ehrlich SD, Zucker JD, Rizkalla SW, Clément K. Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PLOS ONE. 2014;9:e109434. doi: 10.1371/journal.pone.0109434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol. 2014;426:3838–3850. doi: 10.1016/j.jmb.2014.07.028. [The authors introduce the concept of ‘discrete structure’ of dietary fibers that can be correlated to enrichment of specific gut microbial populations based on their genome-encoded glycobiome and hierarchical substrate preference. This mechanistic framework will be valuable for tailoring dietary fibers for targeted manipulation of gut microbiota and desired functional outcomes.] [DOI] [PubMed] [Google Scholar]
- 40•.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [This paper matched several dozen gene clusters in two common human gut symbionts, B. thetaiotaomicron and B. ovatus, with utilization of a variety of polysaccharides from plant origin and compared their structure and gene content to gene clusters involved in host glycan degradation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, Klinter S, Pudlo NA, Urs K, Koropatkin NM, et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature. 2014;506:498–502. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517:165–169. doi: 10.1038/nature13995. [This study identified three gene clusters in B. thetaiotaomicron that are responsible for metabolizing the sterically-hindered yeast cell wall polysaccharide, a-mannan. Genetic, gnotobiotic mouse, and metagenomic experiments provided a broader perspective of the importance of amannan digestion for the human gut microbiota.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Baslé A, Morland C, Day AM, Zheng H, Rogers TE, Thompson P, Hawkins AR, Yadav MP, Henrissat B, Martens EC, Dupree P, Gilbert HJ, Bolam DN. Glycan complexity dictates microbial resource allocation in the large intestine. Nat Commun. 2015;6 doi: 10.1038/ncomms8481. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward RE, Niñonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res. 2007;51:1398–1405. doi: 10.1002/mnfr.200700150. [DOI] [PubMed] [Google Scholar]
- 45.Shanahan F. Fiber man meets microbial man. Am J Clin Nutr. 2015;101:1–2. doi: 10.3945/ajcn.114.101550. [DOI] [PubMed] [Google Scholar]
- 46.Shanahan F, Quigley EMM. Manipulation of the microbiota for treatment of IBS and IBD — challenges and controversies. Gastroenterology. 2014;146:1554–1563. doi: 10.1053/j.gastro.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 47.Martens EC, Kelly AG, Tauzin AS, Brumer H. The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J Mol Biol. 2014;426:3851–3865. doi: 10.1016/j.jmb.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frese S, Mills DA. Should infants cry over spilled milk? Fecal glycomics as an indicator of a healthy infant gut microbiome. J Pediatr Gastroenterol Nutr. 2015 doi: 10.1097/MPG.0000000000000798. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaillancourt J. Ending the war metaphor: the changing agenda for unraveling the host-microbe relationship. Regulating pre- and probiotics: a U.S. FDA perspective. Institute of Medicine of the National Academies. National Academies Press; Washington, DC: 2006. pp. 229–237. [PubMed] [Google Scholar]
- 53.van Loveren H, Sanz Y, Salminen S. Health claims in Europe: probiotics and prebiotics as case examples. Annu Rev Food Sci Technol. 2012;3:247–261. doi: 10.1146/annurev-food-022811-101206. [DOI] [PubMed] [Google Scholar]
- 54.FDA guidance for industry on complementary and alternative medicine products and their regulation by the food and drug administration. 2006 http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM145405.pdf.
- 55.Kumar H, Salminen S, Verhagen H, Rowland I, Heimbach J, Banares S, Young T, Nomoto K, Lalonde M. Novel probiotics and prebiotics: road to the market. Curr Opin Biotechnol. 2015;32:99–103. doi: 10.1016/j.copbio.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 56.EFSA: Scientific Opinion on the substantiation of health claims related to various food(s)/food constituents(s) and increasing numbers of gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905), and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 760, 761, 779, 780, 779, 1905) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J. 2010;8:1809. [Google Scholar]
- 57.EFSA: Scientific opinion on the substantiation of a health claim related to non-digestible carbohydrates and a reduction of post-prandial glycaemic responses pursuant to Article 13(5) of Regulation (EC) No 1924/20061. EFSA J. 2014;12:3513. [Google Scholar]
- 58.EFSA: Scientific Opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency pursuant to Article 13.5 of Regulation (EC) No 1924/2006. EFSA J. 2015;13:3951. [Google Scholar]
- 59.Canada Food Inspection Agency Health claims, use of the term “Prebiotic(s)”. http://inspection.gc.ca/food/labelling/food-labelling-for-industry/healthclaims/eng/1392834838383/1392834887794?chap=10.
- 60.Japanese Ministry of Health Labour and Welfare: Food for Specified Health Uses (FOSHU) 2010 http://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html.
- 61.Zhang Y, Sun Y, Xie B. Quality of health information for consumers on the web: a systematic review of indicators, criteria, tools, and evaluation results. J Assoc Inf Sci Technol. 2015 [Google Scholar]
- 62.Herbert VG, Frings A, Rehatschek H, Gisbert R, Leithner A. Wikipedia challenges and new horizons in enhancing medical education. BMC Med Educ. 2015;15:32–37. doi: 10.1186/s12909-015-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Loo J, Coussement P, DeLeenheer L, Hoebregs H, Smits G. On the presence of inulin and oligofructose as natural ingredients in the Western diet. Crit Rev Food Sci Nutr. 1995;35:525–552. doi: 10.1080/10408399509527714. [DOI] [PubMed] [Google Scholar]
- 64.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]