Summary
Long non-coding (lnc)RNAs, once thought to merely represent noise from imprecise transcription initiation, have now emerged as major regulatory entities in all eukaryotes. In contrast to the rapidly expanding identification of individual lncRNAs, mechanistic characterization has lagged behind. Here we provide evidence that the GAL lncRNAs in the budding yeast S. cerevisiae promote transcriptional induction in trans by formation of lncRNA-DNA hybrids or R-loops. The evolutionarily conserved RNA helicase Dbp2 regulates formation of these R-loops as genomic deletion or nuclear depletion results in accumulation of these structures across the GAL cluster gene promoters and coding regions. Enhanced transcriptional induction is manifested by lncRNA-dependent displacement of the Cyc8 co-repressor and subsequent gene looping, suggesting that these lncRNAs promote induction by altering chromatin architecture. Moreover, the GAL lncRNAs confer a competitive fitness advantage to yeast cells as expression of these non-coding molecules correlates with faster adaptation in response to an environmental switch.
Introduction
Eukaryotic genomes are pervasively transcribed, but only a small fraction of these transcripts are translated into proteins. Instead, most of this activity corresponds to non-coding RNAs that encompass a wide variety of functional classes, including ribosomal RNAs, transfer RNAs, microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). LncRNAs are an abundant class of non-coding RNAs predominantly transcribed by RNA polymerase II, which range in size from 200 to 10,000 nts and have been implicated in protein-coding gene regulation, largely at the level of transcription initiation (Rinn and Chang, 2012). This regulation modulates cell cycle progression, imprinting, cell differentiation and development in response to cellular and/or developmental signals (Rinn and Chang, 2012). Well-studied examples include the mammalian Air and HOTAIR lncRNAs, which may promote transcriptional silencing by recruiting specific histone modifying complexes to targeted gene loci (Khalil et al., 2009; Nagano et al., 2008; Tsai et al., 2010). Other examples include numerous enhancer-associated lncRNAs (eRNAs) that are thought to facilitate activation via promoter-enhancer gene looping (Li et al., 2013) and Firre that bridges trans-chromosomal interactions to alter the 3D architecture of the genome (Hacisuleyman et al., 2014). Thus, an emerging theme in lncRNA-dependent transcriptional regulation is modulation of chromatin structure, both at the local and global level.
In contrast to the diverse mechanisms for lncRNA function, limited knowledge exists for how lncRNAs recognize specific genomic loci in vivo. The most well studied example is XIST, which mediates X chromosome inactivation (Augui et al., 2011); YY1 has been shown to nucleate XIST at the X chromosome activation center (Jeon and Lee, 2011) whereas the DNA helicase ATRX recruits PRC2 to XIST and facilitates spreading along the chromosome (Engreitz et al., 2013; Sarma et al., 2014). In other cases, lncRNAs, such as the telomeric TERRA lncRNAs, directly recognize gene targets through base pairing to form an RNA-DNA hybrid structure termed an R-loop (Cusanelli and Chartrand, 2015). Although R-loops have been primarily associated with defects in elongation and mRNA-protein packaging, correlating with increased genomic instability and DNA damage (Bhatia et al., 2014; Dominguez-Sanchez et al., 2011; Gomez-Gonzalez et al., 2011; Hatchi et al., 2015), recent studies demonstrate that these structures also perform beneficial functions in wild type cells (Boque-Sastre et al., 2015; Ginno et al., 2012; Skourti-Stathaki and Proudfoot, 2014; Sun et al., 2013). This dichotomy suggests that the formation of functional R-loops is tightly controlled to prevent potentially harmful events.
The GAL gene cluster in the budding yeast S. cerevisiae has been extensively studied as a model for inducible gene regulation. Under repressed conditions, promoters are bound by glucose-dependent transcriptional repressors, such as Mig1, as well as co-repressors Cyc8 and Tup1 (De Vit et al., 1997; Johnston et al., 1994; Papamichos-Chronakis et al., 2004). Two, Reb1-dependent long non-coding RNAs (lncRNAs) are also transcribed from the 3’ end of GAL10 in repressed conditions; the GAL10 lncRNA is a 4.0 kb transcript that overlaps GAL10 and GAL1 and the GAL10s lncRNA is a 0.5 kb transcript that overlaps the promoter of GAL7 (Houseley et al., 2008; Pinskaya et al., 2009; van Dijk et al., 2011). Initial studies reported that the GAL lncRNAs weakly attenuate transcription (Houseley et al., 2008), a finding corroborated by recent single cell microscopy studies (Lenstra et al., 2015). However, the GAL lncRNAs have also been shown to promote induction from a repressed state, an activity that would have biological relevance to metabolic adaptation (Cloutier et al., 2013; Wang and Tran, 2013). Interestingly, induction occurs even faster in cells lacking the evolutionarily conserved DEAD-box RNA helicase DBP2 (Cloutier et al., 2013). The fact that Dbp2 is exported from the nucleus during the shift from glucose to galactose-containing media (Beck et al., 2014), suggests that loss of nuclear Dbp2 may prime the GAL genes for rapid, lncRNA-dependent induction. Here we provide the mechanism and physiological role for the GAL lncRNAs in transcriptional induction from a transcriptionally repressed state in S. cerevisiae and demonstrate that Dbp2 regulates this process by controlling formation of GAL lncRNA-dependent R-loops. These studies underscore an emerging role for R-loops in lncRNA-dependent gene regulation and provide evidence that lncRNAs enable rapid adaptation by temporally modulating transcriptional activation.
Results
GAL LncRNAs Function in Trans to Enhance Activation of the GAL Cluster Genes
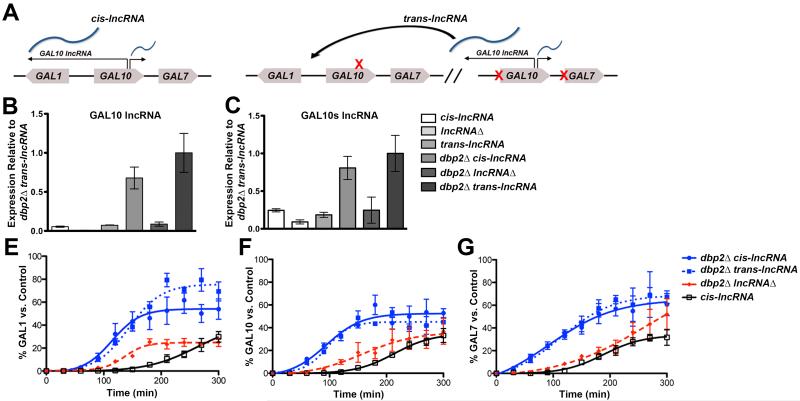
The GAL lncRNAs promote transcriptional induction during a transition from repressive to transcriptionally active conditions (Cloutier et al., 2013). To determine if lncRNA-dependent induction occurs in cis, we conducted transcriptional induction assays of the GAL genes when the GAL lncRNAs were encoded in cis or in trans (Figure 1A). These studies were conducted in the context of the dbp2Δ strain, because loss of DBP2 exacerbates GAL lncRNA-dependent induction. To construct the dbp2Δ trans-lncRNA strain, we designed a GAL10-GAL7 construct with mutations in the Gal4-binding sites within the bidirectional GAL1-GAL10 and GAL7 promoter (MacIsaac et al., 2006; Rhee and Pugh, 2011) to prevent transcription of the sense GAL10 and GAL7 genes without impacting synthesis of the GAL lncRNAs. This trans-lncRNA construct was subsequently integrated into the dbp2Δ lncRNAΔ strain, which harbors silent mutations of the Reb1 binding sites, preventing synthesis of the GAL10 and GAL10s lncRNA (Cloutier et al., 2013; Houseley et al., 2008). Strand-specific RT-qPCR (ssRT-qPCR) confirmed expression of the trans-lncRNAs in both wild type and dbp2Δ cells and showed that the expression levels are similar to the cis- encoded transcripts (Figures 1B and 1C). Moreover, both the cis and trans- lncRNA constructs exhibited overabundance of the GAL lncRNAs in dbp2Δ cells consistent with prior studies (Cloutier et al., 2012; Cloutier et al., 2013).
Figure 1. GAL lncRNAs function in trans to promote induction of the GAL cluster genes.
(A) A schematic of “cis-lncRNAs” and “trans-lncRNAs” at the GAL cluster. (B-C) The trans-lncRNA strain expresses the GAL lncRNAs at similar levels to cis-encoded strains. Levels of the antisense GAL10 lncRNA (B) and GAL10s lncRNA (C) were assayed in wild type, lncRNAΔ, dbp2Δ, dbp2Δ lncRNAΔ, trans-lncRNA and dbp2Δ trans-lncRNAΔ cells by strand-specific RT-qPCR (ssRT-qPCR) as described (Beck et al., 2014) using cells grown in repressive (+glucose) conditions and primers listed in Table S5. Results are presented as the mean of 3 biological replicates relative to ACT1 with S.E.M. (D-F) Graphical representation of GAL gene induction of isogenic wild type, dbp2Δ, and dbp2Δ lncRNAΔ and dbp2Δ “trans-lncRNA” strains. Transcriptional induction of GAL1 (D), GAL10 (E) and GAL7 (F) transcripts during a shift from repressive (+glucose) to activated conditions (+galactose) was assayed by Northern blotting, quantified with respect to sCR1, and plotted as a percentage of a fully induced wild type “control” as previously described (Cloutier et al., 2013). Results are presented as the mean with SEM of three biological replicates. Also see Figure S1.
We then conducted transcriptional induction assays by analyzing GAL1, GAL10 and GAL7 transcript levels in wild type, dbp2Δ, dbp2Δ trans-lncRNA, and dbp2Δ lncRNAΔ strains by northern blotting RNAs isolated at time points during a 300 min glucose to galactose (repressed to activated) shift. Consistent with previous studies (Cloutier et al., 2013), dbp2Δ cells with the GAL lncRNAs in cis exhibited rapid induction of GAL1, GAL10 and GAL7 genes, which was ~5 fold faster than either wild type or the dbp2Δ lncRNAΔ cells (Figures 1D-1F and S1). Expression of the GAL lncRNAs in trans in dbp2Δ cells also promoted rapid induction of all three protein-coding GAL genes (Figures 1D-1F and S1). In fact, induction of GAL1, GAL10 and GAL7 in dbp2Δ cells was identical regardless of whether the GAL lncRNA was encoded in cis or trans (Figures 1D-1F and S1). This suggests that the lncRNA molecule itself, rather than the act of transcription, promotes transcriptional induction.
Ectopic Expression of RNase H1 Represses Rapid Induction in dbp2Δ Cells
RNA-DNA hybrids, termed R-loops, have recently been implicated in lncRNA-dependent gene activation in mammalian cells (Boque-Sastre et al., 2015; Pefanis et al., 2015). To determine if the rapid, lncRNA-dependent induction in dbp2Δ cells is due to formation of R-loops, we conducted transcription induction assays with strains expressing plasmid-encoded human RNase H1 (pRNH1), an enzyme that degrades the RNA within an RNA-DNA hybrid, or empty vector (Wahba et al., 2011). Unexpectedly, use of selective growth media resulted in a reduced transcriptional induction of the GAL genes in both wild type and dbp2Δ strains lacking the GAL lncRNAs (dbp2Δ lncRNAΔ) (Figures 2A-2C and S2A-S2C). However, when comparing the profiles of RNase H1-expressing dbp2Δ cells to vector alone, an RNase H1-dependent reduction in the induction kinetics of GAL1 and GAL10 was clearly seen (Figures 2A, 2B, S2A and S2B) with a more modest reduction in GAL7 (Figures 2C and S2C). Reduced transcriptional induction was most evident when comparing later time points in the time course (180-300 min), as RNase H1 did not appreciably alter the lag time to induction or repress the GAL genes in dbp2Δ cells to levels seen in dbp2Δ lncRNAΔ strain. Induction of the INO1 gene by inositol depletion revealed that RNase H1 expression has no effect on transcriptional induction in dbp2Δ cells of a gene lacking overlapping, annotated lncRNAs (Figures 2D and S2D), suggesting that the effect of RNase H1 is specific for lncRNA-targeted genes. RNase H1 expression also reduced induction of wild type cells to that of lncRNAΔ cells, as revealed by usage of a longer induction time course (Figure S3).
Figure 2. Ectopic expression of RNase H1 suppresses rapid, GAL lncRNA-dependent induction in dbp2Δ cells.
Graphical representation of GAL1 (A), GAL10 (B) and GAL7 (C) gene induction assays of wild type, dbp2Δ and dbp2Δ lncRNAΔ cells harboring a plasmid encoding the human RNase H1 (pRNH1) gene or empty vector. Strains were grown as in Figure 1 but in –LEU selective media for plasmid maintenance with transcript induction and graphical representation as above across three biological replicates. (D) Graphical representation of INO1 induction assays in dbp2Δ cells expressing pRNH1 or empty vector. Induction assays were performed by growing dbp2Δ cells with vector or pRNH1 to mid-log in synthetic complete media (SC) + inositol, before shifting to SC – inositol to induce INO1 expression. (E-F) RNase H1 expression reduces the levels of the GAL10 (E) and GAL10s (F) lncRNAs in dbp2Δ cells. Wild type, lncRNAΔ, dbp2Δ and dbp2Δ lncRNAΔ cells harboring either empty vector or pRNH1 were grown in SC-LEU + glucose and transcript abundance was assayed by ssRT-qPCR as in Figure 1. * = p < 0.05. See also Figures S2 and S3.
To determine if ectopic expression of RNase H1 reduces the levels of the GAL lncRNAs, suggesting that these molecules are degraded in vivo, we conducted ssRT-qPCR (Figures 2E and 2F). This revealed a slight but statistically significant decrease in the GAL10 lncRNA with a much larger decrease in the GAL10s lncRNA in dbp2Δ cells expressing RNase H1 as compared to empty vector (Figures 2E and 2F). We speculate that this differential sensitivity is due to the distinct processing mechanisms for these two lncRNAs (Tuck and Tollervey, 2013; van Dijk et al., 2011; Xu et al., 2009). In contrast to dbp2Δ cells, an RNase H1-dependent decrease in GAL lncRNA levels was not evident in wild type cells, most likely due to technical limitations from the very low levels of the GAL lncRNAs across a cell population (Houseley et al., 2008) and the fact that only a fraction is likely to be chromatin-bound at any one time. Regardless, this suggests that the reduced, lncRNA-dependent induction is manifested by removal of lncRNA R-loops upon ectopic expression of RNase H1.
DBP2-deficient Cells Accumulate LncRNA-dependent R-loops Across the GAL Cluster
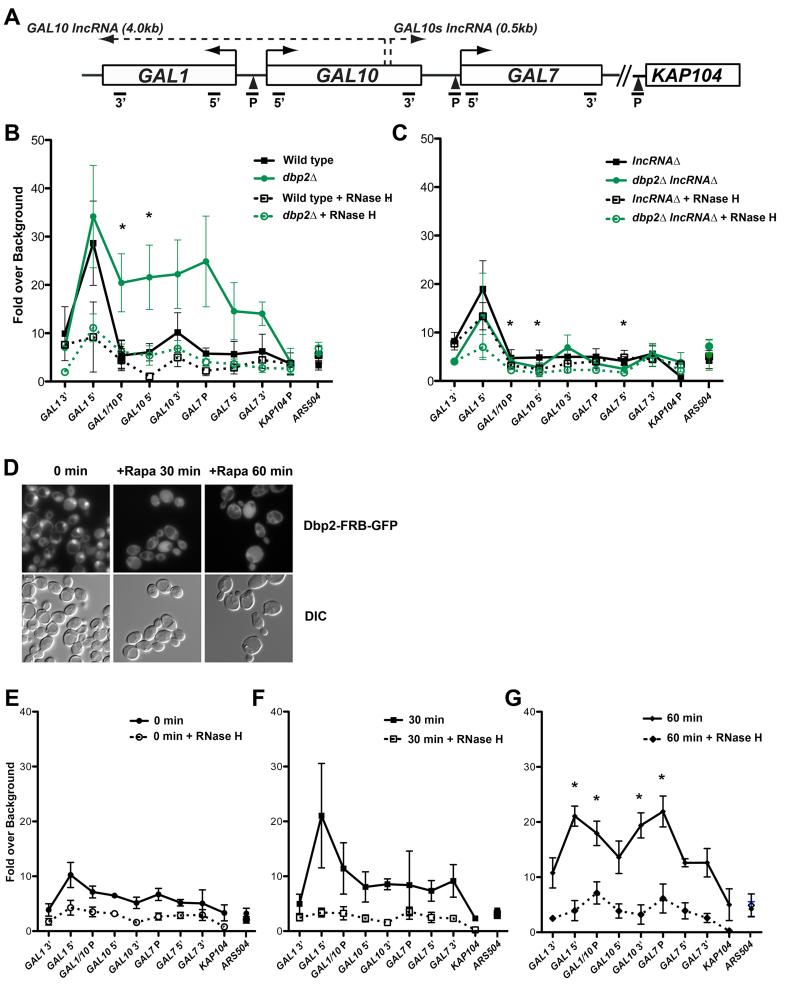
To determine if the GAL lncRNAs form R-loops at the GAL cluster, we performed a DNA-RNA immunoprecipitation (DRIP) assay using the S9.6 RNA-DNA hybrid antibody (Boguslawski et al., 1986; El Hage et al., 2010; Hu et al., 2006). Briefly, genomic DNA was isolated from wild type, lncRNAΔ, dbp2Δ and dbp2Δ lncRNAΔ cells grown under repressive (glucose) conditions and subjected to DRIP. Immunoprecipitated DNA was then measured by qPCR using primer sets across the GAL cluster promoters and coding regions (Figure 3A). A recent genome-wide analysis reported detection of R-loops at the 5’ end of the GAL1 ORF in S. cerevisiae (Chan et al., 2014). Consistently, we observed R-loops at the 5’ end of the GAL1 ORF (GAL1 5’) in both wild type and also in dbp2Δ cells, as evidenced by a qPCR signal 3-fold above RNase H-treated samples (Figure 3B). In dbp2Δ cells, we also detected accumulation of R-loops across distal regions of the GAL cluster, including the bidirectional GAL1/10 promoter (GAL1/10 P) and the GAL7 promoter (GAL7 P), but not at the KAP104 promoter region immediately downstream of GAL7 (Figures 3A and 3B). This accumulation was abolished upon deletion of the GAL lncRNAs (Figure 3C), indicating that R-loop formation across the GAL cluster in dbp2Δ cells is lncRNA-dependent. This suggests that the R-loops are formed by the GAL lncRNAs themselves and is consistent with expression of a 3’ extended GAL10s lncRNA in dbp2Δ cells, which extends to the 3’ end of GAL7 (Cloutier et al., 2012). In contrast, the 5’ region of GAL1 exhibited a more modest reduction in R-loop accumulation in lncRNAΔ and dbp2Δ lncRNAΔ cells (Figure 3C).
Figure 3. Loss of Dbp2 promotes accumulation of GAL lncRNA R-loops across the GAL cluster.
(A) Schematic of the GAL gene cluster showing coding regions, GAL10 and GAL10s lncRNA loci, and qPCR primer-probe positions. (B-C) DNA-RNA-immunoprecipitation (DRIP) of wild type and dbp2Δ (B) or lncRNAΔ and dbp2Δ lncRNAΔ (C) cells grown under transcriptionally repressive (+glucose) conditions. DRIP was conducted using the S9.6 antibody (Boguslawski et al., 1986; El Hage et al., 2010; Hu et al., 2006) or beads alone (no antibody control) followed by qPCR using the indicated primer-probe sets (A) or the non-protein coding region ARS504, which lacks both RNase H-dependent R-loops (Chan et al., 2014) and Dbp2-dependent transcripts (Beck et al., 2014). R-loop levels were determined across 5 biological replicates as the increase in signal of above the no antibody control presented as the mean and S.E.M. * = p < 0.05. (D) Rapamycin-dependent cytoplasmic localization of Dbp2-GFP via anchor away. Dbp2-FRB-GFP was visualized by fluorescent microscopy 0 min (-Rapa), 30 min or 60 min after treatment with 10μg/mL rapamycin. (E-G) Cytoplasmic relocalization of Dbp2 promotes time-dependent accumulation and spreading of R-loops across the GAL cluster. DRIP was conducted as in B with cells following 0, 30 or 60 min rapamycin treatment. * = p < 0.05 with respect to 0 min.
The predominantly nuclear Dbp2 protein is rapidly exported to the cytoplasm upon a switch from glucose to galactose in the media (Beck et al., 2014; Cloutier et al., 2012). To shed light on the kinetics of R-loop accumulation and determine if cytoplasmic re-localization of Dbp2 is sufficient, we used an “anchor away” strategy so Dbp2 could be depleted from the nucleus without the confounding transcriptional and physiological changes that occur upon glucose removal. The anchor away method exploits the inducible interaction between FK506-binding protein (FKBP12) and the FKBP12-rapamycin-binding (FRB) domain, which causes rapid, rapamycin-dependent localization of FRB-tagged proteins due to fusion of FKBP12 (via RNP13A) to the ribosome (Haruki et al., 2008). To deplete nuclear Dbp2, we constructed a DBP2-FRB-GFP at the endogenous DBP2 locus and subsequently confirmed rapamycin-dependent relocalization of the Dbp2-FRB-GFP chimera by fluorescent microscopy (Figure 3D). We then conducted DRIP to analyze accumulation of R loops at 0, 30, and 60 min following rapamycin treatment (Figures 3E-3G). Strikingly, this revealed that nuclear depletion of Dbp2 is sufficient to induce R-loop accumulation, forming initially at the 5’ end of GAL1 (Figures 3E and 3F) followed by accumulation and spreading downstream (Figure 3G) upon redistribution of Dbp2 to the cytoplasm. This suggests that cytoplasmic redistribution of Dbp2 results in a time-dependent increase in R-loops at the GAL cluster.
Comparison of dbp2Δ RNA-Seq and Genome-wide R-loop Detection Reveals Enrichment in Nutrient Utilization Genes
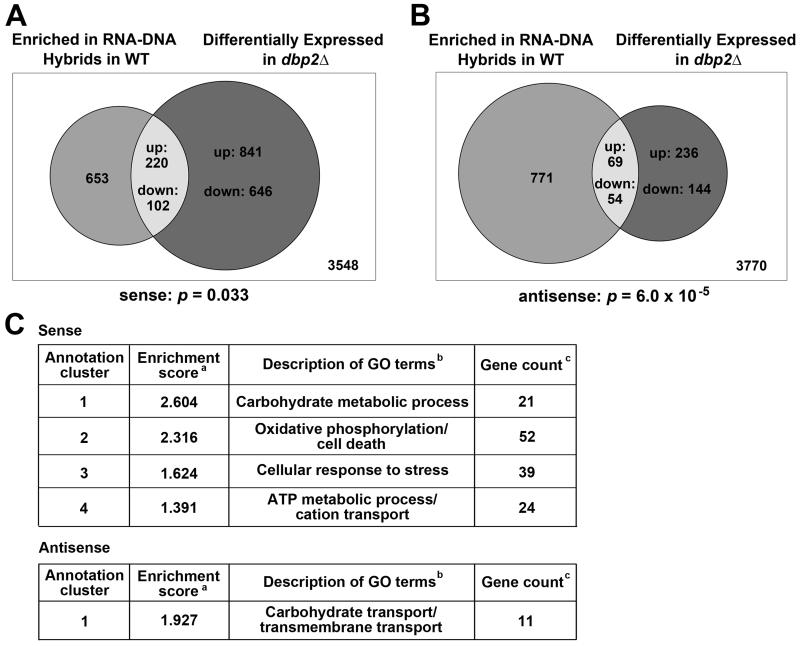
To determine the degree of overlap between genes with a propensity to form R-loops and genes whose expression is affected by loss of DBP2, we compared published genome-wide data sets of DRIP in wild-type cells to the RNA sequencing results of dbp2Δ cells (Beck et al., 2014; Chan et al., 2014). This revealed that differentially expressed, protein-coding, sense transcripts in dbp2Δ have a tendency to be associated with R-loops (two-sided Fisher’s test, p = 0.033)(Figure 4A). Moreover, 220 out of 322 (68.3%) of these genes were up-regulated in dbp2Δ (X-squared=14.6, p=1.31×10−4) (Table S8), correlating DBP2-dependent repression with the propensity to form R-loops. We also found co-occurrence of R-loops at regions associated with DBP2-dependent antisense transcripts (two-sided Fisher’s test, p = 6.0×10−5) (Figure 4B), however, there was no difference in the proportion of up- or down-regulated antisense RNAs for genes associated with R-loops (Table S8).
Figure 4. Functional analysis of genes that are both differentially expressed in dbp2Δ and form R loops in wild type cells.
(A-B) Venn diagrams showing the numbers of R loop-enriched ORFs in wild type (Chan et al., 2014) with differentially expressed sense (A) or antisense (B) transcripts in dbp2Δ (this study and (Beck et al., 2014)). Numbers (inset) correspond to the total up- or down-regulated transcripts in dbp2Δ or ORFs with R-loops in wild type cells. The number of ORFs that are neither category are shown in the box. (C) Functional annotation clustering for sense and antisense transcripts that are both DBP2-dependent and whose ORFs form R loops in wild type cells. aORF groups with significant enrichment score of at least 1.3 (p = or < 0.05). bGO term descriptions. cThe number of unique ORFs in each cluster. See Tables S8-S9. See Figure S4 and Table S10 for comparison to hpr1Δ.
To determine if the sense genes that accumulate in dbp2Δ cells and form R-loops in wild type cells fall into common biological categories, we conducted functional annotation clustering analysis using DAVID (Huang da et al., 2009a, b). This revealed over representation of four significant clusters including energy homeostasis, carbohydrate metabolism, oxidative phosphorylation and cellular responses to stress (Figure 4C, top; Table S9). The same analysis with antisense transcripts revealed carbohydrate transport as the sole cluster with significant enrichment (Figure 4C, bottom; Table S9). This suggests that Dbp2 may regulate formation of R-loops at specific genes involved in nutrient utilization and environmental responses.
Loss of DBP2 Does Not Result in Significant Hyper-recombination but May Mimic hpr1Δ at Some Loci
Hpr1 is a member of the THO complex required for efficient elongation whose loss causes widespread R-loop formation and hyper-recombination (Chan et al., 2014; Chavez and Aguilera, 1997; Huertas and Aguilera, 2003). To determine if loss of DBP2 results in hyper-recombination, we measured recombination frequencies in isogenic wild type, dbp2Δ or hpr1Δ cells (Figure S4A). HPR1- deficient cells displayed the expected hyper-recombination phenotype (Figure S4B). Loss of DBP2 also resulted in a length-dependent increase in recombination, but at levels significantly below the definition for hyper-recombination (Prado et al., 1997) (Figure S4B). This suggests that loss of DBP2 either does not cause widespread R loop accumulation or that the LEU2 gene within the hyper-recombination reporter is not a Dbp2 target.
We then used bioinformatics to ask if Dbp2-dependent transcripts correlate with genes that exhibit increased R-loops in other mutant strains (Chan et al., 2014). This revealed that genes with significantly enriched R-loop formation in hpr1Δ tend to coincide with up-regulated genes in dbp2Δ (two-sided Fisher’s test, p = 7.3×10−3), but not the RNase H-deficient rnh1Δrnh201Δ strain or in cells harboring a sen1-1 termination mutation (Chan et al., 2014); Table S10). This suggests that Dbp2 may have a role in repressing transcription-dependent R-loop formation at some loci similar to Hpr1.
The GAL lncRNAs Promote Gene Looping at the GAL Cluster
Gene loops are higher order chromatin structures that promote interaction between distant gene elements (Hampsey et al., 2011). GAL10 has been shown to form gene loops upon transcriptional activation of the gene in S. cerevisiae (Laine et al., 2009). To determine if the GAL lncRNAs promote gene looping, we utilized chromatin confirmation capture or 3C (Ansari and Hampsey, 2005; Laine et al., 2009; Medler et al., 2011). Consistent with prior characterization of GAL10 (Laine et al., 2009), we observed a time-dependent increase in promoter-terminator looping following transcriptional activation in wild type cells (Figures 5B and 5C). Analysis of dbp2Δ cells revealed a slight but detectible increase in GAL10 looping at early time points post-activation, representing a ~50% and ~25% increase as compared to wild type cells at 90 and 180 min (Figures 5B and 5C). In contrast, neither the lncRNAΔ nor the dbp2Δ lncRNAΔ strain exhibited any detectible increase in the P1/T1 PCR product above the 0 min time point, indicating that the GAL lncRNAs, and/or the Reb1-binding sites, are necessary for GAL10 looping and are epistatic to DBP2.
Figure 5. GAL10 forms lncRNA-dependent gene loops.
(A) Schematic of GAL10 showing restriction sites (vertical lines) and primers for Chromosome Conformation Capture (3C) (arrows). P1 and T1 correspond to the GAL1/10 promoter and GAL10 terminator, respectively, whereas F and R are internal control primers. (B-C) 3C of GAL10 in wild type, lncRNAΔ, dbp2Δ and dbp2Δ lncRNAΔ cells during a glucose to galactose shift. Samples were quantified across three biological replicates relative to the control PCR with error bars representing SEM. (B) Ethidium bromide-stained agarose gel showing representative PCR products. (C) Quantification of looping at 0, 90 and 180 min post-induction. (D-E) Chromatin immunoprecipitation (ChIP) of 3XFLAG-tagged Cyc8 in wild type, lncRNAΔ, dbp2Δ and dbp2Δ lncRNAΔ strains grown under transcriptionally repressive (+glucose) conditions. ChIP-qPCR was performed as described (Cloutier et al., 2013). Results are presented as the mean percent of input with SEM. * = p < 0.05 with respect to the lncRNAΔ strain. (F) Western blot of Cyc8-3XFLAG. Whole cell lysate was extracted and resolved by SDS-PAGE. Cyc8, G6PDH and Pgk1 were detected using antibodies specific to FLAG, G6PDH and Pgk1, respectively, from the indicted strains.
To determine if the GAL lncRNAs alter the association of trans-acting factors with the GAL cluster, we conducted chromatin immunoprecipitation (ChIP) of the Cyc8 co-repressor in wild type, lncRNAΔ, dbp2Δ and dbp2Δ lncRNAΔ cells grown in transcriptionally repressive conditions. Consistent with our prior studies, we observed reduced association of Cyc8-FLAG with the GAL1/10 and GAL7 promoter in dbp2Δ cells as compared to wild type ((Cloutier et al., 2013); Figures 5D and 5E). Interestingly, removal of the GAL lncRNAs in dbp2Δ cells restored Cyc8 binding to wild type levels, an effect that is not due to altered abundance of Cyc8 (Figure 5F). This suggests that the GAL lncRNAs reduce repressor binding at GAL promoters, a necessary step prior to activation and gene looping.
The GAL lncRNAs Promote Faster Adaptation to A Nutritional Shift
The GAL cluster genes are part of a network whose induction is necessary to metabolize galactose as an alternative carbon source from glucose (Lohr et al., 1995). To determine if the GAL lncRNAs provide a fitness advantage to yeast cells when challenged with adaptation to galactose, we measured the growth of wild type and lncRNAΔ strains grown in synthetic complete media over the course of a glucose to galactose or a mock carbon source shift (Figure 6). Interestingly, we observed an ~30 min faster doubling time for wild type cells as compared to the lncRNAΔ strain (Figure 6A). Faster growth was nutrient-specific as a mock shift from glucose to glucose resulted in superimposable growth curves for the two strains (Figure 6B). We then conducted a growth assay in wild type and lncRNAΔ cells expressing human RNase H1 or an empty vector to determine if loss of R-loops abolished the growth advantage of wild type cells in a carbon shift. Strikingly, expression of RNase H1 in wild type cells caused a profound increase in lag time of ~6 hours when cells were shifted to galactose media as compared to vector alone (Figure 6C). This lag time was similar to lncRNAΔ cells harboring empty vector or pRNH1 However, both wild type and lncRNAΔ cells expressing RNase H1 showed reduced doubling times overall, suggesting that overexpression of RNase H1 has other effects on cell growth (Figure 6C).
Figure 6. GAL lncRNAs promote faster growth and adaptation to a changing environment.
(A) Growth curves of wild type and lncRNAΔ cells during a carbon source switch. Cell density was measured by absorbance (A600nm) across a glucose to galactose switch in synthetic complete (SC) media. Results are presented as the mean of 6 biological replicates with the SEM. (B) Growth curves of wild type and lncRNAΔ cells during a mock (glucose to glucose) shift. Doubling times (t2) were determined by fitting to an exponential growth curve in GraphPad Prism whereas lag times were calculated using DMFit (Baranyi and Roberts, 1994) (http://www.combase.cc/tools/). (C) RNase H1 represses growth of wild type cells during a glucose to galactose shift. Doubling and lag times of cells were determined as above. (D-E) Competition assay of wild type and lncRNAΔ cells during a carbon source switch. A TRP1 allele was integrated into the trp1-63 locus of the lncRNAΔ strain to differentiate from wild type. Colony forming units (c.f.u.’s) were determined on both rich and selective media at each time point with the proportion of wild type or lncRNAΔ cells in the culture corresponding to the number of trp- or trp+ cells over the total. Results represent the mean of 3 biological replicates with SEM. (F-G) Competition assays of trans-lncRNA versus lncRNAΔ (F) and cis-versus trans-lncRNA strains (G) reveals equal benefit of trans versus cis-encoded GAL lncRNAs during a carbon source shift. Competition assays were performed as above.
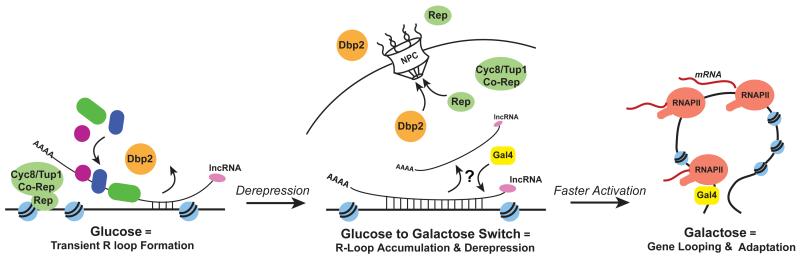
To determine if cells expressing the GAL lncRNAs can better compete for food sources in a diverse population, we conducted a competition assay using a mixed culture of wild type and lncRNAΔ cells. This revealed that wild type cells markedly outcompete lncRNAΔ cells in a mixed genetic population when challenged with a carbon source switch (Figure 6D). In fact, wild type cells represented ~60% and 95% of the culture within 12 and 24 hours after the shift to galactose, respectively. In contrast, the two strains remained largely equal through the duration of a mock shift (Figure 6E). The GAL lncRNAs encoded in trans (trans-lncRNA) also conferred a competitive advantage over lncRNAΔ cells during the nutritional switch (Figure 6F), whereas we saw no difference in fitness between the cis versus trans-encoded GAL lncRNA strains (Figure 6G). Thus, the GAL lncRNAs promote a fitness advantage to yeast cells in response to changing nutritional availability irrespective of genomic location (cis versus trans). Taken together, our work suggests that Dbp2 regulates formation of lncRNA-DNA hybrids in response to nutrients, thereby facilitating rapid transcriptional induction for environmental adaptation (Figure 7).
Figure 7. Regulation of Dbp2 stimulates transcriptional induction by promoting formation of transient GAL lncRNA-DNA hybrids.
Loss of Dbp2 from the nucleus upon removal of glucose promotes formation of GAL lncRNA-DNA hybrids, or R-loops, likely by preventing Dbp2-dependent lncRNA-protein complex assembly (Ma et al., 2013). These R-loops promote derepression and subsequent assembly of transcriptional activators, acting in concert with nutrient-responsive transcription factors and signaling pathways (Sellick et al., 2008). GAL lncRNA R-loops are presumably cleared by the Gal4 activator and subsequent transcriptional interference from active GAL gene expression and/or re-import of Dbp2 in the nucleus upon prolonged exposure to galactose (Beck et al., 2014). This rapid activation promotes gene looping and faster metabolic adaptation to a new sugar source.
Discussion
R-loops have been shown to function as transcriptional regulators at the level of initiation, elongation and termination, as well as etiological agents of DNA damage (Costantino and Koshland, 2015). As transcriptional regulators, R-loops protect CpG-containing promoters from transcriptional silencing by blocking recruitment of the DNA methyltransferase DNMT3B1 (Ginno et al., 2013; Ginno et al., 2012), promote efficient termination by pausing RNA polymerase (Skourti-Stathaki et al., 2011), and repress transcription by promoter occlusion (Sun et al., 2013). Our results now show that R-loops formed with the GAL lncRNAs promote gene induction, thus positively regulating transcription. Consistently, lncRNA-dependent R-loops in mammalian cells have very recently been shown to promote activation of the vimentin (VIM) gene (Boque-Sastre et al., 2015). This suggests a conserved mechanism for R-loops in lncRNA-dependent gene activation.
There are two proposed mechanisms for formation of R loops in vivo (Costantino and Koshland, 2015). The first envisions that nascent RNA emerging from RNA polymerase folds back onto the DNA, forming an R-loop in cis from defects in elongation, termination and mRNA-protein packaging (Costantino and Koshland, 2015; Dominguez-Sanchez et al., 2011; Gomez-Gonzalez et al., 2011; Stirling et al., 2012). The second mechanism follows the observation that R-loops can form in trans through the action of homologous recombination machinery (Wahba et al., 2013). Interestingly, the fact that the GAL lncRNAs function both in cis and in trans upon loss of DBP2 suggests that RNA-protein assembly may be a determinant of R-loop formation in both cases. This finding also suggests that many cis-acting lncRNAs may be much more similar mechanistically to trans- acting molecules than previously thought. If this is the case, the prevalence of cis-encoded lncRNAs near targeted gene loci (Necsulea et al., 2014) may reflect evolutionary constraints to maintain base pairing between the target DNA and lncRNA, rather than a reflection of molecular function.
Export of the RNA helicase Dbp2 promotes lncRNA-dependent R-loop spreading, addressing a long-standing question regarding how functional R-loops may be regulated. Interestingly, this regulation appears to be specific for carbon source metabolic genes (Beck et al., 2014). Because Dbp2 is required for efficient 3’ end formation and assembly of RNA-binding proteins (Ma et al., 2013), factors that also associate with lncRNAs (Tuck and Tollervey, 2013), this suggests that specificity may arise from differential requirements for Dbp2 in RNA processing and packaging. Structured elements in the nascent RNA that require Dbp2 for resolution are the most likely determinants, as Dbp2 is an efficient RNA helicase in vitro and structured RNA may be refractory to mRNP/lncRNP assembly (Ma et al., 2013). Failure to properly assemble nascent mRNPs/lncRNPs would be consistent with co-occurrence of derepressed genes in dbp2Δ cells with ones that form R-loops in hpr1Δ cells, as Hpr1 is a member of the THO/TREX complex required for mRNP packaging, transcriptional elongation, and mRNA export (Gomez-Gonzalez et al., 2011; Strasser et al., 2002; Zenklusen et al., 2002). Alternatively, loss of Dbp2 may stabilize lncRNAs in the nucleus. The latter is consistent with evidence that the exosome component Rrp6 prevents R-loop formation at enhancers in mouse B cells and ESCs (Pefanis et al., 2015) and the fact that simultaneous loss of DBP2 and RRP6 is lethal in S. cerevisiae (Cloutier et al., 2012).
Consistent with promoting activation, the GAL lncRNAs are also essential for gene looping at GAL10. Alternatively, gene looping may be mediated by the Reb1-binding sites in the DNA. Regardless, this is reminiscent of mammalian enhancers and associated non-coding RNAs, which have recently been shown to form R-loops (Lai et al., 2013; Orom et al., 2010; Pefanis et al., 2015). However, whereas GAL1 and GAL10 form gene loops (Laine et al., 2009; Tan-Wong et al., 2009), we have not detected these structures at the GAL7 gene (data not shown). This may be a technical limitation or reflective of an alternative mechanism for enhanced induction at this locus. Indeed, recruitment of the GAL genes to the nuclear periphery facilitates deSUMOylation of Cyc8 and subsequent derepression (Texari et al., 2013), suggesting that the GAL lncRNAs and/or R-loops may also promote rearrangements within the 3-dimensional nuclear space.
Our observations appear to be in conflict with previous studies noting a role for the GAL lncRNAs in transcriptional repression and it is important to integrate our results with the field (Geisler et al., 2012; Houseley et al., 2008; Lenstra et al., 2015; Pinskaya et al., 2009). Our data and model suggest that lncRNA-dependent induction only occurs in the context of a repressive (glucose) to activated (galactose) switch, consistent with our prior studies (Cloutier et al., 2013) and a recent report published during submission of this study showing no role for the GAL lncRNAs from a non-induced (raffinose) state (Lenstra et al., 2015). However, the latter does not establish that the GAL lncRNAs have no positive role as concluded (Lenstra et al., 2015), as raffinose is not a transcriptionally repressed state (Sellick et al., 2008). Another point of confusion are conclusions from studies of the RNA decapping factor DCP2 that the GAL lncRNAs are strongly repressive from the same non-inducing conditions (Geisler et al., 2012). In this case, the strong transcriptional attenuation appears to be specific for dcp2Δ cells (Cloutier et al., 2013; Geisler et al., 2012), a finding that may be explained by a role for Dcp2 in transcription itself (Haimovich et al., 2013), as well as the use of a strain harboring a genomic deletion of GAL10 to analyze GAL lncRNA-dependent repression of GAL1 (Geisler et al., 2012).
Regardless, several lines of evidence suggest that transcriptional repression does occur to prevent low level, leaky expression of galactose metabolic genes in the context of mixed sugars (i.e., glucose and galactose) (Houseley et al., 2008; Lenstra et al., 2015). These findings are fully compatible with our results and suggest a dual role for the GAL lncRNAs: one in transcriptional repression in cis by transcriptional interference and one in derepression/enhanced induction in trans by the lncRNA molecule itself (see Graphical Abstract). Importantly, our work shows that the GAL lncRNAs confer a strong competitive advantage over otherwise genetically identical yeast cells during a glucose to galactose switch, establishing a biologically beneficial role for these molecules that outweighs the subtle transcriptional benefit in wild type cells (Cloutier et al., 2013). This has widespread implications as investigators pursue functions of individual lncRNAs by underscoring the value of simple model organisms and utilization of mutant strains to tease apart complex molecular processes.
Experimental Procedures
For detailed protocols, see supplemental experimental procedures.
Plasmids, Cloning and Yeast Strain Construction
Plasmids are listed in Table S1. Yeast strains were constructed using classical yeast genetics (Table S2). Oligos for homologous recombination are listed in Table S3.
Northern Blotting
Northern blotting was performed as described previously (Cloutier et al., 2013). Probes were generated from PCR products using plasmids and primers in Tables S1 and S4.
Strand-specific Reverse Transcriptase-quantitative PCR (ssRT-qPCR)
Strand-specific cDNA preparation and qPCR) were performed as described (Beck et al., 2014) using oligos listed in Table S5.
DNA-RNA Immunoprecipitation (DRIP)
DRIP was conducted similar to prior studies (El Hage et al., 2010) using the S9.6 antibody (Boguslawski et al., 1986; Hu et al., 2006). Primer/probes for qPCR are in Table S6.
Determination of Recombination Frequencies
Strains were transformed with direct repeat constructs pRS314-L or pSCh204 to assay the recombination frequencies as described previously (Chavez and Aguilera, 1997; Prado et al., 1997).
RNA-seq Bioinformatics
RNA-Seq data was previously deposited in the Gene Expression Omnibus (GSE58097, (Beck et al., 2014)). Bioinformatics was conducted as detailed in Supplemental Experimental Procedures.
Chromosome Conformation Capture (3C)
3C was performed as described previously (El Kaderi et al., 2012). Primers are listed in Table S7.
Chromatin Immunoprecipitation (ChIP)
ChIP and qPCR were performed as described (Cloutier et al., 2013) using primer/probes listed in Table S6.
Growth Curves and Competition Assay
Cell density was assessed by absorbance (600nm) over 30 hours of growth in synthetic complete media and indicated carbon source. For competition assays, equal amounts of wild type and lncRNAΔ TRP1, wild type and trans-lncRNA, or lncRNAΔ and trans-lncRNA cells were mixed and resuspended in liquid culture and subjected to a carbon shift followed by plating on selective media.
Supplementary Material
Acknowledgements
We thank S. Leppla (NIH) for the kind gift of the S9.6 antibody. This work was supported by NIH R01GM097332-01 to E.J.T., MCB1020911 to A. A., and the Purdue DNA sequencing facility supported by P30 CA023168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
S. C. C. conducted transcriptional analysis, DRIP, genetic manipulations, and growth assays. W.K.M. performed ss-RT-qPCR. S. W. and P.E.P. conducted bioinformatic and statistical analysis. N. A. H. and Z. D. conducted 3C. This manuscript was written by S. C. C. and E. J. T. with input from A. Z., P. E. P.
References
- Ansari A, Hampsey M. A role for the CPF 3’-end processing machinery in RNAP II-dependent gene looping. Genes Dev. 2005;19:2969–2978. doi: 10.1101/gad.1362305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augui S, Nora EP, Heard E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet. 2011;12:429–442. doi: 10.1038/nrg2987. [DOI] [PubMed] [Google Scholar]
- Baranyi J, Roberts TA. A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol. 1994;23:277–294. doi: 10.1016/0168-1605(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Beck ZT, Cloutier SC, Schipma MJ, Petell CJ, Ma WK, Tran EJ. Regulation of glucose-dependent gene expression by the RNA helicase Dbp2 in Saccharomyces cerevisiae. Genetics. 2014;198:1001–1014. doi: 10.1534/genetics.114.170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, Barroso SI, Garcia-Rubio ML, Tumini E, Herrera-Moyano E, Aguilera A. BRCA2 prevents R-loop accumulation and associates with TREX-2 mRNA export factor PCID2. Nature. 2014;511:362–365. doi: 10.1038/nature13374. [DOI] [PubMed] [Google Scholar]
- Boguslawski SJ, Smith DE, Michalak MA, Mickelson KE, Yehle CO, Patterson WL, Carrico RJ. Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J Immunol Methods. 1986;89:123–130. doi: 10.1016/0022-1759(86)90040-2. [DOI] [PubMed] [Google Scholar]
- Boque-Sastre R, Soler M, Oliveira-Mateos C, Portela A, Moutinho C, Sayols S, Villanueva A, Esteller M, Guil S. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc Natl Acad Sci U S A. 2015;112:5785–5790. doi: 10.1073/pnas.1421197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YA, Aristizabal MJ, Lu PY, Luo Z, Hamza A, Kobor MS, Stirling PC, Hieter P. Genome-wide profiling of yeast DNA:RNA hybrid prone sites with DRIP-chip. PLoS Genet. 2014;10:e1004288. doi: 10.1371/journal.pgen.1004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez S, Aguilera A. The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome instability. Genes Dev. 1997;11:3459–3470. doi: 10.1101/gad.11.24.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier SC, Ma WK, Nguyen LT, Tran EJ. The DEAD-box RNA helicase Dbp2 connects RNA quality control with repression of aberrant transcription. J Biol Chem. 2012;287:26155–26166. doi: 10.1074/jbc.M112.383075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier SC, Wang S, Ma WK, Petell CJ, Tran EJ. Long Noncoding RNAs Promote Transcriptional Poising of Inducible Genes. PLoS Biol. 2013;11:e1001715. doi: 10.1371/journal.pbio.1001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino L, Koshland D. The Yin and Yang of R-loop biology. Curr Opin Cell Biol. 2015;34:39–45. doi: 10.1016/j.ceb.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanelli E, Chartrand P. Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity. Frontiers in genetics. 2015;6:143. doi: 10.3389/fgene.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vit MJ, Waddle JA, Johnston M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sanchez MS, Barroso S, Gomez-Gonzalez B, Luna R, Aguilera A. Genome instability and transcription elongation impairment in human cells depleted of THO/TREX. PLoS Genet. 2011;7:e1002386. doi: 10.1371/journal.pgen.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A, French SL, Beyer AL, Tollervey D. Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes Dev. 2010;24:1546–1558. doi: 10.1101/gad.573310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaderi B, Medler S, Ansari A. Analysis of interactions between genomic loci through Chromosome Conformation Capture (3C) In: Bonifacino Juan S, et al., editors. Current protocols in cell biology / editorial board. 2012. Chapter 22, Unit22 15. [DOI] [PubMed] [Google Scholar]
- Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science. 2013;341:1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Lojek L, Khalil AM, Baker KE, Coller J. Decapping of long noncoding RNAs regulates inducible genes. Mol Cell. 2012;45:279–291. doi: 10.1016/j.molcel.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginno PA, Lim YW, Lott PL, Korf I, Chedin F. GC skew at the 5’ and 3’ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 2013;23:1590–1600. doi: 10.1101/gr.158436.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginno PA, Lott PL, Christensen HC, Korf I, Chedin F. R-loop formation is a distinctive characteristic of unmethylated human CpG island promoters. Mol Cell. 2012;45:814–825. doi: 10.1016/j.molcel.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Garcia-Rubio M, Bermejo R, Gaillard H, Shirahige K, Marin A, Foiani M, Aguilera A. Genome-wide function of THO/TREX in active genes prevents R-loop-dependent replication obstacles. Embo J. 2011;30:3106–3119. doi: 10.1038/emboj.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacisuleyman E, Goff LA, Trapnell C, Williams A, Henao-Mejia J, Sun L, McClanahan P, Hendrickson DG, Sauvageau M, Kelley DR, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21:198–206. doi: 10.1038/nsmb.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich G, Medina DA, Causse SZ, Garber M, Millan-Zambrano G, Barkai O, Chavez S, Perez-Ortin JE, Darzacq X, Choder M. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell. 2013;153:1000–1011. doi: 10.1016/j.cell.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Hampsey M, Singh BN, Ansari A, Laine JP, Krishnamurthy S. Control of eukaryotic gene expression: gene loops and transcriptional memory. Adv Enzyme Regul. 2011;51:118–125. doi: 10.1016/j.advenzreg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Hatchi E, Skourti-Stathaki K, Ventz S, Pinello L, Yen A, Kamieniarz-Gdula K, Dimitrov S, Pathania S, McKinney KM, Eaton ML, et al. BRCA1 recruitment to transcriptional pause sites is required for R-loop-driven DNA damage repair. Mol Cell. 2015;57:636–647. doi: 10.1016/j.molcel.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, Rubbi L, Grunstein M, Tollervey D, Vogelauer M. A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Mol Cell. 2008;32:685–695. doi: 10.1016/j.molcel.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhang A, Storz G, Gottesman S, Leppla SH. An antibody-based microarray assay for small RNA detection. Nucleic Acids Res. 2006;34:e52. doi: 10.1093/nar/gkl142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009a;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009b;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. 2003;12:711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Jeon Y, Lee JT. YY1 tethers Xist RNA to the inactive X nucleation center. Cell. 2011;146:119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M, Flick JS, Pexton T. Multiple mechanisms provide rapid and stringent glucose repression of GAL gene expression in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3834–3841. doi: 10.1128/mcb.14.6.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, Shiekhattar R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JP, Singh BN, Krishnamurthy S, Hampsey M. A physiological role for gene loops in yeast. Genes Dev. 2009;23:2604–2609. doi: 10.1101/gad.1823609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra TL, Coulon A, Chow CC, Larson DR. Single-Molecule Imaging Reveals a Switch between Spurious and Functional ncRNA Transcription. Mol Cell. 2015;60:597–610. doi: 10.1016/j.molcel.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D, Venkov P, Zlatanova J. Transcriptional regulation in the yeast GAL gene family: a complex genetic network. FASEB J. 1995;9:777–787. doi: 10.1096/fasebj.9.9.7601342. [DOI] [PubMed] [Google Scholar]
- Ma WK, Cloutier SC, Tran EJ. The DEAD-box Protein Dbp2 Functions with the RNA-Binding Protein Yra1 to Promote mRNP Assembly. J Mol Biol. 2013 doi: 10.1016/j.jmb.2013.05.016. doi: 10.1016/j.jmb.2013.1005.1016. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIsaac KD, Wang T, Gordon DB, Gifford DK, Stormo GD, Fraenkel E. An improved map of conserved regulatory sites for Saccharomyces cerevisiae. BMC Bioinformatics. 2006;7:113. doi: 10.1186/1471-2105-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler S, Al Husini N, Raghunayakula S, Mukundan B, Aldea A, Ansari A. Evidence for a complex of transcription factor IIB with poly(A) polymerase and cleavage factor 1 subunits required for gene looping. J Biol Chem. 2011;286:33709–33718. doi: 10.1074/jbc.M110.193870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- Necsulea A, Soumillon M, Warnefors M, Liechti A, Daish T, Zeller U, Baker JC, Grutzner F, Kaessmann H. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature. 2014;505:635–640. doi: 10.1038/nature12943. [DOI] [PubMed] [Google Scholar]
- Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M, Gligoris T, Tzamarias D. The Snf1 kinase controls glucose repression in yeast by modulating interactions between the Mig1 repressor and the Cyc8-Tup1 co-repressor. EMBO Rep. 2004;5:368–372. doi: 10.1038/sj.embor.7400120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J, Federation A, Chao J, Elliott O, Liu ZP, et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161:774–789. doi: 10.1016/j.cell.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinskaya M, Gourvennec S, Morillon A. H3 lysine 4 di- and tri-methylation deposited by cryptic transcription attenuates promoter activation. Embo J. 2009;28:1697–1707. doi: 10.1038/emboj.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F, Piruat JI, Aguilera A. Recombination between DNA repeats in yeast hpr1delta cells is linked to transcription elongation. Embo J. 1997;16:2826–2835. doi: 10.1093/emboj/16.10.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell. 2011;147:1408–1419. doi: 10.1016/j.cell.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma K, Cifuentes-Rojas C, Ergun A, Del Rosario A, Jeon Y, White F, Sadreyev R, Lee JT. ATRX directs binding of PRC2 to Xist RNA and Polycomb targets. Cell. 2014;159:869–883. doi: 10.1016/j.cell.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick CA, Campbell RN, Reece RJ. Galactose metabolism in yeast-structure and regulation of the leloir pathway enzymes and the genes encoding them. Int Rev Cell Mol Biol. 2008;269:111–150. doi: 10.1016/S1937-6448(08)01003-4. [DOI] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Proudfoot NJ. A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014;28:1384–1396. doi: 10.1101/gad.242990.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki K, Proudfoot NJ, Gromak N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell. 2011;42:794–805. doi: 10.1016/j.molcel.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling PC, Chan YA, Minaker SW, Aristizabal MJ, Barrett I, Sipahimalani P, Kobor MS, Hieter P. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 2012;26:163–175. doi: 10.1101/gad.179721.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- Sun Q, Csorba T, Skourti-Stathaki K, Proudfoot NJ, Dean C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science. 2013;340:619–621. doi: 10.1126/science.1234848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Wong SM, Wijayatilake HD, Proudfoot NJ. Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 2009;23:2610–2624. doi: 10.1101/gad.1823209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texari L, Dieppois G, Vinciguerra P, Contreras MP, Groner A, Letourneau A, Stutz F. The nuclear pore regulates GAL1 gene transcription by controlling the localization of the SUMO protease Ulp1. Mol Cell. 2013;51:807–818. doi: 10.1016/j.molcel.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck AC, Tollervey D. A Transcriptome-wide Atlas of RNP Composition Reveals Diverse Classes of mRNAs and lncRNAs. Cell. 2013;154:996–1009. doi: 10.1016/j.cell.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EL, Chen CL, d’Aubenton-Carafa Y, Gourvennec S, Kwapisz M, Roche V, Bertrand C, Silvain M, Legoix-Ne P, Loeillet S, et al. XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature. 2011;475:114–117. doi: 10.1038/nature10118. [DOI] [PubMed] [Google Scholar]
- Wahba L, Amon JD, Koshland D, Vuica-Ross M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol Cell. 2011;44:978–988. doi: 10.1016/j.molcel.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L, Gore SK, Koshland D. The homologous recombination machinery modulates the formation of RNA-DNA hybrids and associated chromosome instability. eLife. 2013;2:e00505. doi: 10.7554/eLife.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tran EJ. Unexpected functions of lncRNAs in gene regulation. Commun Integr Biol. 2013;6:e27610. doi: 10.4161/cib.27610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wei W, Gagneur J, Perocchi F, Clauder-Munster S, Camblong J, Guffanti E, Stutz F, Huber W, Steinmetz LM. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457:1033–1037. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen D, Vinciguerra P, Wyss JC, Stutz F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol. 2002;22:8241–8253. doi: 10.1128/MCB.22.23.8241-8253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.