Abstract
Objectives
To characterize the epidemiology of sarcoidosis from 1946 to 2013.
Patients and Methods
An inception cohort of patients with incident sarcoidosis in 1976–2013 in Olmsted County, Minnesota was identified based on comprehensive individual medical record review. Inclusion required physician diagnosis supported by histopathological confirmation, radiologic features of intrathoracic sarcoidosis and compatible clinical presentation. Data were collected on demographic characteristics, clinical presentation, laboratory investigations and mortality. The data were augmented with a previously identified cohort of Olmsted County, Minnesota residents diagnosed with sarcoidosis in 1946–1975. Incidence rates were age and sex adjusted to the US white 2010 population.
Results
A total of 448 incident cases of sarcoidosis were identified (mean age 44.2 years and 52% female). The annual incidence of sarcoidosis was 10.0 per 100,000 population. The incidence of sarcoidosis increased among females from 1950–1960, but otherwise there were no significant calendar year trends. However, the peak age at incidence among females shifted from 40–59 years in 1950 to 50–69 years in 2010. Similarly, the peak age at incidence for males shifted from 30–49 years in 1950 to 40–59 years in 2010.
97% of cases had intrathoracic involvement but only 43% of patients had respiratory symptoms. The overall mortality of patients with sarcoidosis was not different from general population (standardized mortality ratio: 0.90; 95% CI, 0.74–1.08).
Conclusions
Sarcoidosis occurred in about 10 persons per 100,000 per year. Most of the patients had intra-thoracic involvement, although less than half had respiratory symptoms. Overall mortality was not different from general population.
Keywords: Sarcoidosis, Epidemiology, Incidence, Mortality
Introduction
Sarcoidosis is a multi-system disorder of unclear etiology typically affecting the lymphatic system and the lungs. The presence of non-caseating granuloma is the histopathological hallmark of the disease. The clinical course of the disease can range from an acute self-limited process to progressive organ dysfunction with significant morbidity and mortality. Sarcoidosis is generally categorized into two subgroups, intra-thoracic and extra-thoracic, based on the site of involvement.1
The epidemiology of sarcoidosis, especially in the United States, is not well-described, as a limited number of epidemiological studies have been conducted.2–5 The first epidemiological study of sarcoidosis in the United States was published in 1986.2 This study used the resource of Rochester Epidemiology Project to identify all the incident cases of sarcoidosis in Olmsted County, Minnesota between 1946 and 1975. The age- and sex-adjusted incidence of sarcoidosis was 6.1 per 100,000 person-years. Incidence figures from subsequent studies were very variable, ranging from 9.6 to 71 per 100,000 person-years.3, 4 Ethnic/racial differences were also observed.3 Studies on mortality found age-adjusted mortality rates varied from 1.3 to 28.0 per 1,000,000, depending on sex, ethnicity/race and calendar year.5–7
The objective of this study was to characterize the epidemiology of sarcoidosis, with emphasis on annual incidence and mortality, from 1946 to 2013, in a geographically well - defined population of patients.
Patients and Methods
Data source and study population
Through the resources of the Rochester Epidemiology Project, the population of Olmsted County, Minnesota, in which resides the city of Rochester, is well suited for investigation of the epidemiology of sarcoidosis because comprehensive and complete medical records for all residents seeking medical care for over six decades are available. A record linkage system allows ready access to the medical records from all health care providers for the local population, including the Mayo Clinic and the Olmsted Medical Center and their affiliated hospitals, local nursing homes, and the few private practitioners. The potential of this data system for use in population-based studies has previously been described.8, 9 This system ensures virtually complete ascertainment of all clinically recognized cases of sarcoidosis among the residents of Olmsted County, Minnesota.
Approval for this study was obtained from the Mayo Clinic and Olmsted Medical Center institutional review boards and the need for informed consent was waived.
Study design
A cohort containing Olmsted County, Minnesota residents diagnosed with sarcoidosis between January 1, 1976 and December 31, 2013 was identified to augment the previously identified cohort of Olmsted County, Minnesota residents diagnosed with sarcoidosis in 1946–1975.2 All patients with diagnosis codes related to sarcoid, sarcoidosis, and contextual noncaseating granuloma were screened for inclusion in the cohort based on comprehensive individual medical record review. Inclusion required physician diagnosis supported by histopathology and radiologic features of intrathoracic sarcoidosis, compatible clinical presentation, and exclusion of other granulomatous diseases. Tissue samples were considered positive if they demonstrated non-caseating granuloma without evidence of acid-fast bacilli or fungi. The only exception to the requirement of histopathological confirmation was stage I pulmonary sarcoidosis that required only radiographic evidence of symmetric bilateral hilar adenopathy with or without mediastinal lymphadenopathy in the absence of symptoms or identifiable causes. Isolated granulomatous disease of a specific organ except for the skin was also included if there was no better alternative diagnosis. Cases with a diagnosis of sarcoidosis prior to residency in Olmsted County were not included.10, 11
A standardized data extraction form was utilized to record the following information: date of diagnosis, age at diagnosis, sex, self-reported ethnicity, smoking status, date of last follow up, status at last follow up (death or alive), cause of death, presence of intrathoracic disease, presence of symptoms related to intrathoracic disease, radiologic findings, presence of extrathoracic disease, angiotensin converting enzyme level and calcium level. Data on self-reported ethnicity were collected to investigate the possible racial difference of the incidence of sarcoidosis.
Case verification and data extraction was conducted by the first author (P.U.). To ensure the accuracy of our data, 10% of medical records were randomly selected and reviewed by the second author (E.M.C.) and the senior author (E.L.M.).
Statistical analysis
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Age-and sex-specific incidence rates were calculated by using the number of incident cases as the numerator and population estimates for adults (age ≥ 18 years) based on decennial census counts as the denominator, with linear interpolation used to estimate population size for intercensal years. Overall incidence rates were age- and/or sex-adjusted to the 2010 white population of the United States. In order to compute 95% confidence intervals (95% CI) for incidence rates, it was assumed that the number of incident cases followed a Poisson distribution. Trends in incidence rates were examined using Poisson regression methods with smoothing splines for age and calendar year. The annual incidence rates were graphically illustrated using a 3-year, centered, moving average to reduce the random fluctuations over time.
Mortality rates were estimated using the Kaplan-Meier method and compared to expected mortality for persons of the same age, sex and calendar year estimated using Minnesota population life tables. The standardized mortality ratio (SMR) was estimated as the ratio of the observed and expected number of deaths. Ninety-five percent confidence intervals for the SMR were calculated assuming that the expected rates are fixed and the observed rates followed a Poisson distribution. Univariable Cox models adjusted for age, sex and calendar year of sarcoidosis incidence were used to identify prognostic factors of death.
Results
The combined cohort included a total of 448 adults (age ≥ 18 years) with incident sarcoidosis (103 from 1946–1975 and 345 from 1976–2013). One patient diagnosed in 1970 at age 16 was excluded from our analysis. The mean age of our cohort was 44.2 years (52% were female). Table 1 describes the demographic characteristics of the study population. The average age at diagnosis was older among patients diagnosed in 1976–2013 compared to those diagnosed in 1946–1975 (45.6 vs 35.4 P<.001).
Table 1.
Demography of 448 adults (age ≥ 18 years) with incident sarcoidosis in 1946–2013 in Olmsted County, Minnesota
| 1946–1975 cohort (N=103) | 1976–2013 cohort (N=345) | Total (N=448) | p-value | |
|---|---|---|---|---|
| Age at diagnosis, years (±SD) | 35.4 (±13.6) | 45.6 (±13.6) | 44.2 (±13.8) | <0.001 |
| Female sex, n(%) | 61 (59%) | 174 (50%) | 235 (52%) | 0.12 |
| Race, n(%) | 0.13 | |||
| Caucasian | 101 (98%) | 301 (90%) | 402 (92%) | |
| African-American | 1 (1%) | 18 (5%) | 19 (4%) | |
| Asian | 0 (0%) | 6 (2%) | 6 (1.5%) | |
| Native American | 0 (0%) | 2 (1%) | 2 (0.5%) | |
| Other | 1 (1%) | 7 (2%) | 8 (2%) | |
| Unknown | 0 | 11 | 11 | |
| Length of follow-up, years (±SD) | 32.1 (±17.9) | 14.5 (±10.4) | 18.5 (±14.5) | -- |
| Smoking status at diagnosis, n(%) | ||||
| Never | 198 (60%) | -- | ||
| Ex-smoker | 71 (21%) | |||
| Current smoker | 63 (19%) | |||
| Data not available | 102 | 13 |
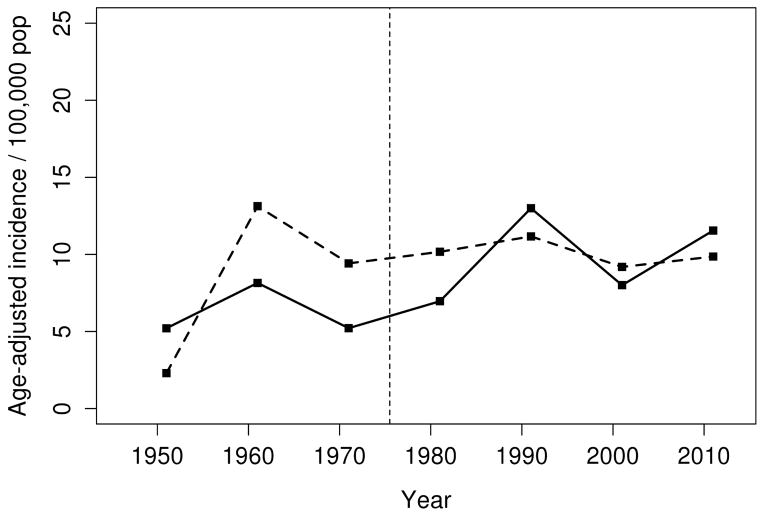
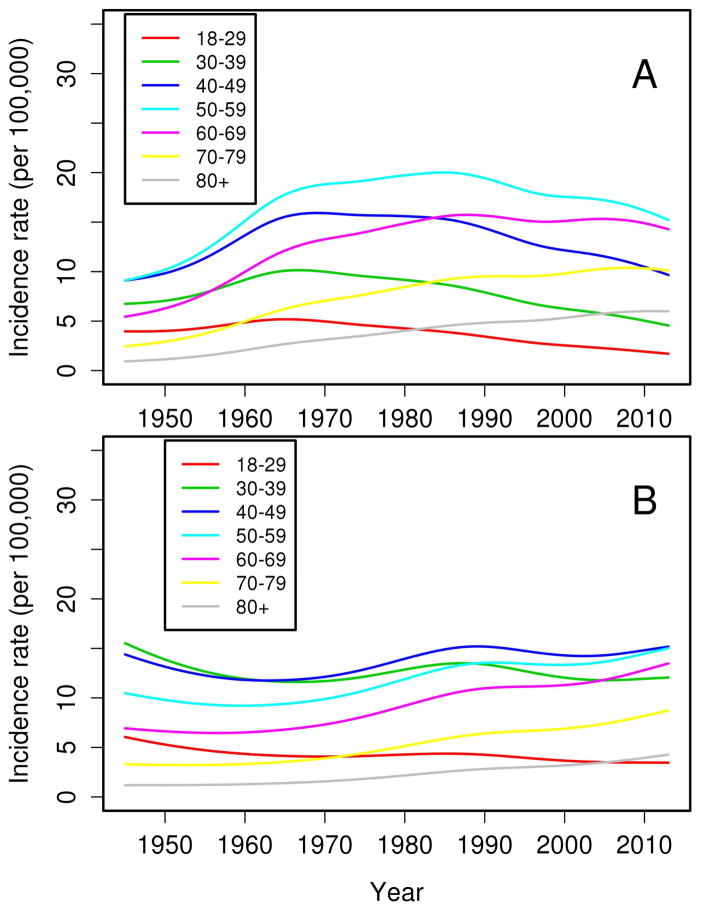
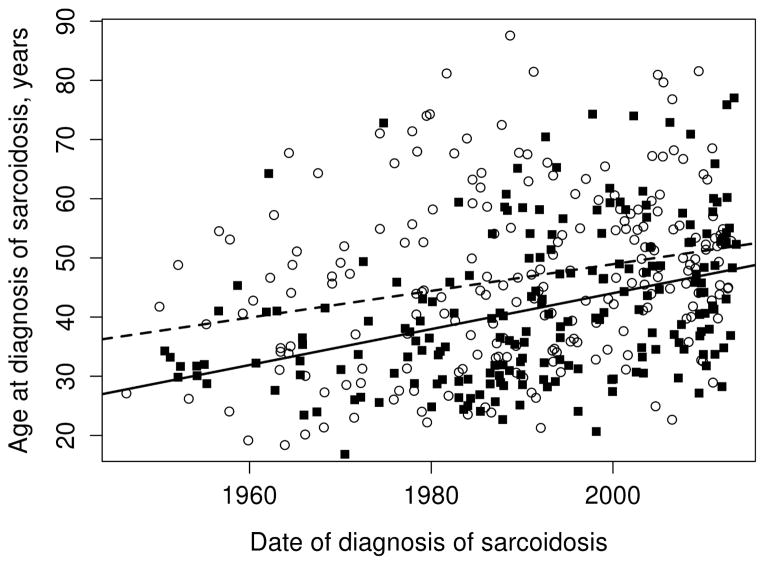
Overall, the annual incidence of sarcoidosis among adults in 1946–2013 was 10.0 per 100,000 population (10.5 per 100,000 population among females and 9.4 per 100,000 population among males; Table 2). Figure 1 demonstrates the annual incidences of sarcoidosis per calendar year by sex for the entire time period. As previously mentioned, the incidence of sarcoidosis increased among women from 1950–1960, but otherwise there were no significant calendar year trends in incidence for either females or males. However, significant age by calendar year trends in incidence rates were noted for both females (P=.001) and males (P=.02). The age-year interaction is depicted in Figure 2. Panel A demonstrates the incidence of sarcoidosis decreased over time after 1960 among females ages 18–29, 30–39, and 40–49 years, was relatively stable after 1960 among females age 50–59 years and increased among females ages 60–69, 70–79 and 80+ years. The peak age at incidence among women shifted from 40–59 years in 1950 to 50–69 years in 2010. Panel B shows the incidence of sarcoidosis among males decreased over time for ages 18–29 years, and was stable for ages 30–39 and 40–49 years, and increased over time for ages 50–59, 60–69, 70–79 and 80+ years. Similarly, the peak age at incidence for males shifted from 30–49 years in 1950 to 40–59 years in 2010. The change in age at diagnosis over time is further demonstrated in Figure 3 wherein linear relationships between calendar year and age were noted for both sexes with similar increases in the mean age at diagnosis of sarcoidosis in both sexes (2.3 years for females and 2.8 years for males per decade of calendar time).
Table 2.
Incidence of sarcoidosis in 1946–2013 among adult (age ≥ 18 years) residents of Olmsted County, MN, by sex and age
| Females | Males | Total | ||||
|---|---|---|---|---|---|---|
| Age group | N | Rate | N | Rate | N | Rate |
| 18–29 | 31 | 4.9 | 41 | 7.7 | 72 | 6.1 |
| 30–39 | 48 | 9.9 | 68 | 14.1 | 116 | 12.0 |
| 40–49 | 63 | 15.2 | 51 | 12.7 | 114 | 14.0 |
| 50–59 | 54 | 16.3 | 35 | 11.2 | 89 | 13.8 |
| 60–69 | 26 | 11.1 | 10 | 4.9 | 36 | 8.2 |
| 70–79 | 8 | 4.9 | 8 | 6.7 | 16 | 5.6 |
| 80+ | 5 | 4.3 | 0 | 0.0 | 5 | 2.9 |
| Overall (95% CI) | 235 | 10.5* (9.1, 11.9) | 213 | 9.4* (8.1, 10.7) | 448 | 10.0** (9.0, 10.9) |
Age- adjusted to the US white 2010 population
Age- and sex- adjusted to the US white 2010 population
N = number; CI=confidence interval
Figure 1.
Incidence of sarcoidosis among residents of Olmsted County, Minnesota in 1946–2013 age-adjusted to the 2010 US white population according to sex (males= solid line, females=dashed line)
Figure 2.
Trends in incidence of sarcoidosis among residents of Olmsted County, Minnesota in 1946–2013 for females (panel A) and males (panel B) according to age groups
Figure 3.
Trends in age at diagnosis of sarcoidosis among residents of Olmsted County, Minnesota in 1946–2013 by sex: females (open circles and dashed lines) and males (black squares and solid line)
In the 1976–2013 cohort, intrathoracic involvement was present in 97% of cases. In 87% of cases this consisted of intrathoracic lymphadenopathy, and 50% had evidence of pulmonary parenchymal infiltration, but only 43% had respiratory symptoms. The most common extra-thoracic manifestations were skin rash (18%) followed by arthralgia (12%), ophthalmologic involvement (7%), hepatic involvement (6%), splenomegaly (4%), renal involvement (3%), neurological involvement (3%), extra-thoracic lymphadenopathy (3%), exocrine gland involvement (2%), upper respiratory tract involvement (2%) and cardiac involvement (1%). Bone involvement was observed in one patient. Isolated extrapulmonary sarcoidosis was diagnosed in 10 (3% of patients).
At diagnosis, angiotensin converting enzyme level was obtained in 241 patients and was elevated in 104 (41%) while total calcium was elevated in only a minority of patients (16%; 46 of 295 tested patients).
During a median follow-up of 18.5 years (8289 total person-years) in the total cohort, 110 patients died. The overall mortality of patients with sarcoidosis was not different from the general population (SMR: 0.90; 95% CI, 0.74–1.08). No significant changes in mortality over time were observed. Potential risk factors for mortality were examined in the 1976–2013 cohort. Age (hazard ratio [HR]: 2.48 per 10 year increase; 95% confidence interval [CI]: 1.97 – 3.13), male sex (HR: 1.98; 95% CI: 1.04 – 3.79), absence of intrathoracic disease (HR: 3.57; 95% CI: 1.08 – 12.5), as well as hepatic (HR: 5.37; 95% CI: 1.90, 15.2), cardiac (HR: 12.4; 2.76 – 55.2), splenic (HR: 11.5; 95% CI: 4.05 – 32.9) or neurological (HR: 4.18; 95% CI: 1.44 – 12.1) involvement in conjunction with intrathoracic disease were associated with increased for mortality in univariable models after adjustment for age, sex and calendar year of sarcoidosis diagnosis.
Discussion
In this study of the epidemiology of sarcoidosis in a geographically well-defined population, the age and sex-adjusted annual incidence was 10.0 per 100,000 population. The incidence was similar in males and females.
Previous studies have demonstrated a varying incidence of sarcoidosis in different populations.3, 4, 12–19 The annual incidence was reported to be as low as 0.73 per 100,000 in Japanese males12 to as high as 71 per 100,000 in African-American females (4). Our results are comparable with other studies with predominant Caucasian populations.3, 14, 15, 17–19
Despite extensive research effort, the etiology and pathogenesis of sarcoidosis remains poorly understood. The widely accepted hypothesis is that of a complex interaction between environmental triggers and genetic predisposition.20 A recent study of New York City’s firefighters who were exposed to World Trade Center-disaster dust has identified 13 new cases of pathology-confirmed sarcoidosis (most of them Caucasian males) within a year after the exposure.21 This finding corresponded to an estimated incidence rate of 86 per 100,000 population, which is substantially higher the historical incidence in that population of 12.9 per 100,000 person-years22 and the incidence estimate from the current study, supporting a role of environmental triggers in the pathogenesis of sarcoidosis.
An increase in the mean age at diagnosis was detected in this study (2.3 years for females and 2.8 years for males per decade of calendar time). A similar finding was previously reported from a Japanese coding-based study that utilized the national administrative database.12 Although we are not able to evaluate exposures, this increase in the mean age at diagnosis may provide indirect evidence to support the role of environmental factors in the development of sarcoidosis as it suggest that environmental triggers are less ample in more recent years, and thus it may take longer for a cumulative exposure burden to occur and reach the disease threshold. Nonetheless, the increase in the mean age at diagnosis could be secondary to several factors such as changes in practice patterns, use of diagnostic imaging technology or other as yet unidentified secular trends.
Data on mortality of sarcoidosis compared with general population are limited. The mortality rate of our sarcoidosis cohort was not significantly different from that of the general Minnesota population. This is in contrast to the report of a significant increase in the mortality rate from a British population-based study, with hazard ratio of 2.09 (95% CI, 1.59–2.74),14 and an African-American female prospective cohort study, with hazard ratio of 2.44 (95% CI, 2.03–2.93).23 This discrepancy may reflect differences in management in different geographical regions or the difference of sarcoidosis severity among different ethnic groups. For example, a coding-based study using the U.S. death certificates demonstrated that the age-adjusted mortality rates differed by sex and ethnic groups. The highest mortality rate was observed among African-American females while the lowest rate was observed among White males. 6
In the current study, 97% the patients had intrathoracic involvement even though only half of them were symptomatic from intrathoracic disease. This finding underscores the importance of chest radiographic imaging when the diagnosis of sarcoidosis is suspected, even in the clinically apparently asymptomatic patient.
Even though sarcoidosis is widely regarded as a multi-system disorder, our study found that extra-thoracic manifestations were seen in a minority of patients with skin rash, joint pain and ophthalmologic involvement being the most common. Nonetheless, the presence of extra-thoracic manifestations might reflect a more extensive and severe disease as the presence of some extra-thoracic manifestations, including hepatic, cardiac, splenic or neurological involvement were predictive factors for mortality after adjustment for age, sex and calendar year of sarcoidosis diagnosis.
The major strengths of this study are that it is a population-based study with a long follow-up period of over four decades. Our comprehensive record-linkage system allows capture of nearly all the cases of sarcoidosis in the community with verification of diagnosis by medical record review. This approach minimizes referral bias and the likelihood of misclassification, a common concern in coding-based studies, is very low. The major limitations are those inherent in the retrospective study design as the case ascertainment depends on diagnosis being made by the health-care providers. Therefore, the exact burden of undiagnosed disease remains unclear. Also, all the pertinent information might not be recorded. The results of this study might not be generalizable as the population of Olmsted County is predominately of Northern European ancestry and less diverse than other US populations. There is also a higher proportion of health-care workers and correspondingly higher education level and socioeconomic status in this population.
Conclusion
In conclusion, we found that sarcoidosis occurred in about 10 persons per 100,000 per year. Most of the patients had intra-thoracic involvement, although less than half had respiratory symptoms. Overall mortality was not different from that of the general population.
Acknowledgments
Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 95% CI
95% confidence interval
- SMR
standardized mortality ratio
- HR
hazard ratio
Footnotes
Disclosure: All authors have disclosed no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas KW, Hunninghake GW. Sarcoidosis. JAMA. 2003;289(24):3300–3303. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 2.Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT. The epidemiology of sarcoidosis in Rochester Minnesota: A population-based study of incidence and survival. Am J Epidemiol. 1986;123(5):840–845. doi: 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 3.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 4.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: Data from the black women’s health study. Chest. 2011;139(1):144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gideon NM, Mannino DM. A sarcoidosis mortality in the United States, 1979–1991: An analysis of multiple-cause mortality data. Am J Med. 1996;100(4):423–427. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 6.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirsaedi M, Machado RF, Schraufnagel D, Swess NJ, Baughman RP. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147(2):438–449. doi: 10.1378/chest.14-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., III History of the Rochester Epidemiology Project: Half a century of medical records linkage in a U.S. population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Access research group. Design of a case control etiologic study of sarcoidosis (ACCESS) J Clin Epidemiol. 1999;52(12):1173–1186. doi: 10.1016/s0895-4356(99)00142-0. [DOI] [PubMed] [Google Scholar]
- 11.Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14(4):735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Resp J. 2008;31(2):372–329. doi: 10.1183/09031936.00075307. [DOI] [PubMed] [Google Scholar]
- 13.Coquart N, Cadelis G, Tressieres B, Cordel N. Epidemiology of sarcoidosis in Afro-Caribbean people: a 7-year retrospective study in Guadeloupe. Int J Dermatol. 2015;54(2):188–192. doi: 10.1111/ijd.12633. [DOI] [PubMed] [Google Scholar]
- 14.Gribbin J, Hubbard RB, Jeune IL, Smith CJP, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61(11):980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillerdal G, Nou E, Osterman K, Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Ann Rev Respir Dis. 1984;130(1):29–32. doi: 10.1164/arrd.1984.130.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Kowalska M, Niewiadomska, Zejda JE. Epidemiology of sarcoidosis recorded in 2006–2010 in the Silesian voivodeship on the basis of routine medical reporting. Ann Agric Environ Med. 2014;21(1):55–58. [PubMed] [Google Scholar]
- 17.Parkes SA, Baker SB, Bourdillon RE, et al. Incidence of sarcoidosis in the Isle of Man. Thorax. 1985;40(4):284–287. doi: 10.1136/thx.40.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazzi P, Solfanelli S, Di Pede F, et al. Sarcoidosis in Tuscany. A preliminary report. Sarcoidosis. 1992;9(2):123–126. [PubMed] [Google Scholar]
- 19.Poukkula A, Huthi E, Lilja M, et al. Incidence and clinical picture of sarcoidosis in a circumscribed geographical area. Br J Dis Chest. 1986;80(2):138–147. doi: 10.1016/0007-0971(86)90034-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29(3):365–377. doi: 10.1016/j.ccm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131(5):1414–1423. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 22.Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence and severity of sarcoidosis in New York City firefighters. Chest. 1999;116(5):1183–1193. doi: 10.1378/chest.116.5.1183. [DOI] [PubMed] [Google Scholar]
- 23.Tukey MH, Berman JS, Boggs DA, White LF, Rosenberg L, Cozier YC. Mortality among African American women with sarcoidosis: data from the Black Women’s Health Study. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(2):128–133. [PMC free article] [PubMed] [Google Scholar]