Abstract
Objective
The primary preclinical model of peripheral artery disease (PAD), which involves acute limb ischemia (ALI), can result in appreciable muscle injury that is attributed to the acuity of the ischemic injury. A less acute model of murine limb ischemia using ameroid constrictors (AC) has been developed in an attempt to mimic the chronic nature of human disease. However, there is currently little understanding of how genetics influence muscle injury following subacute arterial occlusion in the mouse.
Methods
We investigated the influence of mouse genetics on skeletal muscle tissue survival, blood flow, and vascular density by subjecting two different mouse strains, C57BL/6 (BL6) and BALB/c, to ALI or subacute limb ischemia (SLI) using single (1AC) or double (2AC) ameroid constrictor placement on the femoral artery.
Results
Similar to ALI, the 2AC model resulted in significant tissue necrosis and limb perfusion deficits in genetically susceptible BALB/c but not BL6 mice. In the 1AC model, no outward evidence of tissue necrosis was observed, and there were no differences in limb blood flow between BL6 and BALB/c. However, BALB/c mice displayed significantly greater muscle injury, as evidenced by increased inflammation and myofiber atrophy, despite having no differences in CD31+ and SMA+ vascular density and area. BALB/c mice also displayed significantly greater centralized myonuclei, indicating increased muscle regeneration.
Conclusions
The susceptibility of skeletal muscle to ischemia-induced injury is at least partly independent of muscle blood flow and vascular density, consistent with a muscle cell autonomous response that is genetically determined. Further development of preclinical models of PAD that more accurately reflect the nature of the human disease may allow more accurate identification of genetic targets for therapeutic intervention.
Introduction
Critical limb ischemia (CLI) is the most severe manifestation of peripheral artery disease (PAD) and results in appreciable morbidity and mortality as a result of rest pain and tissue necrosis or gangrene, which often necessitates limb amputation1. Surgical and percutaneous revascularization approaches are generally unsuccessful at improving limb salvage2 and investigational approaches derived from preclinical studies (e.g., pro-angiogenic and stem cell-based therapies) have also been largely ineffective3–5. Moreover, it remains unclear why some individuals develop CLI while others with essentially the same vascular anatomy and similar limb perfusion based on angiography and ankle-brachial index (ABI) develop only intermittent claudication (IC, exertional pain without tissue necrosis)6. A potentially important but often overlooked determinant of tissue survival in CLI is the skeletal muscle cellular response to ischemia7–10. Recent evidence from our group has demonstrated that the responses of isolated skeletal muscle cells to experimental ischemia vary significantly depending on their genetic background, consistent with the possibility that tissue survival in CLI is at least partly a muscle cell autonomous process11.
The most commonly used animal model of PAD involves surgical ligation and excision of a segment of the femoral artery to induce hind limb ischemia (HLI)12. This model has been used not only to understand the pathogenic mechanisms involved in limb ischemia but also to investigate potential therapies, particularly those promoting vascular growth and remodeling13–16. A criticism of this model is that it does not accurately represent the natural history of human PAD because it induces acute limb ischemia (ALI), which in humans typically results in skeletal muscle infarction depending on the presence and distribution of collateral arteries. In mice the overall tissue response to ALI is highly variable depending on the animals’ genetic background17–19. Some inbred mouse strains (e.g., BALB/c) display marked tissue necrosis in response to ALI, often resulting in limb auto-amputation which is consistent with the response expected in humans after acute arterial occlusion17. Other strains (e.g., C57BL/6 [BL6]) show little to no tissue injury and typically display near complete recovery of limb perfusion within 3–4 weeks post-operatively. The genetic regulation of limb tissue survival after ALI has been mapped to a quantitative trait locus (QTL) on mouse chromosome 7 called Lsq-1 17, although the precise gene(s) responsible for this differential response are not yet known. Furthermore, whether any of the genes in Lsq-1 regulate tissue survival in humans with CLI remains unclear.
In an attempt to develop a better model of CLI, one group has reported a modified model of hind limb ischemia in both rats and mice utilizing ameroid constrictors to induce gradual femoral artery occlusion 20, 21. Ameroid constrictors (ACs) were used as early as the 1950s to study myocardial ischemia and collateral artery development following gradual coronary artery occlusion 22, 23. These devices consist of an outer metal sleeve encasing an inner cylinder of a hygroscopic substance, usually casein. When placed around an artery, absorption of moisture from the surrounding tissues results in gradual constriction and, ultimately, complete occlusion of the vessel lumen. Compared to acute occlusion, ameroid constrictor occlusion in mice resulted in lower expression of shear stress-dependent and inflammatory genes, lower blood flow recovery 4–5 weeks post-operatively, and less muscle necrosis 21. Notably, in that study Yang et al examined effects only in C57BL/6 mice 21, which are relatively ischemia-tolerant, and the response of more ischemia-susceptible strains to gradual femoral artery occlusion remains unknown.
Here we report two modifications of the ameroid constrictor model of femoral artery occlusion to induce less severe, subacute hind limb ischemia (SLI). We demonstrate that skeletal muscle necrosis is a major component of the ischemic response in BALB/c mice, but not BL6 mice, even after SLI and in the absence of differences in vascular density; further supporting of the idea that susceptibility to ischemic skeletal myopathy is genetically determined.
Materials and Methods
Detailed information can be found in Appendix I. Supplemental Material
Animals
Experiments were conducted on adult C57/BL6 (N=51), BALB/c (N=58) mice. All mice were ≥12 weeks old and the study was approved by the Institutional Review Committees of Duke University Medical Center and/or East Carolina University.
Animal Models of Ischemic Peripheral Artery Disease
Acute hindlimb ischemia (ALI)17 was induced by ligation and resection of the femoral artery proximally inferior to the inguinal ligament and immediately proximal to the superficial caudal epigastric artery, and distally immediately proximal to the bifurcation of the popliteal and saphenous arteries. Subacute limb ischemia (SLI) was performed by placement of ameroid constrictors (ACs) on the femoral artery in one of two ways: 1) An AC was placed on both the proximal and distal femoral artery (2AC model) in a modification of the approach described previously by Yang et al 21. The proximal and distal ameroid constrictors were placed around the femoral artery in identical anatomical locations as the resections for the ALI model; and 2) A single AC was placed on the femoral artery immediately proximal to the origin of the superficial caudal epigastric artery (1AC model). To minimize the potential for acute ischemia, the inferior epigastric, lateral circumflex, and superficial epigastric artery branches were left intact in all models (ALI, 2AC, 1AC). The extent of necrosis, if any, in ischemic limbs was recorded post-operatively using the previously described semi-quantitative scale 17.
Assessment of Limb Perfusion
To quantify blood flow in both acute and subacute limb ischemia models, laser Doppler perfusion image (LDPI) scanning was performed using a Moor Instruments LDI2-High Resolution (830nM) System (Moor, Axminster, UK).
Histological Analysis
Skeletal muscle morphology, vessel density, macrophage infiltration, and markers of muscle regeneration were assessed by standard light microscopy and immunofluorescence microscopy. Vessel density was expressed as the number of CD31+ or SMA+ cells per µm2 of muscle analyzed. The size of SMA+ vessels was also determined and expressed as the percentage of SMA+ vessels per total muscle area (in µm2) analyzed. Macrophage immunostaining was quantified as the percentage of total TA muscle area Mac-1+.
Limb Muscle Morphology and Regeneration
Sections from muscle samples were stained with H&E, and digital images were obtained for the analysis of non-contractile tissue (NCT), total myofiber cross sectional area (CSA, µm2) and centralized myofiber nuclei.
Statistical Analysis
Statistical analyses were carried out using StatPlus:mac (v. 2009) statistical analysis software, Vassarstats (www.vassarstats.net) or Prism 6 (v. 6.0d). Non-parametric necrosis score data are presented as proportions and compared using Mann-Whitney U tests. Fiber size distributions were compared with the χ2 test. All other data were compared using ANOVA and Student’s 2-tailed t-test. In all cases, P< 0.05 was considered statistically significant.
Results
Marked tissue necrosis occurs in BALB/c mice after subacute limb ischemia in the double ameroid constrictor model
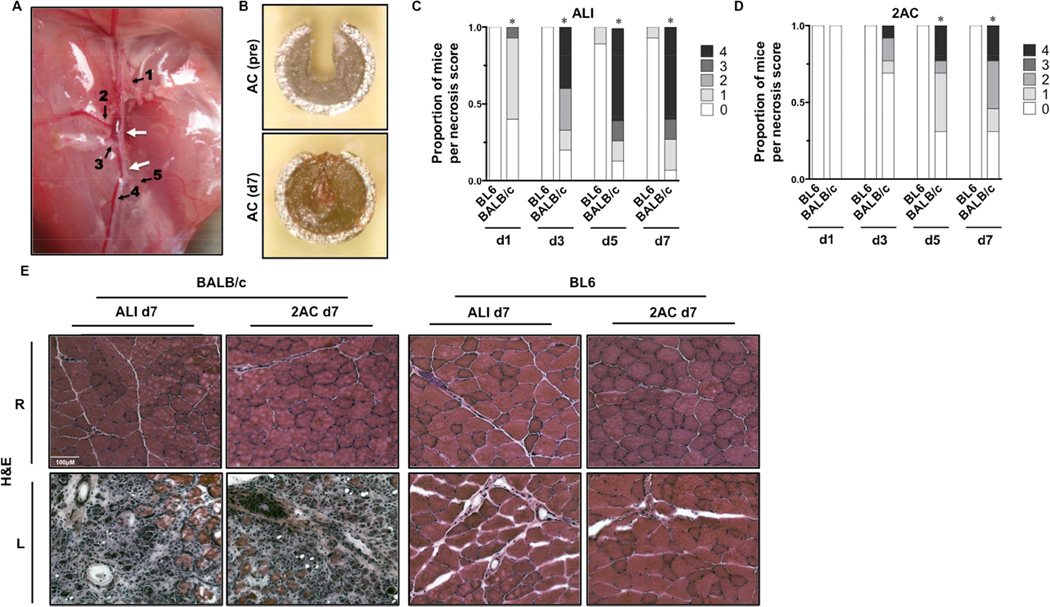
Because the genetic background of patients with PAD varies widely, it is essential to consider how genetics influences outcomes in developing any model of this disease. We compared muscle tissue responses in BL6 mice to those in ischemia-susceptible BALB/c mice after ALI induced by femoral artery ligation and excision and following SLI using a modified model of femoral artery occlusion. In our first modification of the SLI model, we placed ACs on the proximal and distal femoral artery (2AC) without any other manipulation of the collateral vessels (Figure 1A) and compared the response to ischemia between genetically ischemia resistant BL6 and ischemia susceptible BALB/c mice. Detailed surgical methodology can be found in Appendix I: Data Supplement. Mice were monitored for gross limb tissue necrosis and complete closure of the ACs was verified at sacrifice (Figure 1B). Similar to previously published results, necrosis scores in BALB/c mice after ALI were significantly worse than in BL6 mice at all time points throughout the first week post-operatively (Fig 1C) 17, 24. Strikingly, overt tissue necrosis became evident in BALB/c mice in the 2AC model by postoperative day 3, and necrosis scores were significantly worse than in BL6 mice on post-op days 5 and 7 (Fig 1D). Although BALB/c mice displayed significantly less necrosis in the 2AC model than after ALI, muscle tissue from these groups was virtually indistinguishable histologically by post-op day 7, as both models demonstrated appreciable inflammation and myofiber necrosis (Fig 1E). Consistent with previous results, BL6 mice remained resistant to skeletal myopathy regardless of the model of ischemia utilized (Fig 1 C–E).
Figure 1. Tissue pathology after subacute femoral artery occlusion with two ameroid constrictors.
Acute hindlimb ischemia (ALI) or sub-acute femoral artery occlusion using 2ACs was performed in BL6 and BALB/c mice, and animals were monitored for 7-days (ALI N=16 per strain; 2AC, N=8 BL6 – N=13 BALB/c). A, Representative image and schematic of sites of ligation/resection for ALI or 2AC placement on the femoral artery. Arrows indicate sites of constrictor placement or resection. 1 Lateral Circumflex Femoral. 2 Proximal Caudal Femoral. 3 Superficial Caudal Epigastric. 4 Saphenous. 5 Popliteal.B Ex vivo image of an AC after removal at day 7, demonstrating complete occlusion. C, Distribution of limb necrosis scores during the week of ischemia (d1, d3, d5, d7) induced by ALI in BL6 and BALB/c mice. *P<0.05 vs. day matched BL6. D, Distribution of limb necrosis scores during the week of ischemia (d1, d3, d5, d7) induced by 2AC in BL6 and BALB/c mice. *P<0.05 vs. day matched BL6. E, Representative H&E sections demonstrating TA morphology 7 days after ALI and 2AC surgery; R, right (non-surgical limb); L, left (surgical limb).
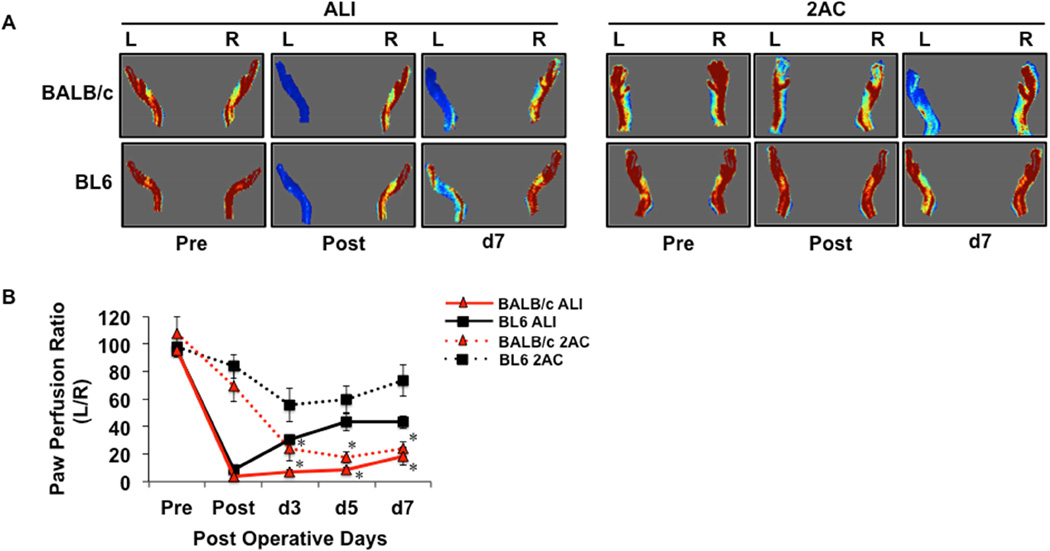
To assess effects on limb blood flow we performed LDPI analysis in BL6 and BALB/c mice in both of these models (Fig 2A). As expected, ALI resulted in an immediate postoperative loss of blood flow in both strains, while reductions in blood flow occurred gradually and to varying degrees in the 2AC model (Fig 2B). There were no strain-dependent differences in the immediate post-op limb blood flow in either model. BL6 mice demonstrated a significant perfusion recovery by post-op day 3 in the ALI model, and they showed no significant loss of blood flow in the 2AC model throughout the first week post-operatively (Fig 2B). These findings support a genetic contribution to ischemia-induced muscle necrosis, even after subacute limb ischemia. However, they do not rule out the possibility that differences in blood flow after SLI were responsible for the greater necrosis observed in BALB/c mice.
Figure 2. Comparison of limb blood flow between BALB/c and BL6 mice after either acute limb ischemia or subacute femoral artery occlusion with two ameroid constrictors.
A, Representative images from laser Doppler perfusion imaging in BALB/c and BL6 mice before and after ALI or 2AC placement up to 7 days post-operatively. B, Quantitation of paw perfusion, represented as a ratio of ischemic (L) to non-ischemic (R) limb perfusion (ALI, N=10 per strain; 2AC, N=8 per strain).. * P<0.05 vs. day and ischemic model matched BL6.
Blood flow does not differ between BL6 and BALB/c mice after subacute limb ischemia with a single ameroid constrictor
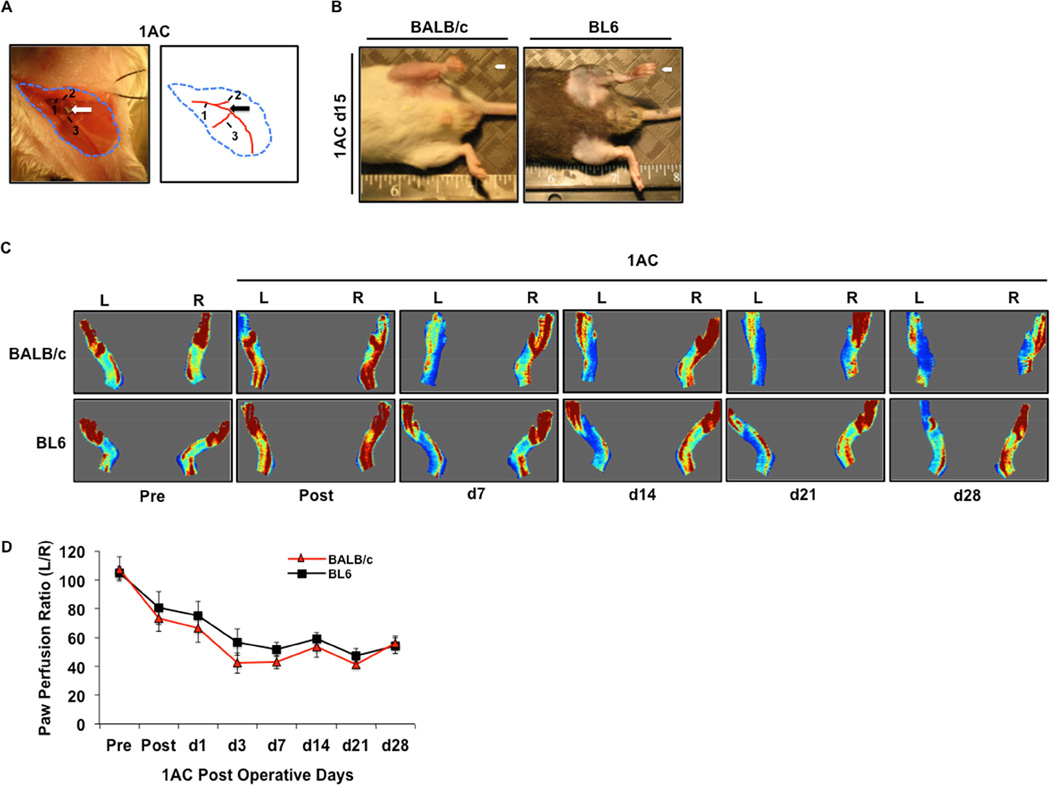
To determine the effects of an even less severe model of subacute limb ischemia, we assessed the effects of a single, proximal femoral artery constrictor (1AC model, Fig 3A) on blood flow and muscle injury. In contrast to the double constrictor model, neither BALB/c nor BL6 mice displayed any outward evidence of limb necrosis after single constrictor placement up to our designated 15- or 28-day endpoints (data not shown) (Fig 3B). Moreover, there were no differences in limb blood flow between the two strains in the 1AC model over 28 days post-operatively (Fig 3C,D).
Figure 3. Comparison of BALB/c and BL6 pathology and blood flow after further refinement of subacute femoral artery occlusion by placement a single ameroid constrictor.
A, Representative image of a BALB/c mouse after placement of a single AC (1AC) on the proximal portion of the femoral artery, immediately proximal to the epigastric arterial branch. Arrow indicates site of constrictor placement. 1 Femoral artery. 2 Lateral Circumflex Femoral. 3 Superficial caudal epigastric artery. B, Representative gross anatomy photos and photomicrographs of TA muscle from BALB/c and BL6 mice subjected to subacute femoral artery occlusion via the placement of a single AC on the proximal femoral artery (1AC) for 15-days. C, Representative images from laser Doppler perfusion imaging in BALB/c and BL6 mice before and after 1AC placement up to 28 days post-operatively. D, Quantitation of paw perfusion in BALB/c (N=29) and BL6 (N=27) mice, represented as a ratio of ischemic (L) to non-ischemic (R) limb perfusion. * P<0.05 vs. day and ischemic model matched BL6.
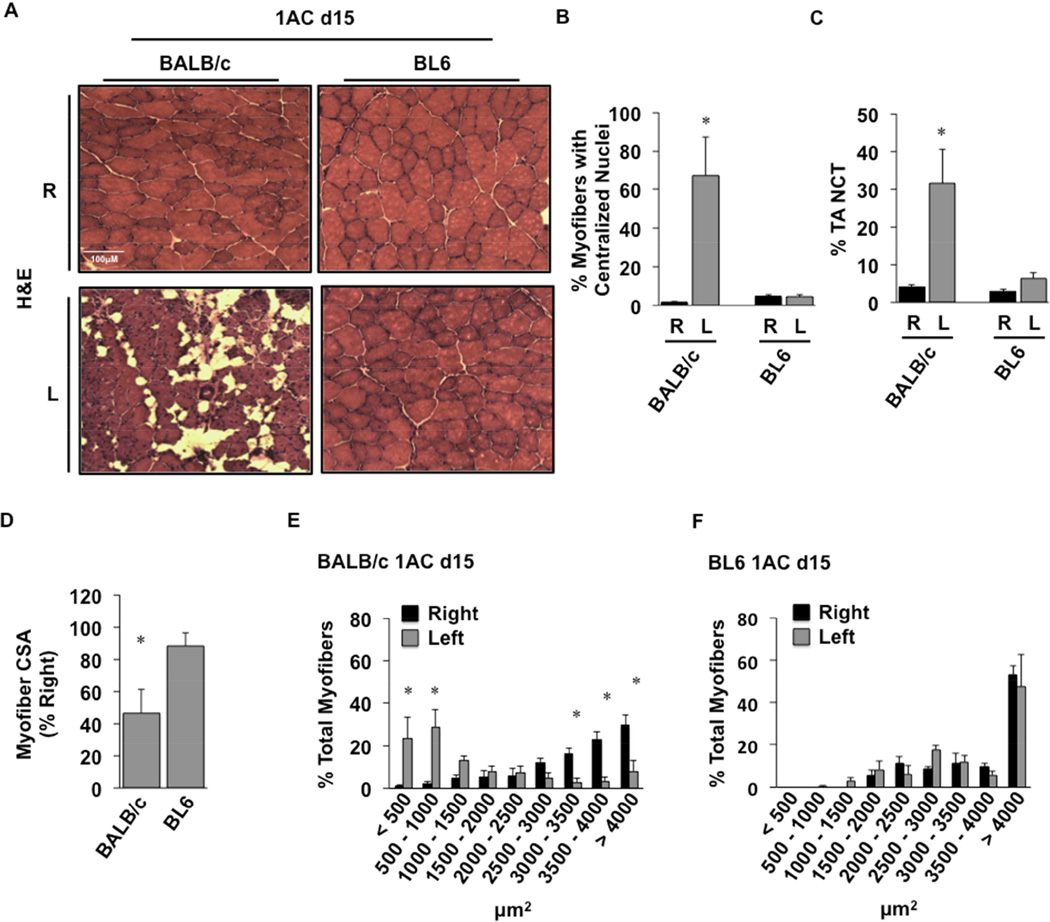
The single constrictor model of SLI causes strain-dependent inflammation and muscle injury
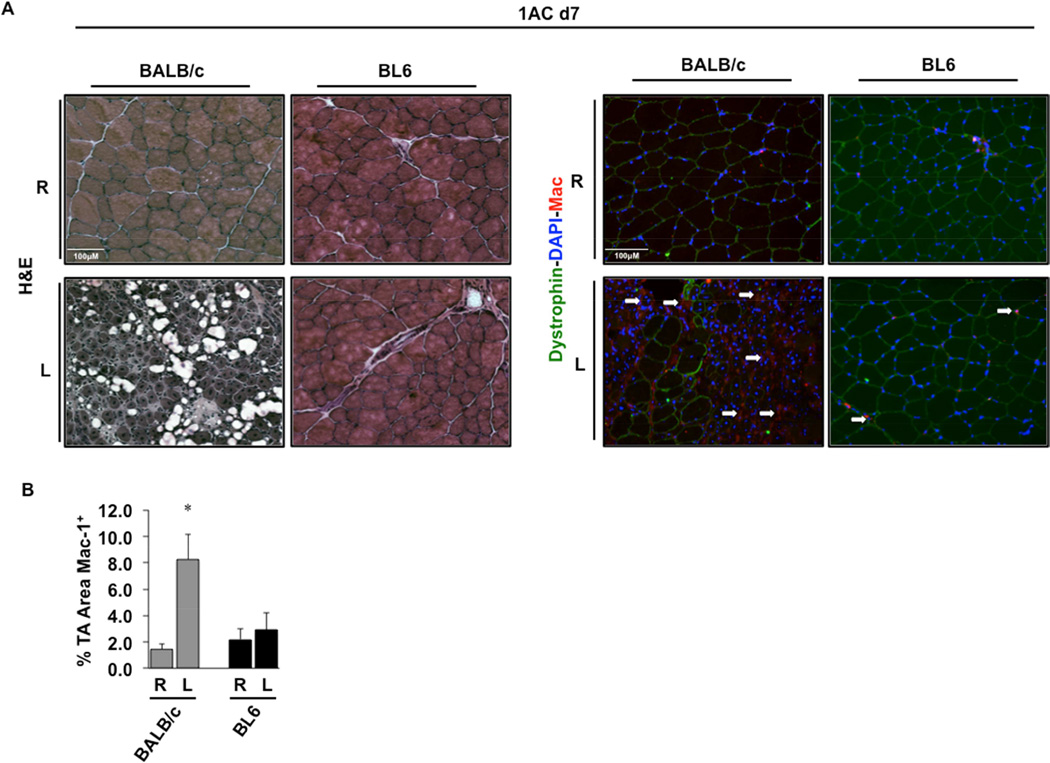
Histological analysis of TA muscles from these mice after single constrictor placement revealed fascicular architectural abnormalities at day 7 only in BALB/c mice (Fig 4A, left panel). Immunofluorescence microscopy revealed a significant (P=0.005) increase in macrophage staining of BALB/c limb muscle 7 days post-operatively (Fig 4A,B). Macrophage infiltration was unchanged in BL6 muscle, which also appeared essentially unchanged morphologically compared to control, non-ischemic muscle (Fig 4A,B). Further histological analysis of tibialis anterior (TA) muscle 15 days after single constrictor placement revealed sustained myofiber abnormalities only in BALB/c mice (Fig 5A). There were significant increases in centralized myofiber nuclei (Fig 5B; P=0.01), consistent with myofiber regeneration, and non-contractile tissue (NCT; Fig 5C; P=0.05) in muscle from BALB/c but not BL6 mice. Myofiber cross-sectional area (CSA) was also decreased in the ischemic limbs of BALB/c mice (Fig 5D; P=0.009). Similarly, myofiber size distribution was significantly shifted toward smaller myofibers (<500 µm2, P=0.0001; 500–1000 µm2, P=0.0001; 3000–3500 µm2, P=0.003; 3500–4000 µm2, P=0.00003; >4000 µm2, P=0.0001) in BALB/c (Fig 5E) but not BL6 mice (Fig 5F).
Figure 4. Skeletal muscle pathology and macrophage infiltration after 1AC-mediated subacute ischemia.
A, Representative H&E sections demonstrating TA morphology and immunostaining of macrophages (white arrows) after 7 days of 1AC subacute ischemia; R, right (non-surgical limb); L, left (surgical limb). B, Quantitation of the percentage total TA area immunostaining positive for macrophages (% TA Area Mac1+). *P<0.05 v strain specific contralateral (R) control.
Figure 5. Genetic divergence of ischemic skeletal muscle myofiber morphology after 1AC-mediated subacute ischemia.
BALB/c and BL6 mice were subjected to subacute femoral artery occlusion via the placement of a single AC on the proximal femoral artery (1AC) for 15-days. A. Representative H&E sections demonstrating TA morphology after 15 days of 1AC subacute ischemia; R, right (non-surgical limb; black bar); L, left (surgical limb; grey bar). B, Percentage TA myofibers with centralized nuclei. C, Percentage TA noncontractile tissue (NCT). Values are presented as means ± SEM. * P<0.05 v strain specific contralateral (R) control. D, Myofiber cross-sectional area (CSA) in BALB/c and BL6 TA. Mean CSA are presented as means ± SEM % change from contralateral (R) control. *P<0.05 vs. BL6. Distribution of skeletal muscle myofiber size was calculated in (E) BALB/c and (F) BL6 TA muscles. Values are presented as means ± SEM. *P<0.05 v strain specific contralateral (R) control.
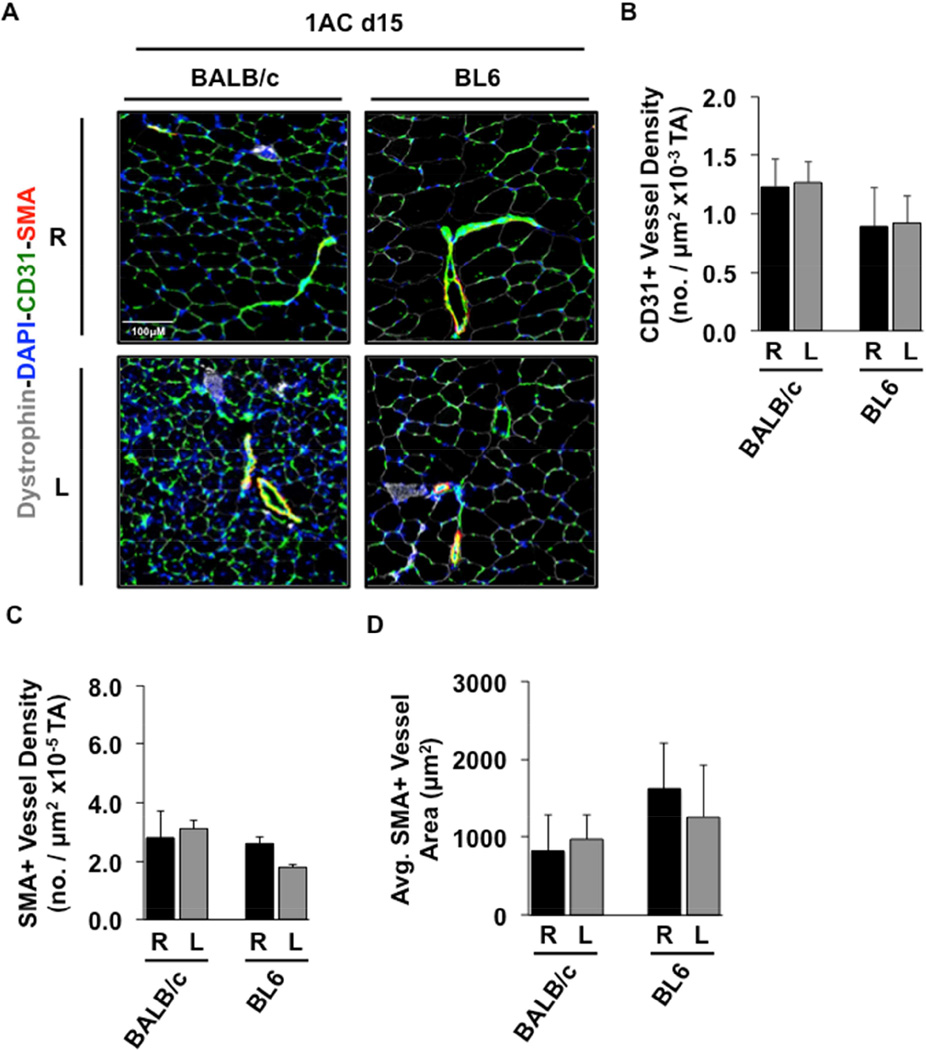
Single ameroid constrictor-induced SLI does not alter vascular density
Immunofluorescence microscopy of TA muscle was used to quantify vessel density and size (Fig 6A). CD31+ vascular density was unchanged after single constrictor-mediated SLI, regardless of genetic background (Fig 6B). There were also no significant alterations in TA muscle SMA+ vessel density (Fig 6C) or SMA+ vessel area (Fig 6D). Overall, these data demonstrate that vascular density of BALB/c muscle in the single constrictor model is no different from that of BL6 muscle, supporting the idea that a muscle-specific response to subacute limb ischemia is at least partly independent of pre-existing vascular differences and those that might develop during ischemia (e.g., collateral density).
Figure 6. Skeletal muscle vascular density after 1AC-induced subacute limb ischemia.
A, TA muscle sections from BALB/c and BL6 mice 15 days after 1AC-induced SLI were stained as indicated for the visualization of myofibers (dystrophin), capillaries/endothelial cells (CD31), intermediate vessels (SMA), and nuclei (DAPI). Representative sections are shown. B, TA CD31+ capillary vessel density. C, TA SMA+ vessel density. D, Average TA SMA+ vessel area. Values are presented as means ± SEM; R, right (non-surgical limb; black); L, left (surgical limb; grey).
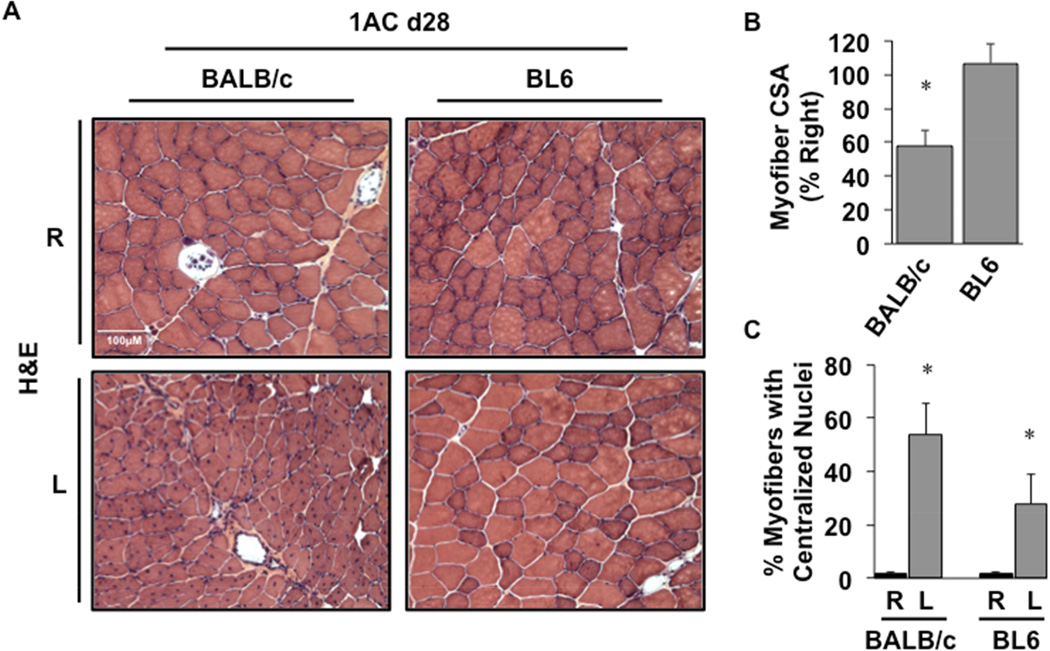
Despite similar limb blood flow between the two strains in the single constrictor model, BALB/c mice displayed sustained myofiber atrophy up to 28 days post-operatively (Fig 7A,B P=0.01), consistent with the changes observed in that strain at day 15. The presence of centralized myonuclei as a marker of muscle regeneration was also sustained up to 28 days post-operatively in BALB/c muscle (Fig 7C, P=0.004). Although centralized myonuclei were also observed in BL6 mice 28 days after single constrictor placement, there was no evidence of muscle injury or atrophy as seen in BALB/c mice. Taken together, these results are consistent with the notion that the susceptibility to develop ischemic skeletal myopathy is genetically determined and at least partly independent of blood flow and vascular density.
Figure 7. Ischemic skeletal muscle regeneration after sustained 1AC-mediated subacute ischemia.
A, Representative H&E-stained sections demonstrating TA morphology 28 days after 1AC placement. Sections of TA muscle were analyzed for mean myofiber CSA at day 28 and presented as a percentage of the right, non-surgical limb (B). *P<0.05 vs. BL6. C, The percentages of total myofibers with centralized nuclei. *P<0.05 vs. strain-specific contralateral (R) control.
All values are presented as means ± SEM.
Discussion
In this study, we found that subacute limb ischemia in mice induced by occlusion of the femoral artery with one or two ameroid constrictors results in skeletal muscle myopathy that is determined by genetic background, similar to that which has been described after acute limb ischemia 11, 17, 18. In the original description of ameroid constrictor induced limb ischemia it was found that muscle injury was diminished relative to acute ischemia, however that report examined effects only in ischemia tolerant C57BL/6 mice 21. We observed similar strain-dependent responses to SLI using two different modifications of the approach to further diminish the acuity of the ischemic insult. First, we placed two ACs on the femoral artery (one proximal and one distal) but all the major collateral branches were left intact. Strikingly, this modification was insufficient to rescue the substantial tissue necrosis seen in BALB/c mice, which has been attributed previously to the acute and profound nature of the onset of ischemia. Further modification of the surgical model to a single ameroid constrictor rescued the overt limb tissue necrosis in BALB/c mice but revealed the susceptibility of BALB/c muscle tissue to ischemic myopathy. The significantly greater muscle injury and subsequent regenerative hallmarks observed in BALB/c mice argues strongly for a genetic determinant of muscle injury in PAD. This injury appears to be independent of limb blood flow and vascular adaptation, as Doppler perfusion imaging and vascular density were not different between the two strains in this refined model. Importantly, these findings support our previous assertion that the response of skeletal muscle cells in the distal limb is a critical determinant of the overall myopathy of limb ischemia, at least in mice11.
Although it is well known that different inbred strains of mice display distinct phenotypic responses to limb ischemia, improved understanding of the mechanisms responsible for these differences has been complicated by the fact that the methods for inducing HLI are not well standardized among laboratories. For example, our laboratory and others have reported rapid tissue loss within 7 days after ALI in BALB/c mice 11, 17, 25, whereas other laboratories have reported very little tissue necrosis up to several weeks after the onset of ischemia in this same strain19. These differences may be due to subtle variations in the surgical model, which are often not detailed methodologically 26. The most common mouse model of PAD involves ligation and resection of a segment of the femoral artery, but a concern about this technique is that it more likely models a different disease entity altogether, i.e., ALI. Clinically, ALI is most commonly caused by acute arterial embolization, whereas PAD is the result of progressive atherosclerosis and arterial stenosis27, 28. Subacute occlusion of the femoral artery with ameroid constrictors holds the potential to mimic the progressive nature of PAD.
It should be noted that the original report of this model by Yang et al21 employed a hybrid technique that included an acute ischemic component, as those investigators ligated all branches of the femoral artery in addition to placing two constrictors on the artery. Our initial surgical strategy was less aggressive, as the only intervention was constrictor placement, and we left all branches of the femoral artery intact. Even with this modification the more ischemia-susceptible BALB/c mice still developed profound necrosis. The development of tissue necrosis and loss of blood flow in the double constrictor model occurred on a timeline similar to that observed for ALI, suggesting that this model might be more akin to ALI than previously anticipated and might not reflect the typical insidious course of PAD.
Although the AC model was originally described as one of “gradual” arterial occlusion, it is perhaps better described as a model of subacute ischemia, as the time to complete occlusion is not immediate but may be shorter than suspected. Among the original uses of ACs was to model chronic coronary ischemia in dogs. In those studies ACs typically had inner diameters of 3–4 mm, and one in vitro study demonstrated complete occlusion after approximately 30 days23. Assuming the rate of closure is linear, one would expect the ACs used in our study (inner diameter of 0.25 mm) to close within several days. This possibility is supported by our observation that appreciable necrosis occurred in the 2AC model within 5 days. Furthermore, our data suggests that limb perfusion reaches its lowest level by d3 post-1AC placement. Yang et al21 found that limb perfusion was identical at day 7 in their gradual and acute occlusion models. Although our approach likely provides a better model of human PAD, additional modifications might be necessary to provide an optimal model of true chronic limb ischemia, which mimics atherosclerotic lesion formation.
As might be expected, the vast majority of studies examining susceptibility to tissue necrosis in limb ischemia have focused on vascular contributions, such as angiogenesis and collateral artery growth 17–19, 29, 30. In these studies muscle survival is logically assumed to be dependent on perfusion and subsequent oxygen and nutrient delivery. Consistent with this idea, comparisons of inbred strains of mice have shown that BL6 mice have greater baseline collateral artery density and better limb perfusion after HLI than BALB/c mice, which would appear to explain the relative differences in muscle necrosis 17, 31, 32. Similar to differences in surgical model, subtle differences in LDPI methodology (supine versus prone positioning, animal temperature, and methods of anesthesia) can result in significant differences in blood flow. In the current study, we exclusively assessed blood flow in mice in the supine position after ketamine/xylazine anesthesia while maintaining body temperature. Our methods were sensitive enough to reproduce previously reported differences between BL6 and BALB/c limb blood flow with ALI and they demonstrated strain-specific differences in the double constrictor model. The lack of strain-specific differences in blood flow after single constrictor placement does not rule out a role for pre-existing collaterals in limb tissue survival, however the lack of differences in vascular density further support a muscle cell autonomous response to ischemia that is unique to the genetic background of the mouse. The distinct muscle cell responses may reflect differences in the ability of endogenous muscle progenitor cells, e.g., satellite cells and pericytes, to promote skeletal muscle regeneration or recovery from ischemia 33–36.
A critical component of the ability of muscle to regenerate after ischemic injury is the inflammatory response 37–40. Type 2 cytokine production (high IL-4) is altered in BALB/c tissues and results in delays in immune cell phagocytosis and muscle satellite cell activation 41. We previously demonstrated that macrophages infiltrate both BL6 and BALB/c muscle tissue at a similar time after acute ischemia, however they persist longer in BALB/c muscle24. Sustained ischemic degeneration of BALB/c muscle would necessitate the continued presence of inflammatory cells. In the present study, this persistent inflammation was recapitulated in the single constrictor model of subacute limb ischemia and corresponded with histological evidence of persistent muscle degeneration. BL6 muscle displayed no evidence of either degeneration or macrophage infiltration in that model despite similar limb blood flow. These findings suggest immune cell infiltration is dictated by the level of ischemic muscle injury, although we cannot rule out a genetic contribution to the inflammatory response similar to that which appears to influence muscle necrosis. In the context of ischemic myopathy, skeletal muscle cells’ ability to survive a severe ischemic insult could be a form of evolutionary adaptation to facilitate distal tissue survival until blood flow can be restored by angiogenesis and/or collateral vessel formation. Variation in genes that regulate muscle cell plasticity, including those related to inflammatory cell function or the resolution of macrophage infiltration, could lead to maladaptive muscle regenerative responses that ultimately result in a CLI phenotype.
Conclusion
The results of this study are consistent with previous reports demonstrating that the specific surgical method for inducing limb ischemia can significantly alter outcomes with respect to tissue injury. Perhaps more importantly, our results demonstrate that genetic background is a critical determinant of the overall outcome following either acute or subacute limb ischemia. It will be important in future studies to examine the myopathic effects of ischemia in other inbred strains with the goal of identifying specific genes responsible for these differences 17–19, 42. The specific combination of surgical model and inbred strain of mouse utilized here allowed us to examine the muscle morphological response to ischemia without the severity of injury observed commonly in the ALI model. Currently, all interventional therapies for PAD are aimed at restoring or improving perfusion by surgical or percutaneous approaches to circumvent obstructive atherosclerotic lesions, but they are largely ineffective long-term. By solidifying the skeletal muscle cell’s contribution to overall pathological outcomes in CLI, our results may lead to novel therapies targeted directly at this compartment, which could be used alone or in combination with reperfusion strategies to improve clinical care.
Supplementary Material
Clinical Relevance.
The transition of therapies from the bench to the clinic is highly dependent on accurate animal models of human disease. The specific combination of surgical model and inbred strains of mice utilized allowed us to examine pathological changes that mirror the range of responses seen in patients with CLI. Importantly, this facilitated our ability to solidify the skeletal muscle myopathic response as a critical genetically controlled component of the overall ischemic limb response. Our results may lead to novel therapies targeted directly at this compartment, which could be used alone or in combination with reperfusion strategies to improve clinical care.
Acknowledgement
The authors would like to thank Sarah B. Mueller for assistance with figures and statistical analysis.
Sources of Funding
This study was supported in part by a Research Fellowship Award from the Association for Academic Surgery to KWS; and by NIH grants K99/R00HL103797 to JMM and R21HL118661 and R56HL124444 to CDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No competing interest declared
References
- 1.Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12(2):142–147. [PubMed] [Google Scholar]
- 2.Taylor SM, Cull DL, Kalbaugh CA, Senter HF, Langan EM, 3rd, Carsten CG, 3rd, et al. Comparison of interventional outcomes according to preoperative indication: a single center analysis of 2,240 limb revascularizations. Journal of the American College of Surgeons. 2009;208(5):770–778. doi: 10.1016/j.jamcollsurg.2009.01.025. discussion 8–80. [DOI] [PubMed] [Google Scholar]
- 3.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359(9323):2053–208. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108(16):1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 5.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209(1):10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Matzke S, Lepantalo M. Claudication does not always precede critical leg ischemia. Vascular medicine. 2001;6(2):77–80. [PubMed] [Google Scholar]
- 7.Bhat HK, Hiatt WR, Hoppel CL, Brass EP. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation. 1999;99(6):807–812. doi: 10.1161/01.cir.99.6.807. [DOI] [PubMed] [Google Scholar]
- 8.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5(1):55–59. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 9.van Weel V, Deckers MML, Grimbergen JM, van Leuven KJM, Lardenoye JHP, Schlingemann RO, et al. Vascular Endothelial Growth Factor Overexpression in Ischemic Skeletal Muscle Enhances Myoglobin Expression In Vivo. Circ Res. 2004;95(1):58–66. doi: 10.1161/01.RES.0000133247.69803.c3. [DOI] [PubMed] [Google Scholar]
- 10.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, et al. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160(4):1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClung JM, McCord TJ, Keum S, Johnson S, Annex BH, Marchuk DA, et al. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am J Pathol. 2012;180(5):2156–2169. doi: 10.1016/j.ajpath.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. The American journal of pathology. 1998;152(6):1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 13.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Hazarika S, Xie D, Pippen AM, Kontos CD, Annex BH. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes. 2007;56(3):656–665. doi: 10.2337/db06-0999. [DOI] [PubMed] [Google Scholar]
- 15.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation. 1999;99(24):3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 16.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. The American journal of pathology. 1999;154(2):355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 2008;117(9):1207–1215. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiological genomics. 2007;30(2):179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 19.Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiological genomics. 2010;42(3):469–479. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. Journal of Vascular Surgery. 2005;41(2):312–320. doi: 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Tang G, Yan J, Park B, Hoffman A, Tie G, et al. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. Journal of Vascular Surgery. 2008;48(6):1546–1558. doi: 10.1016/j.jvs.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvak J, Siderides LE, Vineberg AM. The experimental production of coronary artery insufficiency and occlusion. Am Heart J. 1957;53(4):505–518. doi: 10.1016/0002-8703(57)90359-9. [DOI] [PubMed] [Google Scholar]
- 23.Bredee JJ. An improved ameroid constrictor. Preliminary communication. J Surg Res. 1969;9(2):107–112. doi: 10.1016/0022-4804(69)90039-0. [DOI] [PubMed] [Google Scholar]
- 24.McClung JM, McCord TJ, Keum S, Johnson S, Annex BH, Marchuk DA, et al. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. The American journal of pathology. 2012;180(5):2156–2169. doi: 10.1016/j.ajpath.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keum S, Marchuk DA. A locus mapping to mouse chromosome 7 determines infarct volume in a mouse model of ischemic stroke. Circulation Cardiovascular genetics. 2009;2(6):591–598. doi: 10.1161/CIRCGENETICS.109.883231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellingman AA, Bastiaansen AJ, de Vries MR, Seghers L, Lijkwan MA, Lowik CW, et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg. 2010;40(6):796–803. doi: 10.1016/j.ejvs.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366(23):2198–2206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 28.Bartholomew JR, Olin JW. Pathophysiology of peripheral arterial disease and risk factors for its development. Cleveland Clinic journal of medicine. 2006;73(Suppl 4):S8–S14. doi: 10.3949/ccjm.73.suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Zhang H, Dai X, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circulation research. 2010;107(4):558–568. doi: 10.1161/CIRCRESAHA.110.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sealock R, Zhang H, Lucitti JL, Moore SM, Faber JE. Congenic Fine-Mapping Identifies a Major Causal Locus for Variation in the Native Collateral Circulation and Ischemic Injury in Brain and Lower Extremity. Circulation Research. 2013 doi: 10.1161/CIRCRESAHA.114.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(3):520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 32.Sealock R, Zhang H, Lucitti JL, Moore SM, Faber JE. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circ Res. 2014;114(4):660–671. doi: 10.1161/CIRCRESAHA.114.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nature reviews Molecular cell biology. 2012;13(2):127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- 34.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiological reviews. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 35.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 36.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 37.Sachdev U, Cui X, Xu J, Xu J, Tzeng E. MyD88 and TRIF mediate divergent inflammatory and regenerative responses to skeletal muscle ischemia. Physiol Rep. 2014;2(5) doi: 10.14814/phy2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEnaney RM, Shukla A, Madigan MC, Sachdev U, Tzeng E. P2Y nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.06.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachdev U, Cui X, McEnaney R, Wang T, Benabou K, Tzeng E. TLR2 and TLR4 mediate differential responses to limb ischemia through MyD88-dependent and independent pathways. PloS one. 2012;7(11):e50654. doi: 10.1371/journal.pone.0050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachdev U, Cui X, Hong G, Namkoong S, Karlsson JM, Baty CJ, et al. High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury. J Vasc Surg. 2012;55(1):180–191. doi: 10.1016/j.jvs.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagrota-Candido J, Canella I, Pinheiro DF, Santos-Silva LP, Ferreira RS, Guimaraes-Joca FJ, et al. Characteristic pattern of skeletal muscle remodelling in different mouse strains. International journal of experimental pathology. 2010;91(6):522–529. doi: 10.1111/j.1365-2613.2010.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Zhang H, Wiltshire T, Sealock R, Faber JE. Genetic dissection of the Canq1 locus governing variation in extent of the collateral circulation. PloS one. 2012;7(3):e31910. doi: 10.1371/journal.pone.0031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.