Abstract
Provided that care is taken in adjusting the WATERGATE element of a 1H-15N TROSY-HSQC experiment, such that neither the water magnetization nor the 1Hα protons are inverted by its final 180° pulse, 3JHNHα couplings can be measured directly from splittings in the 1H dimension of the spectrum. With band-selective 1H decoupling, very high 15N resolution can be achieved. A complete set of 3JHNHα values, ranging from 3.4 to 10.1 Hz was measured for the 56-residue third domain of IgG-binding protein G (GB3). Using the H-N-Cα-Hα dihedral angles extracted from a RDC-refined structure of GB3, 3JHNHα values predicted by a previously parameterized Karplus equation agree to within a root-mean-square deviation (rmsd) of 0.37 Hz with the experimental data. Values measured for the Alzheimer's implicated Aβ1-40 peptide fit to within an rmsd of 0.45 Hz to random coil 3JHNHα values.
Keywords: IDP, Karplus curve, random coil, protein NMR
3JHNHα values have long been recognized as important parameters for defining the backbone torsion angles, ϕ, in peptides and proteins (Bystrov 1976)(Pardi et al. 1984). Parameterization of best-fitting Karplus curves typically has been carried out using 3JHNHα couplings measured for small proteins and dihedral angles taken from the corresponding high resolution X-ray structures, yielding root-mean-square deviations (rmsd's) in the 0.8–1.0 Hz range (Pardi et al. 1984)(Ludvigsen et al. 1991)(Vuister and Bax 1993). Considering that in such X-ray structures, the electron density of the hydrogens is often ill defined, these atoms are typically model-built into the structure, assuming idealized geometry. Alternatively, the H-N-Cα-Hα dihedral angles needed as input to the Karplus equation are directly calculated from ϕi − 60°, where ϕi is the backbone torsion angle of residue i, defined by the backbone atoms C′i−1, Ni, Cαi, and C′i. The residual rmsd in the fit was initially believed to result from difficulties in measuring 3JHNHα at an accuracy better than about 0.8 Hz, but a large number of conceptually rather different methods, ranging from addition and subtraction of in-phase and anti-phase 1HN-{1Hα} doublets (Ludvigsen et al. 1991), E.COSY measurements (Griesinger et al. 1987)(Wang and Bax 1996)(Montelione and Wagner 1989), quantitative J correlation methods (Vuister and Bax 1993)(Lohr et al. 1999), fitting of JHH-modulated 2D HSQC and HMQC spectra (Billeter et al. 1992)(Kuboniwa et al. 1994), to multiple quantum methods (Rexroth et al. 1995), were unable to go significantly below this 0.8 Hz threshold. Only when using the actual H-N-Cα-Hα dihedral angles, with 1H positions determined by measurement of residual dipolar couplings (RDCs), as input to the Karplus equation, were rmsd's well below 0.5 Hz obtained (Vogeli et al. 2007)(Maltsev et al. 2014). This result indicates that the earlier, higher rmsd values were dominated by deviations from idealized geometries, in particular the assumption of the amide hydrogen being located in the peptide plane.
Here we demonstrate that for small or intrinsically disordered proteins 3JHNHα can be measured conveniently and at high accuracy from a 2D 1H-15N TROSY-HSQC spectrum (Pervushin et al. 1997)(Pervushin et al. 1998)(Schulte-Herbruggen and Sorensen 2000). However, as discussed below, care must be taken in adjustment of the pulses that normally have a “water flip-back” function, such that they also invert the 1Hα resonances. Appropriate choice of the flip back pulses will suppress 3JHNHα-modulation during the last phase of the magnetization transfer from 15N to 1H, preventing an antiphase contribution to the 1HN-{1Hα} doublet line shape. When 1H-15N TROSY-HSQC spectra are recorded on protonated proteins, unresolvable 2JHαN, 3JHαN, and 3JHβN splittings can limit the attainable resolution in small or intrinsically disordered protein, and it then is useful to remove such splittings by insertion of a band-selective decoupling pulse at the mid-point of the 15N evolution period.
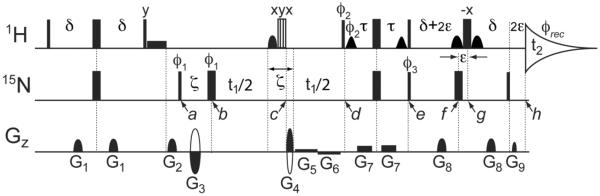
The pulse scheme of the water-flip-back 1H-15N TROSY-HSQC experiment is shown in Figure 1 and it is essentially the same as the original 1H-15N TROSY-HSQC experiment (Pervushin et al. 1998)(Schulte-Herbruggen and Sorensen 2000), except for the decoupling pulse applied to the aliphatic protons at time point c. Note that this decoupling pulse is implemented as a combination of an HN-selective 180° pulse, using an IBURP2 shape (Geen and Freeman 1991), followed by a non-selective offset-compensated composite 180° pulse (Levitt and Freeman 1981). Together, this pulse pair inverts all 1H except for 1HN, yielding a convenient and effective method for decoupling aliphatic protons during 15N evolution (Bruschweiler et al. 1988). In order to permit 15N coherence encoding without introducing the requirement of a large frequency-dependent phase correction in the 15N dimension, a short gradient encoding period (using gradients G3 and G4, separated by a 15N 180° pulse at time point b is inserted in the standard manner (Kay et al. 1992). To avoid the need for a frequency-dependent phase correction in the 1H dimension, the last pair of 1H/15N 180° pulses are slightly offset relative to one another: By applying the last non-selective 1H 180° pulse (time point g) a short time, ε≈150 μs, after the 15N 180° pulse (time point f), full 1HN chemical shift refocusing after the last simultaneous pair of 1H/15N 90° pulses (time point e) is delayed by 2ε, whereas 1JNH rephasing is active for the regular duration, 2δ = 1/(21JNH) ≈5.3 ms. This permits insertion of the last pulsed field gradient decoding pulse after the last 15N 90° pulse, before 1HN chemical shifts are refocused at time point h, which completes the ST2-PT polarization transfer (Pervushin et al. 1998). Water suppression is very effective with this pulse scheme, also on cryogenic probeheads, due to the combined effects of water-flip-back (Grzesiek and Bax 1993), the WATERGATE element (Piotto et al. 1992b), and coherence selection (Kay et al. 1992).
Fig. 1.
Pulse scheme of the water-flip-back 1H-15N TROSY-HSQC experiment. The narrow and wide filled bars represent the hard 90° and 180° pulses, while the vertically hatched open bar is a 90x210y90x composite 180° 1H pulse. The filled rectangular box before the G2 gradient pulse is a 1- ms rectangular water-flipback pulse that returns the solvent magnetization to +z after the INEPT transfer. The shaped pulse applied for the band selective decoupling in the middle of t1 has an IBURP2 (Geen and Freeman) shape (1.1 ms duration at 800 MHz), is applied to HN and centered 3.6 ppm downfield from the H2O resonance. Two water-flipback pulses (center lobe of a sinc profile, 1.0 ms) are applied between time points d and e to ensure the water magnetization returns to +z at time point e. At time point g, two sine-bell shaped pulses (600 μs) flanking the non-selective 180° 1H pulse are used to keep the water magnetization at +z, and to refocus 3JHNHα evolution during the 2δ+4ε delay. All pulses are applied along x unless otherwise indicated. Durations of all shaped pulses are for a 1H frequency of 800 MHz and should be scaled inversely relative to this frequency if applied at higher or lower magnetic fields. Delay durations: δ = 2.65 ms,; ε = 150 μs; ζ = 2081 μs; τ ≈ 2.1 ms (shorter than 1/(41JNH) to minimize the 15N anti-TROSY component (Schulte-Herbruggen and Sorensen 2000)). Phase cycling: ϕ1 = y, x, −y, −x; ϕ2 = y; ϕ3 = y; ϕrec = y, −x, −y, x. To obtain the second FID for the echo-antiecho quadrature detection, the following phase cycling is used together with inversion of the G3 and G4 gradient pulses: ϕ1 = y, −x, −y, x; ϕ2 = −y; ϕ3 = −y. These phase parameters pertain to Bruker spectrometers; for Varian/Agilent spectrometers y and −y should be interchanged. Gradients are sine-bell or rectangular shaped, as marked in the figure, with durations: G1,2,3,4,5,6,7,8,9 = 0.977, 1.2, 1.1, 0.9, t1/4, t1/4, 1.086, 0.977, 0.202 ms, and strengths of 21.7, 28.7, −41.3, 41.3, 0.91, −0.91, 2.1, 25.9, 41.3 G/cm.
3JHNHα dephasing during the initial INEPT magnetization transfer from 1HN to 15N (prior to time point a) and during the first half of the final ST2-PT element, between time points d and e, only results in very slight attenuation of the final signal intensity at time point h (by a factor of ca. cos[2π 3JHNHα τ]{1+ cos[2π 3JHNHα τ]}/2) and creation of multiple quantum 1HN-1Hα coherences that will remain invisible during detection. This dephasing prior to time point e therefore may be safely ignored. However, 3JHNHα dephasing between time points e and h will remain intact if only the non-selective 1H 180° pulse were applied at time point g. Refocusing of the 3JHNHα dephasing at time point h is achieved if only the 1HN is inverted by the 180° pulse at time point g. In practice, this can be accomplished by again using the combination of a non-selective 180° pulse, followed or preceded by a selective 1Hα inversion pulse, which forms the basis for band-selective homonuclear (BASH) decoupling (Bruschweiler et al. 1988)(Ying et al. 2014). In Pervushin's ST2-PT implementation of the TROSY experiment, this last non-selective 1H 180° pulse is already surrounded by two water-selective 90° flip-back pulses, ensuring that the water magnetization is minimally impacted by this combination of 1H pulses, essentially constituting a WATERGATE element when surrounded by the standard pair of pulsed field gradients (Piotto et al. 1992a). 1Hα spins that resonate close to the H2O resonance therefore are also minimally affected by the soft/hard pulse combination, centered at time point g.
The width of the 1Hα region not affected by the soft/hard pulse combination is remarkably wide, due to the offset-compensating effect of this symmetric 90°x, 180°−x, 90°x composite pulse (Levitt and Freeman 1979; Shaka and Freeman 1983). For example, Figure 2 compares the inversion profile for the case where the non-selective 180°−x pulse is applied just prior to two back-to-back sine-bell-shaped 600-μs 90°x pulses with the profile obtained when the 180°−x is applied in between these two pulses, as in the TROSY scheme of Figure 1. As can be seen, the non-inversion profile of the 90°x, 180°−x, 90°x pulse combination has a favorable shape, leaving z magnetization above 85% (i.e., inverting less than ~7.5% of the Hα spins) for offsets up to ±640 Hz, and >90% inversion of z magnetization (i.e., inverting >95% of the 1H spins) at offsets ≥2100 Hz. These bandwidths are inversely proportional to the duration of the shaped 90°x pulses. Pulse shapes different from a sine bell, e.g. Gaussian shapes, can be used to fine tune the inversion profile. Even a single sine-bell shaped soft 180°−x pulse, with a non-selective 1H 180° pulse superimposed at its mid-point gives a desirable inversion profile (red line in Figure 2). In practice, we use the two sine-bell shaped 90°x pulses separated by the non-selective 180° pulse as a compromise between an optimal inversion profile and a minimal total duration of this pulse combination. In principle, a REBURP 180° 1HN pulse (Geen and Freeman 1991) could be used instead of the 90°x, 180°−x, 90°x pulse combination, but in practice we find the use of such a pulse less effective at optimizing suppression of the H2O signal.
Fig. 2.
Inversion profile, Mz/Mo, as a function of resonance offset for different hard-soft pulse combinations, all using a 20 μs hard 180° pulse. Black line: 90°x, 180°−x, 90°x, where the 90°x pulses are sine-bell shaped with a duration of 1.5 ms each. Blue: 90°x, 180°−x, 90°x, where the 90°x pulses are sine-bell shaped with a duration of 0.6 ms each. Red; 90°x, 180°−x, 90°x, where the first and second 90°x pulse have the shape of the left half and of the right half of a sine-bell, respectively, and a duration of 0.6 ms each. For comparison, the green line corresponds to the inversion profile of a 90°x, 90°x, 180°−x combination, with 0.6-ms sine-bell shaped 90°x pulses.
In the absence of selective 1Hα inversion, the phase of the two 1HN-{1Hα} doublet components at time point h in Figure 1 is given by ±2π 3JHNHα(δ+2ε). Although relatively small (ca ±k° for a 3JHNHα coupling of k Hz and 2δ+4ε = 5.9 ms), such an antiphase dispersive contribution, which is not easily visible to the eye, will increase the apparent splitting, as measured from the maximum height position of each doublet component, by ca k×LW/90, where LW is the average line width of each doublet component (Fig. 3). So, the error introduced in the measured 3JHNHα coupling when J-modulation is not suppressed is approximately proportional to both the size of the 3JHNHα coupling and to the 1HN line width. For a typical 3JHNHα coupling of 7.5 Hz and a digitally enhanced line width of 3 Hz, this therefore would result in an overestimate of the measured coupling by ca 0.25 Hz. However, as described above, this contribution is easily suppressed by adjusting the duration of the shaped 90°x 1H pulses such that the 1Hα spins are not inverted. Note that the small error introduced by J modulation is distinct from the apparent decrease caused by the finite lifetime of the 1Hα spin states, i.e., by the dependence on their selective inversion recovery rates (Harbison 1993). This latter effect can become quite significant in larger proteins, where zero frequency spectral density and therefore 1H-1H spin flip rates are high. It then needs to be accounted for either computationally (Vuister and Bax 1993)(Ball et al. 2013), or by altering the pulse scheme (Rexroth et al. 1995). For the smaller systems to which the TROSY-HSQC 3JHNHα measurement is applicable, such as GB3, the effect tends to be minor, resulting in a small systematic difference of only ~0.12 Hz relative to the values measured previously (Vogeli et al. 2007) with a multi-quantum pulse scheme that was designed to suppress the effect.
Fig. 3.

Illustration of the effect of 3JHH phase modulation prior to the start of 1H data acquisition on the apparent splitting. Simulated data are shown for a doublet with 3JHH = 6 Hz, marked by the dashed lines. The bottom doublet corresponds to the absence of phase modulation, with vertically displaced doublets having phase errors of ±10, ±20, and ±30°. The apparent splitting, as measured from the maxima of the two components, increases approximately linearly with the phase error. Note that the phase error introduced by the ca 5.5-ms 3JHH modulation during the final half of the ST2-PT transfer for a 6 Hz 3JHH coupling is only ±6°, but is responsible for the differences in apparent 3JHH shown in Fig. 5b.
Figure 4 shows three small regions of the 1H-15N TROSY-HSQC spectra of GB3, the Aβ1-40 peptide, and α-synuclein. For the latter two, the 1Hα spins all resonate upfield of the 1H2O water resonance and the 1H carrier is shifted upfield by 0.3 ppm during application of the final 90°x, 180°−x, 90°x pulse combination, such as to shift the band where 1Hα spins are not inverted upfield relative to the H2O resonance. For GB3 at 293 K, where H2O resonates approximately at the center of the Hα region, no such frequency shift was used. As can be seen from the spectra, well resolved doublets are readily obtained for these proteins, whose 1HN line widths are significantly reduced by the TROSY effect (ca 1.5 to 2-fold at 800 MHz 1H frequency) over regular HSQC spectra, i.e. by relaxation interference between the 1HN chemical shift anisotropy (CSA) and the 15N-1H dipolar relaxation mechanisms.
Fig. 4.
Expanded small regions of the 800 MHz 1H-15N TROSY-HSQC spectra recorded for samples of (a) 1.2 mM GB3 in 100 mM NaCl, 25 mM sodium phosphate, pH 6.4, 293 K and (b) 150 μM Aβ1-40 in 20 mM sodium phosphate, pH 7.0, 277 K and (c) 300 μM α-synuclein in 25 mM sodium phosphate, pH 6.0, 288 K. Spectra were recorded with acquisition times of 284 ms (t2) and 128 ms (t1) for Aβ1-40; 393 ms (t2) and 74 ms (t1) for GB3; and 393 ms (t2) and 1016 ms (t1) for α-synuclein, all using 0.6-ms sine-bell shaped pulses surrounding the last non-selective 180° pulse. For Aβ1-40 and α-synuclein the carrier frequency was moved upfield by 0.3 ppm relative to the H2O resonance position only during application of the last 1H 90°x, 180°−x, 90°x pulse combination, and for the remainder of the pulse sequences the 1H carrier was positioned on the H2O resonance. Spectra were resolution-enhanced in the 1H dimension using a Lorentzian-to-Gaussian enhancement function, and processed and peak-picked with the NMRPipe software package (Delaglio et al. 1995).
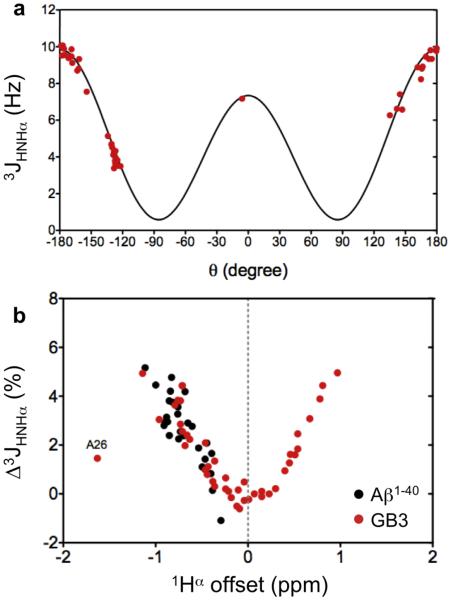
With an rmsd of only 0.37 Hz, 3JHNHα couplings measured for GB3 from the TROSY-HSQC spectrum (Supplementary Table S1) agree very well with the previously parameterized Karplus equation (Vogeli et al. 2007) when using a recent RDC-refined structure of this protein (PDB entry 2N7J) to derive the dihedral angles, confirming the accuracy of our 3JHNHα measurements (Fig. 5a).
Fig. 5.
Measurement of 3JHNHα couplings from the HSQC-TROSY spectra of Figure 4. (a) Plot of the GB3 3JHNHα values (Table S1) as a function of the H-N-Cα-Hα dihedral angle, θ, derived from a RDC-refined structure (PDB entry 2N7J), using the Karplus equation parameterization 3JHNHα = 7.97×cos2θ – 1.26×cosθ + 0.63 (Vogeli et al. 2007). Mobile residues L12 and D40 were not included in the fit. (b) Fractional difference Δ3JHNHα = [3JHNHα(1.5 ms) - 3JHNHα(0.6 ms)]/3JHNHα(0.6 ms), where 3JHNHα(1.5 ms) and 3JHNHα(0.6 ms) are the values measured using 1.5-ms and 0.6-ms duration 90° water-flip back pulses around the last 1H 180° pulse. Δ3JHNHα values are plotted as a function of the 1Hα chemical shift offset relative to the frequency at which the radiofrequency carrier is positioned during the final 90°x, 180°−x, 90°x pulse combination. Δ3JHNHα values measured for Aβ1-40 are in black, GB3 values in red. Note that for the far upfield shifted GB3 A26 resonance, neither measurement suppresses 3JHNHα modulation, and both yield a slight over-estimate of the true coupling, and therefore a small Δ3JHNHα.
As mentioned above, the error in the apparent 3JHNHα splitting that exists when J modulation is not suppressed is proportional to the size of 3JHNHα. Figure 5b shows the fractional difference (as a percentage of the actually measured 3JHNHα coupling) between values measured from two 1H-15N TROSY-HSQC spectra: one using 1.5 ms 90° shaped sine bell pulses and one using 0.6-ms pulses. The first measurement corresponds to 50% inversion of the 1Hα spins at resonance offsets of ~±505 Hz relative to the carrier, and nearly complete suppression of J modulation over a ~±260 Hz bandwidth. For the measurement with 0.6-ms pulses, these bandwidths are 2.5 times larger. As can be seen in Figure 5b, when the J modulation is fully suppressed in both measurements, i.e., at small offsets, the fractional difference between the two sets is essentially zero. However, at ±810 Hz the fractional difference reaches a maximum, before again decreasing for large offsets (e.g. residue A26 at 3.19 ppm) where neither measurement was effective at suppressing J modulation.
Measurements of 3JHNHα couplings are quite useful in the analysis of residual structure in IDPs, where they represent a particularly unambiguous reporter on the time- or ensemble-average of the ϕ angles.
We note that 1H-15N TROSY-HSQC spectra that are free of 3JHNHα phase modulation errors can also be generated with the powerful BEST-TROSY pulse scheme, which then additionally yields sensitivity enhancement over the scheme of Figure 1 (Favier and Brutscher 2011; Solyom et al. 2013). However, addition of aliphatic 1H decoupling during 15N evolution (Fig. 1) is somewhat incompatible with the spirit of the BEST type experiments, which aim to avoid inversion of the aliphatic protons. Achieving the highest possible 15N resolution is often paramount, as illustrated for the most crowded region of the α-synuclein spectrum (Fig. 4c), and we therefore opted to modify the standard TROSY-HSQC pulse sequence instead. Note that, as applies for most experiments for 3JHNHα measurement, hydrogen exchange with water causes line broadening in the 1HN dimension (and for TROSY type experiments such as the present one also in the 15N dimension) by an amount kex/π Hz, which can become a factor limiting the smallest couplings that can be measured.
A number of computational studies (Sgourakis et al. 2011)(Ball et al. 2011; Ball et al. 2013; Ball et al. 2014)(Rosenman et al. 2013) strongly rely on a set of relatively incomplete 3JHNHα values measured for Aβ1-40 and Aβ1-42 by various groups (Hou et al. 2004)(Yan et al. 2008)(Sgourakis et al. 2007)(Waelti et al. 2015). Here, we have demonstrated that these couplings can be measured at very high precision and good accuracy in such systems, at a very high level of completeness. The only missing values are for the N-terminal residue and two His residues, which exhibit rapid exchange and concomitant broadening of their resonances, and the Gly residues, for which the 3JHNHα2 and 3JHNHα3 couplings give rise to triplet splitting patterns from which only the sum of their two 3JHNHα couplings can be measured. Although, with a Pearson's correlation coefficient of RP = 0.8, the values reported here for Aβ1-40 (Supplementary Table S2) correlate well with random coil 3JHNHα values (Maltsev et al. 2012) derived for IDP α-synuclein (Fig. 6), significant deviations are also seen. For example, values for V12 (6.78 Hz) and V24 (6.82 Hz) fall well below the 7.6-Hz random coil value of this β-branched residue, whereas V18 (8.30 Hz) and V39 (7.94 Hz) show significantly elevated values. Analysis of these couplings, in concert with other types of J couplings, chemical shifts, and NOEs, is currently on-going in our group in an effort to obtain quantitative, residue-specific ϕ/ψ distributions using the recently introduced MERA program (Mantsyzov et al. 2015).
Fig. 6.
Plot of 3JHNHα values measured for Aβ1-40 against “random coil” values, derived from the residue-specific averages observed for IDP α-synuclein (Maltsev et al. 2012). The Aβ1-40 3JHNHα couplings are reported in SI Table S2. The rmsd between observed and random coil 3JHNHα couplings equals 0.45 Hz, and the Pearson's correlation coefficient, RP is 0.8.
Supplementary Material
Acknowledgment
We thank Dennis A. Torchia for useful discussions, and Jung Ho Lee for preparing the sample used for Fig. 4c. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and the Intramural Antiviral Target Program of the Office of the Director, NIH, and by the Max Planck Society.
References
- Ball KA, Phillips AH, Nerenberg PS, Fawzi NL, Wemmer DE, Head-Gordon T. Homogeneous and Heterogeneous Tertiary Structure Ensembles of Amyloid-beta Peptides. Biochemistry. 2011;50:7612–7628. doi: 10.1021/bi200732x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KA, Phillips AH, Wemmer DE, Head-Gordon T. Differences in beta-strand Populations of Monomeric A beta 40 and A beta 42. Biophys J. 2013;104:2714–2724. doi: 10.1016/j.bpj.2013.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KA, Wemmer DE, Head-Gordon T. Comparison of Structure Determination Methods for Intrinsically Disordered Amyloid-beta Peptides. J Phys Chem B. 2014;118:6405–6416. doi: 10.1021/jp410275y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M, Neri D, Otting G, Qian YQ, Wuthrich K. Precise Vicinal Coupling-Constants 3J(HN-HA) in Proteins From Nonlinear Fits of J-Modulated [15N,1H]-COSY Experiments. J Biomol NMR. 1992;2:257–274. doi: 10.1007/BF01875320. [DOI] [PubMed] [Google Scholar]
- Bruschweiler R, Griesinger C, Sørensen OW, Ernst RR. Combined use of hard and soft pulses for omega-1 decoupling in two-dimensional NMR spectroscopy. J Magn Reson. 1988;78:178–185. [Google Scholar]
- Bystrov VF. Spin-spin couplings and the conformational states of peptide systems. Prog NMR Spectrosc. 1976;10:41–81. [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, feifer J, Bax A. NMRpipe - a multidimensional spectral processing system based on Unix pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Favier A, Brutscher B. Recovering lost magnetization: polarization enhancement in biomolecular NMR. J Biomol NMR. 2011;49:9–15. doi: 10.1007/s10858-010-9461-5. [DOI] [PubMed] [Google Scholar]
- Geen H, Freeman R. Band-selective radiofrequency pulses. J Magn Reson. 1991;93:93–141. [Google Scholar]
- Griesinger C, Sørensen OW, Ernst RR. Practical aspects of the E.COSY technique. Measurement of scalar spin-spin coupling constants in peptides. J Magn Reson. 1987;75:474–492. [Google Scholar]
- Grzesiek S, Bax A. The Importance of Not Saturating H2O in Protein NMR. Application to Sensitivity Enhancement and NOE Measurement. J Am Chem Soc. 1993;115:12593–12593. [Google Scholar]
- Harbison GS. Interference Between J-Couplings and Cross-Relaxation in Solution NMR-Spectroscopy - Consequences For Macromolecular Structure Determination. J Am Chem Soc. 1993;115:3026–3027. [Google Scholar]
- Hou LM, Shao HY, Zhang YB, Li H, Menon NK, Neuhaus EB, Brewer JM, Byeon IJL, Ray DG, Vitek MP, Iwashita T, Makula RA, Przybyla AB, Zagorski MG. Solution NMR studies of the A beta(1-40) and A beta(1-42) peptides establish that the met35 oxidation state affects the mechanism of amyloid formation. J Am Chem Soc. 2004;126:1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- Kay LE, Keifer P, Saarinen T. Pure Absorption Gradient Enhanced Heteronuclear Single Quantum Correlation Spectroscopy with Improved Sensitivity. J Am Chem Soc. 1992;114:10663–10665. [Google Scholar]
- Kuboniwa H, Grzesiek S, Delaglio F, Bax A. Measurement of HN-Hα J couplings in calcium-free calmodulin using new 2D and 3D water-flip-back methods. J Biomol NMR. 1994;4:871–878. doi: 10.1007/BF00398416. [DOI] [PubMed] [Google Scholar]
- Levitt MH, Freeman R. NMR Population-Inversion Using a Composite Pulse. J Magn Reson. 1979;33:473–476. doi: 10.1016/j.jmr.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Levitt MH, Freeman R. Compensation for pulse imperfections in NMR spin-echo experiments. J Magn Reson. 1981;43:65–80. [Google Scholar]
- Lohr F, Schmidt JM, Ruterjans H. Simultaneous measurement of (3)J(HN,H alpha) and (3)J(H alpha,H beta) coupling constants in C-13,N-15-labeled proteins. J Am Chem Soc. 1999;121:11821–11826. [Google Scholar]
- Ludvigsen S, Andersen KV, Poulsen FM. Accurate Measurements of Coupling-Constants From 2-Dimensional Nuclear-Magnetic-Resonance Spectra of Proteins and Determination of Phi-Angles. J Mol Biol. 1991;217:731–736. doi: 10.1016/0022-2836(91)90529-f. [DOI] [PubMed] [Google Scholar]
- Maltsev AS, Grishaev A, Roche J, Zasloff M, Bax A. Improved Cross Validation of a Static Ubiquitin Structure Derived from High Precision Residual Dipolar Couplings Measured in a Drug-Based Liquid Crystalline Phase. J Am Chem Soc. 2014;136:3752–3755. doi: 10.1021/ja4132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev AS, Ying JF, Bax A. Impact of N-Terminal Acetylation of α-Synuclein on Its Random Coil and Lipid Binding Properties. Biochemistry. 2012;51:5004–5013. doi: 10.1021/bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsyzov AB, Shen Y, Lee JH, Hummer G, Bax A. MERA: A webserver for evaluating backbone torsion angle distributions in dynamic and disordered proteins from NMR data. J Biomol NMR. 2015;63:85–95. doi: 10.1007/s10858-015-9971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelione GT, Wagner G. Accurate Measurements Of Homonuclear H-N-H-Alpha Coupling-Constants In Polypeptides Using Heteronuclear 2d Nmr Experiments. J Am Chem Soc. 1989;111:5474–5475. [Google Scholar]
- Pardi A, Billeter M, Wüthrich K. Calibration of the angular dependence of the amide proton-Cα proton coupling constants, 3JHNα, in a globular protein: Use of 3JHNα for identification of helical secondary structure. J Mol Biol. 1984;180:741–751. doi: 10.1016/0022-2836(84)90035-4. [DOI] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T-2 relaxation by mutual cancellation of dipole- dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin KV, Wider G, Wuthrich K. Single transition-to-single transition polarization transfer (ST2-PT) in [N15, H1]-TROSY. J Biomol NMR. 1998;12:345–348. doi: 10.1023/A:1008268930690. [DOI] [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenar V. Gradient-Tailored Excitation for Single-Quantum Nmr-Spectroscopy of Aqueous-Solutions. J Biomol NMR. 1992a;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenár V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous sloutions. J Biomol NMR. 1992b;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Rexroth A, Schmidt P, Szalma S, Geppert T, Schwalbe H, Griesinger C. New Principle For The Determination Of Coupling-Constants That Largely Suppresses Differential Relaxation Effects. J Am Chem Soc. 1995;117:10389–10390. [Google Scholar]
- Rosenman DJ, Connors CR, Chen W, Wang C, Garcia AE. A beta Monomers Transiently Sample Oligomer and Fibril-Like Configurations: Ensemble Characterization Using a Combined MD/NMR Approach. J Mol Biol. 2013;425:3338–3359. doi: 10.1016/j.jmb.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Herbruggen T, Sorensen OW. Clean TROSY: Compensation for relaxation-induced artifacts. J Magn Reson. 2000;144:123–128. doi: 10.1006/jmre.2000.2020. [DOI] [PubMed] [Google Scholar]
- Sgourakis NG, Merced-Serrano M, Boutsidis C, Drineas P, Du Z, Wang C, Garcia AE. Atomic-Level Characterization of the Ensemble of the A beta(1-42) Monomer in Water Using Unbiased Molecular Dynamics Simulations and Spectral Algorithms. J Mol Biol. 2011;405:570–583. doi: 10.1016/j.jmb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgourakis NG, Yan Y, McCallum SA, Wang C, Garcia AE. The Alzheimer's peptides A beta 40 and 42 adopt distinct conformations in water: A combined MD/NMR study. J Mol Biol. 2007;368:1448–1457. doi: 10.1016/j.jmb.2007.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaka AJ, Freeman R. Composite Pulses with Dual Compensation. J Magn Reson. 1983;55:487–493. [Google Scholar]
- Solyom Z, Schwarten M, Geist L, Konrat R, Willbold D, Brutscher B. BEST-TROSY experiments for time-efficient sequential resonance assignment of large disordered proteins. J Biomol NMR. 2013;55:311–321. doi: 10.1007/s10858-013-9715-0. [DOI] [PubMed] [Google Scholar]
- Vogeli B, Ying JF, Grishaev A, Bax A. Limits on variations in protein backbone dynamics from precise measurements of scalar couplings. J Am Chem Soc. 2007;129:9377–9385. doi: 10.1021/ja070324o. [DOI] [PubMed] [Google Scholar]
- Vuister GW, Bax A. Quantitative J correlation: A new approach for measuring homonuclear three-bond J(HNHα) coupling constants in 15N-enriched proteins. J Am Chem Soc. 1993;115:7772–7777. [Google Scholar]
- Waelti MA, Orts J, Voegeli B, Campioni S, Riek R. Solution NMR Studies of Recombinant A beta(1-42): From the Presence of a Micellar Entity to Residual beta-Sheet Structure in the Soluble Species. ChemBioChem. 2015;16:659–669. doi: 10.1002/cbic.201402595. [DOI] [PubMed] [Google Scholar]
- Wang AC, Bax A. Determination of the backbone dihedral angles phi in human ubiquitin from reparametrized empirical Karplus equations. J Am Chem Soc. 1996;118:2483–2494. [Google Scholar]
- Yan Y, McCallum SA, Wang C. M35 oxidation induces A beta 40-like structural and dynamical changes in A beta 42. J Am Chem Soc. 2008;130:5394–5395. doi: 10.1021/ja711189c. [DOI] [PubMed] [Google Scholar]
- Ying J, Roche J, Bax A. Homonuclear decoupling for enhancing resolution and sensitivity in NOE and RDC measurements of peptides and proteins. J Magn Reson. 2014;241:97–102. doi: 10.1016/j.jmr.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.