Abstract
Cocaine, a commonly used drug of abuse, has been shown to cause neuropathological dysfunction and damage in the human brain. However, the role of autophagy in this process is not defined. Autophagy generally protective in nature, can also be destructive leading to autophagic cell death. This study was designed to investigate whether cocaine induces autophagy in the cells of CNS origin. We employed astrocyte, the most abundant cell in the CNS, to define the effects of cocaine on autophagy. We measured levels of the autophagic marker protein LC3II in SVGA astrocytes after exposure with cocaine. The results showed that cocaine caused an increase in LC3II level in a dose- and time-dependent manner, with the peak observed at 1 mM cocaine after 6 hours exposure. This result was also confirmed by detecting LC3II in SVGA astrocytes using confocal microscopy and transmission electron microscopy. Next, we sought to explore the mechanism by which cocaine induces the autophagic response. We found that cocaine-induced autophagy was mediated by sigma 1 receptor, and autophagy signaling proteins p-mTOR, Atg5, Atg7 and p-Bcl-2/Beclin-1 were also involved and this was confirmed by using selective inhibitors and siRNAs. In addition, we found that chronic treatment with cocaine resulted in cell death, which is caspase-3 independent, and can be ameliorated by autophagy inhibitor. Therefore, this study demonstrated that cocaine induces autophagy in astrocytes and is associated with autophagic cell death.
Introduction
Cocaine is one of the most popular recreational drugs in United States. It is a strong central nervous system stimulant, and its chronic use causes a long-term change of the brain’s reward system leading to addiction [1]. In addition, use of cocaine causes constriction of blood vessels, hallucinations, and paranoia [2,3]. Furthermore, repeated use of cocaine also leads to brain impairment (deficits in cognition, motivation, insight and attention) [4]. Clinical and pre-clinical studies have demonstrated the occurrence of learning and memory impairment and movement disorders in cocaine abusers, even after a long period of drug withdrawal [5,6]. A recent meta-analysis study involving 46 studies showed that cocaine abuse predominantly affects attention, impulsivity, verbal learning/memory, and working memory [7]. The mechanism by which cocaine causes cellular damage is complex, and has been associated with ER stress [8,9], apoptosis [10,11] and oxidative stress [12,13]. However, the exact mechanism of cocaine-induced neurotoxicity remains an area of interest. Several studies have suggested that brain impairment caused by drugs of abuse induced neurocognitive disorder is correlated with apoptotic cell death [14,15].
Astrocytes are the most abundant cell type in the brain. Astrocytes are present throughout the central nervous system, and they play supportive role for neurons. They provide structural and metabolic support to the neurons, and are involved in blood brain barrier maintenance, immune/inflammatory response modulation, and are known to play an important role in neurotransmission [16]. Since astrocytes play an important role in the brain, the interaction between astrocytes and neurons is essential for neuronal survival. It is well established that any impairment in these astrocyte functions can negatively impact normal function and survival of neurons [17]. Apart from that, increased number of apoptotic astrocytes has been found in HIV-associated dementia [18] and other neurological disorders [19,20]. Based on these findings, it is reasonable to establish the correlation between astrocytic cell death and cocaine-induced brain injury.
Autophagy is a process in eukaryotic cells that is characterized by the sequestration of cytoplasmic organelles within an autophagosome and degradation by lysosomal enzymes [21]. It is a regulated degradative pathway in which cytoplasmic cargo is delivered into lysosomes. Under stress conditions such as nutrient depletion and oxidative stress, autophagy is activated to protect the cells from dying. Autophagy is negatively regulated by mammalian target of rapamycin (mTOR). mTOR is deactivated under stress condition, leading to activation of downstream cascade of autophagy-related signaling proteins such as Atg1, Beclin-1, Atg5, and Atg7 [22]. Autophagy process is strongly inhibited by Bcl-2, and the interaction between Bcl-2 and Beclin-1 plays an important role in the regulation of autophagy process [23]. Most assays for autophagy modulators use an autophagy marker protein microtubule-associated protein 1 light chain 3 (MAP-LC3) as the indicator of autophagy activity.
In general, autophagy is considered to be protective for cells, for the fact that autophagosome-lysosome degradation of cytoplasmic components can provide amino acids to maintain cellular energy. However, in contrast with its protective role, excessive autophagy could cause cell death, which is known as “autophagic cell death” or “type II programmed cell death”. The relationship between autophagy and apoptosis is complex, and they can be triggered by same stimuli. While autophagy is an early response stimulated by stress, it often occurs prior to apoptosis. In certain cases when apoptotic machinery is inhibited, autophagy is preferred in promoting cell death [24,25]. In several neurodegenerative diseases, autophagy has been found to be involved in cell death. For example, accumulation of autophagic vacuoles has been reported in brains of Alzheimer’s Disease patients [26]; and in Parkinson’s Disease, dopaminergic neurons die through both apoptotic and autophagic process [27]. Similarly, commonly abused controlled substance like methamphetamine and morphine has been reported to be involved in lysosomal vacuolization and autophagosome formation [28-31], Methamphetamine has also been shown to be associated with autophagic cell death in cardiomyocytes [32], and cells of CNS origin [33,34]. These findings represent another role of autophagy, as a suicide mechanism other than apoptosis.
In this study, we sought to address the mechanism of cocaine-mediated astrocyte death via autophagy. We also sought to determine the pathways involved in cocaine-mediated autophagy. We specifically focused on the activation and involvement of autophagy related protein mTOR, Beclin-1, Atg5/7, and upstream pathway activity, including sigma 1 receptor activation.
Results
Cocaine induces autophagy in astrocytes in dose and time-dependent manner
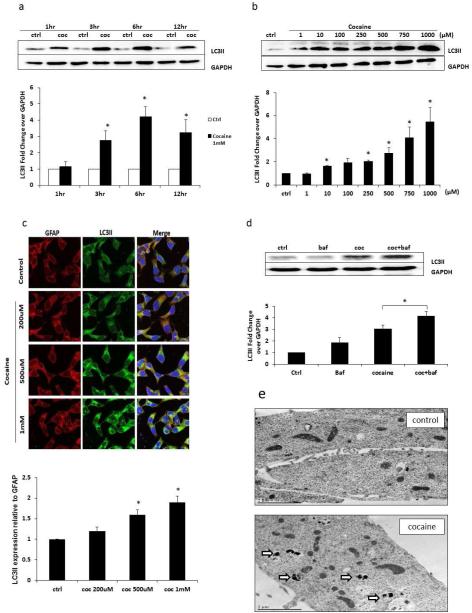
During autophagy, the cytosolic form of LC3 (LC3I) is conjugated to phosphatidylethanolamine to form LC3I-phosphatidylethanolamine conjugate (LC3II), which is considered to be a specific marker for autophagic activity. To determine whether cocaine can induce autophagy in astrocytes, we treated the SVGA astrocytes with cocaine at 1 mM concentration for 1, 3, 6 and 12 hours. The LC3II level was detected in whole cell lysate using western blot analysis. Cocaine did not cause any change in LC3II level after 1 hour exposure but 3, 6 and 12 hour exposure caused substantial increase with 6 hour exposure showing peak LC3II expression (Figure 1a). In the next set of experiments the astrocytes were exposed for 6 hour with varying concentrations of cocaine ranging from 1 μM to 1 mM followed by detection of LC3II in whole cell lysate. The response was dose dependent with 1 mM concentration causing maximum change in LC3II level (Figure 1b). Therefore, all other experiments were performed using cocaine at 1mM concentration with 6 hour exposure time. These results were also confirmed in immunocytochemistry using 200, 500 μM and 1 mM cocaine (Figure 1c). The green punctuate dots indicate LC3II-positive immunostaining in astrocytes. The LC3II expression was once again dose-dependent as seen in immunocytochemistry and was analyzed using ImageJ software. We then wanted to determine whether this increased LC3II was due to increased autophagosome formation or a defect in the fusion process. We, therefore, treated the cells with a lysosomal inhibitor, bafilomycin A1, which prevents the fusion between autophagosomes and lysosomes. The results from this experiment clearly showed that bafilomycin treatment significantly increased LC3II levels (Figure 1d). As autophagy is characterized by autophagosome formation, we further examined the ultrastructure of cocaine-treated and control astrocytes using transmission electron microscopy. The images of transmission electron microscopy are shown in Figure 1e. The cocaine treated astrocytes showed numerous double-membrane vacuoles. However, there was no autophagosome in the control cells. Taken together, these results clearly demonstrated that cocaine induced autophagy in dose and time-dependent manner in astrocytes.
Fig. 1.
Cocaine induces autophagy in SVGA cells, in a time- and dose-dependent manner. Results are shown as mean ± S.E. for 3 separate experiments. * p< 0.05 as compared with control. (a) SGVA cells were exposed to 1 mM of cocaine for the indicated times, and then were subject to western blot analysis with anti-LC3II antibody. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (b) SVGA cells were exposed to different doses of cocaine as indicated for 6 hours, and were then subject to western blot analysis with anti-LC3II antibody. The data quantified by AlphaEase FC software are shown on the bottom of the panel. (c) Punctate LC3II dots in cocaine-treated SVGA cells. SVGA cells were treated with 200 μM, 500 μM, or 1 mM of cocaine for 6 hours. Cells were fixed with methanol and immunostained with anti-LC3II antibody. Cells were examined by confocal microscopy (Leica TCS SP5 II). (d) SVGA cells were exposed to 1mM of cocaine for 6 hours, with or without 20 nM of bafilomycin A1 and followed by western blot analysis with anti-LC3II antibody. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (e) Electron micrographs showing the ultrastructure of cocaine-treated SVGA cells. Control indicates no treatment with cocaine but process otherwise in same fashion. The other panel (cocaine) shows SVGA cells treated with 1 mM of cocaine for 6 hours. Arrows in the electron micrograph denote presence of autophagosomes.
Cocaine induces autophagy through sigma 1 receptor-mediated pathway
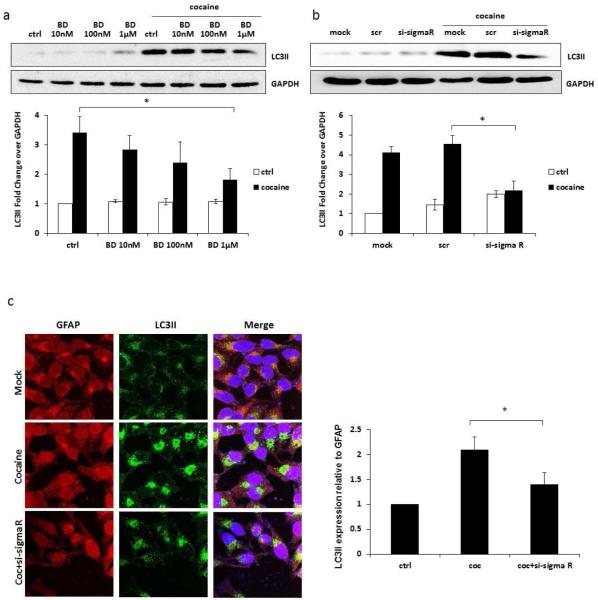
Sigma 1 receptor has been well documented in the literature as a receptor for cocaine and has been shown to be involved in the toxic and stimulant properties of the cocaine [35,36]. We therefore examined whether cocaine-induced autophagy was mediated by sigma 1 receptor. The involvement of sigma 1 receptor was determined using both a pharmacological antagonist and siRNA for sigma 1 receptor. We first used BD1047, a selective sigma 1 receptor antagonist, which has been previously shown to attenuate cocaine-induced toxicity [37]. The BD1047 was tested at 10 nM, 100 nM and 1 μM. The inhibitor blocked cocaine-induced LC3II expression in dose dependent manner and the decline was statistically significant at highest concentration (Figure 2a). We then used a sigma 1 receptor-specific siRNA with scrambled siRNA as control. The scrambled siRNA did not affect LC3II expression but sigma 1 receptor-specific siRNA down-regulated cocaine-induced LC3II expression by >60% (Figure 2b). These results were also confirmed in immunocytochemistry (Figure 2c), and ImageJ analysis showed significant reduction in the level of cocaine-induced LC3II when the cells were transfected with specific siRNA. These results provide clear evidence of the involvement of sigma 1 receptor in cocaine-induced autophagy.
Fig. 2.
Cocaine induces autophagy through sigma 1 receptor-mediated pathway. Results are shown as mean ± S.E. for 3 separate experiments. * p< 0.05 as compared to control. (a) SVGA cells were treated with or without indicated doses of BD1047 and then treated with 1 mM of cocaine for 6 hours. LC3II was detected by western blot and the band intensity was quantified by AlphaEase FC software, shown at the bottom of the panel. (b) SVGA cells transfected with a sigma 1 receptor or a scrambled siRNA were treated with 1mM cocaine for 6 hours. LC3II was detected by western blot. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (c) SVGA cells transfected with a sigma 1 receptor siRNA or ascrambled siRNA were treated with 1mM of cocaine for 6 hours. Cells were fixed with methanol and immunostained with anti-LC3II antibody. Cells were examined using a confocal microscope (Leica TCS SP5 II). The intensity of the staining in each cell was quantified by using ImageJ software.
Signaling proteins Beclin-1, Bcl-2, mTOR, and Atg5/7 are involved in cocaine-induced autophagy
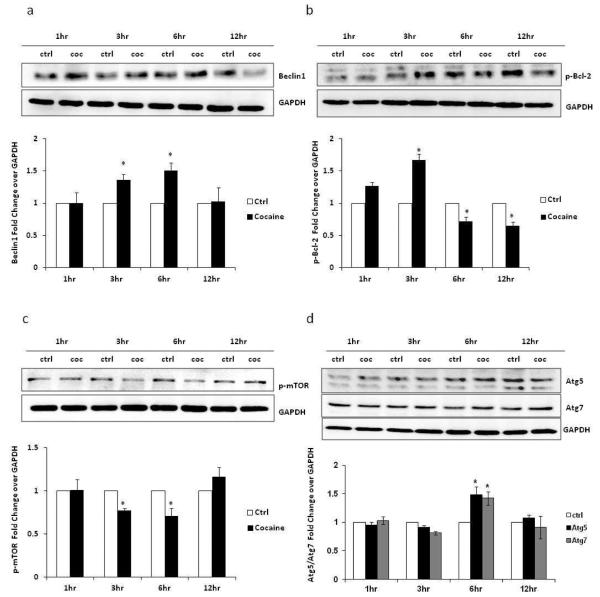
Beclin-1 is one of the m ost important signaling proteins in autophagosome formation. Therefore, we hypothesized that cocaine-induced autophagy is mediated through Beclin-1. Astrocytes were treated with 1 mM cocaine and the cell lysate was prepared after 1, 3, 6 and 12 hours of exposure. These lysates were stained for Beclin-1, phosphorylated Bcl-2, phosphorylated mTOR, Atg5 and Atg7. Consistent with our hypothesis, we found that Beclin-1 was significantly upregulated in cocaine-treated cells (Figure 3a). The Beclin-1 was significantly up-regulated after 3 and 6 hours of exposure followed by normalization of Beclin-1 level. Further, phosphorylation of Bcl-2 dissociates Bcl-2/Beclin-1 complex and releases Beclin-1 to participate in the autophagy process. In cocaine-treated cells, we found increased level of phosphorylated Bcl-2, which further provides evidence of the involvement of Beclin-1 in the initiation of autophagy (Figure 3b). The maximum Bcl-2 phosphorylation was observed at 3 hours followed by normalization. mTOR is a negative regulator of autophagy, and activation of mTOR suppresses autophagy. Upon starvation, mTOR is dephosphorylated leading to initiation of autophagy. Consistent with this, we found significant downregulation of p-mTOR level at 3 and 6 hours in cells treated with cocaine (Figure 3c). Finally, in addition to Beclin-1 and Bcl-2, Atg 5 and Atg7 have also been reported to regulate autophagy. In cocaine-treated cells, both Atg5 and Atg7 were induced compared with control, suggesting the involvement of Atg5 and Atg7 in cocaine-mediated autophagy process (Figure 3d).
Fig. 3.
Cocaine induces autophagy through mTOR, Beclin-1, and Atg5/7 pathways. The results are shown as average ± S.E. for 3 independent experiments with each experiment performed in triplicate. * p< 0.05 as compared to control. (a) SVGA cells were treated with 1 mM of cocaine for the indicated times and were then subjected to western blotting for presence of Beclin-1. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (b) SVGA cells were treated with 1 mM of cocaine for the indicated times, followed by western blot analysis using anti-p-Bcl2 antibody. The results quantified by AlphaEase FC software are shown at the bottom of the panel. (c) SVGA cells were treated with 1 mM of cocaine for the indicated times. p-mTOR was detected in each sample using anti-p-mTOR antibody. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (d) SVGA cells were treated with 1 mM of cocaine for 1, 3, 6 and 12 hours. The Atg5/7 was detected in western blotting assay using anti-Atg5 and anti-Atg7 antibodies. The band intensity was quantified by using AlphaEase FC software and is shown at the bottom of the panel.
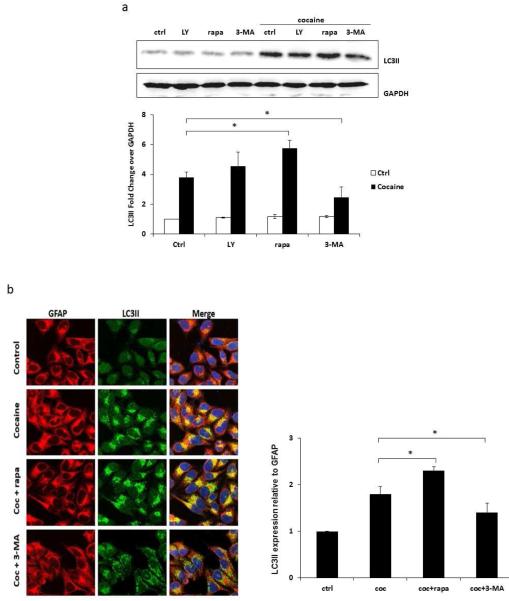
To further determine cocaine-induced autophagic pathway, we used 3-methyladenine (3-MA) that inhibits PI3K I and III [38]. Beclin-1/PI3KIII complex is one of the key regulators of autophagy, and short term treatment with 3-MA inhibits autophagy by blocking autophagosome formation via the inhibition of class III PI3K. As shown in Figure 4a, 3-MA significantly decreased the expression of cocaine-induced LC3II (34% reduction) whereas another PI3K inhibitor LY294002 did not block autophagy. On the contrary, it increased LC3II level by 19% although this increase was statistically not significant. As expected, pretreatment of rapamycin (mTOR inhibitor) caused 51% increase in the level of LC3II over cells treated with 1 mM cocaine. These results were further confirmed in immunocytochemistry using confocal microscopy (Figure 4b). The astrocytes growing on coverslips were pretreated with 3-MA or rapamycin followed by 1 mM cocaine for 6 hours. These cells were then fixed and stained for LC3II. 3-MA significantly decreased level of cocaine-induced LC3II (23% reduction over cocaine-treated astrocytes), whereas rapamycin increased the LC3II expression by 27% in the cells compared to the cells only treated with cocaine. The ImageJ analysis showed approximately 80% increase exhibited by cocaine which was further potentiated by rapamycin (130% increase over control).
Fig. 4.
Cocaine-induced increase in LC3II level is attenuated by inhibition of PI3K. Results are shown as average ± S.E. for 3 separate experiments. * p< 0.05 as compared to control. (a) SVGA cells were treated with LY294002, 3-MA or rapamycin, and then treated with 1 mM of cocaine for 6 hours. Cells were then subjected to western blot analysis with anti-LC3II antibody. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (b) SVGA cells were treated with 3-MA or rapamycin, and then treated with 1 mM of cocaine for 6 hours. Cells were fixed with methanol and immunostained with anti-LC3II antibody. Cells were examined by confocal microscopy (Leica TCS SP5 II).
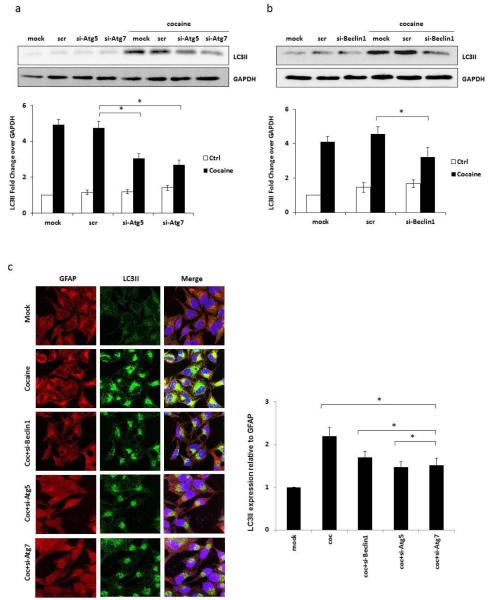
Furthermore, to determine the role of Beclin-1, Atg5 and Atg7 in cocaine-induced autophagy, we used specific siRNA against these signaling proteins to confirm the direct involvement of these proteins in the cocaine-induced autophagy. The SVG astrocytes were transfected with specific siRNA 48 hours before they were exposed with cocaine. These cocaine-treated cells were used for immunocytochemistry and also for the preparation of whole cell lysate after 6 hours cocaine exposure. The results from these experiments are shown in Figure 5. The Atg5 and Atg7 siRNA reduced the cocaine-mediated LC3II expression by 39 and 47%, respectively, whereas scrambled siRNA did not cause any significant change (Figure 5a). Likewise Beclin-1 siRNA caused ~30% reduction in cocaine-mediated LC3II with scrambled siRNA showing only marginal increase over cocaine-mediated LC3II expression that was statistically not significant (Figure 5b). These results were confirmed in immunocytochemistry where ImageJ analysis showed 23, 33 and 31% decline in LC3II expression caused by Beclin-1, Atg5 and Atg7 siRNA, respectively (Figure 5c).
Fig. 5.
Cocaine-induced increase in LC3II level is attenuated by silencing of Beclin-1, Atg5 and Atg7. Results are shown as average ± S.E. for 3 separate experiments. * p< 0.05 as compared to control. (a) SVGA cells transfected with Atg5, Atg7 or a scrambled siRNA were treated with 1 mM of cocaine for 6 hours. LC3II was detected in whole cell lysate by western blot. The band intensity quantified by AlphaEase FC software is shown at the bottom of the panel. (b) SVGA cells transfected with Beclin-1 or a scrambled siRNA were treated with 1 mM of cocaine for 6 hours. LC3II was detected by western blot. The data quantified by AlphaEase FC software are shown at the bottom of the panel. (c) SVGA cells transfected with Beclin-1, Atg5 or Atg7 or a scrambled siRNA were treated with 1 mM of cocaine for 6 hours. Cells were fixed with methanol and immunostained with anti-LC3II antibody. Cells were examined by confocal microscopy (Leica TCS SP5 II).
Cocaine-induced autophagy leads to cell death in SVGA
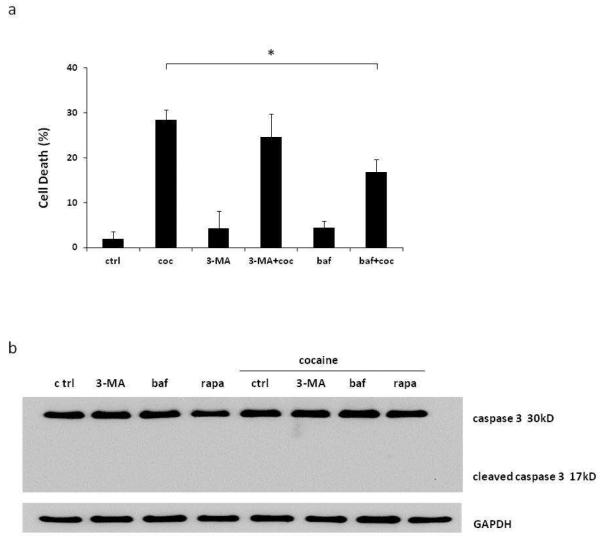
The role of autophagy in cell death is controversial. Autophagy is generally considered to be a mechanism for cell survival. However, extensive autophagy can lead to programmed cell death, called autophagic cell death. We therefore examined the role of autophagy in cocaine-treated astrocytes. In preliminary experiments we found that 6 hour treatment was not enough to induce cocaine-mediated cell death, and we required 48 hours with cocaine exposure at every 6 hour to cause significant cell death (results of experimental protocol optimization not shown). SVGA cells were pretreated with inhibitors 3-MA and bafilomycin A1, followed by treatment with 1 mM cocaine every 6 hours for 48 hours. Upon termination of the experiment, MTT assay was performed to measure the viability of the cells. We found that cocaine treatment caused 29.4±3.7% cell death compared with control untreated cells (Figure 6a). 3-MA caused only marginal decline in cocaine-induced cell death (29.4±3.7 vs 25.6±8.9) which was statistically not significant. However, pretreatment with another autophagy inhibitor, bafilomycin A1, significantly prevented the cells from cocaine-induced cell death. The cells pretreated with bafilomycin A1 showed only 17.8±4.8 cell death as opposed to 29.4±3.7% cell death caused by cocaine and the difference was statistically significant. To rule out the possibility of apoptotic cell death, we performed western blot analysis using antibody against full/cleaved caspase-3. The results showed that cocaine did not induce cleavage of caspase-3, which clearly suggested that apoptosis was not involved in cocaine-induced cell death (Figure 6b). Taken together, our results clearly suggest that multiple cocaine exposure induces autophagic cell death that could be partially reversed by autophagy inhibitor. The Figure 7 depicts a pathway involved in cocaine-mediated autophagy. In summary, the cocaine functions through sigma 1 receptor and inhibits mTOR, causing autophagy. The phosphorylation of Bcl-2 helps in dissociation of Beclin-1/Bcl-2 complex and free Beclin-1 induces autophagy with involvement of Atg5 and Atg7.
Fig. 6.
Cocaine induces caspase-3 independent cell death, which is decreased by inhibition of autophagy. Results are shown as mean ± SE for 3 separate experiments. *p<0.05 as compared to control. (a) SVGA cells were treated with 3-MA or bafilomycin, and then treated with 1 mM of cocaine every 6 hours for 48 hours. Then cell viability was measured in a MTT assay. (b) SVGA cells were treated with 3-MA, bafilomycin or rapamycin, and then treated with 1 mM of cocaine for 6 hours. Cells were subject to western blot analysis with anti-“cleaved caspase 3” antibody.
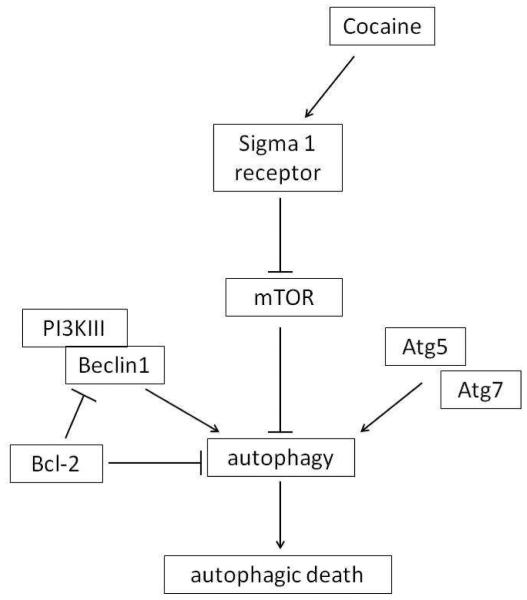
Fig. 7.
Schematic diagram showing the signaling pathways leading to cocaine-induced autophagy. Cocaine induces autophagy through sigma 1 receptor. Subsequently, cocaine inhibits downstream autophagy regulator mTOR, and activates Beclin-1, Atg5 and Atg7, leading to autophagy.
Discussion
Cocaine, as one of the most popular recreational drugs in United States and many other parts of the world, can cause addiction and neurological impairment [1]. However, role of autophagy in cocaine-mediated deleterious effect in the brain remains poorly understood. Several recent studies with other psychostimulants suggest involvement of autophagy in neurotoxicity [30,33]. For example, morphine-mediated autophagy is responsible for the development of morphine antinociceptive tolerance [39] and methamphetamine has been reported to induce cell death via autophagy [40]. This study revealed for the first time that cocaine exposure induces autophagy in SVGA astrocytes through sigma 1 receptor and mTOR, and PI3KIII/Beclin-1 pathways are involved in cocaine-mediated autophagic process. This study also demonstrated that cocaine-mediated cell death in SVGA astrocytes involves autophagy, and the cell death was partially reversed by inhibiting autophagy. Our data also suggests that autophagy is an early response to cocaine-induced stress. However, chronic exposure to cocaine leads to excessive autophagy, which may damage the cellular components leading to cell death. This is the first study describing cocaine-induced autophagy in astrocytic cells, which may help to explain how chronic exposure of cocaine may contribute to CNS injury.
The concentration of cocaine for use in this study was determined based on input from earlier studies. Earlier reports have indicated mean plasma cocaine concentration of 0.26±0.5 μM from a group of 111 cocaine users even though it could be as high as 120 μM [41] In another report Patel showed in 1996, that in a fatal case because of overdosing blood cocaine concentration was found to be 0.31 mM [42]. In yet another report of fatal case, >1mM cocaine was found to be present in blood [43]. Based on earlier studies, cocaine it is also known that CNS concentration could be as high as up to 155 times higher than that present in blood [44]. This justifies our choice of using 1 mM cocaine concentration.
The pharmacological effect of cocaine on the central nervous system involves multiple neurotransmitter proteins [45-47]. Cocaine blocks dopamine transporter resulting in dopamine increase in the synapse [1]. In addition, cocaine binds to sigma 1 receptor, which is highly expressed in the brain [47]. The modulation of sigma 1 receptor has been found to be associated with cytoprotective effects in astrocytes [48]. However, it is unknown whether sigma 1 receptor correlates with autophagic activity in astrocytes. Our study that utilized sigma 1 receptor inhibitor BD1047 and siRNA showed that sigma 1 receptor is involved in cocaine-induced autophagy, and blocking sigma 1 receptor significantly inhibited autophagy. Sigma receptors have earlier been shown to be involved in the stimulant actions and toxicity of cocaine [49]. Earlier studies have also shown that inhibition of sigma 1 receptor attenuated the convulsive and locomotor stimulatory effect of cocaine in mice [50,51]. Therefore, it would be interesting to study the underlying mechanism in these anomalies and whether reversal of autophagy will attenuate the effect in vivo.
During autophagy, LC3I (cytosolic form of protein LC3) is converted to LC3II, which is expressed on the membranes of autophagosomes. Among different isoforms of LC3, LC3II is the one that correlates with increase in autophagy activity level. Therefore, anti-LC3II antibody is recommended for western blot analysis. Previously, the ratio between LC3I and LC3II has been used to determine change in the extent of autophagy. However, the immunoreactivity of LC3I and LC3II defers. In most cases, the sensitivity of detection for LC3II is much higher than that for LC3I. Due to the difference in the affinity of LC3II antibodies to LC3I and LC3II, comparison of LC3II between different samples is considered to be a more accurate method [52,53]. To rule out the possibility of degradation of LC3II during autophagy, it is important to use a lysosomal protease inhibitor, such as bafilomycin A1. Bafilomycin A1 inhibits the fusion of autophagosomes and lysosomes. Thus, autophagic activity in cells can be represented more accurately by comparing the levels of LC3II with or without bafilomycin A1 treatment [53]. Consistent with this, we found that the level of cocaine-induced LC3II is further increased by bafilomycin A1. This result further strengthens our hypothesis that cocaine induces autophagic activity in astrocytes.
Several signaling pathways are involved in the induction of autophagy. The expression level of Beclin-1 is a critical inducer of autophagy. Bcl-2 and Beclin-1 are key determinants in the regulation of apoptosis-autophagy switch [54]. Reduced level of Beclin-1 has been correlated with a defect/lack in removing damaged organelles whereas Bcl-2 inhibits both apoptosis and autophagy-associated cell death [55]. Bcl-2 binds to Beclin-1 and prevents assembling of the pre-autophagosomal structure, resulting in inhibition of autophagy [56]. During autophagy, phosphorylation of Bcl-2 dissociates Beclin-1 from the Bcl-2:Beclin-1 complex facilitating autophagy. Previous studies with other drugs of abuse such as methamphetamine and morphine have shown that downregulation of Bcl-2 releases Beclin-1 to promote autophagy [40,29]. Consistent with previous findings, our results also showed an increase in the levels of Beclin-1 and phosphorylated Bcl-2 by cocaine exposure, which clearly suggest the involvement of Beclin-1 and Bcl-2 in cocaine-induced autophagy. The findings were further supported byknockdown of Beclin-1 by siRNA, resulting in reduction in autophagy.
In addition to Beclin-1, mammalian target of rapamycin (mTOR) has also been shown to be a key regulator of autophagic activity in eukaryotic cells [57]. Consistent with this, our study clearly demonstrated the role of mTOR pathway in cocaine-mediated autophagy. Specifically, we showed that level of phosphorylated mTOR, a negative regulator of autophagy, is significantly reduced with cocaine treatment. Although level of total mTOR is not determined in this study, phosphorylation of mTOR is widely used as an indicator of mTOR activity level in cells or tissues [58]. During autophagy, many Atg proteins are localized to membrane structure as an initial sequestering compartment, which contribute to the formation of the double membrane structure of autophagosome [59]. Consistent with this, cocaine treatment in our study significantly increased levels of Atg5 and Atg7, and knocking down of Atg5 and Atg7 reduced the level of cocaine-mediated LC3II expression. Taken together, these results suggest that cocaine-induced autophagy involves mTOR, Beclin-1, and Atg5/7 in astrocytes.
The definition of autophagic cell death is mainly morphologic, such as cell death associated with formation of autophagosomes [60]. There is no study that provides specific mechanism for autophagic cell death. However, previous studies have shown that cells with autophagic morphology often exist in regions where there is programmed cell death, an evidence for the existence of autophagic cell death [61]. Many recent reports suggest that autophagy-associated cell death, or type II programmed cell death, can occur in variety of cell types [62-66]. For example, anti-microbial drug clioquinol has been found to disrupt the mTOR signaling pathway, and induce autophagic cell death in leukemia and myeloma cells [67]; morphinone, a narcotic analgesic, has been proved to induce non-apoptotic cell death with autophagic feature (formation of autophagosomes), which is associated with functional loss of mitochondria and depletion of ATP [68]. In our study, the morphological features of cells exposed to cocaine are typical of autophagy, which is evident from increase in the number of LC3II punctuate dots using confocal microscopy and formation of autophagosome using transmission electron microscopy (Figure 1). The increase in LC3II protein levels also supports the induction of autophagy in SVGA cells. In addition, we observed significant cell death with cocaine treatment, which is ameliorated by autophagy inhibition (Figure 5). Two forms of programmed cell death, apoptosis and autophagy, can be induced by the same stimuli, and there may be a crosstalk between autophagy and apoptosis. In our study, we didn’t find morphological features of apoptosis, such as granulation of cells. In addition, the cell death was caspase-3 independent, which ruled out the possibility of apoptotic cell death. Furthermore, the use of bafilomycin partially brought back the cell viability. Taken together, it is reasonable to assume that under our condition with cocaine treatment, cells have gone through autophagic cell death. However, due to the fact that none of the autophagy inhibitors used in the study completely reversed the cell death caused by cocaine, we cannot rule out the possibility of an alternative cell death mechanism that is independent of autophagy and apoptosis.
We observed opposing effect of two PI3K inhibitors. LY294002 caused increased autophagy as evident by LC3II expression whereas 3-MA inhibited cocaine-induced autophagy. There are 3 types of PI3K (class I, class II and class III). The class I PI3K/AKT has been reported to be involved in autophagy inhibition [69,70] whereas class III PI3K/AKT is known promoter of autophagy [71]. Although both inhibitors are known to interfere with PI3KI and III [71] but it is likely that LY294002 is a more potent inhibitor of PI3KI whereas 3-MA inhibits PI3KIII more effectively than PI3KI. This is one possible explanation why cocaine-induced LC3II is reduced by 3-MA, but not LY294002.
In summary, this study showed that cocaine induces autophagy in SVGA astrocytes and Beclin-1, mTOR, and Atg5/7 are involved in the autophagy signaling pathway. Our study further suggests that cocaine, at least in part, induces autophagic cell death. This study is clinically relevant because cocaine is one of the common substances of abuse. Further studies are needed to determine the mechanism of chronic cocaine use on autophagic cell death and whether inhibitors of autophagy would have therapeutic value.
Materials and Methods
Cell culture and reagents
SVGA cells [Astroglial cells modified from simian virus 40 (SV40)-transformed human glial cells (SVG)] was generously provided by Dr. Avindra Nath. Cells were grown in Dulbecco's Modified Eagle Medium (DMEM) (Cellgro, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum, 1% non-essential amino acids, 1% sodium bicarbonate, 1% L-glutamine, and 50 μg/ml of gentamycin. The cells were maintained in an incubator at 37°C and humidified air with 5% CO 2. Cocaine and sigma 1 receptor antagonist (BD1047) were obtained from Sigma (Sigma-Aldrich, St. Louis, MO). The inhibitors of PI3K (3-methyladenine, LY294002) and mTOR (rapamycin) pathways were obtained from Cayman Chemicals (Cayman Chemicals, Ann Arbor, MI). Specific antibodies against LC3II, Atg5, Atg7, OPRS1, p-Bcl-2, p-mTOR, and GAPDH were obtained from Cell Signaling Technology (Cell Signaling Technology, Beverly, MA). Vectashield Mounting Medium with DAPI was obtained from Vector laboratories (Vector Laboratories, Burlingame, CA). Specific siRNA of sigma 1 receptor, Beclin-1, Atg5 and Atg7, and control siRNAs were purchased from Ambion Inc (Ambion, Carlsbad, CA).
The cells were seeded in 6-well plates at a density of 0.8 ×106 per well in 2 ml media and allowed to adhere overnight. The cells were treated with cocaine for specified lengths of time as specified in the figures. Furthermore, treatments for 6 hrs were performed with 1mM cocaine.
Transfection of astrocytes with siRNA
Cells were plated at 0.6 × 106 cells/well in a 6 well plate. The cells were allowed to adhere overnight before transfection with 50 nM siRNA. Transfection of SVGA was performed using Lipofectamine™ 2000 as per the manufacturer’s instructions (Invitrogen Inc., Carlsbad, CA). Briefly, complete medium was removed from plates, and cells were washed twice with PBS before addition of serum free medium. Transfection reagent as mixtures of Opti-MEM, lipofectamine, and siRNAs against sigma 1 receptor, Beclin-1, Atg5, and Atg7 were prepared and added into wells. After 24 hours, the transfection reagent was replaced with fresh complete medium. Cells were trypsinized 10 hours later and re-seeded into 6-well plates at a density of 0.8 × 106 per well. Cocaine treatment was performed the next day as described above. Transfection with 50nM of scrambled siRNA was used as the negative control.
Western blotting
SVGA cells were harvested at indicated time points in RIPA buffer (Boston BioProducts, Ashland, MA). The whole-cell lysates were homogenized and centrifuged for 15 minutes at 14,000 rpm to remove debris. The concentration of protein was measured using BCA assay, and 40μg of protein was loaded in each well of 12% polyacrylamide gel for electrophoresis. The proteins were separated at 90V for 2.5 hours and transferred onto PVDF membrane at 350mA for 90 minutes. The membranes were probed with appropriate primary and secondary antibodies for LC3II, Beclin-1, P-Bcl-2, p-mTOR, Atg5, and Atg7 to measure their expression levels. The bands were detected using BM Chemiluminescence Western Blotting Substrate (POD) (Roche Applied Sciences, Indianapolis, IN). The bands were analyzed using FluorChem HD2 software (Alpha Innotech, San Leandro, CA), and the intensities of bands were normalized using GAPDH.
Immunocytochemistry
SVGA cells were seeded at 0.8 × 106 on 1.5mm cover slips followed by treatment with cocaine as before. After termination of treatment, the cells were fixed with 1: 1 ice-cold methanol and acetone solution for 20 minutes at −20°C. The wells were air dried followed by blocking and permeabilization with 1% BSA in PBS with 0.1% Triton for 30 minutes. After blocking, the cells were incubated with a cocktail of rabbit anti-LC3II antibody (1:2000) and a mouse anti-GFAP antibody (1:1500) (Abcam, Cambridge, MA) overnight in a humidified chamber. After 3 washes with 0.1% Triton in PBS, the cells were incubated in the dark chamber for 1 hour with an anti-mouse antibody conjugated with Alexa Fluor 555 (1:2000) and an anti-rabbit antibody conjugated with Alexa Fluor 488 (1:2000) (Cell Signaling, Beverly, MA) followed by 3 washes with 0.1% Triton in PBS. All the antibodies were diluted in 1% BSA in PBS. Finally, the cover slips were transferred onto glass slides with 10μl of Vectashield mounting reagent with DAPI. The fluorescence microscopy was performed using a Leica TCS SP5 II Laser Scanning Confocal microscope. The images were captured using a 40X zoom lens and ImageJ software was used to analyze the images and calculate the intensity values by using GFAP as housekeeping protein.
Transmission electron microscopy
SVGA cells were treated as indicated and fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylade buffer at 4°C for overnight. Sections were observed under transmission electron microscopy in Electron Microscopy Laboratory in School of Dentistry, UMKC.
Cell proliferation assay
Cells were plated in 12-well plates and treated with cocaine for indicated times. Upon termination of the experiment, medium was removed, and cells were washed with PBS. Cells were incubated with 500μl of 0.2mg/ml MTT solution at 37°C for 3 hours. The supernatants were discarded and 500μl per well dimethyl sulfoxide (DMSO) was added. The MTT assay was performed to measure cell viability using a microplate reader. Absorbance was read at 570nm with a reference filter at 630nm.
Statistical analysis
The statistical analysis was performed to represent the data in mean ± S.E. values. Results were based on at least three independent experiments with individual experiments performed in triplicates. For the comparison between two groups, statistical analysis was using two-tailed Student's t-test to calculate p-values, and p-value ≤0.05 was considered statistically significant.
Acknowledgements
The work was supported by grants from National Institute on Drug Abuse (DA025528 and DA025011). We also acknowledge use of the confocal microscope in the University Missouri, Kansas City School of Dentistry Confocal Microscopy Core. This facility is supported by the UMKC Office of Research Services, UMKC Center of Excellence in Dental and Musculoskeletal Tissues, and NIH grant S10RR027668.
Footnotes
Author contributions:
LC, MW, SK, MF and AK conceptualized and designed the project. LC did all the experiments and analyzed the data. LC wrote the first draft of the manuscript and AK finalized the manuscript for submission.
Conflict of interest:
Authors declare no conflict of interest.
Reference
- 1.Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251(5001):1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 2.Flores ED, Lange RA, Cigarroa RG, Hillis LD. Effect of cocaine on coronary artery dimensions in atherosclerotic coronary artery disease: enhanced vasoconstriction at sites of significant stenoses. Journal of the American College of Cardiology. 1990;16(1):74–79. doi: 10.1016/0735-1097(90)90459-3. [DOI] [PubMed] [Google Scholar]
- 3.Satel SL, Southwick SM, Gawin FH. Clinical features of cocaine-induced paranoia. The American journal of psychiatry. 1991;148(4):495–498. doi: 10.1176/ajp.148.4.495. [DOI] [PubMed] [Google Scholar]
- 4.Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, Bolla KI, Quednow BB. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention-deficit hyperactivity disorder, craving and early age at onset. The British journal of psychiatry : the journal of mental science. 2013;203(1):35–43. doi: 10.1192/bjp.bp.112.118091. doi:10.1192/bjp.bp.112.118091. [DOI] [PubMed] [Google Scholar]
- 5.Morrow CE, Culbertson JL, Accornero VH, Xue L, Anthony JC, Bandstra ES. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Developmental neuropsychology. 2006;30(3):905–931. doi: 10.1207/s15326942dn3003_8. doi:10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serper MR, Bergman A, Copersino ML, Chou JC, Richarme D, Cancro R. Learning and memory impairment in cocaine-dependent and comorbid schizophrenic patients. Psychiatry research. 2000;93(1):21–32. doi: 10.1016/s0165-1781(99)00122-5. [DOI] [PubMed] [Google Scholar]
- 7.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8(5):368–376. doi: 10.1097/ADM.0000000000000066. doi:10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 8.Pavlovsky AA, Boehning D, Li D, Zhang Y, Fan X, Green TA. Psychological stress, cocaine and natural reward each induce endoplasmic reticulum stress genes in rat brain. Neuroscience. 2013;246:160–169. doi: 10.1016/j.neuroscience.2013.04.057. doi:10.1016/j.neuroscience.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin EH, Bian S, Shim YB, Rahman MA, Chung KT, Kim JY, Wang JQ, Choe ES. Cocaine increases endoplasmic reticulum stress protein expression in striatal neurons. Neuroscience. 2007;145(2):621–630. doi: 10.1016/j.neuroscience.2006.12.013. doi:10.1016/j.neuroscience.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Pandhare J, Addai AB, Mantri CK, Hager C, Smith RM, Barnett L, Villalta F, Kalams SA, Dash C. Cocaine enhances HIV-1-induced CD4(+) T-cell apoptosis: implications in disease progression in cocaine-abusing HIV-1 patients. Am J Pathol. 2014;184(4):927–936. doi: 10.1016/j.ajpath.2013.12.004. doi:10.1016/j.ajpath.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein PS, Shah A, Weemhoff J, Kumar S, Singh DP, Kumar A. HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Curr HIV Res. 2012;10(5):369–383. doi: 10.2174/157016212802138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sordi AO, Pechansky F, Kessler FH, Kapczinski F, Pfaffenseller B, Gubert C, de Aguiar BW, de Magalhaes Narvaez JC, Ornell F, von Diemen L. Oxidative stress and BDNF as possible markers for the severity of crack cocaine use in early withdrawal. Psychopharmacology (Berl) 2014;231(20):4031–4039. doi: 10.1007/s00213-014-3542-1. doi:10.1007/s00213-014-3542-1. [DOI] [PubMed] [Google Scholar]
- 13.Cerretani D, Fineschi V, Bello S, Riezzo I, Turillazzi E, Neri M. Role of oxidative stress in cocaine-induced cardiotoxicity and cocaine-related death. Curr Med Chem. 2012;19(33):5619–5623. doi: 10.2174/092986712803988785. [DOI] [PubMed] [Google Scholar]
- 14.Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain pathology. 2002;12(4):488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(17):7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norenberg MD. Astrocyte responses to CNS injury. Journal of neuropathology and experimental neurology. 1994;53(3):213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Swanson RA. Astrocytes and brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23(2):137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 18.Thompson KA, McArthur JC, Wesselingh SL. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Annals of neurology. 2001;49(6):745–752. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Yao SY, Whetsell WO, Jr., Sriram S. Astrogliopathy and oligodendrogliopathy are early events in CNS demyelination. Glia. 2013;61(8):1261–1273. doi: 10.1002/glia.22513. doi:10.1002/glia.22513. [DOI] [PubMed] [Google Scholar]
- 20.Jo WK, Law AC, Chung SK. The neglected co-star in the dementia drama: the putative roles of astrocytes in the pathogeneses of major neurocognitive disorders. Mol Psychiatry. 2014;19(2):159–167. doi: 10.1038/mp.2013.171. doi:10.1038/mp.2013.171. [DOI] [PubMed] [Google Scholar]
- 21.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Developmental cell. 2004;6(4):463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 22.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annual review of genetics. 2009;43(67):93. doi: 10.1146/annurev-genet-102808-114910. doi:10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. doi:10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nature reviews Molecular cell biology. 2014;15(2):81–94. doi: 10.1038/nrm3735. doi:10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature reviews Molecular cell biology. 2007;8(9):741–752. doi: 10.1038/nrm2239. doi:10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 26.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(27):6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. doi:10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histology and histopathology. 1997;12(1):25–31. [PubMed] [Google Scholar]
- 28.Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(20):8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, Zhu Y, Wang D, Chen M, Gao P, Xiao W, Rao G, Wang X, Jin H, Xu L, Sui N, Chen Q. Morphine induces Beclin 1- and ATG5-dependent autophagy in human neuroblastoma SH-SY5Y cells and in the rat hippocampus. Autophagy. 2010;6(3):386–394. doi: 10.4161/auto.6.3.11289. [DOI] [PubMed] [Google Scholar]
- 30.El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ. HIV-1 and morphine regulation of autophagy in microglia: Limited interactions in the context of HIV-1 infection and opioid abuse. Journal of virology. 2014 doi: 10.1128/JVI.02022-14. doi:10.1128/JVI.02022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Wan J, Meng J, Banerjee S, Ramakrishnan S, Roy S. Methamphetamine induces autophagy as a pro-survival response against apoptotic endothelial cell death through the Kappa opioid receptor. Cell Death Dis. 2014;5:e1099. doi: 10.1038/cddis.2014.64. doi:10.1038/cddis.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funakoshi-Hirose I, Aki T, Unuma K, Funakoshi T, Noritake K, Uemura K. Distinct effects of methamphetamine on autophagy-lysosome and ubiquitin-proteasome systems in HL-1 cultured mouse atrial cardiomyocytes. Toxicology. 2013;312(74):82. doi: 10.1016/j.tox.2013.07.016. doi:10.1016/j.tox.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Hu Z, Chen B, Bu Q, Lu W, Deng Y, Zhu R, Shao X, Hou J, Zhao J, Li H, Zhang B, Huang Y, Lv L, Zhao Y, Cen X. Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicology letters. 2012;215(1):1–7. doi: 10.1016/j.toxlet.2012.09.019. doi:10.1016/j.toxlet.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Chandramani Shivalingappa P, Jin H, Anantharam V, Kanthasamy A. N-Acetyl Cysteine Protects against Methamphetamine-Induced Dopaminergic Neurodegeneration via Modulation of Redox Status and Autophagy in Dopaminergic Cells. Parkinson's disease. 2012;2012:424285. doi: 10.1155/2012/424285. doi:10.1155/2012/424285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan S, Mesangeau C, Poupaert JH, McCurdy CR. Sigma receptors and cocaine abuse. Curr Top Med Chem. 2011;11(9):1128–1150. doi: 10.2174/156802611795371323. [DOI] [PubMed] [Google Scholar]
- 36.Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152(1-2):236–247. doi: 10.1016/j.cell.2012.12.004. doi:10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol. 1999;370(3):225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 38.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285(14):10850–10861. doi: 10.1074/jbc.M109.080796. doi:10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi Y, Koga Y, Zhang X, Peters C, Yanagawa Y, Wu Z, Yokoyama T, Nakanishi H. Autophagy in superficial spinal dorsal horn accelerates the cathepsin B-dependent morphine antinociceptive tolerance. Neuroscience. 2014;275(384):394. doi: 10.1016/j.neuroscience.2014.06.037. doi:10.1016/j.neuroscience.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 40.Nopparat C, Porter JE, Ebadi M, Govitrapong P. The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. Journal of pineal research. 2010;49(4):382–389. doi: 10.1111/j.1600-079X.2010.00805.x. doi:10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 41.Blaho K, Logan B, Winbery S, Park L, Schwilke E. Blood cocaine and metabolite concentrations, clinical findings, and outcome of patients presenting to an ED. The American journal of emergency medicine. 2000;18(5):593–598. doi: 10.1053/ajem.2000.9282. doi:10.1053/ajem.2000.9282. [DOI] [PubMed] [Google Scholar]
- 42.Patel F. A high fatal postmortem blood concentration of cocaine in a drug courier. Forensic science international. 1996;79(3):167–174. doi: 10.1016/0379-0738(96)01914-7. [DOI] [PubMed] [Google Scholar]
- 43.Peretti FJ, Isenschmid DS, Levine B, Caplan YH, Smialek JE. Cocaine fatality: an unexplained blood concentration in a fatal overdose. Forensic science international. 1990;48(2):135–138. doi: 10.1016/0379-0738(90)90105-8. [DOI] [PubMed] [Google Scholar]
- 44.Heard K, Palmer R, Zahniser NR. Mechanisms of acute cocaine toxicity. Open Pharmacol J. 2008;2(9):70–78. doi: 10.2174/1874143600802010070. doi:10.2174/1874143600802010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 46.Little KY, Patel UN, Clark TB, Butts JD. Alteration of brain dopamine and serotonin levels in cocaine users: a preliminary report. The American journal of psychiatry. 1996;153(9):1216–1218. doi: 10.1176/ajp.153.9.1216. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T, Su T. The sigma receptor: evolution of the concept in neuropsychopharmacology. Current neuropharmacology. 2005;3(4):267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klouz A, Tillement JP, Boussard MF, Wierzbicki M, Berezowski V, Cecchelli R, Labidalle S, Onteniente B, Morin D. [3H]BHDP as a novel and selective ligand for sigma1 receptors in liver mitochondria and brain synaptosomes of the rat. FEBS letters. 2003;553(1-2):157–162. doi: 10.1016/s0014-5793(03)01011-1. [DOI] [PubMed] [Google Scholar]
- 49.Xu YT, Kaushal N, Shaikh J, Wilson LL, Mesangeau C, McCurdy CR, Matsumoto RR. A novel substituted piperazine, CM156, attenuates the stimulant and toxic effects of cocaine in mice. The Journal of pharmacology and experimental therapeutics. 2010;333(2):491–500. doi: 10.1124/jpet.109.161398. doi:10.1124/jpet.109.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto RR, McCracken KA, Pouw B, Miller J, Bowen WD, Williams W, De Costa BR. N-alkyl substituted analogs of the sigma receptor ligand BD1008 and traditional sigma receptor ligands affect cocaine-induced convulsions and lethality in mice. European journal of pharmacology. 2001;411(3):261–273. doi: 10.1016/s0014-2999(00)00917-1. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42(8):1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 52.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221(2):117–124. doi: 10.1002/path.2694. doi:10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 54.Marquez RT, Xu L. Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. American journal of cancer research. 2012;2(2):214–221. [PMC free article] [PubMed] [Google Scholar]
- 55.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4(5):600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. The EMBO journal. 2007;26(10):2527–2539. doi: 10.1038/sj.emboj.7601689. doi:10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS letters. 2010;584(7):1287–1295. doi: 10.1016/j.febslet.2010.01.017. doi:10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280(27):25485–25490. doi: 10.1074/jbc.M501707200. doi:10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 59.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annual review of cell and developmental biology. 2011;27(107):132. doi: 10.1146/annurev-cellbio-092910-154005. doi:10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 60.Levine B, Yuan J. Autophagy in cell death: an innocent convict? The Journal of clinical investigation. 2005;115(10):2679–2688. doi: 10.1172/JCI26390. doi:10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell death and differentiation. 2001;8(6):569–581. doi: 10.1038/sj.cdd.4400852. doi:10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 62.Gozuacik D, Kimchi A. Autophagy and cell death. Current topics in developmental biology. 2007;78(217):245. doi: 10.1016/S0070-2153(06)78006-1. doi:10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- 63.Baehrecke EH. Autophagy: dual roles in life and death? Nature reviews Molecular cell biology. 2005;6(6):505–510. doi: 10.1038/nrm1666. doi:10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 64.Duan Y, Ke J, Zhang H, He Y, Sun G, Sun X. Autophagic cell death of human hepatoma G2 cells mediated by procyanidins from Castanea mollissima Bl. Shell-induced reactive oxygen species generation. Chemico-biological interactions. 2014 doi: 10.1016/j.cbi.2014.09.021. doi:10.1016/j.cbi.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 65.Gao Q, Liu H, Yao Y, Geng L, Zhang X, Jiang L, Shi B, Yang F. Carnosic acid induces autophagic cell death through inhibition of the Akt/mTOR pathway in human hepatoma cells. Journal of applied toxicology : JAT. 2014 doi: 10.1002/jat.3049. doi:10.1002/jat.3049. [DOI] [PubMed] [Google Scholar]
- 66.Chen YJ, Chi CW, Su WC, Huang HL. Lapatinib induces autophagic cell death and inhibits growth of human hepatocellular carcinoma. Oncotarget. 2014;5(13):4845–4854. doi: 10.18632/oncotarget.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao B, Li J, Zhou X, Juan J, Han K, Zhang Z, Kong Y, Wang J, Mao X. Clioquinol induces pro-death autophagy in leukemia and myeloma cells by disrupting the mTOR signaling pathway. Scientific reports. 2014;4:5749. doi: 10.1038/srep05749. doi:10.1038/srep05749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeuchi R, Hoshijima H, Nagasaka H, Chowdhury SA, Kikuchi H, Kanda Y, Kunii S, Kawase M, Sakagami H. Induction of non-apoptotic cell death by morphinone in human promyelocytic leukemia HL-60 cells. Anticancer research. 2006;26(5A):3343–3348. [PubMed] [Google Scholar]
- 69.Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276(38):35243–35246. doi: 10.1074/jbc.C100319200. doi:10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- 70.Xing C, Zhu B, Liu H, Yao H, Zhang L. Class I phosphatidylinositol 3-kinase inhibitor LY294002 activates autophagy and induces apoptosis through p53 pathway in gastric cancer cell line SGC7901. Acta Biochim Biophys Sin (Shanghai) 2008;40(3):194–201. doi: 10.1111/j.1745-7270.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- 71.F OF, Rusten TE, Stenmark H. Phosphoinositide 3-kinases as accelerators and brakes of autophagy. FEBS J. 2013;280(24):6322–6337. doi: 10.1111/febs.12486. doi:10.1111/febs.12486. [DOI] [PubMed] [Google Scholar]