Abstract
The NG2 proteoglycan promotes tumor growth as a component of both tumor and stromal cells. Using intracranial, NG2-negative B16F10 melanomas, we have investigated the importance of pericyte and macrophage NG2 in brain tumor progression. Reduced melanoma growth in myeloid-specific NG2 null (Mac-NG2ko) and pericyte-specific NG2 null (PC-NG2ko) mice demonstrates the importance of NG2 in both stromal compartments. In each genotype, loss of pericyte-endothelial cell interaction diminishes formation of endothelial junctions and assembly of the basal lamina. Tumor vessels in Mac-NG2ko mice have smaller diameters, reduced patency, and increased leakiness compared to PC-NG2ko mice, thus decreasing tumor blood supply and increasing hypoxia. While reduced pericyte interaction with endothelial cells in PC-NG2ko mice results from loss of pericyte activation of β1 integrin signaling in endothelial cells, reduced pericyte-endothelial cell interaction in Mac-NG2ko mice results from 90% reduced macrophage recruitment. The absence of macrophage-derived signals in Mac-NG2ko mice causes loss of pericyte association with endothelial cells. Reduced macrophage recruitment may be due to diminished activation of integrins in the absence of NG2, causing decreased macrophage interaction with endothelial adhesion molecules that are needed for extravasation. These results reflect the complex interplay that occurs between macrophages, pericytes, and endothelial cells during tumor vascularization.
Keywords: macrophage recruitment, NG2 proteoglycan, pericyte-endothelial cell interaction, tumor vascularization, tumor microenvironment, tumor growth
Introduction
The progression of solid tumors is determined both by factors that are intrinsic to the tumor cells and by extrinsic factors present in the host-derived microenvironment. While the importance of oncogenes and other tumor-autonomous factors is well-established, the effects of stromal factors are proving to be surprisingly strong in determining the ability of tumors to thrive and spread to distant sites. Numerous studies show that interactions between tumor cells and components of the host microenvironment are essential for supporting tumor growth and progression [36,66,72,73,89,93]. One of the most widely-recognized elements of the host-derived stroma is the tumor vasculature, which is required in order for neoplasms to expand beyond a very small size, to develop into full-blown tumors, and to metastasize to secondary tissues [5,25,26,37,51]. Vascular hyperplasia, involving aggressive recruitment of pericytes and endothelial cells, is an especially prominent characteristic of brain tumors [24,66]. There is a very large literature on the role of endothelial cells in forming the vascular lumen during blood vessel development, resulting in extensive knowledge regarding molecular mechanisms underlying endothelial cell biology [18,21,32,40,70,71]. Accordingly, anti-angiogenic approaches to cancer therapy have largely relied on attacking vascular endothelial cells [20,23,27,60]. In contrast, pericytes have often been considered to play mostly stabilizing roles during vessel maturation, with little impact during early stages of vessel formation. In reality, however, although pericytes are indeed important for vessel maturation, they are also increasingly recognized as early players in vascular morphogenesis [58,63,85,88], and mechanisms that regulate interactions between pericytes and endothelial cells have been the subject of intensive investigation [2,3,6,31]. Moreover, dual targeting of endothelial cells and pericytes has been proposed as a means of increasing the effectiveness of anti-angiogenic therapy for tumors [5,7,52,74].
It is important to emphasize that pericytes and endothelial cells do not act in isolation from the rest of the tumor stroma, but can be impacted by other stromal components. For example, the role of macrophages in promoting tumor vascularization is unexpectedly large [15,17,69]. Initially recruited as part of the host immune surveillance mechanism, macrophages are efficiently subverted by tumor cells to performing a variety of tumor-promoting functions, including vascularization, immune suppression, and promotion of tumor cell migration, invasion, vascular intravasation and extravasation, and survival in metastatic sites [59,68]. In this latter context, macrophages are also thought to be responsible for conditioning of pre-metastatic sites [38,45]. A growing body of evidence suggests that different macrophage subpopulations are responsible for these varied functions, although these subpopulations are for the most part not yet well-defined.
The NG2 proteoglycan, also known as chondroitin sulfate proteoglycan-4 (CSPG-4), is of considerable interest in the context of tumor biology because of its expression not only by tumor cells, but also by several stromal cell types in the tumor microenvironment. As a frequent component of glioma, melanoma, and sarcoma cells, increased NG2 expression by tumor cells is thought to contribute to aggressive tumor growth and to poor patient outcome [1,10,13,55,65,67,77,82,86,87]. While NG2 itself is not known to be a major mediator of transmembrane signaling, the proteoglycan is able to promote tumor cell proliferation, migration, and resistance to apoptosis via its interactions with integrins, growth factor receptors, and extracellular matrix components [78].
In addition to its expression on some types of tumor cells, NG2 is a frequent, possibly universal, component of the stroma of solid tumors. Several reports from our laboratory have focused on pericyte function during tumor vascularization, especially with regard to the role of NG2 in mediating pericyte recruitment [11,64] and pericyte interaction with endothelial cells [34,39,90-92]. Because pericytes are best defined and identified by their extremely intimate, perivascular association with vascular endothelial cells [2], it is essential to have immunohistochemical markers that distinguish pericytes from endothelial cells. Although there is no single marker for adequate recognition of pericytes, partly due to their extremely plastic nature, use of NG2 as a marker to help with pericyte recognition has been a significant factor in recognizing the early role of pericytes in vessel development [58,61-63,75,85]. Unlike previously-used markers such as α-smooth muscle actin that are preferentially expressed in rodents by mature pericytes, NG2 expression by immature pericytes has allowed us to demonstrate the presence of these cells at very early stages of vessel development. Some degree of confidence in recognizing pericytes by immunohistochemistry can be achieved by dual use of antibodies against NG2 and PDGFRβ, the latter being an important functional receptor responsible for the ability of pericytes to respond to PDGF-BB [3]. Use of NG2 as a marker has also led to the realization that NG2 is expressed not only by pericytes, but also by macrophages (characterized by markers such as F4/80, CD11b, and CD18) that are often closely associated with developing vasculature [83]. Macrophage expression of NG2 has been noted not only in the context of tumors [34], but also in several other pathological contexts [9,16,43,46,49].
The expression of NG2 by several cell types has made it difficult in several cases to clearly interpret the results of experiments carried out with germline NG2 null mice [35]. Skin development [44], obesity [12], spinal cord demyelination [46], and tumor progression [34,39] all reflect the effects of germline NG2 ablation in more than one cell population. This has necessitated development of an NG2 floxed mouse [12] that can be used in conjunction with specific Cre driver mice for cell type-specific ablation of NG2. To date we have crossed the NG2 floxed mouse with the Olig2-Cre mouse [76] for specific NG2 ablation in the oligodendrocyte lineage [12], with the Pdgfrb-Cre mouse [80] for specific NG2 ablation in pericytes [92], and with LysM-Cre mice [81] for specific NG2 ablation in myeloid cells [47,90]. This review will focus on the use of pericyte-specific NG2 null (PC-NG2ko) mice and myeloid-specific NG2 null (Mac-NG2ko) mice to define the NG2-dependent roles of pericytes and macrophages in brain tumor progression. An additional point of emphasis will be on methods we have developed for quantifying vascular structure and function and for correlating these phenomena with tumor progression. These measurements provide much a more extensive and informative characterization of tumor vessels than mere quantification of vascular density. Because comparisons of pericyte and macrophage contributions to the formation of functional vessels have been studied most extensively in two of our recent publications [90,92], these papers receive what may seem to be a disproportionate amount of attention in the review. In all cases, however, attempts have been made to set these recent results in the context of previous knowledge about NG2 and tumor vascularization.
Brain tumor progression in germline NG2 null mice
We have used intracranial microinjection of B16F10 melanoma cells into wild type and germline NG2 null mice to study NG2-dependent effects of the host stroma on brain tumor progression [39]. The C57Bl/6 origin of B16F10 cells makes them compatible with the C57Bl/6 background of our engineered mouse lines. B16F10 cells are NG2-negative, so that NG2 is expressed exclusively in the stroma of these tumors by pericytes associated with tumor blood vessels and by macrophages recruited from the circulation. Germline ablation abolishes NG2 expression in both of these stromal elements. Tumors in wild type and NG2 null mice begin to diverge in size as early as 5 days post-injection. At 17 days post-injection, tumors in NG2 null mice are roughly one-third the volume of tumors in wild type mice [39], illustrating the powerful influence of the host-derived microenvironment on tumor progression.
Deficits in the tumor vasculature provide at least a partial explanation for the reduced tumor growth seen in NG2 null mice. Spatial overlap between PDGFRβ-positive pericytes and CD31-positive endothelial cells is reduced in tumors in germline NG2 null mice, and substantial numbers of pericytes lack any association with endothelial cells in these mice. Overall, the extent of pericyte ensheathment of endothelial cells is reduced 2-fold by germline NG2 ablation [39]. This loss of pericyte-endothelial cell interaction has a substantial impact on assembly of the vascular basement membrane, a process that requires the participation of both vascular cell types. Basement membrane assembly, assessed by immunolabeling for the abundant basal lamina component collagen IV, is reduced 4-fold by germline NG2 ablation [39]. In addition to its role in stimulating endothelial cell synthesis of basal lamina components, the ability of NG2 to serve as a cell surface receptor for collagen VI [78] allows the proteoglycan to function as a point of anchorage for basal lamina components, including collagen IV. Collagen VI is a unique collagen species that acts as a link between cell surface molecules and fibrillar collagens [48]. As expected, collagen VI deposition is reduced in the basal lamina of tumor vessels in germline NG2 null mice, supporting the possibility of a direct role for NG2 in basal lamina assembly.
These changes in pericyte-endothelial cell interaction and the resulting changes in basal lamina deposition have important consequences for the function of tumor blood vessels. Perfusion with FITC-labeled tomato lectin (FITC-LEA) and perfusion with FITC-dextran can be used to quantify tumor vessels that are functionally connected to the circulation and the leakiness of tumor vessels, respectively. Whereas almost 100% of tumor vessels in wild type mice are patent, vessel patency falls to about 50% in germline NG2 null mice. At the same time, the leakiness of functional tumor vessels increases 4-fold in tumors in NG2 null mice. These changes in vessel function lead to a 20-fold increase in intratumoral hypoxia in NG2 null mice, as determined via use of a pimonidazole hypoxia probe [39]. These functional deficits in tumor blood vessels in germline NG2 null mice are first detectable at 5 days after establishment of the B16F10 tumors in the brain, correlating with the time at which tumors in the NG2 null mice begin to diverge in size from tumors in wild type mice. This may be regarded as an NG2-dependent delay in activation of the angiogenic switch in the NG2 null mice, although the germline nature of the NG2 ablation does not allow us to attribute the delay to pericyte versus macrophage loss of NG2.
Brain tumor progression in pericyte-and myeloid-specific NG2 null mice
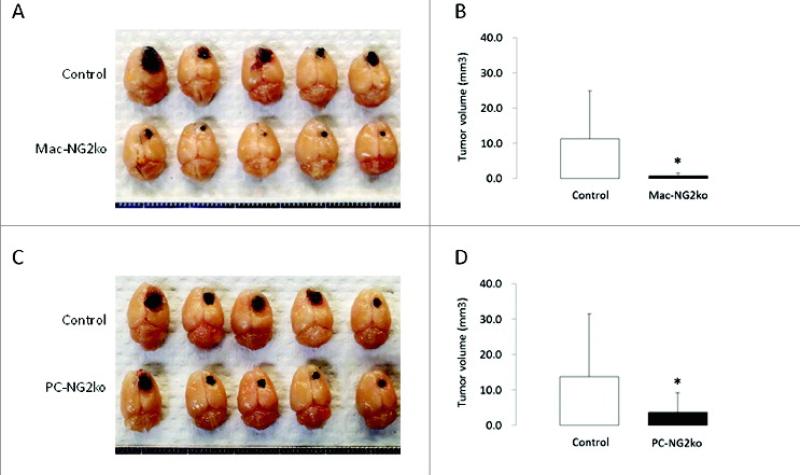
In order to dissect the pericyte and macrophage-specific roles of NG2 in tumor vascularization and growth, it has been necessary to ablate NG2 specifically in these two cell types. For pericyte-specific NG2 ablation (PC-NG2ko), NG2 floxed mice [12] are crossed with pdgfrb-Cre transgenic mice [28,80]. For myeloid-specific NG2 ablation (Mac-NG2ko), the NG2 floxed mice are crossed with LysM-Cre transgenic mice [14,81]. To allow comparison with our results obtained in germline NG2 null mice, we have continued to utilize intracranial microinjection of B16F10 cells to establish brain tumors in control, PC-NG2ko, and Mac-NG2ko mice [90]. As shown in Figure 1A and C, the pigmentation of B16F10 cells allows clear visualization of tumor size. At 10 days post-injection, tumor volumes are markedly reduced in both PC-NG2ko and Mac-NG2ko mice, although the reduction appears to be somewhat greater in Mac-NG2ko mice (Fig 1B and D).
Figure 1. Decreased brain tumor progression in myeloid- and pericyte-specific NG2 null mice.
B16F10 tumors in control versus Mac-NG2ko mice (A) and in control versus PC-NG2ko mice (C) at 10 days after tumor initiation. Graphs quantify tumor volumes in control versus Mac-NG2ko mice (B) and in control versus PC-NG2ko mice (D). Data in B represent 21 control and 20 Mac-NG2ko mice. Data in D represent 22 control and 18 PC-NG2ko mice. *P<0.01 compared to controls. Figure taken from ref (90).
As judged by double labeling for NG2 and PDGFRβ, the efficiency of pdgfrb-Cre mediated NG2 ablation in pericytes appears to be at least 95% [92]. NG2 is still expressed by macrophages in PC-NG2ko mice, based on double labeling for NG2 and F4/80. The primary effect of pericyte-specific NG2 ablation is reduced pericyte coverage of endothelial cells, along with associated structural and functional deficits that will be discussed below. The extent of pericyte ensheathment of endothelial cells in brain tumors in PC-NG2ko mice is diminished by about 30%, compared to tumors in control mice [92]. Macrophage recruitment to tumors is unaffected in PC-NG2ko mice.
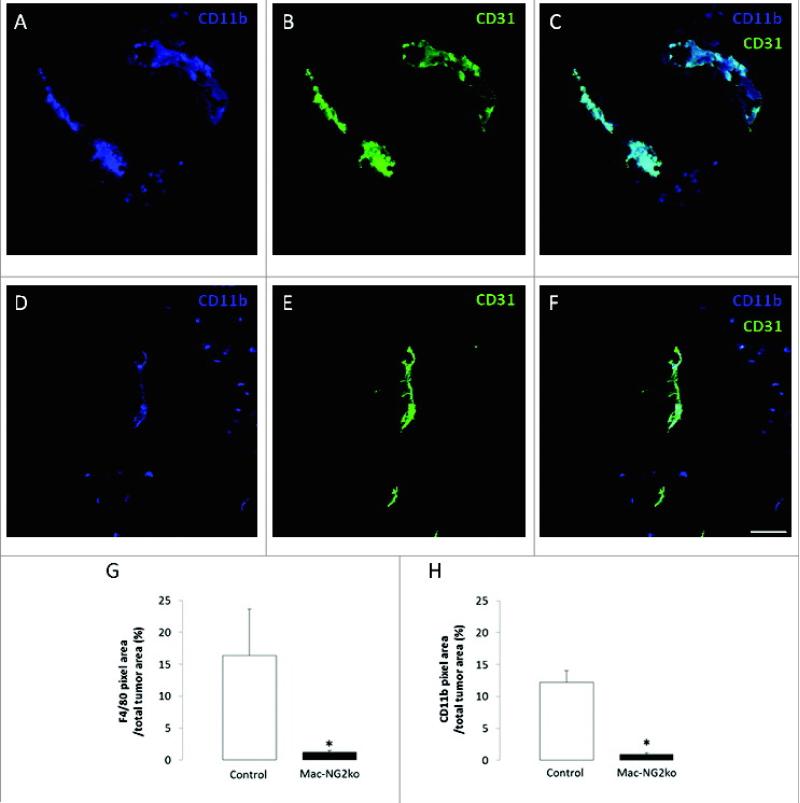
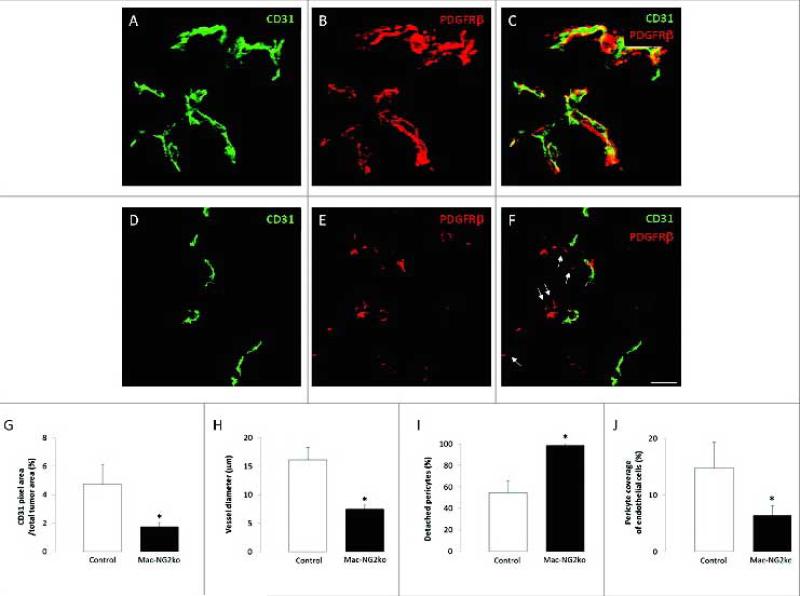
The efficiency of LysM-Cre mediated NG2 ablation in macrophages varies between 70-90%, depending on which marker is used to identify macrophages (F4/80, CD11b, CD18). The transient nature of NG2 expression on macrophages has also complicated the precise determination of these values. Nevertheless, regardless of which marker is used for macrophage identification, macrophage recruitment to B16F10 tumors is dramatically reduced in Mac-NG2ko mice [90]. Figure 2A-F demonstrates the reduced abundance of CD11b-positive macrophages in tumors in Mac-NG2ko mice. Macrophage abundance is quantified for CD11b-positive and F4/80-positive macrophages in Fig 2G,H. Use of mice transplanted with either NG2-positive or NG2-negative EGFP-positive bone marrow [34] confirms the NG2 dependence of this macrophage deficit, and also indicates that the large majority of macrophages in these tumors are recruited from bone marrow (as opposed to being tissue resident myeloid cells) [90]. Along with this loss of macrophage recruitment, there is also a large loss of pericyte association with endothelial cells in the Mac-NG2ko mice, even though the pericytes still express NG2. This is shown in Fig 3A-F by double immunolabeling for PDGFRβ and CD31, with quantification shown in Figure 3I. As summarized in Table 1, this loss of pericyte association with endothelial cells is one of three phenomena (along with decreased vessel diameter and decreased N-cadherin expression) that occur in Mac-NG2ko mice, but are not observed in PC-NG2ko mice. The absence of pericyte interaction with endothelial cells is reminiscent of the loss of pericyte-endothelial cell interaction seen in germline NG2 null mice. The resulting decrease in pericyte ensheathment of endothelial cells is on the order of 50% in Mac-NG2ko mice (Fig 3J), compared to 33% in PC-NG2ko mice.
Figure 2. Myeloid-specific NG2 ablation impairs macrophage recruitment to tumors.
Double immunostaining for CD11b (blue) and CD31 (green) in tumor sections from control (A-C) and Mac-NG2ko mice (D-F). Areas of overlap in z-stacks appear as pale blue (C,F). Quantification of macrophage abundance in tumors using the markers F4/80 (G) and CD11b (H). Data are plotted as the percentage of total tumor area occupied by marker pixels in 10-day tumors (G,H). Scale bars = 20 μm. *P<0.01 compared to controls. Figure taken from ref (90).
Figure 3. Loss of pericyte-endothelial cell interaction following myeloid-specific NG2 ablation.
Double immunostaining for PDGFRβ (red) and CD31 (green) was used to quantify pericyte and endothelial cell abundance, along with pericyte ensheathment of endothelial cells, in tumor sections from control (A-C) and Mac-NG2ko mice (D-F). Z-stacks of confocal images were used to generate three-dimensional reconstructions for this purpose. 10-day tumors in Mac-NG2ko mice exhibit decreased CD31 pixel density (G) due to diminished vessel diameter (H). In addition, the percentage of pericytes lacking physical contact with endothelial cells (I; arrows in F) is increased, resulting in diminished pericyte ensheathment of endothelial cells (J). Scale bar = 20 μm. *P<0.01 compared to controls. Figure taken from ref (90).
Table 1.
Differences in vessel structure and function between control and NG2 null mice.
| Change (versus control mice) |
|||
|---|---|---|---|
| Parameter | Germline-NG2ko mice | PC-NG2ko mice | Mac-NG2ko mice |
| Vessel structure | |||
| Pericyte coverage of endothelial cells | 45% decrease | 33% decrease | 47% decrease |
| Detached pericytes | 200% increase | Unchanged | 181% increase |
| Vessel diameter | 20% decrease* | Unchanged | 53% decrease |
| Basal lamina assembly | 73% decrease | 31% decrease | 72% decrease |
| N-cadherin (endothelial cell) | N.D. | Unchanged | 74% decrease |
| N-cadherin (pericyte) | N.D. | Unchanged | 97% decrease |
| Vessel function | |||
| Vessel patency | 50% decrease | 43% decrease | 48% decrease |
| Vessel leakiness | 400% increase | 270% increase | 416% increase |
| Intratumoral hypoxia | 2000% increase | 560% increase | 1580% increase |
Effects of NG2 ablation on the structure of tumor blood vessels
As expected from our studies of brain tumors in germline NG2 null mice [39], pericyte-specific and macrophage-specific ablation of NG2 both have effects on the structure of tumor blood vessels. Although the underlying mechanisms differ, in both cases these structural deficits appear to result from the loss of interaction between pericytes and endothelial cells. However, because the decrease in pericyte-endothelial cell interaction is larger in Mac-NG2ko mice, the magnitude of structural changes is also greater in these mice. Most striking is the 53% decrease in tumor vessel diameter observed in Mac-NG2ko mice (Fig 3G,H; Table 1), compared to unchanged vessel diameter in PC-NG2ko mice (see also Fig 2B,E). An important contributing factor to reduced vessel diameter in Mac-NG2ko mice is likely to be the greatly reduced assembly of the collagen IV-positive vascular basement membrane. The basal lamina deficit in Mac-NG2ko mice is 72%, compared to 31% in PC-NG2ko mice (Table 1). This loss of perivascular extracellular matrix results in diminished perivascular sequestration of VEGF-A. Although total VEGF-A levels increase almost 4-fold in Mac-NG2ko mice, due to increased hypoxia in these mice (see below), levels of perivascular VEGF-A are decreased 3-fold in these mice. We have previously observed a similar phenomenon in B16F10 brain tumors in collagen VI null mice [91] and in MMTV-PyMT mammary tumors in NG2 null mice [34]. As in the case of Mac-NG2ko mice, the loss of perivascular VEGF localization in these previous studies was correlated with greatly reduced basal lamina assembly. In each case, reduced vessel diameter is the most dramatic structural manifestation of decreased vascular VEGF localization. These results echo the previously-reported importance of perivascular, ECM-bound VEGF in vascular endothelial cell development [41].
Our studies of Mac-NG2ko mice have yielded additional details of NG2-dependent structural changes in tumor blood vessels, emphasizing the observation that diminished pericyte-endothelial cell interactions have deleterious effects on both vascular cell populations [90]. Pericyte maturation, assessed by quantifying the percentage of PDGFRβ-positive pericytes that express the maturation marker αSMA, is reduced by 80% in Mac-NG2ko mice. In parallel, the formation of endothelial cell junctions, determined by quantifying VE-cadherin and ZO-1 expression in CD31-positive endothelial cells, is diminished by 50% in Mac-NG2ko mice. These changes in pericytes and endothelial cells underlie the structural changes discussed above, and provide explanations for the functional changes discussed below.
Effects of NG2 ablation on the function of tumor blood vessels
As seen previously in brain tumors in germline NG2 null mice [39], NG2-dependent changes in vessel structure caused by diminished pericyte-endothelial cell interaction lead to changes in vessel function in both PC-NG2ko and Mac-NG2ko mice (Table 1). Since macrophages are associated more peripherally with microvessels than pericytes [83], macrophages with altered properties would appear to be in a less advantageous position to affect microvessel function. Nevertheless, macrophage-specific ablation of NG2 results in more serious microvascular deficits than pericyte-specific NG2 ablation. Tumor vessel patency falls to 43% of control levels in PC-NG2ko mice, compared to 48% in Mac-NG2ko mice. Vessel leakiness increases almost 3-fold in PC-NG2ko mice, and rises more than 4-fold in Mac-NG2ko mice. As a result of these deficits, intratumoral hypoxia is increased almost 6-fold in PC-NG2ko mice, compared to more than 15-fold in Mac-NG2ko mice. The larger increase in hypoxia in Mac-NG2ko mice probably reflects not only decreased vessel patency and increased vessel leakiness, but also the 2-fold decrease in vessel diameter seen in these mice. All of these factors combine to reduce the tumor blood supply.
The sum of these qualitative and quantitative alterations in tumor vessels may at least partially explain why tumor growth is impacted more severely in Mac-NG2ko mice than in PC-NG2ko mice. It is important to emphasize that our current studies focus on early stages of tumor growth and vascularization, a critical period during which successful tumor vascularization is a major factor in determining progression from hyperplasia to neoplasia [5]. During this period, there may be a fairly direct correlation between effective tumor vascularization and tumor growth.
NG2-dependent mechanisms of pericyte function
NG2 is important for both pericyte recruitment [64] and pericyte interaction with endothelial cells [30]. Because of several pieces of evidence supporting the ability of NG2 to promote activation of β1 integrin signaling [13,30,56], we have focused initially on the importance of this mechanism in pericyte biology. In the case of pericyte recruitment, an in vitro approach was used to demonstrate the involvement of NG2 in β1 integrin activation in pericytes. Treatment of human microvascular brain pericytes with NG2 siRNA results in greater than 90% knockdown of NG2, accompanied by almost 70% reductions in pericyte proliferation and migration [92]. The involvement of β1 integrin activation in these phenomena is suggested by a 2-fold decrease in binding of the activation-dependent β1 monoclonal antibody HUTS-21 [54] to NG2 knockdown pericytes. In addition, NG2 knockdown pericytes exhibit reduced phosphorylation of FAK, a downstream target of β1 integrin signaling.
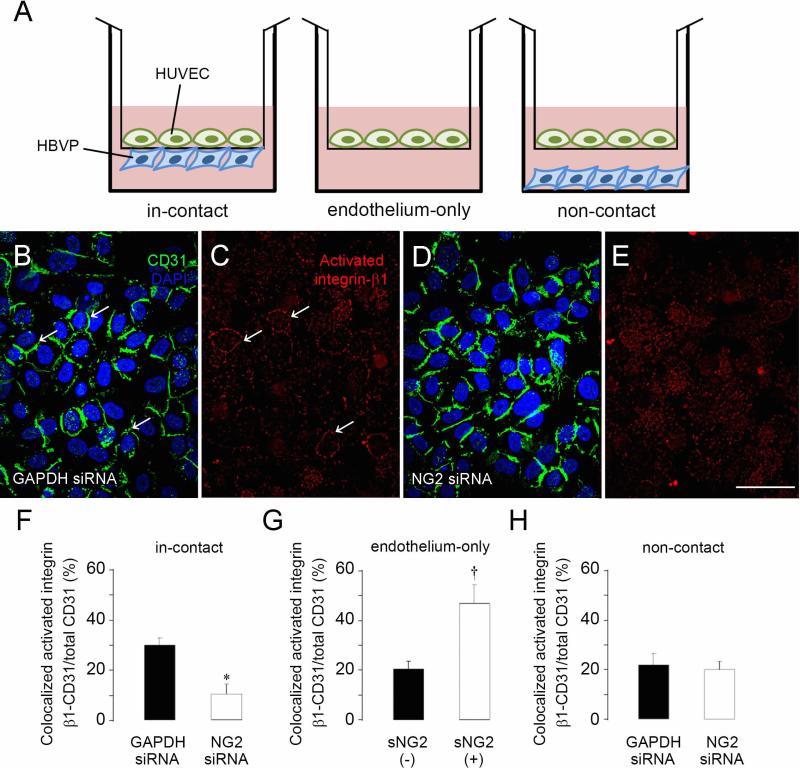
Interestingly, NG2 appears to be able to activate β1 integrin signaling not only in this cis type of format on pericytes, but also in a trans format between pericytes and endothelial cells. Culturing pericytes and endothelial cells on opposite sides of a Transwell membrane with 0.4 μm pores (Fig 4A) shows that physical contact between pericyte and endothelial cell processes is responsible for increased β1 integrin activation in the endothelial cells, leading to enhanced formation of endothelial cell junctions and increased barrier function of the endothelial cell monolayer [92]. NG2 knockdown in the pericyte population reduces β1 integrin signaling in the endothelial cells (Fig 4B-E and F-H), resulting in reduced junction formation and barrier function. In vivo, although NG2-deficient pericytes still interact with endothelial cells, the association is less intimate, as judged by decreased physical overlap between the two cell types. This impairs the development of both cell types, leading to deficits in the assembly of the vascular basal lamina, increased vessel leakiness, and decreased vessel patency [92].
Figure 4. NG2 down-regulation in pericytes decreases activation of β1 integrin in endothelial cells.
(A) Three different in vitro culture formats were used to investigate interactions between human umbilical vein endothelial cells (HUVECs) and human brain vascular pericytes (HBVP). HUVECs and pericytes were cultured on opposite sides of a transwell membrane (in-contact); HUVECs alone were cultured on a transwell membrane (endothelium-only); HUVECs were cultured on a transwell membrane, and pericytes were cultured on the well bottom (non-contact). The in-contact model was used to examine pericyte-mediated activation of endothelial cell β1. Membranes were immunostained for CD31 (green) and activated β1 integrin (red), and confocal microscopy was used to examine the endothelial cell monolayer. CD31 labeling is similar in endothelial cell monolayers co-cultured with either control (B) or NG2 knockdown pericytes (D). However, activated β1 is more prominent and more closely co-localized with CD31 on the surfaces of HUVECs co-cultured with control pericytes (C) than on HUVECs co-cultured with NG2 knockdown pericytes (E, quantified in F). In the endothelium-only model, β1 integrin activation is increased by addition of soluble NG2 (sNG2)(G). In the non-contact model, activation of β1 integrin is not affected by treatment with control or NG2 siRNA (H). Levels of total β1 integrin are not affected in any of the three models (not shown). *P<0.05 compared to GAPDH siRNA (F). †P<0.05 compared to absence of sNG2 (G). Scale bar = 120 μm (B-E). Figure taken from ref (92).
NG2-dependent mechanisms of macrophage function
NG2 does not seem to be expressed by naïve or quiescent monocytes/macrophages. Macrophage expression of NG2 appears to be induced by inflammatory signaling that originates from pathological sites. In vitro, NG2 expression can be induced in monocyte cell lines and in peritoneal or bone marrow-derived macrophages by treatment with PMA or with toll-like receptor agonists such as LPS or poly-I:C . The greatly-reduced macrophage recruitment to tumors after NG2 ablation, in the absence of changes in levels of circulating myeloid cells, suggests that up-regulation of NG2 by inflammatory signals may be important for the ability of macrophages to extravasate from the circulation into the tumor tissue. This idea is bolstered by the fact that at least two integrin-dependent mechanisms are involved in the extravasation process, providing a possible means of NG2 involvement. While the cascade of leukocyte rolling, arrest, crawling, and membrane transmigration is extremely complex, the importance of macrophage α4β1 and αMβ2 integrin interaction with endothelial cell VCAM-1 and ICAM1, respectively, in this process [29,42,79,84] is well-established. Ablation of NG2 might diminish α4β1 and/or αMβ2 activation in macrophages, weakening interactions with endothelial VCAM-1 and/or ICAM-1 to the extent that extravasation is impaired.
The potential validity of this hypothesis is supported by the finding that NG2 promotes activation of both β1 and β2 integrins on human THP-1 cells. NG2 expression is induced in THP-1 cells by treatment with PMA, followed by treatment of the NG2-positive macrophages with control and NG2 siRNA to produce parallel populations of NG2-positive and NG2-negative macrophages. Immunofluorescence with HUTS-21 [54] and mAb24 [19] monoclonal antibodies is then used to quantify activation of β1 and β2 integrins, respectively. Figure 5 shows that levels of activated β1 and β2 are both reduced by NG2 knockdown in the PMA-treated THP-1 cells. Parallel staining for total β1 and β2 indicate that NG2 ablation affects integrin activation without affecting integrin expression levels. Additional work is required to determine if trans-endothelial migration of macrophages is reduced by these effects of NG2 knockdown on integrin activation.
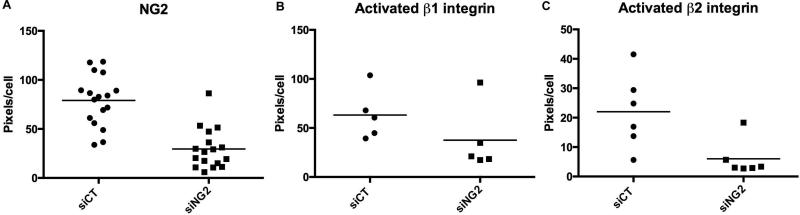
Figure 5. NG2-dependent activation of β1 and β2 integrins in THP-1 macrophages.
Human THP-1 monocytes were differentiated via PMA treatment and then transfected with control siRNA (siCT) or NG2 siRNA (siNG2). NG2 expression (A) was quantified by immunofluorescence with rabbit anti-NG2 antibody, revealing a 70% decrease in NG2 expression in NG2 knockdown cells. Activation of β1 integrin (B) was evaluated by immunolabeling with HUTS-21 monoclonal antibody, revealing a 50% decrease in activation in NG2 knockdown cells. Activation of β2 integrin (C) was investigated by immunolabeling with mAb-24 monoclonal antibody, revealing a 75% decrease in activation in NG2 knockdown cells. Labeling is quantified as pixels per cell. In B and C, levels of total β1 and β2 expression were not significantly altered by NG2 knockdown (not shown). Each data point is obtained from a separate culture well, and numerical values represent the number of pixels per cell averaged from 4 fields in each well. Thus in A, n = 17 wells, in B, n = 5 wells, and in C, n = 6 wells.
Although the forgoing results are relevant to the influence of NG2 on macrophage recruitment, they do not shed any light on mechanisms by which macrophages promote pericyte interaction with endothelial cells. One point to consider in this respect is the possibility that NG2 may only affect macrophage recruitment (i.e. the abundance of tumor macrophages), without affecting mechanisms by which pericyte-endothelial cell interaction are influenced by macrophages that successfully extravasate from the circulation. One clue concerning the effects of macrophages on pericyte-endothelial interaction comes from our observation that levels of N-cadherin expression on both vascular cell types is greatly reduced in tumors in Mac-NG2ko mice [90]. Since N-cadherin expression is not affected in PC-NG2ko mice, reduced N-cadherin expression in Mac-NG2ko mice must be a cause of decreased pericyte-endothelial cell interaction, rather than a result of this decreased interaction. N-cadherin is known to be important for pericyte interaction with endothelial cells [3,33], and is also implicated in up-regulation of VE-cadherin-mediated junction formation by endothelial cells [53]. The extent of pericyte ensheathment of endothelial cells in Mac-NG2ko mice is even lower than that observed in PC-NG2ko mice, possibly due to the loss of N-cadherin expression. These changes are likely to underlie the more extreme changes in microvascular characteristics seen in the Mac-NG2ko mice. These include decreased pericyte maturation, reduced endothelial cell sprouting and junction formation, and diminished assembly of the vascular basement membrane. Additional work will be required to understand fully the impact of lost N-cadherin-mediated interactions between pericytes and endothelial cells. The crosstalk known to exist between N-cadherin and β1 integrin signaling [4,57] makes this an especially intriguing topic in light of the role of NG2 in promoting β1 integrin activation in endothelial cells. Information is also needed regarding the macrophage-dependent signals that control N-cadherin expression in the two microvascular cell types. An interesting candidate in this regard is TGF-β, a product of tumor-associated macrophages [8,22] that has the ability to activate Smad4 in endothelial cells, resulting in upregulation of N-cadherin and stabilized endothelial cell interaction with pericytes [50].
Since we have only examined vascular function in these studies, we must consider that other NG2-dependent macrophage functions may also be important in promoting tumor progression. For example, in the polyoma middle T transgenic mouse model of breast cancer, we have demonstrated that germline ablation of NG2 affects not only tumor vascularization, but also aspects of macrophage phenotype that may be relevant to M1-M2 polarization [34]. We have also reported that NG2 ablation affects the expression of pro-inflammatory and anti-inflammatory cytokines in a tissue-specific manner [34,46]. Classically-activated M1 macrophages produce pro-inflammatory cytokines and can have anti-tumor functions. Conversely, tumor-associated macrophages frequently exhibit an alternatively-activated M2 phenotype, producing anti-inflammatory cytokines that promote tumor growth [68]. Effects of NG2 on macrophage polarization are therefore likely to be highly relevant to macrophage impact on tumor progression. These possibilities remain to be investigated.
Significance
The importance of vascularization for tumor growth along with the expression/activity of NG2 in both pericytes and macrophages suggests that the proteoglycan can be a key stromal factor in the development of all solid tumors. NG2 expression by pericytes is low in quiescent vasculature, but is quickly up-regulated in any situation that involves vascular remodeling, including tumor growth. Although NG2 expression by macrophages is poorly understood, our experience suggests that the proteoglycan is not expressed by quiescent monocytes/macrophages, but is quickly up-regulated upon activation of myeloid cells by signals such as ligand binding to toll-like receptors. This suggests that NG2 may be a very useful marker for activated macrophages. Even though transient, NG2 expression is nevertheless sufficient to transform the properties of these cells during early stages of recruitment and extravasation. The universality of macrophage infiltration in all types of inflammatory situations indicates that NG2 may represent an important aspect of macrophage recruitment in multiple pathologies. An additional example can be found in our work on CNS demyelination and remyelination, in which macrophage-specific ablation of NG2 also greatly diminishes macrophage recruitment to demyelinated lesions [47]. Taken together, these findings emphasize the possible value of targeting pericytes and macrophages as a means of improving therapies for cancer and other pathologies with inflammatory components.
Perspectives
The development of tumor blood vessels depends on intimate interactions between endothelial cells and pericytes that lead to the formation of patent vessels with tight endothelial junctions and a mature vascular basement membrane. The NG2 proteoglycan mediates two sets of interactions that are important for pericyte-endothelial cell communication: (1) direct pericyte-mediated activation of β1 integrin signaling in endothelial cells, and (2) β1 and β2 integrin-mediated recruitment of tumor macrophages that provide additional signals required to promote pericyte-endothelial cell interaction. NG2 expression thus characterizes and underlies the function of two key stromal cell populations (pericytes and macrophages) that play critical supporting roles in the tumor microenvironment.
ACKNOWLEDGEMENTS
We thank Ms. Regina Kapono for assistance with manuscript preparation. NG2 floxed mice were generously provided by Dr. Kenji Sakimura. This work was supported by funding from NIH R01 CA095287, NIH P30 CA030199 pilot project, and the Sanford Burnham Prebys Laboratory Funding Initiative.
LIST OF ABBREVIATIONS
- ECM
Extracellular matrix
- EGFP
Enhanced green fluorescent protein
- FAK
Focal adhesion kinase
- FITC
Fluorescein isothiocyanate
- Floxed
Flanked by LoxP sites
- HIF
Hypoxia-inducible factor
- ICAM-1
Intercellular adhesion molecule 1
- LEA
Lycopersicon esculentum agglutinin
- LPS
Lipopolysaccharide
- LysM
Lysozyme M
- Mac
Macrophage
- MMTV-PyMT
Polyoma virus middle T oncogene driven by mouse mammary tumor virus promoter
- NG2
Neuron-glia antigen 2
- PC
Pericyte
- PDGFRβ
Platelet-derived growth factor receptor β
- ΠMA
Πηoρbol myristate acetate
- Poly I:C
Poly-inosine:cytosine
- TAM
Tumor associated macrophage
- TGFβ
Transforming growth factor receptor β
- TEM
Tie2-expressing monocyte
- VCAM-1
Vascular cell adhesion molecule 1
- VEGF
Vascular endothelial growth factor
REFERENCES
- 1.Al-Mayhani MT, Grenfell R, Narita M, Piccirillo S, Kenney-Herbert E, Fawcett JW, Collins VP, Ichimura K, Watts C. NG2 expression in glioblastoma identifies an actively proliferating population with an aggressive molecular signature. Neuro-oncology. 2011;13:830–845. doi: 10.1093/neuonc/nor088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells, tissues, organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 3.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 4.Arregui C, Pathre P, Lilien J, Balsamo J. The nonreceptor tyrosine kinase fer mediates cross-talk between N-cadherin and beta1-integrins. The Journal of cell biology. 2000;149:1263–1274. doi: 10.1083/jcb.149.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature reviews. Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 6.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. The Journal of clinical investigation. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- 10.Cattaruzza S, Nicolosi PA, Braghetta P, Pazzaglia L, Benassi MS, Picci P, Lacrima K, Zanocco D, Rizzo E, Stallcup WB, Colombatti A, Perris R. NG2/CSPG4-collagen type VI interplays putatively involved in the microenvironmental control of tumour engraftment and local expansion. Journal of molecular cell biology. 2013;5:176–193. doi: 10.1093/jmcb/mjt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaruzza S, Ozerdem U, Denzel M, Ranscht B, Bulian P, Cavallaro U, Zanocco D, Colombatti A, Stallcup WB, Perris R. Multivalent proteoglycan modulation of FGF mitogenic responses in perivascular cells. Angiogenesis. 2013;16:309–327. doi: 10.1007/s10456-012-9316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang Y, She ZG, Sakimura K, Roberts A, Kucharova K, Rowitch DH, Stallcup WB. Ablation of NG2 proteoglycan leads to deficits in brown fat function and to adult onset obesity. PLoS One. 2012;7:e30637. doi: 10.1371/journal.pone.0030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chekenya M, Krakstad C, Svendsen A, Netland IA, Staalesen V, Tysnes BB, Selheim F, Wang J, Sakariassen PO, Sandal T, Lonning PE, Flatmark T, Enger PO, Bjerkvig R, Sioud M, Stallcup WB. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 15.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochimica et biophysica acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.de Castro R, Jr., Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 17.De Palma M, Naldini L. Tie2-expressing monocytes (TEMs): novel targets and vehicles of anticancer therapy? Biochimica et biophysica acta. 2009;1796:5–10. doi: 10.1016/j.bbcan.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 19.Dransfield I, Cabanas C, Barrett J, Hogg N. Interaction of leukocyte integrins with ligand is necessary but not sufficient for function. The Journal of cell biology. 1992;116:1527–1535. doi: 10.1083/jcb.116.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Druker BJ. STI571 (Gleevec) as a paradigm for cancer therapy. Trends in molecular medicine. 2002;8:S14–18. doi: 10.1016/s1471-4914(02)02305-5. [DOI] [PubMed] [Google Scholar]
- 21.Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer letters. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Seminars in oncology. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 24.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 27.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer research. 1999;59:99–106. [PubMed] [Google Scholar]
- 28.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Forlow SB, White EJ, Barlow SC, Feldman SH, Lu H, Bagby GJ, Beaudet AL, Bullard DC, Ley K. Severe inflammatory defect and reduced viability in CD18 and E-selectin double-mutant mice. The Journal of clinical investigation. 2000;106:1457–1466. doi: 10.1172/JCI10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 32.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. The Journal of cell biology. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Gibby K, You WK, Kadoya K, Helgadottir H, Young LJ, Ellies LG, Chang Y, Cardiff RD, Stallcup WB. Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the neuron-glial antigen 2 proteoglycan. Breast Cancer Res. 2012;14:R67. doi: 10.1186/bcr3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112(Pt 6):905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 38.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature cell biology. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 39.Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes S, Chang-Ling T. Roles of endothelial cell migration and apoptosis in vascular remodeling during development of the central nervous system. Microcirculation. 2000;7:317–333. [PubMed] [Google Scholar]
- 41.Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003;17:1520–1522. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 42.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer research. 2006;66:2146–2152. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 43.Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadoya K, Fukushi J, Matsumoto Y, Yamaguchi Y, Stallcup WB. NG2 proteoglycan expression in mouse skin: altered postnatal skin development in the NG2 null mouse. J Histochem Cytochem. 2008;56:295–303. doi: 10.1369/jhc.7A7349.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer research. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB. Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. J Neuroinflammation. 2011;8:158. doi: 10.1186/1742-2094-8-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucharova K, Stallcup WB. NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair. J Neuroinflammation. 2015;12:161. doi: 10.1186/s12974-015-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272:26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- 49.Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Developmental cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 52.Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, Spannuth W, Arumugam T, Han LY, Jennings NB, Logsdon C, Jaffe RB, Coleman RL, Sood AK. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin Cancer Res. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 53.Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. The Journal of cell biology. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 55.Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer research. 2008;68:8066–8075. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makagiansar IT, Williams S, Mustelin T, Stallcup WB. Differential phosphorylation of NG2 proteoglycan by ERK and PKCalpha helps balance cell proliferation and migration. The Journal of cell biology. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monier-Gavelle F, Duband JL. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. The Journal of cell biology. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 59.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 62.Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- 63.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Persson AI, Petritsch C, Swartling FJ, Itsara M, Sim FJ, Auvergne R, Goldenberg DD, Vandenberg SR, Nguyen KN, Yakovenko S, Ayers-Ringler J, Nishiyama A, Stallcup WB, Berger MS, Bergers G, McKnight TR, Goldman SA, Weiss WA. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18:669–682. doi: 10.1016/j.ccr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 67.Price MA, Colvin Wanshura LE, Yang J, Carlson J, Xiang B, Li G, Ferrone S, Dudek AZ, Turley EA, McCarthy JB. CSPG4, a potential therapeutic target, facilitates malignant progression of melanoma. Pigment cell & melanoma research. 2011;24:1148–1157. doi: 10.1111/j.1755-148X.2011.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribatti D, Crivellato E. Immune cells and angiogenesis. Journal of cellular and molecular medicine. 2009;13:2822–2833. doi: 10.1111/j.1582-4934.2009.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 71.Risau W, Flamme I. Vasculogenesis. Annual review of cell and developmental biology. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 72.Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubin JB. Only in congenial soil: the microenvironment in brain tumorigenesis. Brain Pathol. 2009;19:144–149. doi: 10.1111/j.1750-3639.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saharinen P, Alitalo K. Double target for tumor mass destruction. The Journal of clinical investigation. 2003;111:1277–1280. doi: 10.1172/JCI18539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlingemann RO, Rietveld FJ, de Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990;136:1393–1405. [PMC free article] [PubMed] [Google Scholar]
- 76.Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith FO, Rauch C, Williams DE, March CJ, Arthur D, Hilden J, Lampkin BC, Buckley JD, Buckley CV, Woods WG, Dinndorf PA, Sorensen P, Kersey J, Hammond D, Bernstein ID. The human homologue of rat NG2, a chondroitin sulfate proteoglycan, is not expressed on the cell surface of normal hematopoietic cells but is expressed by acute myeloid leukemia blasts from poor-prognosis patients with abnormalities of chromosome band 11q23. Blood. 1996;87:1123–1133. [PubMed] [Google Scholar]
- 78.Stallcup WB, Huang FJ. A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr. 2008;2:192–201. doi: 10.4161/cam.2.3.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nature immunology. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 80.Stenzel D, Nye E, Nisancioglu M, Adams RH, Yamaguchi Y, Gerhardt H. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–924. doi: 10.1182/blood-2008-10-186239. [DOI] [PubMed] [Google Scholar]
- 81.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Hanecker P, Ayers-Ringler J, Phillips J, Siu J, Lim DA, Vandenberg S, Stallcup W, Berger MS, Bergers G, Weiss WA, Petritsch C. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20:328–340. doi: 10.1016/j.ccr.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 84.Ulyanova T, Priestley GV, Banerjee ER, Papayannopoulou T. Unique and redundant roles of alpha4 and beta2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Experimental hematology. 2007;35:1256–1265. doi: 10.1016/j.exphem.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Svendsen A, Kmiecik J, Immervoll H, Skaftnesmo KO, Planaguma J, Reed RK, Bjerkvig R, Miletic H, Enger PO, Rygh CB, Chekenya M. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS One. 2011;6:e23062. doi: 10.1371/journal.pone.0023062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Osada T, Wang Y, Yu L, Sakakura K, Katayama A, McCarthy JB, Brufsky A, Chivukula M, Khoury T, Hsu DS, Barry WT, Lyerly HK, Clay TM, Ferrone S. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst. 2010;102:1496–1512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wesseling P, Schlingemann RO, Rietveld FJ, Link M, Burger PC, Ruiter DJ. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol. 1995;54:304–310. doi: 10.1097/00005072-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yotsumoto F, You WK, Cejudo-Martin P, Kucharova K, Sakimura K, Stallcup WB. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology. 2015;4:e1001204. doi: 10.1080/2162402X.2014.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.You WK, Bonaldo P, Stallcup WB. Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina. Am J Pathol. 2012;180:1145–1158. doi: 10.1016/j.ajpath.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.You WK, Yotsumoto F, Sakimura K, Adams RH, Stallcup WB. NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function. Angiogenesis. 2014;17:61–76. doi: 10.1007/s10456-013-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan L, Siegel M, Choi K, Khosla C, Miller CR, Jackson EN, Piwnica-Worms D, Rich KM. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene. 2007;26:2563–2573. doi: 10.1038/sj.onc.1210048. [DOI] [PubMed] [Google Scholar]