Abstract
Objective
To further understand the association between semen quality and cancer risk using well-defined semen parameters.
Design
Retrospective cohort study.
Setting
Subfertility Heath and Assisted Reproduction (SHARE) study in Utah from 1994 to 2011.
Patients
20,433 men from that underwent semen analysis (SA) and a sample of 20,433 fertile controls matched on age and birth year
Interventions
none.
Main Outcome Measures
Risk of all cancers, as well as site-specific results for prostate, testicular, and melanoma.
Results
Relative to fertile men, men with SA have an increased risk of testicular cancer (Hazard Rate Ratio (HR) =3.3). When the characterization of infertility is refined using individual semen parameters, we find that oligozoospermic men have an increased risk of cancer relative to fertile controls. This association is particularly strong for testicular cancer, with increased risk in men with oligozoospermia based on concentration (HR=11.9) and sperm count (HR=10.3). Men in the in the lowest quartile of motility (HR=4.1), viability (HR=6.6), morphology (HR=4.2) or total motile count (HR=6.9) have higher risk of testicular compared to fertile men. Men with sperm concentration and count in the 90th percentile of the distribution (≥178 M/ml and ≥579, respectively) and total motile count (TMC) have an increased risk of melanoma (HRConcentration=2.1; HRCount=2.7; HRTMC=2.0). We find no differences in cancer risk between azoospermic and fertile men.
Conclusions
Men with SA have an increased risk of testicular cancer that varies by semen quality. Unlike prior work, we did not find an association between azoospermia and increased cancer or testicular cancer risk.
Capsule
Subfertile men have an increased risk of testicular cancer that varies by semen quality. We did not find an association between azoospermia and increased cancer or testicular cancer risk.
Keywords: semen quality, cancer, testicular cancer, infertility
Introduction
Male infertility evaluation is common, with an estimated 7.5% of American men reporting at least one visit to an assisted reproduction clinic during their lifetime. Multiple cohort studies have identified an association between infertility and increased risk of testicular and prostate cancer (1-3). There is emerging evidence that multiple mechanisms may be responsible for the increased risk of cancer in the subfertile male. In brief, Y-chromosome deletions, epigenetic hypermethylation, DNA mismatch repair gene deletions/mutations, and aneuploidy are all mechanisms identified in cell culture, animal models, and human tissues, which have been shown to contribute to infertility and cancer (4-9). A more complete understanding of the association between cancer and infertility will help elucidate the underlying mechanisms, and contribute to our understanding of male somatic health.
Previous studies addressing this question have examined cancer risk by broadly categorizing men with male factor infertility or azoospermia. Only one study has reported cancer risk based on sperm concentration and motility(10) and no studies to date have explored the association between viability, morphology and cancer risk. We also improve upon previous studies by comparing the risk of cancer in men seen in a fertility clinic, subfertile men, to fertile men not seeking care for fertility problems.
The Subfertility Heath and Assisted Reproduction (SHARE) study combines medical, genealogical, and administrative data with biospecimen data to create a unique data resource that can be used to evaluate the association between fertility and morbidity and mortality. SHARE combined the University of Utah and Intermountain Health Care’s semen analysis (SA) measures collected from 1996 - 2011 with longitudinal health and cancer information in the Utah Population Database (UPDB) and the Utah Cancer Registry to retrospectively examine semen characteristics and subsequent risk of cancer. We sought to further understand the risk of cancer in men with SA relative to an age- and birth year-matched cohort of fertile men using multiple semen parameters, including sperm concentration, count, motility, viability, total motile count (TMC), and morphology. We hypothesize that men with SA have an increased risk of all cancers as well as testicular and prostate cancer and melanoma. We also hypothesize that variation in risk exists among men with SA that can be partially explained by several measures of semen quality.
Materials and Methods
Data
Measures of semen quality were extracted from two healthcare systems in Utah that when combined capture an estimated 90% of all semen analyses performed in the state since 2004. The data were used to perform a retrospective cohort analysis of cancer risk in men with SA relative to an age-matched fertile male cohort for those age 18 or older. First, all men presenting for infertility at the University of Utah Andrology Clinic from 1996 – 2011 with semen samples were selected for the analysis. Due to a change in database systems, data from August of 2001 to September of 2002 were not available; however, this omission is a random event and unrelated to individual characteristics, cancer incidence, or technologies designed to measure semen quality, and therefore does not introduce any systematic bias into our analysis. Second, we selected men with SA parameters stored in Intermountain Healthcare’s Sunquest system from 2002 to 2011. The Sunquest Lab database is part of Intermountain Healthcare Analytic Health Repository and stores all lab data from Intermountain Healthcare facilities in the state of Utah.
This study required the integration of demographic and follow-up information within the Utah Population Database (UPDB). The UPDB has supported numerous biodemographic, epidemiological, and genetic studies in large part because of its sample size, pedigree complexity, and linkages across data sources, including statewide birth certificate records and cancer diagnoses.(11, 12) Due to longstanding and ongoing efforts to add new sources of data and update records as they become available, the full UPDB contains data on nearly 8 million individuals.(13, 14) Of the 22,456 men with semen samples collected during this period, 94% (N=21,214) were linked to the UPDB. We excluded men who were not Utah residents or were lacking adequate follow-up information (N=370). Men with any cancer diagnosis prior to the semen analyses were excluded (N=389) and men with poor data quality in the UPDB were also excluded (N=25). This yielded a final sample of 20,433 men with measured semen parameters; 8,186 men with samples from the Intermountain Healthcare clinics from 2002 – 2011 and 12,247 men with samples from the University of Utah clinic from 1996 - 2011.
Fertile men were selected from the UPDB by matching men by age at time of semen analysis and birth year using a 1:1 matching ratio. Fertile men were those that had at least one naturally conceived child and were cancer free prior to the time of semen analysis of their matched counterpart, and did not have semen analysis data. They were also required to have adequate follow-up information within the UPDB and a Utah resident. Our final sample comprised 40,866 men allowing us to compare men with SA, i.e. partners in a subfertile couple, to a cohort of fertile men.
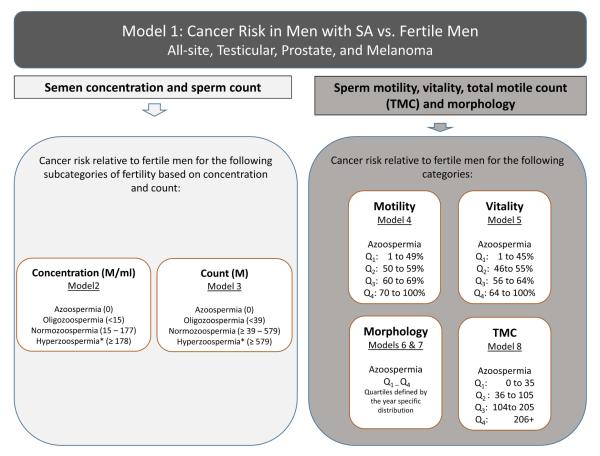
Semen analyses were performed in accordance with the WHO manual for examination and processing of human semen. Figure 1 details the cut-points used for each semen parameter used in the analysis. We analyzed sperm concentration (millions per mL), sperm count (million), and sperm motility (percent of sperm with progressive forward motility). Approximately 17% of men in our final sample had more than one semen sample (range 1 to 10), in which case the average concentration, count, and motility across measures was used. Sensitivity analyses using the minimum and maximum values were also performed. The cut-points for the concentration and count categories are displayed in Figure 1. Measures of viability, TMC and morphology were only available for the samples collected at the University of Utah (n=12,247). Sperm viability and TMC categories were defined either as azoospermia or by quartile defined by the distribution of the measures over the full 16-year period. WHO guidelines and thresholds for morphologically normal spermatozoa have changed over the 16-year span of data (15, 16). To account for this change over time, we classified percent normal heads and tails into quartiles using the distribution for each year of measurement rather than the overall distribution. Like the other measures, this resulted in five categories; azoospermia and Q1 through Q4. This, coupled with our analytic strategy, allowed us to account for the change over time in the measures. Sperm morphology is measured using the percent normal for heads and tails separately. Parity was considered as a possible effect modifier, however, due to the relatively rare event of testicular cancer, distributing the data into smaller categories of risk led to unstable estimates and the results are not reported here.
Figure 1.
Overview of modeling strategy and categorization of semen quality based on measure. Measures of viability, morphology, and total motile count are only available for the data from the University of Utah.
Cancer diagnoses were derived from the Utah State Cancer Registry (UCR), an original member of the National Cancer Institute Surveillance Epidemiology and End Result (SEER) program, which is linked to the UPDB. The UCR data include all cancer diagnoses for Utah residents from 1966 through 2012. Incident cases of cancer are identified based on systematic and routine review of medical records, pathology reports, radiation therapy records, hospital discharge lists, and vital records.
This study was approved by the Institutional Review Boards of the University of Utah and IHC and by the Utah Resource for Genetic and Epidemiologic Research (www.research.utah.edu/rge/), an administrated board that oversees access to the UPDB. IRB_00069711
Statistical Methods
Cox proportional hazard regression models were used to test the association between semen quality and incident cancer diagnosis. Time was measured as months from the date of semen analysis to the time of cancer diagnosis, death, last follow-up date, or end of the observation period. All models were stratified by birth year, year of sample collection, and data source (IH or UU). Fertile men not presenting at a fertility clinic were used as the reference group in all analyses presented in the supplementary table. Ancillary analyses using the normozoospermic men as the reference category were also run and when relevant, the results are presented in the text. Test for trend analyses excluding the fertile controls were conducted to test the hypothesis that cancer risk decreases as semen quality increases. Figure 1 shows the modeling strategy used for the analyses. Analyses were performed for all cancers combined, melanoma, testicular, and prostate cancer (the three most commonly occurring cancers in the sample). In addition, models investigating the risk of all-site cancers excluding testicular, melanoma, and prostate were estimated. Non-proportional models were used to test for the change in the risks over time (1 year, 2 years, and 3+ years) of infertility and semen quality measures.
Results
Table 1 shows the descriptive statistics for the full sample and the number of cancer diagnoses by fertility status. The mean age at semen analysis was 32 and the mean number of semen samples per male was 1.2. On average, men were followed for 7.3 years and a maximum of 18 years. There were 421 total men with a cancer diagnosis in the men with SA and fertile men, and the most common cancers were: melanoma (n=98), testicular (n=40), and prostate (n=58). Overall, there was a slightly higher number of cancer diagnoses for the men with SA compared to the fertile controls (n=218 vs. 203). Men with SA had a higher number of melanoma cases (n=54 vs 44) and three times more testicular cancer diagnoses (n=30 vs. 10) than the fertile group.
Table 1.
Descriptive Statistics
|
Full Sample* (n=40,866)
|
||||
|---|---|---|---|---|
| Variable | Mean | Std Dev |
Min | Max |
| Birth Year | 1973.3 | 7.8 | 1923 | 1990 |
| Year of Semen Analysis | 2005.3 | 4.2 | 1996 | 2011 |
| Age at Time of Semen Analysis | 32.0 | 6.4 | 18 | 77 |
|
Number of Cancer Diagnoses
| |||
|---|---|---|---|
| Full Sample (n=40,866) |
Subfertile (n=20,433) |
Fertile (n=20,433) |
|
| Cancer | N | N | N |
| Any Cancer | 421 | 218 | 203 |
| Melanoma | 98 | 54 | 44 |
| Prostate Cancer | 58 | 27 | 31 |
| Testicular Cancer | 40 | 30 | 10 |
| Other Cancer** | 225 | 107 | 118 |
The subfertile and fertile cohorts are matched using these variables, so there is no variation by fertility status, thus means for the full sample are shown.
Excluding Prostate, Melanoma, and Testicular cancers.
We summarized the analyses performed graphically in Figure 1 and the results are displayed in the supplemental Table 1. When comparing men with SA in our sample to fertile men, we find that men with SA are at increased risk of testicular cancer (HR=3.3, 95% CI 1.6, 6.9). There are no significant differences in cancer risk for the other common sites or overall risk of cancer. Models 2 – 8 in supplemental Table 1 show the risk of cancer by semen characteristic. Results are discussed below by cancer site for these models. All results are presented in supplemental Table 1 unless otherwise noted.
All Cancers
We found that men with oligozoospermia have an increased risk of cancer (all combined sites) (HRConcentration=1.7, 95% CI: 1.1-2.4; and HRCount=1.8, 95% CI: 1.2-2.6) when compared to fertile men. This risk is not shared by the men with normal or high concentration and count. When compared to normozoospermic men, we found similar results with oligozoospermic having and increased risk of cancer (HRConcentration=1.5, 95% CI: 1.1-2.2; and HRCount=1.7, 95% CI: 1.2-2.5) and no difference in risk between normozoospermic men and azoospermic or hyperzoospermic men. Tests for trend excluding the fertile controls were conducted to determine if the risk of cancer increased with decreasing categories of semen quality. We did not find a significant trend for count or concentration. Men in the lowest quartile of motility, viability, and TMC have an increased risk of cancer (HR= 1.5, 95%CI: 1.1-2.0; HR=1.4, 95%CI: 1.0-1.9; and HR=1.6, 95%CI: 1.2-2.1, respectively). However, we did not find an elevated risk of cancer in azoospermic men (HR=1.0; 95%CI: 0.5-2.1). Tests for trend demonstrate an increased risk of cancer with decreasing sperm motility categories (HR=1.19, 95%CI: 1.1-1.4), but not morphology, viability, or TMC (results not shown but available upon request).
Prostate Cancer
We found no association between semen quality and prostate cancer risk in this cohort of men. The majority of men in the sample have not reached the age normally associated with prostate cancer. Therefore, we conducted a series of sensitivity analyses excluding men under the age of 60, men under the age of 50, and men under the age of 40 at their last time of follow-up, which provided us with sample of 655 men, 4,696 men, and 18,290 men, respectively. There was no significant relationship between infertility and prostate cancer risk for these men, likely due to the relatively short follow-up time and young age at baseline.
Melanoma
The relationship between semen quality and melanoma differs from the other cancer sites. There was little association between semen quality and melanoma risk. However, there was a two-fold increase in the risk of melanoma in hyperzoospermic men (HRConcentration =2.1, 95%CI: 1.0-4.4; and HRCount=2.7, 95%CI: 1.4-5.3) relative to fertile controls. However, when compared to normozoospermic men, there is no difference in the risk of melanoma. Men within the highest quartile of TMC have a doubling risk of melanoma (HR=2.0, 95%CI: 1.0-3.87). We saw a similar pattern in the other measures of semen quality; higher categories were associated with an increased risk of melanoma relative to fertile controls, however the differences were not statistically significant. Tests for trend were also insignificant.
Testicular Cancer
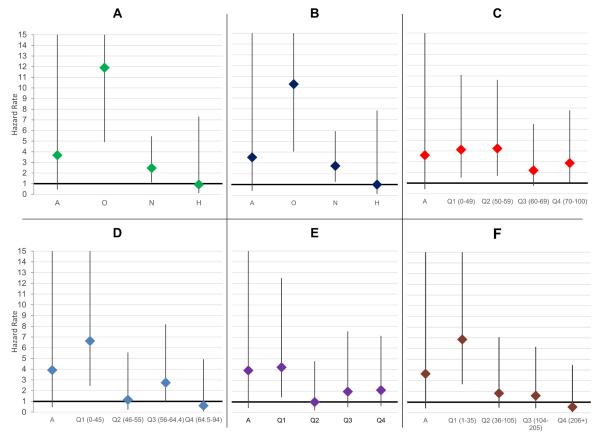
Figure 2 shows the risk of cancer by semen parameter. Poor semen quality was associated with an increased risk of testicular cancer for all measures excluding tail morphology. Azoospermic men were not at significantly higher risk for testicular cancer; however, this may be an artifact of small sample size. Men with oligozoospermia had a greater than ten-fold increase in the risk of testicular cancer (HRConcentration=11.9, 95%CI 4.9-28.8; HRCount=10.3, 95%CI 4.1-26.2) and normozoospermic men presenting for semen analysis had a nearly three-fold increase (HR=2.9; 95%CI 1.2-6.7) relative to fertile men. Compared to normozoospermic men, oligozoospermic men had a nearly four-fold increase in the risk of testicular cancer (HR=3.8; 95%CI 1.6-8.7). Hyperzoospermic men (sperm concentration in the 90th percentile; > 178 M/ml) had testicular cancer rates similar to both the fertile population and the normozoopermic men. For the men with SA, trend tests show a significant increase in testicular cancer risk with a decline in sperm concentration categories (HR=1.6, 95%CI 1.2-2.0). A similar pattern existed with sperm count, with men in lower count categories having a higher risk of testicular cancer (trend test HR=1.5, 95%CI 1.2-2.0) and no difference in risk between hyperzoospermic and fertile men.
Figure 2.
Testicular cancer hazard rates by semen quality: Men with SA vs. Fertile Males. Estimates are also displayed in supplemental table 1. Panel A shows the risk of testicular cancer by semen concentration category. Panel B shows the risk by sperm count. Panel C displays the risk by category of motility. Panel D displays the risk by category of viability. Panel E shows the association between head morphology and cancer risk (tails are not shown). Panel F displays the results for total motile count. A: Azoospermic; O: Oligozoospermic; N: Normozoospermic; H: Hyperzoospermic.
With the exception of azoospermic men, we found an increased risk of testicular cancer as motility, viability, and TMC decline (HR(Motile Trend)=1.3, 95%CI 1.1-1.5; HR(Viability Trend)=1.3, 95%CI 1.1-1.5); and HR(TMC Trend)=1.3, 95%CI 1.1-1.6). There is a less distinct pattern with morphology. We found that men in the lowest quartile of head morphology have an increased risk of testicular cancer (HR=4.2, 95% CI=1.4-12.5) and no significant difference in risk for the other quartile. When we considered tail morphology, we did not find a significant increase in risk for men in the lowest quartile, however men in the second quartile had a five-fold increase in risk (HR=5.3, 95%CI=1.9-14.8).
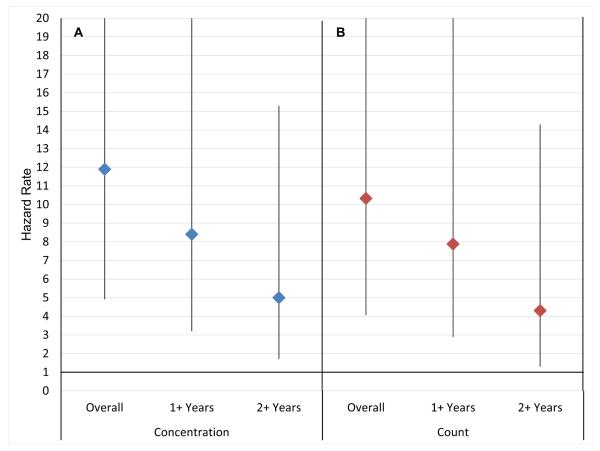
Within Cox regressions, non-proportionality tests show that there was significant attenuation in the magnitude of the risks over time, but that the risks persist one year after semen collection date (results not shown). Figure 3 shows the risk of testicular cancer is highest in the years immediately following the semen collection and declines over time for all semen quality measures. For concentration, we found that the hazard rate one year after semen collection drops to 8.4 (95%CI 3.2-22.1) and then declines further to 5.0 (95%CI 1.7-15.3) two years post collection. There was a similar pattern of attenuation over time with count motility, viability, and TMC. When accounting for non-proportional risks when the parameter of interest is morphology we found that there is a significantly elevated risk of testicular cancer for men in the lower 50% of the distribution of heads and tails (quartiles one and two), and this effect declined over time.
Figure 3.
Testicular cancer hazard rates for oligozoospermic vs. fertile males by time since semen analysis. Panel A shows the risk of testicular cancer when oligozoospermia is measured using concentration. Panel B shows the risk of testicular cancer when oligozoospermia is measured using count.
Other Sites
All-Site models excluding testicular and prostate cancer were estimated to assess the relationship between semen quality and non-sex specific cancer risk. We found that once testicular cancer was removed from the analyses, the relationship between semen quality and all site-cancer risk was attenuated.
Sensitivity Analyses
All models were repeated using the minimum and maximum values of multiple semen samples collected. We did not find substantive differences by value (minimum, maximum, or average) and therefore chose to report the average. Results from these analyses are not displayed here, but are available upon request.
Discussion
This is the first study to calculate the cancer risk of men seen in a fertility clinic compared to known fertile controls not seeking fertility treatment. We found an approximately 50% increase in the risk of cancer for men with poor semen quality but no increased risk for azoospermic men. Testicular cancer is highly associated with abnormal semen parameters across all measures. Interestingly, we did not see any association between azoospermia and the risk of cancer, including testicular cancer.
Epidemiologic study designs have been employed to study the association between male infertility and cancer, however studies are inconsistent in the definition of male infertility, or use of fertile men for comparison. Studies without semen analysis data have used fatherhood as a proxy for male factor infertility and yielded null results (17). Administration claims data have also been used to identify subfertile men when biospecimen data are not available. Eisenberg et al. demonstrated that the diagnosis of male infertility based on Current Procedural Terminology codes increased a man’s risk of being diagnosed with any cancer, prostate, and testicular cancers with hazard ratios of 1.49, 1.78, and 1.99, respectively.(18) While crude measures of infertility hint at an increased risk of cancer in subfertile men, our results show that having well defined measures of semen quality is essential for increasing our knowledge about the relationship between fertility and somatic health in males. A large proportion of men with SA in the study have normal semen parameters. It is important to note that these men themselves may not be subfertile, but they are subfertile at the couple level (i.e., the female partner may have infertility problems). In addition, caution should be practiced when interpreting our results, as we do not have semen measures on our sample of fertile men.
Studies that are able to use semen parameters to define fertility have consistently shown that poor semen quality is associated with an increased risk of cancer. Two studies by Walsh et al. used a cohort of men from California infertility centers, where they had providers report a binary presence of male factor infertility based on semen analyses, and they showed that subfertile men were 2.6 and 2.8 times more likely to be diagnosed with high–grade prostate cancer and testicular cancer, respectively (1, 2). Jacobsen and colleagues linked multiple Danish health registries with a large cohort of men with semen analyses, and the risk of testicular cancer was greater for men with low concentration, poor motility, and abnormal morphology (10). The results presented in this paper highlight the variability of risk within fertile men, with lower semen parameters being associated with higher risk of cancer. Our findings also improve upon previous research by showing that abnormal morphology, viability, and TMC are also associated with an increased risk of testicular cancer. Further, our work compares men with SA to those with proven paternity.
We did not find an association between azoospermia and cancer risk. However, other studies have reported an increased risk of cancer in these men (3, 10). This suggests the processes between total spermotogenic arrest seen in azoospermic men and somatic mutations may be distinct. We hypothesize that the genetic insults in gametes that cause abnormal concentration, count, and TMC must share similar molecular pathways to aberrant cell proliferation in cancer cells. It is also possible that our null result is an artifact of small sample size. Future studies are needed to investigate the risk of cancer in azoospermic men, parity, and the mechanisms driving the association.
The association between high levels of sperm concentration and TMC and melanoma is also a novel finding. It is possible that men with higher sperm counts have higher levels of melanoma cell adhesion molecule (MCAM), which has been shown to be critical for spermatogonial stem cell proliferation in animal models (19) and has been demonstrated to drive melanoma progression (20). These findings will need to be validated in other cohorts.
Although we used fertile, age-matched men to compare the men evaluated in an assisted reproductive clinic there are several notable limitations. First, we were unable to include potential confounders such as medical comorbidities, smoking status, or carcinogenic exposure. Second, men with semen analysis are seen at the University of Utah or Intermountain Health Care clinics for a fertility evaluation. These men are a select subsample of the population that are experiencing fertility problems at the couple level and have the resources to be evaluated by a physician. Third, we do not have a measure of semen quality for the fertile controls. Fourth, We chose to categorize morphology into equally distributed quartiles due to the fact that the WHO threshold for normal motility has changed multiple times during our study period. This study also involves men living in Utah, which has less racial and ethnic diversity compared to other parts of the country. Lastly, this database is relatively young. In the years to come, as the population ages, we may see that the risk of prostate cancer for this cohort displays risk similar to that reported in prior work.
The UPDB affords a unique opportunity to utilize the epidemiologic power to identify novel cohorts and ascertain pedigree data and fertile controls. Along with the clinical data that are linked to these men, we also have over 4,000 linked biospecimens available for future genetic studies. In future studies, we plan to select a cohort of men who later developed testicular cancer after semen analysis to locate associated genetic or epigenetic changes based on semen parameters.
Conclusions
Common biological mechanisms may be underlying the association between semen quality and cancer risk. Men with SA have an increased risk of testicular cancer that varies with semen quality and declines with time since semen analysis. Unlike prior work, we did not find an association between azoospermia and increased cancer or testicular cancer risk.
Supplementary Material
Supplemental Table 1. Semen parameters and cancer risk in men with SA versus fertile men
Acknowledgements
This work was supported by the National Institutes of Health – National Institute of Aging [Grant Numbers 1R21AG036938-01, 2R01 AG022095]. The authors wish to thank the Huntsman Cancer Foundation for database support provided to the Pedigree and Population Resource of the HCI, University of Utah. We also thank Alison Fraser, Justin Berger, Diana Phuong Thai, and Diana Lane Reed for valuable assistance in managing the data. Partial support for all datasets within the UPDB was provided by the HCI Cancer Center Support Grant, P30 CA42014 from National Cancer Institute.
Footnotes
Conflict of Interest: None
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, et al. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010;116:2140–7. doi: 10.1002/cncr.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertility and Sterility. 2013;100:681–5.e1. doi: 10.1016/j.fertnstert.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dada R, Kumar M, Jesudasan R, Fernández J, Gosálvez J, Agarwal A. Epigenetics and its role in male infertility. Journal of assisted reproduction and genetics. 2012;29:213–23. doi: 10.1007/s10815-012-9715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotaling JM, Walsh TJ. Male infertility: a risk factor for testicular cancer. Nature reviews Urology. 2009;6:550–6. doi: 10.1038/nrurol.2009.179. [DOI] [PubMed] [Google Scholar]
- 6.Gonsalves J, Sun F, Schlegel PN, Turek PJ, Hopps CV, Greene C, et al. Defective recombination in infertile men. Hum Mol Genet. 2004;13:2875–83. doi: 10.1093/hmg/ddh302. [DOI] [PubMed] [Google Scholar]
- 7.Maduro MR, Lamb DJ. Understanding New Genetics of Male Infertility. The Journal of urology. 2002;168:2197–205. doi: 10.1016/S0022-5347(05)64355-8. [DOI] [PubMed] [Google Scholar]
- 8.Ji G, Long Y, Zhou Y, Huang C, Gu A, Wang X. Common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. BMC Med. 2012;10:49. doi: 10.1186/1741-7015-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene MH, Kratz CP, Mai PL, Mueller C, Peters JA, Bratslavsky G, et al. Familial testicular germ cell tumors in adults: 2010 summary of genetic risk factors and clinical phenotype. Endocr Relat Cancer. 2010;17:R109–R21. doi: 10.1677/ERC-09-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. Bmj. 2000;321:789–92. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuVall SL, Fraser AM, Rowe K, Thomas A, Mineau GP. Evaluation of record linkage between a large healthcare provider and the Utah Population Database. 2012. [DOI] [PMC free article] [PubMed]
- 12.Hurdle JF, Smith KR, Mineau GP. Mining electronic health records: an additional perspective. Nat Rev Genet. 2013;14:75. doi: 10.1038/nrg3208-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerber RA, O'Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103:1906–15. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- 14.Samadder NJ, Curtin K, Tuohy TMF, Rowe KG, Mineau GP, Smith KR, et al. Increased Risk of Colorectal Neoplasia Among Family Members of Patients With Colorectal Cancer: A Population-Based Study in Utah. Gastroenterology. 2014;147:814–21.e5. doi: 10.1053/j.gastro.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 15.WorldHealthOrganization . WHO laboratory manual for the examination and processing of human semen 5th edition. 4th ed World Health Organization; Geneva: 2010. [Google Scholar]
- 16.Menkveld R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian journal of andrology. 2010;12:47–58. doi: 10.1038/aja.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg ML, Park Y, Brinton LA, Hollenbeck AR, Schatzkin A. Fatherhood and incident prostate cancer in a prospective US cohort. Int J Epidemiol. 2011;40:480–7. doi: 10.1093/ije/dyq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased Risk of Cancer in Infertile Men: Analysis of U.S. Claims Data. The Journal of urology. 2015;193:1596–601. doi: 10.1016/j.juro.2014.11.080. [DOI] [PubMed] [Google Scholar]
- 19.Kanatsu-Shinohara M, Morimoto H, Shinohara T. Enrichment of Mouse Spermatogonial Stem Cells by Melanoma Cell Adhesion Molecule Expression. Biology of reproduction. 2012;87:139, 1–10. doi: 10.1095/biolreprod.112.103861. [DOI] [PubMed] [Google Scholar]
- 20.Lei X, Guan C-W, Song Y, Wang H. The multifaceted role of CD146/MCAM in the promotion of melanoma progression. Cancer Cell International. 2015;15:3. doi: 10.1186/s12935-014-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Semen parameters and cancer risk in men with SA versus fertile men