Abstract
Inflammatory myofibroblastic tumor (IMT) is a rare neoplastic lesion with tendency toward local aggressive behavior and recurrence. It is primarily a visceral and soft tissue tumor; however, involvement of pancreas is extremely unusual. A localization in the pancreas needs differentiation from other tumors and chronic pancreatitis. We report a case of inflammatory myofibroblastic tumor arising in pancreatic head in a 32-year-old female who underwent Whipple’s procedure.
Keywords: Inflammatory myofibroblastic tumor, Pancreas, Rare, Recurrence
Introduction
Inflammatory myofibroblastic tumors (IMTs) (syn. plasma cell granuloma, inflammatory pseudotumor) are solid neoplastic mesenchymal proliferations composed of myofibroblastic spindle cells admixed with the inflammatory infiltrate of plasma cells, lymphocytes, eosinophils, and histiocytes in a collagenous or myxoid matrix [1]. Most of the IMTs often affect children and young adults, with a slight female predominance [2]. The etiology and cellular origin of IMT remains unknown. Human herpes viruses 3 and 8, Eikenella corrodens, and Epstein-Barr virus have been proposed as possible infectious triggers of the IMT [3, 4]. The most common sites of involvement are the lungs, mesentery, and omentum [2]. Pancreatic location is uncommon and can be confused with malignancy clinically and radiologically and needs to be differentiated from other tumors and chronic pancreatitis. Rarity of the location of the tumor prompted us to report this case.
Case Report
A 32-year-old female presented with recurrent localized abdominal pain particularly in the right upper quadrant along with progressive jaundice of 1 month duration and few bouts of vomiting. She had generalized pruritus, severe weight loss, and loss of appetite. Laboratory investigations revealed evidence of obstructive jaundice with serum total bilirubin 346.6 μmol/l, conjugated bilirubin 221.4 μmol/l, and serum alkaline phosphatase 986 U/l. Serum tumor marker assays showed a carbohydrate antigen (CA) 19–9 level of 58 U/ml.
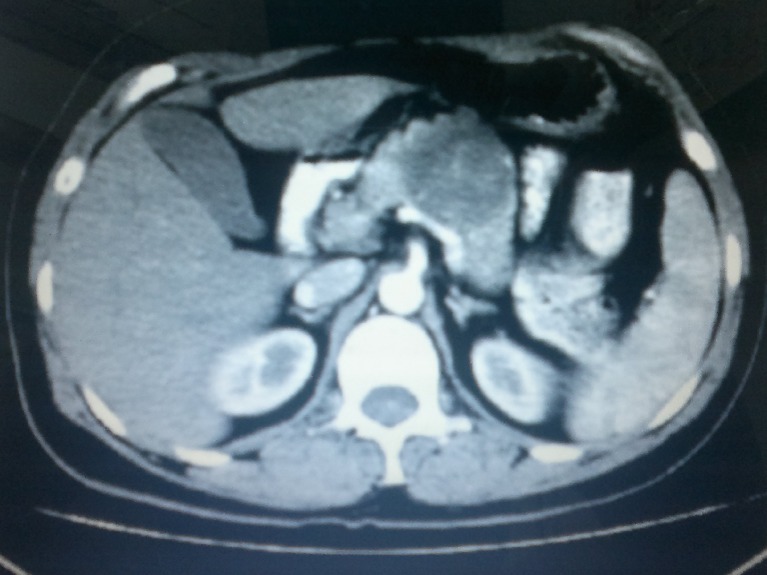
A computed tomography scan of the abdomen revealed a well-defined large heterogeneous mass involving the head of the pancreas pushing the superior mesenteric artery and vein ventrally (Fig. 1). The common bile duct was dilated, the gallbladder was mildly enlarged, but there was no dilatation of intrahepatic bile ducts. No regional lymphadenopathy or liver lesions suggesting metastases were identified. Persistent and progressive increase in serum bilirubin due to the CT-evident SOL led to an obvious diagnosis of adenocarcinoma of the head of the pancreas with involvement of lower common bile duct. The patient was adequately optimized, and pylorus-preserving pancreaticoduodenectomy (PPPD) was undertaken that went ahead without complications. Intraoperatively, the pancreatic head presented as a hard mass with circumscribed necrosis of pancreatic parenchyma.
Fig. 1.
CT scan showing a heterogeneous mass in pancreatic head
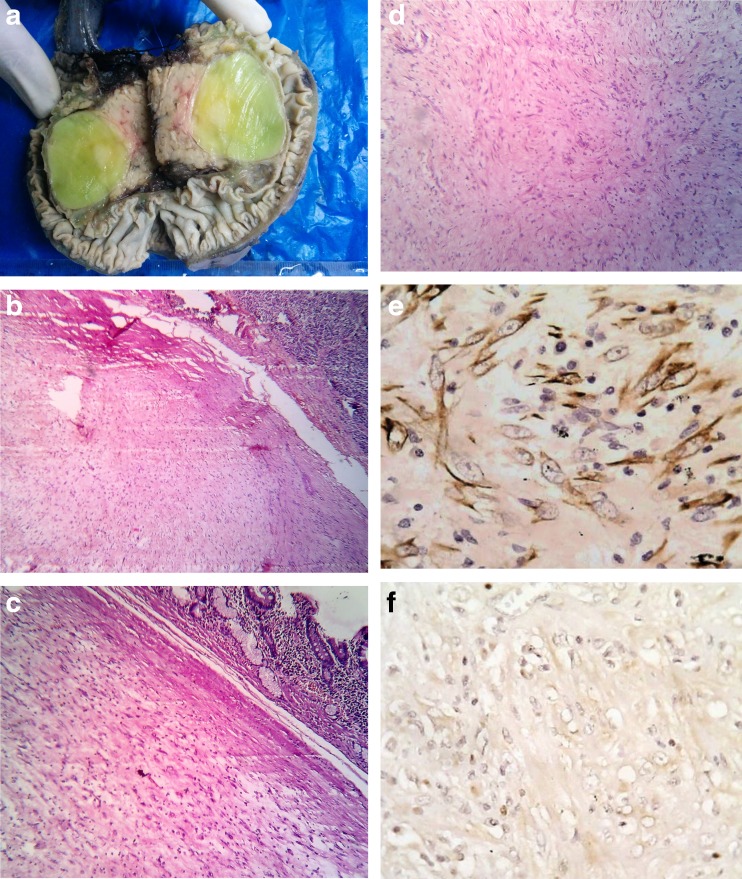
On gross examination, a circumscribed globular mass measuring 48 × 37 mm was noted arising from the pancreatic head. The cut surface was soft and fleshy, and the duodenal mucosa appeared uninvolved (Fig. 2a).
Fig. 2.
a Gross picture of pancreaticoduodenectomy specimen. b IMT with adjacent normal pancreas (H&E ×10). c Tumor mass with adjacent small intestine (H&E ×10). d Myofibroblasts with lymphocytes and plasma cells (H&E ×10). e Smooth muscle actin positivity (×40). f Negative for ALK-1 (×40)
Microscopically, the lesion was composed of fascicles of spindle cells including fibroblasts and myofibroblasts in a myxoid background infiltrated by plasma cells, lymphocytes, and eosinophils. The tumor mass was sharply demarcated from the adjacent pancreatic tissue and small bowel mucosa (Fig. 2b–d).
On immunohistochemistry, the tumor was found to be positive for smooth muscle actin, but negative for anaplastic lymphoma kinase (ALK) (Fig. 2e, f).
Taking into account the above findings, a final diagnosis of inflammatory myofibroblastic tumor was made. The patient was discharged on the 20th postoperative day. There was no clinical or radiological evidence of recurrence over the next 2.5 years of follow up.
Discussion
IMT is a rare benign tumor of viscera and soft tissue that occurs predominantly in the first two decades of life. Originally described in lung in 1939, it can occur throughout the body and the most common sites are the lung, mesentery, and omentum [2]. However, pancreatic IMT is extremely rare, with only 28 cases being reported in English-language literature [5].
Sixty percent of the pancreatic IMTs are located in the head and 40 % in the body and tail of the organ. Obstructive jaundice is noted in 66.7 % of IMTs located in the head of pancreas. It can also obstruct of the main pancreatic duct, resulting in chronic obstructive pancreatitis. CA 19–9 may be elevated in both malignancy and IMT. Imaging appearance also may be deceptive as it presents with mass lesions, biliary radicle dilatations, and even delayed and persistent enhancement with contrast; features which are strikingly similar to pancreatic carcinoma. Histological diagnosis in biopsies or intraoperative frozen sections is difficult and IMTs must be differentiated from chronic pancreatitis, fibrosarcoma, pancreatic carcinoma, and lymphoma [6].
Strong diffuse cytoplasmic reactivity for vimentin is typical for virtually all IMT. Other immunohistochemical markers include ALK, smooth muscle actin, and desmin. ALK-positive cases have a better outcome [2].
The World Health Organization classifies this rare tumor entity as a distinctive neoplasm of intermediate biological potential and it rarely (<5 %) metastasizes [7]. Extrapulmonary IMT has a recurrence rate of approximately 25 % related to location, resectability, and multinodularity. The discovery of cytogenetic aberrations and the recognition of ALK gene rearrangements solidified the concept of IMT as a neoplastic lesion [8]. The prognosis is generally favorable. Although surgery is the principal treatment, regression and response to corticosteroids and nonsteroidal inflammatory agents have been noted in rare cases [2].
Conclusion
IMT of the pancreas is an unusual neoplasm that poses diagnostic dilemma preoperatively. Final diagnosis should be based on histomorphological features and immunohistochemical analysis. Despite being a benign lesion, its potential for recurrence and local invasion requires complete surgical resection.
Acknowledgments
Conflict of Interest
The authors declare that they do not have any conflict of interest.
References
- 1.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: a clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 2.Jones EC, Clement PB, Young RH. Inflammatory pseudotumor of the urinary bladder. A clinicopathological, immunohistochemical, ultrastructural, and flow cytometric study of 13 cases. Am J Surg Pathol. 1993;17:264–274. doi: 10.1097/00000478-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roman JJ, Sanchez-Velasco P, Ocejo-Vinyals G, Hernandez-Nieto E, Leyva-Cobian F, Val-Bernal JF. Human herpesvirus-8 genes are expressed in pulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor) Am J Surg Pathol. 2001;25:624–629. doi: 10.1097/00000478-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JT, Gaffney RL, Casey MB, Farrell MA, Morice WG, Macon WR. Inflammatory pseudotumor of the spleen associated with a clonal Epstein-Barr virus genome. Case report and review of the literature. Am J Clin Pathol. 2003;120:56–61. doi: 10.1309/BUWNMG5RV4D09YYH. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Watanabe K, Nagata M, Tasaki K, Honda I, Watanabe S, Soda H, Takenouti T. Inflammatory myofibroblastic tumor (IMT) of the pancreas. J Hepatobiliary Pancreat Surg. 2002;9:116–119. doi: 10.1007/s005340200013. [DOI] [PubMed] [Google Scholar]
- 6.Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146–1156. doi: 10.1097/00000478-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Coffin CM, Hornick JL, Fletcher CDM. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]