Abstract
The objective of this study is to investigate relationship between the expressions of heparanase and vascular endothelial growth factor-C (VEGF-C) mRNA and tumorigenesis, progression in human lung cancer. The expressions of heparanase and VEGF-C mRNA in 65 cases of lung cancer (31 cases of squamous cell carcinoma, 25 adenocarcinoma, 3 large cell carcinoma, and 6 small cell carcinoma), adjacent tissues of cancer, and normal tissues were tested by reverse transcription-polymerase chain reaction (RT-PCR) and analyzed by clinico-pathological characteristics and prognosis of lung cancer. The rate of expressions of heparanase and VEGF-C mRNA in tumor tissues (55.4, 61.5 %) was significantly higher than that in adjacent tissues of cancer (12.3, 15.4 %) and normal tissues (3.1, 4.6 %) (P < 0.05). It was shown that heparanase and VEGF-C mRNA expressions did not correlate with the pathological type and grade of the tumor (P > 0.05), but they correlated with the clinical stage and survival time of the patients (P < 0.05). Overexpression of heparanase and VEGF-C mRNA in lung cancer tissues perhaps participates in regulation of tumorigenesis and progression. The expressions of heparanase and VEGF-C mRNA should be used as a useful marker of the biological behavior of lung cancer and as an independent prognosis factor for the patient’s survival.
Keywords: Heparanase, Vascular endothelial growth factor-C, mRNA, Lung cancer
Introduction
Metastasis is considered to be a major determinant of the malignant behavior of human lung cancer and related directly to poor prognosis and mortality of patients. The mechanisms of tumor metastasis are not wel1 illuminated so far. However, heparanase has been found that can specifically degrade the extracellular matrix and heparin sulfate proteoglycans of basement membrane and promote metastasis in many tumors [1, 2]. VEGF-C is a specific lymphatic endothelial cell growth factor and has been proved to promoting tumor cell growth and angiogenesis [3]. In addition, degradation of heparin sulfate proteoglycans can result in releasing of growth factors. In this study, heparanase and VEGF-C mRNA expressions and its correlation with tumorigenesis, progression of human lung cancer were studied.
Materials and Methods
Sixty-five cases who were surgically resected with lymph node dissection for lung carcinoma at the Second Hospital of Shandong University between 2003 and 2008 were included in the study, and tumor nearby normal lung tissues and normal lung tissues were also included. The patients included 51 males and 14 females with a mean age of 61 years (range 41–76 years). None of the patients had received preoperative chemotherapy and radiotherapy. The pathological types were 31 squamous cell carcinomas, 25 adenocarcinomas, 3 large cell carcinoma, and 6 small cell carcinoma (well differentiated in 17, moderately in 23, poorly in 16, and undifferentiated in 9). The pathological stages were evaluated as stage I, 12; II, 31; III, 18; and IV, 4. With no hospital death, 65 patients were followed up to December 2012. Four cases were lost, and the follow-up rate was 93.8 %.
Tumor tissues were taken from primary tumor area, avoiding necrosis and inflammatory area. Adjacent tissues were from 2 cm next to the tumor material, and normal lung tissues from 5 cm next to tumor margin; pathological examination showed no carcinoma cells. The specimens were quick-frozen in liquid nitrogen and were preserved at −80 temperature.
Total RNA was extracted from 50 to 100 mg tissue with TRlzol reagent (from Gibco Company) according to the manufacturer’s recommended steps. Its purity and quantitative analysis were studied with UV spectrophotometer (from Bio-Rad Corporation), and RNA integrity was verified on 1 % formaldehyde denaturing gel electrophoresis. Heparanase mRNA expression was detected by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) according to instructions in one-step kit (from Katara Company). Amplified output of the housekeeping gene GAPDH was used as an internal reference. Primers were designed according to the literature [4]. Heparanase, upstream: 5′-TTCG ATCCCAA GAA GAAGGAATCAAC-3′, downstream: 5′-GTAGTGATGCCATGTA ACTG AATC-3′; GAPDH upstream: 5′-TCCTGCACCACCAACTGCTT-3′, downstream: 5′-TCCACCACCCTGTTGCTGTA-3′. The amplification reaction involved 28 cycles of reverse transcriptase at 50 °C for 30 min, denaturation at 94 °C for 45 s, annealing at 60 °C for 45 s, and extension at 72 °C for 5 min. PCR products were scanned and photographed with the Fluor-S multi-functional imaging system after 1.5 % agarose gel electrophoresis.
Specific primers of VEGF-C were synthesized by Life Technologies Corporation, and the fragment length was 183 bp. Upstream: 5′-TGTACAAGTGTCAGCTAAGG-3′, downstream: 5′-CCACATCTATACACACCTCC-3′. Length of ß-actin was 268 bp, upstream: 5′-CAACTTCATCCACGTTGACC-3′, downstream:5′-GAAG AG C CAAGGA CAGGTAC-3′. PCR cycling parameters involved 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 90 s, and extension at 72 °C for 10 min. We used RNA samples and specific primers amplified products in the kit as positive control, RNA amplified products without template as negative control, and ß-actin as internal control. PCR products were observed under ultraviolet light after 2 % agarose gel electrophoresis.
Statistical Methods
Analysis of variance and t test were used for measurement data and multiple linear regression analysis for relationship between multiple measurement data. We used Spearman rank correlation test to determine related trends of indicators, Kaplan-Meier life method to survival analysis, and log-rank test to analyze the difference between the groups. The statistical package SPSS version 12.0 was applied to complete data processing, and values of P < 0.05 were considered statistically significant.
Results
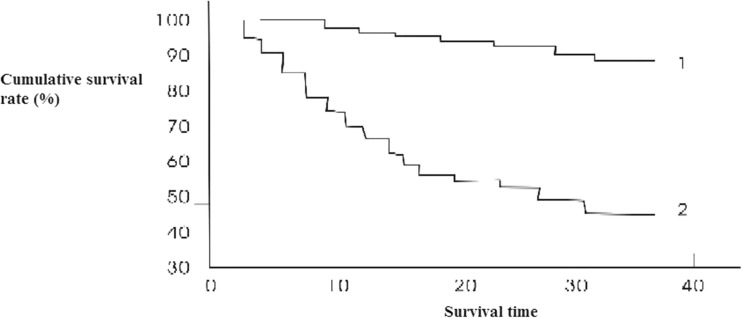
Heparanase mRNA expressed positively in lung cancer (36/65 cases, 55.4 %) compared with the adjacent normal tissue samples (8/65 cases, 12.3 %) and normal lung tissue (2/65 cases, 3.1 %); VEGF-C mRNA expressed positively in lung cancer (40/65 cases, 61.5 %) compared with the adjacent normal tissue samples (10/65 cases, 15.4 %) and normal lung tissue (3/65 cases, 4.6 %). There was a significant difference (P < 0.05). It was shown that heparanase and VEGF-C mRNA expressions did not correlate with the pathological type and grade of the tumor (P > 0.05), but it correlated with the clinical stage and survival time of the patients (P < 0.05). Heparanase and VEGF-C mRNA expressions were significantly higher in stages I and II than that in stages III and IV. Expression rate was higher in patients with more 3 years of survival time than in those with less than 3 years. Survival in patients with high expression of heparanase and VEGF-C mRNA is different from those with low expression. (P < 0.05) (Figs. 1 and 2) (Table 1).
Fig 1.
Kaplan-Meier survival curves of heparanase mRNA expression. Log-rank test χ 2 = 8.42 P = 0.0044, 1 low expression, 2 high expression
Fig 2.
Kaplan-Meier survival curves of VEGF-C mRNA expression. Log-rank test χ 2 = 6.68. P = 0.0078, 1 low expression, 2 high expression
Table 1.
Expression of HPSE and VEGF-C mRNA in different stages
| HPSE mRNA | Positive rate (%) | P | VEGF-C mRNA | Positive rate (%) | P | ||
|---|---|---|---|---|---|---|---|
| Pathological type | n | 0.8302 | 0.3955 | ||||
| Squamous cell carcinoma | 31 | 17 | 48.4 | 19 | 61.3 | ||
| Adenocarcinoma | 25 | 15 | 52 | 15 | 60 | ||
| Large cell carcinoma | 3 | 1 | 66.7 | 2 | 66.7 | ||
| Small cell carcinoma | 6 | 3 | 50 | 4 | 66.7 | ||
| Differentiation | 0.9804 | 0.9832 | |||||
| Well differentiated | 17 | 10 | 58.8 | 10 | 58.8 | ||
| Moderately differentiated | 23 | 12 | 52.2 | 14 | 60.9 | ||
| Poorly differentiated | 16 | 9 | 56.3 | 10 | 62.5 | ||
| Undifferentiated | 9 | 5 | 55.6 | 6 | 66.7 | ||
| Clinical stage | 0.0002 | 0.0001 | |||||
| I | 12 | 5 | 41.7 | 6 | 50 | ||
| II | 31 | 10 | 32.3 | 12 | 38.7 | ||
| III | 18 | 17 | 94.4 | 18 | 100 | ||
| IV | 4 | 4 | 100 | 4 | 100 | ||
| Survival time after operation (year) | 0.0000 | 0.0000 | |||||
| <3 | 25 | 23 | 92 | 24 | 96 | ||
| >3 | 36 | 12 | 33.3 | 16 | 44.4 |
Discussion
Metastasis and invasion are important characteristic of malignant tumors. Prerequisites of tumor growth and metastasis are invasion to extracellular matrix and basement membrane and angiogenesis. Heparan sulfate proteoglycans are essential and ubiquitous macromolecules associated with the cell surface and extracellular matrix of a wide range of cells and tissues. Heparanase is an extracellular matrix degradative enzyme, which degrades the heparan sulfate chains of heparan sulfate proteoglycans at specific intrachain sites. Overexpression of heparanase has been shown in many malignant neoplasms, such as bladder [5], colon [6], prostate, and pancreatic [7]. However, the effects of the expression of Heparanase on human lung cancer have not been fully evaluated. In this study, we detected the heparanase mRNA expression in 65 cases of lung cancer; our result showed that heparanase expression was significantly higher in tumor cells compared with its adjacent tissues and normal lung tissues, and the overexpression of heparanase mRNA was strikingly associated with tumorigenesis, progression, and poor prognosis. VEGF-C is believed to be the only lymphangiogenic factor in the VEGF family; the structure of lymphangiogenesis is in favor of tumor cells into the lymphatic channels, thus the formation of lymphatic metastasis and distant metastasis [8]. We found that VEGF-C mRNA expression levels were higher in lung cancer tissues than that in adjacent tissues and normal lung tissues. The study also found that VEGF-C mRNA expression levels were high in heparanase mRNA positive cases, and low in negative cases. So heparanase expression was positively correlated with VEGF-C expression in tumor tissues. It suggested that a synergistic effect of heparanase and VEGF-C mRNA promote angiogenesis and lymphatic angiogenesis, thus the formation of tumor metastasis and poor prognosis. The clinical analyses suggested that heparanase facilitates tumor lymphangiogenesis which is mediated, possibly, by VEGF-C gene induction [9].
We also found that heparanase and VEGF-C mRNA expression did not correlate with the pathological type and grade of the tumor (P > 0.05); it contradicts the view of correlation between tumor metastasis and pathological type or degree of differentiation. The possible explanation is that formation and development of lung cancer are a complex process. Heparanase and VEGF-C mRNA expression were significantly higher in stages III and IV than that in stages I and II (P < 0.05). In patients with less than 3 years of survival time after operation, heparanase and VEGF-C mRNA expression were higher too (P < 0.05). The results showed that heparanase and VEGF-C mRNA played an important role in promoting the tumor cell invasion and metastasis ability. Zhang [10] reported that heparanase and VEGF-C expression correlated with lymph node metastasis, high concentration of microvascular density, and lymphatic vessel density. So heparanase and VEGF-C mRNA can be used as indicators to determine the biological behavior of lung cancer and prognosis.
Taken together, our findings suggest that HEPE and VEGF-C mRNA were closely involved in lung cancer metastasis and prognosis. They are in also involved in multiple biological activities that include angiogenesis and lymphangiogenesis; however, further studies are necessary to reveal the exact mechanism of HEPE and VEGF-C in lung cancer progression.
Acknowledgments
The authors wish to thank Tian Hui (of the Thoracic Department of Qilu Hospital) for his continued support and major contributions to this research work. The authors also wish to express special thanks to Guo Jiazhong (Division of Thoracic Surgery, Shandong University) for data management and outstanding technical assistance. Regarding funding, this piece of his research was supported by the Science and Technology Agency of Shandong Province, the People’s Republic of China.
Conflict of Interest
The authors declare that they have no competing interest.
References
- 1.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 2.Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108:341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benke EM, Ji Y, Patel V, Wang H, Miyazaki H, Yeudall WA. VEGF-C contributes to head and neck squamous cell carcinoma growth and motility. Oral Oncol. 2010;46:19–24. doi: 10.1016/j.oraloncology.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Aoki Y, Kase H, Tanaka K. Heparanase expression and angiogenesis in endometrial cancer. Gynecol Obstet Investig. 2003;56:77–82. doi: 10.1159/000072821. [DOI] [PubMed] [Google Scholar]
- 5.Shafat I, Pode D, Peretz T, Ilan N, Vlodavsky I, Nisman B. Clinical significance of urine heparanase in bladder cancer progression. Neoplasia. 2008;10:125–130. doi: 10.1593/neo.07875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobuhisa T, Naomoto Y, Ohkawa T, Takaoka M, Ono R, Murata T, Gunduz M, Shirakawa Y, Yamatsuji T, Haisa M, Matsuoka J, Tsujigiwa H, Nagatsuka H, Nakajima M, Tanaka N. Heparanase expression correlates with malignant potential in human colon cancer. J Cancer Res Clin Oncol. 2005;131:229–237. doi: 10.1007/s00432-004-0644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quiros RM, Rao G, Plate J, Harris JE, Brunn GJ, Platt JL, Gattuso P, Prinz RA, Xu X. Elevated serum heparanase-l levels in patients with pancreatic carcinoma are associated with poor surviva1. Cancer. 2006;106:532–540. doi: 10.1002/cncr.21648. [DOI] [PubMed] [Google Scholar]
- 8.Peng C, Sun Q, Fang Y, Cong B, Zhao X. VEGF-C antisense oligoxydeonucleotide suppression of invasive ability of the A-549 lung carcinoma cell line. Asian Pac J Cancer Prev. 2011;12:2097–2099. [PubMed] [Google Scholar]
- 9.Cohen-Kaplan V, Naroditsky I, Zetser A, Ilan N, Vlodavsky I, Doweck I. Heparanase induces VEGF-C and facilitates tumor lymphangiogenesis. Int J Cancer. 2008;123:2566–2573. doi: 10.1002/ijc.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Ming J, Li Y, Zhang S, Li B, Qiu X, Wang E. Heparanase expression correlates with angiogenesis and lymphangiogenesis in human lung cancer. Zhongguo Fei Ai Za Zhi. 2009;12:867–868. doi: 10.3779/j.issn.1009-3419.2009.08.06. [DOI] [PubMed] [Google Scholar]