Abstract
The possible beneficial role of selenium (Se) on the oxidative stress induced by lead (Pb) is still unclear in humans. Therefore, the aim of the present study was to explore the associations among the Se levels, chronic Pb exposure, oxidative stress parameters, and parameters characterizing the function of the antioxidant defense system in men who are occupationally exposed to Pb. Based on the median serum Se concentrations, the 324 study subjects were divided into two subgroups: a subgroup with a low Se level (L-Se) and a subgroup with a high Se level (H-Se). The levels of lead (PbB) and zinc protoporphyrin (ZPP) in the blood and the delta-aminolevulinic acid (ALA) level in the urine served as indices of Pb exposure. The PbB level was significantly lower in the H-Se group compared to that in the L-Se group by 6 %. The levels of 8-hydroxyguanosine and lipofuscin (LPS) and the activity of superoxide dismutase were significantly lower in the H-Se group compared to that in the L-Se group by 17, 19, and 11 %, respectively. However, the glutathione level (GSH) and the activities of glutathione peroxidase (GPx) and catalase were significantly higher by 9, 23, and 3 %. Spearman correlations showed positive associations between the Se level and GPx activity and GSH level. A lower serum Se level in chronically Pb-exposed subjects is associated with higher Pb blood levels and an elevated erythrocyte LPS level, which reflects the intensity of oxidative stress. Besides, in a group of Pb-exposed subjects with lower serum Se level, depleted GSH pool and decreased activity of GPx in erythrocytes were reported. However, the present results are inadequate to recommend Se supplementation for chronic lead exposure at higher doses than would be included in a normal diet except for selenium deficiency.
Keywords: Lead poisoning, Selenium, Oxidative stress, Antioxidant defense system, Glutathione peroxidase
Introduction
Exposure to lead (Pb) is still a major concern for humans worldwide. Exposure to Pb appears from contact with Pb-based paints, automobiles, batteries, and other sources [1, 2]. The respiratory and gastrointestinal tracts are the principal routes that permit Pb to enter the human body. Pb accumulates in the blood, soft tissues, and bones [3]. According to the newest researches, no safe level of exposure to Pb has been found [4]. The symptoms of the early stages of inorganic Pb exposure include a loss of appetite, weight loss, fatigue, irritability, occasional vomiting, and anemia, whereas at high levels, exposure to Pb causes kidney damage, behavioral disturbances, anemia, hypertension, and toxicity to the reproductive system [1, 2].
The toxicity of Pb is largely due to its ability to induce an imbalance in the generation and removal of reactive oxygen species (ROS) in tissue and cellular components, generally called oxidative stress [5]. On the one hand, the presence of Pb may increase the generation of ROS [6, 7]. On the other hand, Pb depletes the antioxidant defenses, such as glutathione (GSH) and modifies activities and expression of antioxidant enzymes, such as superoxide dismutase or glutathione peroxidase (GPx) [8, 9]. Therefore, exposure to Pb results in the oxidative damage to lipids, DNA, and proteins [5]. The toxic action of Pb is also related to its inhibitory effect on delta-aminolevulinic acid dehydratase (ALAD) and ferrochelatase. Consequently, Pb exposure impairs the chain reaction that leads to the formation of heme and results in the accumulation of delta-aminolevulinic acid (ALA), which has pro-oxidative and alkylating properties [5, 6].
It has been well established that Pb interacts and competes with other cations [10]. Among them is selenium (Se). Se is a non-metal element that has been linked to many health benefits in humans and other mammals such as decreasing the incidence of cancer, protecting against cardiovascular diseases, and treating certain muscle disorders. Besides, Se is involved in mammalian development, boosting immune function, tissue respiration, and antioxidant defense [1, 11, 12]. Se is a cofactor for GPx, an antioxidant enzyme, which utilizes hydrogen peroxide to convert reduced glutathione to its oxidized form (GSSG). It has been demonstrated that selenoproteins, through their antioxidant properties, help to eliminate reactive oxygen species induced by metals [1]. The results of several animal studies have shown that Se or the combination of Se and other antioxidants can reduce Pb-induced oxidative stress. On the other hand, the possible beneficial role of Se alone on Pb-induced oxidative stress is still unclear in humans [5].
The current therapeutic approach to Pb poisoning is to increase its excretion by chelation. Chelators have been shown to reduce blood Pb levels; however, their safety and efficacy may be questioned. Consequently, it is necessary to find new agents capable of abating toxic effects of Pb [13]. In light of this, Se supplementation may be considered as a potential therapy for chronic Pb poisoning due to the antioxidant properties of Se. However, too little is known about possible interactions between Pb and Se. Therefore, the aim of the present study was to explore the associations among the Se levels, chronic Pb exposure, oxidative stress parameters, and parameters characterizing the function of the antioxidant defense system in men who were occupationally exposed to Pb.
Materials and Methods
Study Population
The exposed population consisted of 324 occupationally Pb-exposed males who had given informed consent for their participation in this study. All of the study subjects were employed in the zinc and Pb works in the southern region of Poland. They lived near the workplace and had a similar socioeconomic status. The mean concentration of Pb in the air at participants’ workplaces was 0.083 ± 0.12 mg/m3. This mean concentration exceeds the Polish MAC level of 0.050 mg/m3 [14]. Besides, examined subjects were exposed to negligible doses of zinc. The mean concentration of zinc in the air at participants’ workplaces was 0.15 ± 0.13 mg/m3. This value is much lower than the Polish MAC level of 5.0 mg/m3. Examined subjects were not occupationally exposed to selenium. All participants continued to work during the study.
Based on the median serum Se concentrations, the subjects were divided into two subgroups: a subgroup with a low Se level (L-Se) and a subgroup with a high Se level (H-Se). Questionnaire data on age, weight, height, medical history, and smoking habits were obtained. Moreover, blood pressure was measured in all of the study subjects. The study was approved by the Bioethics Committee of the Institute of Occupational Medicine and Environmental Health, Sosnowiec, Poland.
The levels of Pb (PbB) and zinc protoporphyrin (ZPP) in the blood and the ALA level in the urine served as short-term lead-exposure indices. To determine the long-term Pb exposure, the concentrations of Pb and ZPP in the blood samples were determined, on average, every 3 months during the 5 years of observation. From the collected data, the mean blood concentrations of Pb (PbBmean) and ZPP (ZPPmean) were calculated.
Sampling and Laboratory Procedures
Two samples of blood were obtained from the cubital vein into vacuum tubes (Vacuette®, Greiner-Bio, Frickhausen, Germany) containing K3EDTA for whole blood analyses and plain tubes for serum analyses. Blood samples were frozen and stored at −20 °C until the analyses were performed. Midstream morning urine was collected to measure ALA levels.
To measure the ALA and 8-hydroxydeoxyguanosine (8-OHdG) levels, urine was collected on the same day as blood. The assessments of the PbB and serum Se concentrations were performed by graphite furnace atomic absorption spectrometry [15, 16] using an ICE 3400 instrument (Thermo Fisher Scientific Waltham, MA, USA). The laboratory met the requirements of proficiency tests (Lead and Multielement Proficiency—CDC in Atlanta). The ClinCal® Whole Blood Calibrator and ClinCal® Serum Calibrator (Recipe, Germany) were used for calibration of the instrument and control materials. ClinCheck Whole Blood Control Levels I, II, and III and ClinCheck Serum Control Levels I and II were used for quality control. The results were expressed as micrograms per deciliter (Pb) and micrograms per liter (Se). The level of ZPP was measured using an Aviv Biochemical hematofluorometer, model 206. The results were expressed as micrograms per gram of hemoglobin (Hb). The concentration of Hb was determined using the cyanmethemoglobin method.
The level of ALA was estimated spectrophotometrically in urine samples by the method described by Grabecki et al. [17]. The results were expressed as milligrams per deciliter. The 8-OHdG level was measured in urine using a commercially available ELISA-based assay (Cat. RSCN213100R, BioVendor) according to the manufacturer’s instructions. Spectrophotometric readings were obtained using a BIO-TEK Eon microplate reader (BIO-TEK Instruments), and the results were expressed as grams of creatinine in urine.
The levels of serum malondialdehyde (MDA) were determined according to the method described by Ohkawa et al. [18]. The results were recorded as micromoles per liter. The method developed by Södergren et al. [19] was used to measure the concentrations of lipid hydroperoxides (LPH) in the serum. The results were recorded as micromoles per liter. The levels of lipofuscin (LPS) in erythrocytes were measured according to the Jain [20] method. The results were expressed as relative units (RU) per gram of hemoglobin (the fluorescence of a 0.1 mg/ml solution of quinidine sulfate in sulfuric acid is equal to 100 RU). The total oxidant status (TOS) was measured in serum according to the method described by Erel [21]. The data were shown in micromoles per liter.
The total antioxidant capacity (TAC) in millimoles per liter was measured according to the report by Erel [22]. The levels of erythrocyte glutathione (GSH) were measured as described by Pawelski [23]. The obtained concentrations were expressed as micromoles per gram of Hb (μmol/g Hb). Oyanagui’s method [24] was used to measure the activity of superoxide dismutase (SOD) in erythrocytes. The enzymatic activity of SOD was expressed in nitric units. The activity of SOD is equal to 1 nitric unit (NU) when it inhibits nitric ion production by 50 %. The activities of SOD were normalized to milligrams of Hb (NU/mg Hb). The catalase (CAT) in erythrocytes was measured by the Aebi [25] kinetic method. Catalase activity was expressed as international units per milligram of Hb (IU/mg Hb). The erythrocyte glutathione peroxidase (GPx) activity was measured by the kinetic method described by Paglia and Valentine [26]. The activity of GPx was expressed as micromoles of NADPH oxidized per minute normalized to grams of Hb (IU/g Hb). The activity of glutathione reductase (GR) in erythrocytes was measured according to the report by Richterich [27]. The activity of GR was expressed as micromoles of NADPH utilized per minute normalized to 1 gram of Hb (IU/g Hb).
Statistical Analysis
The statistical analysis was performed using the Statistica 9.1 PL software program. The statistical analyses included the means and standard deviations of the data. Shapiro-Wilk’s test was used to verify normality, and Levene’s test was used to verify the homogeneity of variances. Statistical comparisons were made using the t test, t test with separate variance estimates, the Mann-Whitney U test, or the chi-squared test. The Spearman non-parametric correlation was calculated. A value of p < 0.05 was considered to be significant.
Results
There were no significant differences in age, height, weight, number of years worked, or smoking habits between the examined subgroups. The percentages of subjects diagnosed with coronary artery disease, hypertension, and diabetes did not differ between the examined subgroups. There was a tendency for the systolic blood pressure to be lower in the H-Se group compared to that in the L-Se group (p = 0.058), whereas diastolic blood pressure did not differ between the subgroups (Tables 1 and 2).
Table 1.
Epidemiologic parameters and the blood lead level (PbB), mean, maximum and minimum PbB, zinc protoporphyrin concentration in blood (ZPP) mean, maximum and minimum ZPP and delta-aminolevulinic acid (ALA) in urine
| Low Se group n = 162 |
High Se group n = 162 |
Relative change (%) | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 39.0 | 11.0 | 38.0 | 9.78 | −2 | 0.418 |
| Years of work | 13.7 | 11.4 | 12.0 | 10.4 | −13 | 0.157 |
| Height (cm) | 176 | 6.32 | 176 | 5.83 | 0 | 0.608 |
| Weight (kg) | 83.8 | 14.8 | 85.4 | 12.4 | 2 | 0.308 |
| Systolic blood pressure (mmHg) | 121.91 | 5.52 | 121.17 | 5.42 | −1 | 0.058 |
| Diastolic blood pressure (mmHg) | 78.52 | 4.66 | 78.09 | 4.93 | −1 | 0.418 |
| PbB (μg/dl) | 33.83 | 9.43 | 31.85 | 9.87 | −6 | 0.030 |
| PbBmean (μg/dl) | 32.55 | 8.86 | 30.87 | 9.22 | −5 | 0.069 |
| PbBmin (μg/dl) | 20.12 | 9.71 | 19.20 | 9.42 | −5 | 0.397 |
| PbBmax (μg/dl) | 43.18 | 11.47 | 41.45 | 12.12 | −4 | 0.087 |
| ZPP (μg/g Hb) | 4.28 | 2.25 | 4.05 | 1.90 | −5 | 0.324 |
| ZPPmean (μg/g Hb) | 4.81 | 2.38 | 4.50 | 2.26 | −6 | 0.237 |
| ZPPmin (μg/g Hb) | 2.87 | 1.52 | 2.77 | 1.19 | −4 | 0.512 |
| ZPPmax (μg/g Hb) | 7.89 | 4.72 | 7.36 | 5.00 | −7 | 0.089 |
| ALA (mg/l) | 5.38 | 1.73 | 4.77 | 1.95 | −11 | 0.386 |
PbB and ZPP mean levels of Pb and ZPP in the blood determined simultaneously with the biochemical analysis; PbB mean and ZPP mean mean levels of Pb and ZPP in the blood calculated based on the values determined, on average, every 3 months during the 5 years of observation before the study; PbB min, PbB max, ZPP min, and ZPP max mean of the maximal and minimal levels of Pb and ZPP in the blood collected from each worker calculated based on the values determined, on average, every 3 months during the 5 years of observation
Table 2.
Epidemiologic parameters in study population
| Low Se group (%) | High Se group (%) | p value | |
|---|---|---|---|
| Smokers | 42 | 34 | 0.138 |
| Coronary heart disease | 2 | 3 | 0.476 |
| Hypertension | 9 | 10 | 0.711 |
| Diabetes | 2 | 4 | 0.522 |
The PbB level was significantly lower in the H-Se group compared to that in the L-Se group by 6 %. At the same time, there was a tendency for there to be lower maximal values of PbB (PbBmax) and ZPP (ZPPmax) and for the PbBmean in the H-Se group compared to that in the L-Se group. The other Pb-exposure indices did not differ significantly between the subgroups, although the mean values were lower in H-Se group (Table 1, Fig. 1).
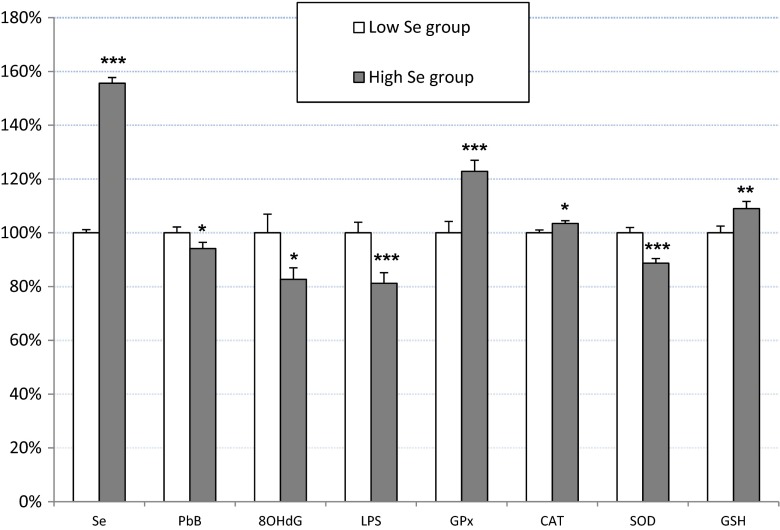
Fig. 1.
The concentration of selenium (Se) in serum, blood lead level (PbB), 8-hydroxydeoxyguanosine (8-OHdG) in urine, lipofuscin (LPS) in erythrocytes, the activity of glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) in erythrocytes and erythrocyte glutathione (GSH) concentration expressed as a percentage of the mean baseline values in low Se group. Data are presented as a mean and SEM. *p < 0.05, **p < 0.01, ***p < 0.001—comparison between the low and high Se groups
The levels of 8-OHdG and LPS and the SOD activity were significantly lower in the H-Se group compared to that in the L-Se group by 17, 19, and 11 %, respectively. However, the GSH level and activities of GPx and CAT were significantly higher by 9, 23, and 3 %, respectively (Table 3, Fig. 1).
Table 3.
Concentration of selenium and parameters related to oxidative stress intensity and antioxidant reserves in blood and urine
| Low Se group n = 162 |
High Se group n = 162 |
Relative change (%) | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Se (μg/l) in serum | 57.9 | 8.62 | 90.1 | 15.6 | 56 | <0.001 |
| TAC (mmol/l) in serum | 0.90 | 0.14 | 0.92 | 0.13 | 3 | 0.123 |
| TOS (μmol/l) in serum | 13.4 | 15.1 | 12.0 | 6.50 | −11 | 0.268 |
| LPH (μmol/l) in serum | 4.47 | 6.07 | 4.93 | 3.13 | 10 | 0.400 |
| MDA (μmol/l) in serum | 2.48 | 1.06 | 2.39 | 1.09 | −3 | 0.469 |
| 8OHdG (ng/g creatinine) in urine | 13.3 | 11.73 | 11.0 | 7.23 | −17 | 0.039 |
| LPS (RF/dl) in erythrocytes | 19.2 | 9.60 | 15.6 | 9.49 | −19 | <0.001 |
| GPx (U/gHb) in erythrocytes | 51.2 | 27.37 | 62.9 | 26.8 | 23 | <0.001 |
| GR (U/g Hb) in erythrocytes | 4.59 | 1.94 | 4.51 | 1.63 | −2 | 0.671 |
| CAT (kU/g Hb) in erythrocytes | 435 | 59.0 | 450 | 57.4 | 3 | 0.023 |
| SOD (NU/mg Hb) in erythrocytes | 10.6 | 2.68 | 9.40 | 2.37 | −11 | <0.001 |
| GSH (μmol/g Hb) in erythrocytes | 3.44 | 1.09 | 3.75 | 1.16 | 9 | 0.006 |
Spearman correlations showed positive correlations between the Se level and GPx activity (R = 0.32, p < 0.001), CAT activity (R = 0.12, p = 0.025), GSH level (R = 0.12, p = 0.035), and LPH level (R = 0.14, p = 0.013). However, negative correlations were reported between the Se level and PbB (R = −0.13, p = 0.019), PbBmean (R = −0.12, p = 0.026), PbBmax (R = −0.14, p = 0.013), LPS level (R = −0.24, p < 0.001), and SOD activity (R = −0.24, p < 0.001) (Table 4).
Table 4.
Correlations between selenium and the blood lead level (PbB), mean, maximum, minimum PbB, zinc protoporphyrin concentration in blood (ZPP) mean, maximum, minimum ZPP, and parameters related to oxidative stress intensity and antioxidant reserves in blood and urine (Spearman R coefficient)
| R | p value | |
|---|---|---|
| Se and PbB | −0.13 | 0.019 |
| Se and PbBmean | −0.12 | 0.026 |
| Se and PbBmin | −0.09 | 0.113 |
| Se and PbBmax | −0.14 | 0.013 |
| Se and ZPP | −0.02 | 0.768 |
| Se and ZPPmean | −0.08 | 0.172 |
| Se and ZPPmax | −0.09 | 0.110 |
| Se and ZPPmin | −0.03 | 0.573 |
| Se and TAC | 0.07 | 0.185 |
| Se and TOS | −0.02 | 0.721 |
| Se and LPH | 0.14 | 0.013 |
| Se and MDA | −0.07 | 0.197 |
| Se and LPS | −0.24 | <0.001 |
| Se and 8OHdG | −0.09 | 0.111 |
| Se and GPx | 0.32 | <0.001 |
| Se and GR | 0.04 | 0.447 |
| Se and CAT | 0.12 | 0.025 |
| Se and SOD | −0.24 | <0.001 |
| Se and GSH | 0.12 | 0.035 |
Discussion
The Se concentration in human serum can be divided into three categories: low Se level below 50–60 μg/l, intermediate level between 60 and 100 μg/l, and high level above 100–120 μg/l [28]. In view of this classification, the Se level in the L-Se group can be classified as rather low (57.9 ± 8.62 μg/l) and in the H-Se group (90.1 ± 15.6 μg/l) as intermediate.
The results of the present study indicate that lower Se levels are associated with slightly higher levels of Pb in the blood. The value of PbB, a short-term marker of exposure, was most significantly related to the Se level. An inverse relationship between the levels of Pb and Se was also reported in other studies. For example, in a study by Kasperczyk et al. [10], a lower level of Se was reported in Pb-exposed workers compared with that in unexposed controls. Similarly, Gustafson et al. [29] showed significantly lower plasma Se levels in Pb smelters than in the control group. There was also a significant negative correlation between the PbB and plasma Se level in this study. Additionally, negative correlations between the levels of Pb and Se were shown in children from urban areas in Poland [30, 31] and Senegal [32]. The above-mentioned associations between Pb and Se may be a result of the formation of inactive Pb-Se complexes (Pb selenide) and alterations of the absorption and tissue distribution of Pb by Se [30, 33].
The antagonistic relationship between Pb and Se has been confirmed in experimental studies on animals. Optimal doses of dietary Se were able to partially ameliorate the toxic effects of Pb in poisoned rats, as estimated by measuring the urine ALA level and ALAD activity and Pb level in selected tissues [34]. Moreover, Se was able to mitigate the toxic effects of Pb on the rat brain [33, 35] and to protect the rat submandibular gland function from Pb-induced adverse effects, which were measured by the levels of calcium (Ca) and protein and the activity of N-acetyl-β-d-glucosaminidase (NAG) in submandibular secretions [35].
The protective effect of Se in Pb poisoning may be due not only to the above-mentioned mechanisms but also to the reduction of oxidative stress through the antioxidant properties of Se. The decreased level of erythrocyte LPS and increased level of erythrocyte GSH in the H-Se group compared to that in the L-Se group support this hypothesis. Moreover, the Se level was negatively correlated with the LPS level and positively correlated with the GSH level. It is well documented that Pb exposure is associated with GSH depletion as a result of elevated oxidative stress, as well as the accumulation of LPS, which is composed of cross-linked aggregates of oxidized proteins and lipids [8, 36]. Therefore, the higher level of GSH and lower level of LPS observed in the H-Se subgroup can be interpreted as effects of the reduced oxidative stress and may be due to the simultaneously elevated activity of GPx in this subgroup. We also observed a significant positive correlation between the Se level and GPx activity, which suggests that Se deficiency may significantly diminish the antioxidant defense. Consistent with our present findings, in the above-mentioned study on Senegalese children [32], a positive correlation was also shown between the Se levels and GPx activity (R = 0.308, p < 0.01).
Nevertheless, we did not observe any positive effects of higher Se levels on oxidative stress markers measured in the serum. We showed that there was only a weak positive correlation between the Se level and serum LPH level (R = 0.14). Moreover, we did not find any significant differences between the serum values of TAC and TOS and the serum levels of MDA and LPH between the examined subgroups. These results suggest that the bioavailability of Se and its protective effects may be higher in erythrocytes than in serum. This hypothesis is supported by a previous in vitro study showing that the presence of Pb results in the accumulation of Se in erythrocytes [37]. The authors of that study postulated the existence of a protein that simultaneously binds Pb and Se in erythrocytes. Supporting that previous study, Chiba et al. [38] showed that the plasma Se level had a tendency to decrease, whereas its concentration in erythrocytes increased significantly, with an increasing blood Pb level in Pb-exposed workers.
The interactions among the Pb and Se levels and activities of SOD, CAT, and GR are more difficult to interpret. The accumulated data suggest that Pb may trigger opposing mechanisms influencing antioxidant enzyme activities. These complex interactions likely resulted in the divergent results of studies on this topic [9]. In a study by Kasperczyk et al. [9] conducted on Pb-exposed workers, it was shown that Pb exposure induced the expression and activity of SOD as a result of a compensatory defense mechanism against oxidative stress. In light of this finding, the lower level of SOD in the H-Se subgroup compared to that in the L-Se subgroup may be due to a lower intensity of oxidative stress due to the simultaneously elevated activity of GPx and CAT.
The results of the present study are generally supported by other studies. Li et al. [39] showed that Se has protective activities against Pb-induced neurotoxicity in Caenorhabditis elegans through its ability to decrease ROS production. Sodium selenite was reported to alleviate oxidative stress injury, as measured by the MDA level induced by Pb exposure, in rats [40]. In other study on rats, Se administration decreased lipid peroxidation in the liver and kidney cells by increasing the activities of the superoxide dismutase and glutathione reductase and the glutathione content [41]. The results of studies on Pb-poisoned fish also confirmed the beneficial effects of Se administration of Pb toxicity [1, 42, 43].
The lower level of urine 8-OHdG in the H-Se subgroup compared to that in the L-Se subgroup in the present study may also be associated with reduced oxidative stress in subjects with higher Se levels. Lead exposure has been linked to elevated DNA damage, including oxidative damage [44]. 8-OHdG is one of the markers of oxidative DNA damage [45], and its lower levels in the H-Se subgroup may be a result of elevated activity of GPx. However, many other mechanisms may be involved, including the influence of Se on DNA repair [46]. Consistent with this possibility, an experimental study on mice reported that the negative effects of Pb on the DNA structure measured in a comet assay were alleviated by the administration of Se [47].
Many studies have shown that Pb can significantly increase arterial blood pressure. This increase may be caused by the influence of ROS on the metabolism of the endothelium and other cells of the circulatory system under Pb-induced oxidative stress conditions [48, 49]. This hypothesis is supported by a study on Pb-exposed workers, which showed that the arterial blood pressure (both systolic and diastolic) was positively correlated with the MDA and Pb levels and negatively correlated with the GPx activity [49]. These results are in agreement with the present findings, because the L-Se subgroup had a tendency to have higher systolic blood pressure compared to the H-Se subgroup. However, data on the associations between the Se level and blood pressure are still inconclusive, and larger-scale studies are needed to clarify these associations [50].
Conclusions
A lower serum Se level in chronically Pb-exposed subjects is associated with higher Pb blood levels and an elevated erythrocyte LPS level, which reflects the intensity of oxidative stress. Besides, in a group of Pb-exposed subjects with lower serum Se level, depleted GSH pool and decreased activity of GPx in erythrocytes were reported. However, the present results are inadequate to recommend Se supplementation for chronic lead exposure at higher doses than would be included in a normal diet except for selenium deficiency.
Acknowledgments
This study was supported by the National Science Center (DEC-2011/03/D/NZ7/05018).
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Tanekhy M. Lead poisoning in Nile tilapia (Oreochromis niloticus): oxidant and antioxidant relationship. Environ Monit Assess. 2015;187:4387. doi: 10.1007/s10661-015-4387-8. [DOI] [PubMed] [Google Scholar]
- 2.Baş H, Kalender Y. Nephrotoxic effects of lead nitrate exposure in diabetic and nondiabetic rats: involvement of oxidative stress and the protective role of sodium selenite. Environ Toxicol. 2015 doi: 10.1002/tox.22130. [DOI] [PubMed] [Google Scholar]
- 3.Malekirad AA, Oryan S, Fani A, Babapor V, Hashemi M, Baeeri M, Bayrami Z, Abdollahi M. Study on clinical and biochemical toxicity biomarkers in a zinc-lead mine workers. Toxicol Ind Health. 2010;26:331–337. doi: 10.1177/0748233710365697. [DOI] [PubMed] [Google Scholar]
- 4.Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu PC, Guo YL. Antioxidant nutrients and lead toxicity. Toxicology. 2002;180:33–44. doi: 10.1016/S0300-483X(02)00380-3. [DOI] [PubMed] [Google Scholar]
- 6.Monterio HP, Bechara EJH, Abdalla DSP. Free radical involvement in neurological porphyries and lead poisoning. Mol Cell Biochem. 1991;103:73–83. doi: 10.1007/BF00229595. [DOI] [PubMed] [Google Scholar]
- 7.Ribarov SR, Bochev PG. Lead-hemoglobin interaction as a possible source of reactive oxygen species—a chemiluminescent study. Arch Biochem Biophys. 1982;213:288–292. doi: 10.1016/0003-9861(82)90464-7. [DOI] [PubMed] [Google Scholar]
- 8.Kasperczyk S, Dobrakowski M, Kasperczyk A, Ostałowska A, Birkner E. The administration of N-acetylcysteine reduces oxidative stress and regulates glutathione metabolism in the blood cells of workers exposed to lead. Clin Toxicol (Phila) 2013;51:480–486. doi: 10.3109/15563650.2013.802797. [DOI] [PubMed] [Google Scholar]
- 9.Kasperczyk A, Machnik G, Dobrakowski M, Sypniewski D, Birkner E, Kasperczyk S. Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead. Toxicology. 2012;301:79–84. doi: 10.1016/j.tox.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Kasperczyk A, Prokopowicz A, Dobrakowski M, Pawlas N, Kasperczyk S. The effect of occupational lead exposure on blood levels of zinc, iron, copper, selenium and related proteins. Biol Trace Elem Res. 2012;150:49–55. doi: 10.1007/s12011-012-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 2014;39:112–120. doi: 10.1016/j.tibs.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurokawa S, Berry MJ. Selenium. Role of the essential metalloid in health. Met Ions Life Sci. 2013;13:499–534. doi: 10.1007/978-94-007-7500-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–945. doi: 10.1016/S0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 14.Trzcinka-Ochocka M, Jakubowski M, Raźniewska G. Assessment of occupational exposure to lead in Poland. Med Pr. 2005;56:395–404. [PubMed] [Google Scholar]
- 15.Stoeppler M, Brandt K, Rains TC. Contribution to automated trace analysis. Part II. Rapid method for the automated determination of lead in whole blood by electrothermal atomic-absorption spectrophotometry. Analyst. 1978;103:714–722. doi: 10.1039/an9780300714. [DOI] [PubMed] [Google Scholar]
- 16.Neve J, Molle L. Direct determination of selenium in human serum by graphite furnace atomic absorption spectroscopy. Improvements due to oxygen ashing in graphite tube and Zeeman effect background correction. Acta Pharmacol Toxicol. 1986;59:606–609. doi: 10.1111/j.1600-0773.1986.tb02836.x. [DOI] [PubMed] [Google Scholar]
- 17.Grabecki J, Haduch T, Urbanowicz H. Die einfachen Bestimmungsmethoden der delta-Aminolavulinsaure im Harn. [Simple determination methods of delta-aminolevulinic acid in urine] Int Arch Arbeitsmed. 1967;23:226–240. [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Södergren E, Nourooz-Zadeh J, Berglund L, Vessby B. Re-evaluation of the ferrous oxidation in xylenol orange assay for the measurement of plasma lipid hydroperoxides. J Biochem Biophys Methods. 1998;37:137–146. doi: 10.1016/S0165-022X(98)00025-6. [DOI] [PubMed] [Google Scholar]
- 20.Jain SK. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hypercoagulability by the lipid peroxidation of erythrocytes in rats. J Clin Invest. 1985;76:281–286. doi: 10.1172/JCI111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Pawelski S. Diagnostyka laboratoryjna w hematologii. Warszawa: PZWL; 1983. [Google Scholar]
- 24.Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984;142:290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 26.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 27.Richterich R. Chemia kliniczna. Warszawa: PZWL; 1971. [Google Scholar]
- 28.Burri J, Haldiman M, Dudler V. Selenium status of the Swiss population: assessment and change over a decade. J Trace Elem Med Biol. 2008;22:112–119. doi: 10.1016/j.jtemb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Gustafson A, Schütz A, Andersson P, Skerfving S. Small effect on plasma selenium level by occupational lead exposure. Sci Total Environ. 1987;66:39–43. doi: 10.1016/0048-9697(87)90075-1. [DOI] [PubMed] [Google Scholar]
- 30.Osman K, Schütz A, Akesson B, Maciag A, Vahter M. Interactions between essential and toxic elements in lead exposed children in Katowice, Poland. Clin Biochem. 1998;31:657–665. doi: 10.1016/S0009-9120(98)00071-X. [DOI] [PubMed] [Google Scholar]
- 31.Gać P, Pawlas N, Poręba R, Poręba M, Pawlas K. The relationship between environmental exposure to cadmium and lead and blood selenium concentration in randomly selected population of children inhabiting industrial regions of Silesian Voivodship (Poland) Hum Exp Toxicol. 2014;33:661–669. doi: 10.1177/0960327113499169. [DOI] [PubMed] [Google Scholar]
- 32.Diouf A, Garçon G, Diop Y, Ndiaye B, Thiaw C, Fall M, Kane-Barry O, Ba D, Haguenoer JM, Shirali P. Environmental lead exposure and its relationship to traffic density among Senegalese children: a cross-sectional study. Hum Exp Toxicol. 2006;25:637–644. doi: 10.1177/0960327106074591. [DOI] [PubMed] [Google Scholar]
- 33.Nehru B, Dua R, Iyer A. Effect of selenium on lead-induced alterations in rat brain. Biol Trace Elem Res. 1997;57:251–258. doi: 10.1007/BF02785293. [DOI] [PubMed] [Google Scholar]
- 34.Cerklewski FL, Forbes RM. Influence of dietary selenium on lead toxicity in the rat. J Nutr. 1976;106:778–783. doi: 10.1093/jn/106.6.778. [DOI] [PubMed] [Google Scholar]
- 35.Sidhu P, Nehru B. Protective effects of selenium to placental lead neurotoxicity in rat pups. Toxicol Mech Methods. 2005;15:419–423. doi: 10.1080/15376520500194775. [DOI] [PubMed] [Google Scholar]
- 36.Kasperczyk A, Słowińska-Łożyńska L, Dobrakowski M, Zalejska-Fiolka J, Kasperczyk S. The effect of lead-induced oxidative stress on blood viscosity and rheological properties of erythrocytes in lead exposed humans. Clin Hemorheol Microcirc. 2014;56:187–195. doi: 10.3233/CH-131678. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Chiba M, Shinohara A, Watanabe H, Inaba Y. Studies on lead-binding protein and interaction between lead and selenium in the human erythrocytes. Ind Health. 1998;36:234–239. doi: 10.2486/indhealth.36.234. [DOI] [PubMed] [Google Scholar]
- 38.Chiba M, Shinohara A, Matsushita K, Watanabe H, Inaba Y. Indices of lead-exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. Tohoku J Exp Med. 1996;178:49–62. doi: 10.1620/tjem.178.49. [DOI] [PubMed] [Google Scholar]
- 39.Li WH, Shi YC, Tseng IL, Liao VH. Protective efficacy of selenite against lead-induced neurotoxicity in Caenorhabditis elegans. PLoS One. 2013;8:e62387. doi: 10.1371/journal.pone.0062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu MC, Xu Y, Chen YM, Li J, Zhao F, Zheng G, Jing JF, Ke T, Chen JY, Luo WJ. The effect of sodium selenite on lead induced cognitive dysfunction. Neurotoxicology. 2013;36:82–88. doi: 10.1016/j.neuro.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Othman AI, El Missiry MA. Role of selenium against lead toxicity in male rats. J Biochem Mol Toxicol. 1998;12:345–349. doi: 10.1002/(SICI)1099-0461(1998)12:6<345::AID-JBT4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Hashish EA, Elgaml SA, El-Murr A, Khalil R (2015) Nephroprotective and antioxidant significance of selenium and α-tocopherol on lead acetate-induced toxicity of Nile Tilapia (Oreochromis niloticus). Fish Physiol Biochem. 2015 Feb 15. [Epub ahead of print] [DOI] [PubMed]
- 43.Özkan-Yilmaz F, Özlüer-Hunt A, Gündüz SG, Berköz M, Yalin S. Effects of dietary selenium of organic form against lead toxicity on the antioxidant system in Cyprinus carpio. Fish Physiol Biochem. 2014;40:355–363. doi: 10.1007/s10695-013-9848-9. [DOI] [PubMed] [Google Scholar]
- 44.García-Lestón J, Méndez J, Pásaro E, Laffon B. Genotoxic effects of lead: an updated review. Environ Int. 2010;36:623–636. doi: 10.1016/j.envint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Dizdaroglu M. Oxidatively induced DNA damage: mechanisms, repair and disease. Cancer Lett. 2012;327:26–47. doi: 10.1016/j.canlet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 46.McKelvey SM, Horgan KA, Murphy RA. Chemical form of selenium differentially influences DNA repair pathways following exposure to lead nitrate. J Trace Elem Med Biol. 2014;29:151–169. doi: 10.1016/j.jtemb.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Yuan X, Tang C. The accumulation effect of lead on DNA damage in mice blood cells of three generations and the protection of selenium. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng. 2001;36:501–508. doi: 10.1081/ESE-100103479. [DOI] [PubMed] [Google Scholar]
- 48.González J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: new insights. World J Cardiol. 2014;6:353–366. doi: 10.4330/wjc.v6.i6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasperczyk S, Kasperczyk J, Ostałowska A, Zalejska-Fiolka J, Wielkoszyński T, Swietochowska E, Birkner E. The role of the antioxidant enzymes in erythrocytes in the development of arterial hypertension among humans exposed to lead. Biol Trace Elem Res. 2009;130:95–106. doi: 10.1007/s12011-009-8323-z. [DOI] [PubMed] [Google Scholar]
- 50.Kuruppu D, Hendrie HC, Yang L, Gao S. Selenium levels and hypertension: a systematic review of the literature. Public Health Nutr. 2014;17:1342–1352. doi: 10.1017/S1368980013000992. [DOI] [PMC free article] [PubMed] [Google Scholar]