Abstract
Inflammasomes are large multiprotein complexes localized in the cytoplasm of the cell. They are responsible for the maturation of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and IL-18 as well as for the activation of inflammatory cell death, the so-called pyroptosis. Inflammasomes assemble in response to cellular infection, cellular stress, or tissue damage; promote inflammatory responses and are of great importance in regulating the innate immune system in chronic inflammatory diseases such as periodontitis and several chronic systemic diseases. In addition to sensing cellular integrity, inflammasomes are involved in the homeostatic mutualism between the indigenous microbiota and the host. There are several types of inflammasomes of which NLRP3 is best characterized in microbial pathogenesis. Many opportunistic bacteria try to evade the innate immune system in order to survive in the host cells. One of these is the periodontopathogen Porphyromonas gingivalis which has been shown to have several mechanisms of modulating innate immunity by limiting the activation of the NLRP3 inflammasome. Among them, ATP-/P2X7- signaling is recently associated not only with periodontitis but also with development of several systemic diseases. The present paper reviews multiple mechanisms through which P. gingivalis can modify innate immunity by affecting inflammasome activity.
Keywords: innate immunity, subversion, inflammasomes, inflammation, Porphyromonas gingivalis, persistence, periodontitis, systemic diseases
The innate immune system is the first line of defense against microbial pathogens. It is initiated by genome-encoded pattern recognition receptors (PRRs) that respond to invading microorganisms. PRRs recognize microbial pathogen-associated molecular patterns (PAMPs). This leads to activation of host defense pathways to clear the infection (1). In addition to microbial components, the receptors can respond to danger-associated molecular patterns (DAMPs) derived from the host (ATP, DNA, cholesterol crystals). Also environmental irritants such as asbestos, silica, alum, and nanoparticles can stimulate inflammasome-driven inflammation (2, 3). In the detection of pathogens, toll-like receptors (TLRs) are of crucial importance. They recognize distinct PAMPs and participate in the first line of defense against invading pathogens, playing a significant role in inflammation and immune cell regulation. Other well characterized evolutionary conserved PRRs involved in innate immune defense are retinoic acid-inducible gene-I (RIG-I) receptors, C-type lectin receptors (CLRs), and nucleotide-binding domain (NOD)-like receptors (4, 5). All these receptors are expressed by several cell types such as macrophages, neutrophils, monocytes, and epithelial cells (6).
The activation of PRRs and their post-receptor signaling can stimulate recruitment of the so-called inflammasome complexes (1, 3, 6–15). Inflammasome is a relatively new concept introduced by Tschopp et al. in 2002 (7). Later, Tschopp also introduced the concept of metabolic syndrome that senses metabolic stress and contributes to the metabolic syndrome associated with obesity and type 2 diabetes (16).
The aims of the present paper are to give a brief description of inflammasome compositions and functions and to systematically review how Porphyromonas gingivalis, a putative keystone pathogen in chronic periodontitis (17), well known for its way to manipulate the innate immune system (18), may carry out/modulate inflammasome activation in chronic periodontitis and certain other chronic systemic infections. The role of inflammasome and danger molecule signaling in the oral cavity was recently reviewed by Yilmaz and Lee (6).
Inflammasomes
Inflammasomes are multiprotein complexes localized within the cytoplasm of the cell. They are engaged in the maturation of pro-inflammatory cytokines such as interleukin-1β (IL1-β) and interleukin-18 (IL-18) (19). After infection or cellular stress, inflammasomes are assembled, activated, and involved in host defense and in the pathophysiology of diseases (20). Inflammasomes follow canonical or non-canonical pathways. A typical functional canonical inflammasome complex consists of a nucleotide-binding leucine-rich repeat (NLR) protein, an adaptor molecule apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) and procaspase-1 (21). A typical non-canonical inflammasome is the one activating caspase-11, which so far is an understudied pro-inflammatory caspase (22).
A unique scaffolding protein (NLR) dictates the formation of inflammasomes. Mutations in members of the NLR family have been linked to various inflammatory diseases consistent with the fact that these molecules play an important role in host–pathogen interactions and the inflammatory response (23). Each inflammasome contains a unique sensor protein of the NLR superfamily or the PYRIN and HIN-200 domain-containing (PYHIN) superfamily (10). In NLRs, signaling is exerted by caspase activation and the so-called caspase activation and recruitment domains (CARDs). These can recruit caspase-1 directly or by PYRIN domains recruiting caspase-1 via the CARD-PYRIN-containing adaptor protein ASC (10). The adaptor protein mediates a critical step in innate immune signaling by bridging the interaction between the pathogen recognition receptors and caspase-1 in inflammasome complexes (24).
Inflammasomes have a critical role in initiating innate immune responses, particularly by acting as platforms for activation of the inflammatory caspase proteases. Among these, caspase-1 initiates innate immune responses by specifically cleaving of pro-IL-1β and pro-IL-18 and mediates their maturations and release (10). These cytokines promote recruitment of phagocytes, angiogenesis, epithelial cell repair, and regulation of cytokines and chemokine production by other immune cells at the site of infection or injury (reviewed by Hao et al. (25)). Inflammasomes also take part in the host defense independent of their classic cytokine targets IL-1β and IL-18.
Caspase-1 and 11 can start a rapid and inflammatory form of cell death, the so-called pyroptosis. They are distinct from caspases classically involved in apoptosis. Pyroptosis, a program of cellular self-destruction that is intrinsically inflammatory, results from osmotic pressure created by caspase-1-dependent formation of membrane pores (26, 27) and is associated with rapid release of cytosolic contents. This process can restrict intracellular replication of invasive bacterial pathogens (28) and probably acts in synergy with the recruitment of neutrophils by IL-1β to restrict replication of bacteria in vivo (10).
Inflammasomes sense cellular integrity and tissue health. When cell homeostasis is disrupted, inflammation is caused by the release of cytokines. A large amount of infectious and noxious insults can assemble these special structures. Thus inflammasomes may have a role in bacterial, parasitic, fungal, and viral infections (29). Inflammasomes also sense products and endogenous signals that indicate loss of cellular homeostasis (10, 15) and can be active both in periodontitis and several systemic diseases (6, 30).
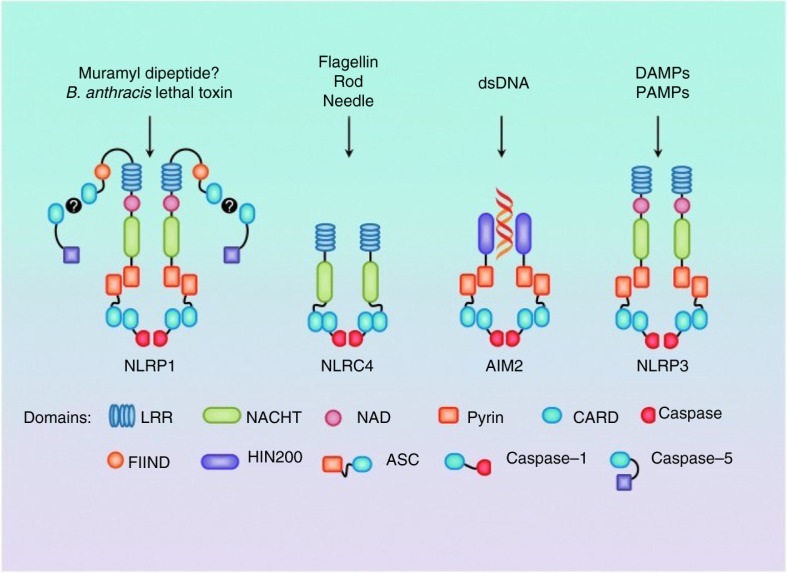
Several distinct inflammasomes have currently been described, each of which is activated by unique stimuli (Fig. 1). Inflammasomes such as NLRP1 (nucleotide-binding domain-like receptor protein) inflammasome1, NLRP3, NLRP4, NLRP6, NLRP/NLRP12, and AIM2 (Absent In Melanoma 2) have been recognized acting in the host defense against intracellularly invading pathogens. Some inflammasomes are particularly well characterized for their role in bacterial recognition such as NLRC4, NLRP3, and AIM2 (Fig. 1). Also there may be more paths to IL-1β and IL-18 generation than via caspase-1 (1). It is likely that other caspase-1-dependent effector cytokines are produced by other proteases during infection (31). Several pathogens have been found to develop strategies to counteract inflammasomes (the so-called pathogenic stealth mechanisms) (32). Thus, Staphylococcus aureus can modify its cell wall peptidoglycan to prevent degradation by lysozymes through peptidoglycan O-acetyl transferase A which also strongly suppresses inflammasome activation and inflammation in vitro and in vivo (33).
Fig. 1.
Major inflammasomes with known stimulators. In NLPR1 muramyl dipeptide and Bacillus anthracis lethal toxin can directly cause caspase-5 processing. NLRC4 activation is mostly related to components of Gram-negative bacteria. In AIM2, double-stranded DNA (dsDNA) binds to the HIN200 domain and requires ASC for processing of caspase-1. Also, NLPR3 requires ASC and caspase-1. It is activated in response to both exogenous and endogenous danger signals. (From ref. 19 with permission.)
While inflammasome activation is important to host defense, excessive inflammasome activation can be detrimental to health. Inflammasome hyperactivation is recently proposed to be the basis for autoinflammatory disease pathogenesis, whereas inflammasome regulated activity is central for appropriate host defense and protection from sepsis (16). Accordingly, there is need for a balance between resolution of infection and excessive inflammation (1). It should also be kept in mind that several pathogenic bacteria, for example, Yersinia pestis, Salmonella, and Listeria monocytogenes activate multiple inflammasomes demonstrating redundancy of inflammatory receptors in vitro and in vivo (34, 35). It is also possible that several NLRs, AIM2s, and caspases co-operate during infections (1), which may be necessary for optimal responses to be obtained.
In inflammasome-mediated cytokine release, a multi-step activation pathway is followed. First, there is an NF-κB-dependent upregulation of the inactive pro-forms of IL-1β and IL-18 and of some NLRs like NLRP3; thereafter, activation of the NLR or AIM2, and inflammasome formation occurs (1). Some cells have simpler activation pathways because they have higher basal levels of the pro-forms of IL-1β and IL-18.
Interestingly, inflammasomes are also involved in the homeostatic mutualism between host and commensals. In the intestine, one inflammasome function is to control the composition of the microbiota (36). The NLCP4 inflammasome, expressed by intestinal phagocytes in particular, plays a major role to discriminate between commensal and pathogenic microbes and initiate a harmful response to the latter (37). Thus, inflammasomes may have a variety of roles regulating homeostasis in the intestinal tract and microbial ecology preventing the emergence of pathobionts (25, 38). Inflammasome-deficient mice exhibited an aberrant microbial community that triggered an enhanced inflammatory reaction in the intestine (39). This microbial dysbiosis affected the physiology and pathophysiology both locally in the intestine and systemically and might contribute to the pathogenesis of intestinal bowel disease (10).
The NLRP3 inflammasome
NLRP3 is part of one of the best-studied inflammasome complexes. It consists of the NLRP3 scaffold, the ASC adaptor, and procaspase-1 (3). Two steps are required to activate the NLRP3 inflammasome (25). The first step is initiated by microbial ligands or endogenous cytokines and is needed to induce upregulation of NLRP3 protein expression (2, 40). NF-кB activation and reactive oxygen species (ROS) are required for this step. The second step is activation of NLRP3 by microbial stimuli or endogenous molecules (25). NLRP3 is activated by several microbial-derived ligands, including toxins (20, 37). The endogenous signals triggering NLRP3 activation include the danger signal ATP, fatty acids, particulate matter, necrosis, and necroptosis (reviewed by Hao et al. (25)). Also K+ efflux, lysosome function, endoplasmic reticulum (ER) stress, intracellular calcium, ubiquitination, microRNAs, and particularly ROS have been proposed (reviewed by Abais et al. (19)) (Fig. 2). ROS may serve a ‘kindling’ or triggering factor for activation of the NLRP3 inflammasome as well as ‘bonfire’ or ‘effector’ molecules leading to pathological processes (19).
Fig. 2.
Activation of the NLRP3 inflammasome as a two-step mechanism. Primary signals come from activation of toll-like receptors (TLRs) which are responsible for the upregulation of NLRP3 and pro-interleukin-1β (IL-1β) in an NF-κB-dependent manner. Secondary signals come from several pathways: K+ efflux via P2X7 receptor activation via ATPe coupling, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, NADPH oxidase, frustrated phagocytosis, and lysosomal rupture pathways. All these primary and secondary signals converge in the production of reactive oxygen species (ROS). (From ref. 19 with permission.)
In monocytes and dendritic cells, TLR stimulation is adequate to induce caspase-1 activation and IL-1β production but not in macrophages (reviewed by Hao et al. (25)). In human monocytes, TLR stimulation promotes extracellular release of ATP which in turn stimulates the purinergic receptor P2X7 needed for activation of the NLRP3 inflammasome. In dendritic cells, however, NLRP3 activation is independent of P2X7 (41). This implies that in some cell types TLR can activate the NLRP3 inflammasome independent of extracellular mediators such as ATP. All NLRP3s have a unique sensor protein of the NLR or the PYHIN superfamily. These proteins seem to possess numerous mechanisms for sensing bacteria and initiating immune mechanisms (10).
The AIM2 inflammasome
AIM2 is a cytosolic binding receptor for double-stranded DNA (1). It is known to form an inflammasome and activate caspase-1 when bacteria and viruses are present (42–44). AIM2 consists of an N-terminal pyrin domain and a C-terminal DNA-binding HIN200 domain. It is the only known HIN200 domain-containing protein with capacity to mature IL-1β and IL-18 through interactions with ASC and caspase-1 (42). The AIM2 inflammasome is particularly important in the defense against intracellular bacterial and viral pathogens (43, 44).
P. gingivalis
Synergy
Several opportunistic pathogens have been shown to develop different mechanisms to inhibit inflammasome activation and function. Similarly, P. gingivalis, a proposed keystone organism in chronic periodontitis (17), has been found to manipulate innate immunity via a number of mechanisms (18). This bacterium has been postulated to suppress inflammasome activation as a mechanism for its low immunostimulatory activity and pathogenic synergy with other periodontal bacteria that are shown to be more immunogenic (45). P. gingivalis can also suppress inflammasome activation by Fusobacterium nucleatum and this may be a contribution from P. gingivalis to the synergy between the two periodontal bacteria (46–49). This specific inhibition appears to affect IL-1β and IL-18 processing and cell death in macrophages of both man and mouse. While F. nucleatum activated IL-1β processing through the NLRP3 inflammasome, P. gingivalis–mediated repression was not related to lowered levels of inflammasome components (45). P. gingivalis infection also influences endocytosis by preferentially suppressing endocytic pathways toward inflammasome activation. This represents a new mechanism of pathogen-mediated inflammasome inhibition (45). It should also be noted that although P. gingivalis inhibits an activation pathway that can kill the microrganism; this may not be the integral part of a general immune suppression strategy as P. gingivalis harnesses acute sustained inflammation that is relatively harmless to the bacterium. Indeed, periodontitis-associated bacteria could benefit from a nutritionally favorable inflammatory environment created by P. gingivalis (50).
Nucleoside-diphosphate kinase
P. gingivalis uses its extracellularly secreted nucleoside-diphosphate kinase homologue (NDK) (51) to inhibit innate immune responses due to stimulation by extracellular ATP (ATPe) (52). ATPe acts as a danger signal that can alert the immune system about a present infection. ATPe binds to P2X7 receptors (see below) and activates an inflammasome and caspase-1. Infection of gingival epithelial cells (GECs) resulted in inhibition of ATP-induced caspase-1 activation (52). ndk-deficient P. gingivalis was less effective in limiting ATP-mediated caspase-1 activation and secretion of IL-1β from infected cells. NDK therefore seems to play an important role in inhibiting P2X7-dependent inflammasome activation. The consequent inhibition of P2X7-mediated apoptosis and extension of the viability of GECs could make P. gingivalis survive for extended periods of time in the gingival epithelium and contribute to disease when other host and bacterial factors participate in tissue destruction. NDK also reduced ATPe-mediated plasma membrane permeabilization of host cells in a dose-dependent manner (53).
In GECs, NDK of P. gingivalis promoted intracellular persistence by inhibiting ATP-induced ROS via P2X7 receptor/NADPH oxidase signaling (54). This implied that GECs produced significant amounts of ROS in response to ATPe and that this depended on P2X7-signaling coupled with membrane-bound NADP oxidase and the mitochondrial respiratory chain. This novel signaling cascade may contribute to successful tissue persistence of this major pathogen.
Also, the secreted multi-functional effector molecule, NDK from P. gingivalis attenuated release of the high-mobility group protein B1 (HMGB1) (52). HMGB1 is a pro-inflammatory danger signal associated with chromatin in healthy cells. Lack of NDK reduced significantly the inhibition of ATP-dependent inflammasome activation and the release of pro-inflammatory cytokines in GECs (52). The findings suggested that NDK could play a significant role in the inhibition of P2X7 -dependent inflammasome activation and HMGB1-release from infected GECs.
The P2X7 and P2X4 receptors
The P2X7 purinergic cell surface receptor, which is expressed on a variety of immune cells, including macrophages, functions as a second signal for assembly of the NLRP3 inflammasome (55). Purinergic signaling is essential for the release of IL-1β from cells infected with P. gingivalis (56). In macrophages P2X7 has a dual role, as it was critical not only for ATPe-induced IL-1β secretion in vitro but also for intracellular pro-IL-1β processing (57). These findings also applied to the in vivo situation since the P2X7 receptor expression was upregulated in a P. gingivalis oral infection model. Further, the P2X7 receptor and NLRP3 transcription were found to be modulated in human chronic periodontitis (57), suggesting that the P2X7 receptor also has a role in periodontal immunopathogenesis. The ability of P. gingivalis to modulate ATP-/P2X7-signaling, to secrete NDK during infection in primary GECs, and its expression of other virulence factors, for example, gingipains and fimbriae, and promotion of peripheral artery disease (PAD) may link this bacterium to periodontitis and other systemic diseases such as rheumatoid arthritis, diabetes, obesity, multiple sclerosis, and pancreatic and kidney diseases (58, 59). Interestingly, gingipains may also affect inflammasome activation. Jung et al. (60) recently showed that the simultaneous protease action of Kgp and Rgps attenuates the caspase-1 activating potential of P. gingivalis in macrophages.
It is well known that infection of GECs with P. gingivalis requires an exogenous danger signal such as ATPe for activation of an inflammasome and caspase-1. This again will induce secretion of IL-1β. Generation of ROS is also stimulated by ATPe. However, the mechanism of ROS generation and the role of purinergic receptors in inflammasome activation were not very clear until Hung et al. (61) demonstrated that the purinergic receptor P2X4 is assembled with P2X7 and its associated pore, pannexin-1. ROS production was induced by ATPe through a complex containing P2X4, P2X7, and pannexin-1. The P2X7-mediated ROS production can activate the NLPR3 inflammasome and caspase-1. Activation by P2X4 alone induced generation of ROS but not inflammasome activation. Depletion or inhibition of P2X4, P2X7, or the pannexin complex markedly blocked IL-1β secretion in P. gingivalis-infected GECs after ATPe treatment (61). Accordingly, ROS is produced by stimulation of the P2X4/P2X7/pannexin-1 complex. This also means that P2X4 acts as a positive regulator of inflammasome activation during infection with P. gingivalis.
A2a adenosine receptor
Danger signals (DS) are molecules like adenosine that exert extracellular signaling derived from autocrine and/or paracrine secretions during inflammation and chronic diseases. Adenosine, which is a metabolite of ATP, has so far been little appreciated as a component of the innate immune system (62). ATPe is produced through a number of enzymatic reactions in normal, stressed, or infected tissues (63). It has recently been shown that P. gingivalis can use A2A adenosine receptor–coupled DS adenosine signaling as a means to proliferate and survive in primary GECs, possibly by down-regulating the pro-inflammatory response (62). P. gingivalis reduces extracellular nucleotide concentrations of ATP and thereby acts as a generator of adenosine which stimulates the bacterium's growth in primary GECs. This may be another anti-inflammatory immune response exerted by P. gingivalis to promote its survival in the oral mucosa.
Phosphatidylserine
During Chlamydia infection of human epithelial, endothelial, granulocyte, or monocytic cells, phosphatidylserine (PS) is translocated from the inner to the outer leaflet and becomes exposed to the external side of the cell (64). PS exposure is an early marker of apoptosis associated with pro-inflammatory triggering of complement activation (65, 66). Yilmaz et al. (67) found that P. gingivalis after establishing itself in the nutritionally rich cytosol of the host cell can protect the infected cell from the host immune defense by reducing the inflammatory response after inducing transient externalization of PS. This would allow multiplication of P. gingivalis inside cells while protecting them from cytotoxic reactions of the immune system. It was also suggested that the bacterium blocks mitochondrion-dependent apoptosis to upkeep its intracellular lifestyle. This may permit successful spreading of P. gingivalis to adjacent and deeper host tissues.
Inflammasome activity in P. gingivalis–induced periodontal disease and defense
GECs are important parts of the immune response to periodontal bacteria. They express a functional NLRP3 inflammasome (68). Much higher levels of inflammasome components were found in the gingival tissues from patients with chronic periodontitis than from healthy controls (14). It therefore seems reasonable to consider the inflammasome as an operational part of innate immunity against periodontitis.
While supragingival biofilm, causing gingivitis, increased the expression of caspase-1, ASC, AIM2, IL-1β, and IL-18 in gingival fibroblasts, subgingival biofilm, promoting periodontitis, enhanced caspase-1, ASC, AIM2, IL-1β, and IL-18 gene expression at lower concentrations, followed by their downregulation at higher concentrations (69). The authors proposed that high concentrations of bacterial virulence factors in sites with affluent immune mechanisms such as the biofilm-tissue interface can down-regulate host defense barriers, while the lower bacterial concentrations deeper in periodontal tissues can have a stimulatory effect on inflammatory responses.
When using a 10-species subgingival biofilm with P. gingivalis present, Belibasakis et al. (70) found a reduction in NLRP3 and IL-1β expression in human gingival fibroblasts after challenge for 6 h. The AIM2 expression was not affected. After exclusion of P. gingivalis from the biofilm, a partial rescue of NLRP3 and IL-1β-expression occurred. It was suggested that subgingival biofilms down-regulate NLRP3 and IL-1β expression partly due to P. gingivalis and that this dampening of the host innate immune responses may favor persistence and survival of biofilm species in periodontal tissues.
It has been recently shown that fimbriae of P. gingivalis inhibit ATPe-induced IL-1β secretion through the P2X7 receptor in macrophages (56). Ramos-Junior et al. (57), however, found that NLRP3 is necessary for ATPe-induced IL-1β secretion as well as for caspase-1 activation irrespective of P. gingivalis fimbriae. Although IL-1β secretion from P. gingivalis–infected macrophages depended on NLRP3, its adaptor protein ASC, or caspase-1, the cleavage of intracellular IL-1β to the mature form occurred independently of NLRP3, its adaptor protein ASC, or caspase-1.
P. gingivalis dampened ATPe-induced IL-1β secretion in macrophages by means of its fimbriae in a purinergic P2X7 receptor-dependent manner (56). In this study, the immune subversion of P. gingivalis was connected with the ability of fimbriae to reduce ATPe-induced macrophage secretion of IL-1β via P2X7 activation. The authors held that this could be another molecular action of subversion of the immune system by P. gingivalis.
In THP-1 (leukemic monocytic) cells, P. gingivalis–induced IL-1β secretion and inflammatory cell death through activation of caspase-1 (14). IL-1β secretion and pyroptic cell death required both NLRP3 and AIM2 inflammasome activation via TLR2 and TLR4 signaling. The activation of the former was mediated by ATP release, the P2X7 receptor, and lysosomal damage (14). These authors also suggested that P. gingivalis–induced NLRP3 activation depends on ATP release, K+ efflux, and cathepsin B.
Underacylated lipopolysaccharide
P. gingivalis can use lipid A phosphatase to alter the lipid A composition of its lipopolysaccharide (LPS). This organism may therefore modulate the immune response by expressing an underacylated LPS (71–73). This LPS could bind but not activate caspase-11 (human caspase-4,5) which results in host cell lysis and reduces survival of bacteria (72, 74). Caspase-4 can be of specific importance to mucosal immunity since caspase-4 expression level and ability to become activated as a response to infection differ markedly from that of caspase-5. Caspase-4 has been suggested to provide key non-redundant discernment into rapid sensing and clearance of bacteria at mucosal tissues (21).
Atherosclerosis
There is mounting evidence that P. gingivalis can invade cardiovascular cells and tissues causing inflammation (75). The NLRP3 inflammasome has been suggested to have an important role in developing vascular inflammation and atherosclerosis (76, 77). In hyperlipidemic animals, P. gingivalis accelerated atherosclerosis (78). Wild-type challenge of apolipoprotein E–deficient, spontaneously hyperlipidemic (Apoeshl) mice with P. gingivalis increased IL-1β, IL-18, and TNF-α production in peritoneal macrophages and gingival or aortic gene expression of the NOD-like receptor family, NLRP3, IL-1β, pro-IL-18 and pro-caspase-1 (78). Fimbriae were found to bring increased tissue invasiveness and pro-inflammatory ability to P. gingivalis. It was also demonstrated that P. gingivalis activates innate immune cells through the NLRP3 inflammasome compared with a KDP136 (gingipain-null) or a KDP150 (FimA-deficient) mutant.
Inflammation-related induction of AIM2 in vascular cells and atherosclerotic lesions has suggested a role for AIM2 in vascular pathogenesis where increased AIM2 expression was seen around the necrotic core of atherosclerotic carotid lesions and in the vasa vasorum of neovasculature of aortic aneurysms (79). The NLRP3 inflammasome and AIM2 may thus have important roles in both P. gingivalis–induced periodontal disease and atherosclerosis through sustained inflammation.
Recently, the CD36/scavenger receptor (SR)-B2 was suggested to play a role at multiple points in P. gingivalis–mediated enhanced atherosclerosis in a mouse model (80). The study suggested that activation of the inflammasome by P. gingivalis is mediated by CD36/SR-B2 and TLR2 which cause systemic release of pro-atherosclerotic IL-1β and macrophage pyroptosis. Systemic IL-1β activates vascular macrophages naïve to P. gingivalis to secrete IL-1β and promotes CD36-mediated uptake of oxLDL and increased formation of foam cells. The presence of oxLDL could inhibit P. gingivalis/P. gingivalis LPS-inflammasome activation and pyroptosis, which would allow greater atherosclerotic plaque to develop. TLR-CD36-/SR-B2-mediated IL-1β generation may thus be important to increase atherosclerotic lesions. Of note is also that cytoplasmic LPS sensing in human cells activates the non-canonical caspase-4-dependent inflammasome. This is a new mechanism of inflammasome activation where direct LPS-binding results in caspase oligomerization and activation leading to induction of IL-1β secretion and pyroptosis (81).
Alzheimer's disease
P. gingivalis may be an important pathogen in Alzheimer's disease (AD) contributing to brain inflammation (82). NLRP3 has been reported in microglial cells that responded to infection and initiation of neuro-degeneration in an Alzheimer's disease model (83). Furthermore, TLR2 and NLRP3 were recently found to co-operate to recognize a functional bacterial amyloid, curli fibers, in the plaques of brains in AD patients (84). Heneka et al. (85) found a strongly activated caspase-1 expression in human mild cognitive impairment and AD brains which suggested a role for the inflammasome in brain degenerative disease. AD brain deposits activated the NLRP3 inflammasome in microglial cells in vitro and in vivo which could lead to progression of AD (85–88). This suggested a role for the inflammasome in this neurodegenerative disease.
Non-alcoholic steatohepatitis
P. gingivalis seems to be a critical risk factor for progression of non-alcoholic steatohepatitis (NASH) through upregulation of the P. gingivalis-LPS-TLR2-pathway and by stimulating inflammasomes (89). These authors found that P. gingivalis exacerbated steatohepatitis induced by diet via induction of inflammasomes and inflammatory cytokines in the liver of mice. P. gingivalis was also demonstrated for the first time in the liver of NASH patients. It was suggested that dental infection with P. gingivalis promotes progression of NASH.
Squamous cell carcinoma
A close association has been found between P. gingivalis and squamous cell carcinoma (90). The fact that P. gingivalis modulates ATP-/P2X7-signaling; secretes the anti-apoptotic enzyme, NDK, during infection of primary OECs; and expresses other virulence factors, such as fimbriae, gingipains, and PAD, that may be potential etiologic links to orodigestive cancers and other chronic diseases (58, 59). Of note is also that IL-1β promotes malignant transformation of tumor aggressiveness in oral cancer (91).
Rheumatoid arthritis
Periodontitis is more prevalent in patients with rheumatoid arthritis (RA) than in those without (92). RA is also prevalent in patients with periodontitis (93). Besides, there is improvement in RA after periodontal treatment and in periodontitis after treatment of RA (94, 95). Furthermore, DNA from a variety of oral bacteria, including P. gingivalis, has been detected in synovial fluid of active RA (96). Bostanci et al. (97) found a positive correlation between NLRP3 and expression of IL-1β and IL-18 in periodontitis, and upregulated levels of NLRP3, AIM2, and caspase-1 have been detected in gingival tissues of patients with periodontitis (14). Probably, P. gingivalis manipulates the host inflammatory responses to be able to survive and prevail within infected periodontal tissues (97), which it may achieve by limiting or controlling the activation of the NLRP3 inflammasome. It seems reasonable to expect similar effects in distant sites, for example, in the joints of rheumatic patients where P. gingivalis can be present.
Concluding remarks
Inflammasomes represent a relatively new concept in innate immunity. There is great variation in inflammasomes and the mechanisms by which they detect and resist pathogens. Many interactions between inflammasomes and the innate immune system are still unknown. It is becoming clear that the inflammasome and its constituents are likely crucial in the initiation of periodontal disease and several chronic systemic diseases associated with periodontitis. Nevertheless, it may be difficult to intervene in inflammasome actions for the purpose of treating disease since interfering with key parts in the complex may have serious local and systemic effects. The ubiquitous distribution and importance of inflammasome activation in many peripheral processes adds to this limitation. There are still not fully understood roles of all players in the inflammasome complex where anti-inflammatory therapies might not be sufficient to treat the roots of the disease. Knowledge about inflammasomes has mainly been retrieved from murine systems. The applicability of some of these results to human cells is unclear because gene products differ between species, and the specificity of ligands is not always conserved. P. gingivalis has a number of ways for suppressing innate immunity and inflammasome activity. Although this subversion probably is important for periodontitis and some related systemic diseases, it remains to see if other parts of the oral microbiota can behave in a similar way and if this subversion can affect players other than F. nucleatum in the dental biofilm. Efforts should also be made to see how inflammasomes can affect the ecology of the dental plaque microbiota.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors. IO acknowledges funding through a grant from the European Commission (FP7-HEALTH-306029 ‘TRIGGER’) and ÖY acknowledges funding through a NIDCR grant R01DE016593.
References
- 1.Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E. Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol. 2013;16:23–31. doi: 10.1016/j.mib.2012.11.008. doi: http://dx.doi.org/10.1016/j.mib.2012.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franchi L, Eigenbrod T, Núñez G. TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–6. doi: 10.4049/jimmunol.0900173. doi: http://dx.doi.org/10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. doi: http://dx.doi.org/10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Sater AA, Saïd-Sadier N, Ojcius DM, Yilmaz O, Kelly KA. Inflammasomes bridge signalling between pathogen identification and the immune response. Drugs Today. 2009;45(Suppl B):105–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz Ö, Lee KL. The inflammasome and danger molecule signaling: at the crossroads of inflammation and pathogen persistence in the oral cavity. Periodontol 2000. 2015;69:83–95. doi: 10.1111/prd.12084. doi: http://dx.doi.org/10.1111/prd.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tshopp J. The inflammasome. A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–22. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. doi: http://dx.doi.org/10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Moltke J, Ayres JS, Kofoed EM, Chavarría-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 11.Baldini C, Rossi C, Ferro F, Santini E, Seccia V, Donati V, et al. The P2X7 receptor–inflammasome complex has a role in modulating the inflammatory response in primary Sjögren's syndrome. J Intern Med. 2013;274:480–9. doi: 10.1111/joim.12115. doi: http://dx.doi.org/10.1111/joim.12115. [DOI] [PubMed] [Google Scholar]
- 12.Bauernfeind F, Hornung V. Of inflammasomes and pathogens – sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5:814–26. doi: 10.1002/emmm.201201771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22. doi: 10.1016/j.cell.2014.04.007. doi: http://dx.doi.org/10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Park E, Na HS, Song Y-R, Shin SY, Chung J. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis . Infect Immun. 2014;82:112–23. doi: 10.1128/IAI.00862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy M, Thaiss CA, Katz MN, Suez J, Elinav E. Inflammasomes and microbiota – partners in the preservation of mucosal homeostasis. Semin Immunopathol. 2015;37:39–46. doi: 10.1007/s00281-014-0451-7. doi: http://dx.doi.org/10.1007/s00281-014-0451-7. [DOI] [PubMed] [Google Scholar]
- 16.Dagenais M, Skeldon A, Saleh M. The inflammasome: in memory of Dr. Jurg Tschopp. Cell Death Differ. 2012;19:5–12. doi: 10.1038/cdd.2011.159. doi: http://dx.doi.org/10.1038/cdd.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajishengallis G, Darveau RP, Curtis MA. The keystone pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–25. doi: 10.1038/nrmicro2873. doi: http://dx.doi.org/10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis . Eur J Immunol. 2014;44:328–38. doi: 10.1002/eji.201344202. doi: http://dx.doi.org/10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abais JM, Xia M, Zhang Y, Boini KM, Li P-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–29. doi: 10.1089/ars.2014.5994. doi: http://dx.doi.org/10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. doi: http://dx.doi.org/10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts JS, Yilmaz Ö. Dangerous liaisons: caspase-11 and reactive oxygen species crosstalk in pathogen elimination. Int J Mol Sci. 2015;16:23337–54. doi: 10.3390/ijms161023337. doi: http://dx.doi.org/10.3390/ijms161023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broz P, Monack DM. Noncanonical inflammasomes: caspase-11 activation and effector mechanisms. PLoS Pathog. 2013;9:e1003144. doi: 10.1371/journal.ppat.1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–28. doi: 10.1111/j.1600-065X.2008.00734.x. doi: http://dx.doi.org/10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar M, Roea K, Orilloa B, Muruve DA, Nerurkar VR, Gale M, Jr., et al. Inflammasome adaptor protein apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in West Nile virus encephalitis. J Virol. 2013;87:3655–67. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao L-Y, Liu X, Franchi L. Inflammasomes in inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2013;29:363–9. doi: 10.1097/MOG.0b013e32836157a4. doi: http://dx.doi.org/10.1097/MOG.0b013e32836157a4. [DOI] [PubMed] [Google Scholar]
- 26.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–25. doi: 10.1111/j.1462-5822.2006.00751.x. doi: http://dx.doi.org/10.1007/s10059-013-0298-0. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 29.Franchi L, Muñoz-Planillo R, Reimer T, Eigenbrod T, Núñez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–15. doi: 10.1002/eji.200940180. doi: http://dx.doi.org/10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 30.Davis BK, Wen H, Ting J-P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. doi: http://dx.doi.org/10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skeldon A, Saleh M. The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Front Microbiol. 2011;2:15. doi: 10.3389/fmicb.2011.00015. doi: http://dx.doi.org/10.3389/fmicb.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taxman DJ, Huang MT-H, Ting JP-Y. Inflammasome inhibition as a pathogenic stealth mechanism. Cell Host Microbe. 2010;8:7–11. doi: 10.1016/j.chom.2010.06.005. doi: http://dx.doi.org/10.1016/j.chom.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, et al. Staphylococcus aureus evades the lysozyme-based digestion of peptidoglycan that links phagocytosis and macrophage IL-1β secretion. Host Cell Microbe. 2010;7:38. doi: 10.1016/j.chom.2009.12.008. doi: http://dx.doi.org/10.1016/j.chom.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella . J Exp Med. 2010;207:1745–55. doi: 10.1084/jem.20100257. doi: http://dx.doi.org/10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, et al. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–51. doi: 10.1002/eji.201040425. doi: http://dx.doi.org/10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elinav E, Thaiss CA, Flavell RA. Analysis of microbiota alterations in inflammasome-deficient mice. In: De Nardo CM, Latz E, editors. The inflammasome: methods and protocols. Methods in molecular biology, Vol. 1040. New York: Springer Science + Business Media; 2013. pp. 185–94. doi: http://dx.doi.org/10.1007/978-1-62703-523-1_14. [DOI] [PubMed] [Google Scholar]
- 37.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes via the inflammasomes. Nat Immunol. 2012;13:325–32. doi: 10.1038/ni.2231. doi: http://dx.doi.org/10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliani N, Palm NW, Flaveli RA. Inflammasome and intestinal homeostasis: regulating and connecting infection, inflammation and the microbiota. Int Immunol. 2014;26:495–9. doi: 10.1093/intimm/dxu066. doi: http://dx.doi.org/10.1093/intimm/dxu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–57. doi: 10.1016/j.cell.2011.04.022. doi: http://dx.doi.org/10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauernfeind F, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. doi: http://dx.doi.org/10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Franchi L, Núñez GT. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo . J Immunol. 2013;190:334–9. doi: 10.4049/jimmunol.1202737. doi: http://dx.doi.org/10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–18. doi: 10.1038/nature07725. doi: http://dx.doi.org/10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandes-Alnemri T, Yu J-W, Juliana C, Solorzano L, Kang S, Wu J, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis . Nat Immunol. 2010;11:385–93. doi: 10.1038/ni.1859. doi: http://dx.doi.org/10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taxman DJ, Swanson KV, Broglie PM, Wen H, Holley-Guthrie E, Huang MT-H, et al. Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J Biol Chem. 2012;287:32791–9. doi: 10.1074/jbc.M112.401737. doi: http://dx.doi.org/10.1074/jbc.M112.401737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feuille F, Ebersole JL, Kesavalu L, Stepfen MJ, Holt SC. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergetic effects on virulence. Infect Immun. 1996;64:2094–100. doi: 10.1128/iai.64.6.2094-2100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebersole JL, Feuille F, Kesavalu L, Holt SC. Host modulation of tissue destruction caused by periodontopathogens: effects on a mixed microbial infection composed of Porphyromonas gingivalis and Fusobacterium nucleatum . Microb Pathog. 1997;23:23–32. doi: 10.1006/mpat.1996.0129. [DOI] [PubMed] [Google Scholar]
- 48.Metzger Z, Lin Y-Y, DiMeo F, Ambrose WW, Trope M, Arnold RR. Synergistic pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model. J Endod. 2009;35:86–94. doi: 10.1016/j.joen.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Polak D, Wilensky A, Shapira L, Halabi A, lzheimerstein D, Weiss EI, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 2009;36:406–10. doi: 10.1111/j.1600-051X.2009.01393.x. doi: http://dx.doi.org/10.1111/j.1600-051X.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 50.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–57. doi: 10.1111/omi.12065. doi: http://dx.doi.org/10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spooner R, Yilmaz Ö. Nucleoside-diphosphate-kinase: a pleiotropic effector in microbial colonization under interdisciplinary characterization. Microbes Infect. 2012;14:228–37. doi: 10.1016/j.micinf.2011.10.002. doi: http://dx.doi.org/10.1016/j.micinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson L, Atanasova KR, Bui PQ, Lee J, Hung S-C, Yilmaz Ö, et al. Porphyromonas gingivalis attenuates ATP-mediated inflammasome activation and HMGB1 release through expression of a nucleoside-diphosphate kinase. Microbes Infect. 2015;17:369–77. doi: 10.1016/j.micinf.2015.03.010. doi: http://dx.doi.org/10.1016/j.micinf.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yilmaz Ö, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, et al. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–75. doi: 10.1111/j.1462-5822.2007.01089.x. doi: http://dx.doi.org/10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi CH, Spooner R, DeGuzman J, Koutouzis T, Ojcius DM, Yilmaz Ö. P. gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signaling and contributes to persistence. Cell Microbiol. 2013;15:961–76. doi: 10.1111/cmi.12089. doi: http://dx.doi.org/10.1111/cmi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. doi: http://dx.doi.org/10.1111/cmi.12089. [DOI] [PubMed] [Google Scholar]
- 56.Morandini AC, Ramos-Junior ES, Potempa J, Nguyen K-A, Oliveira AC, Bellio M, et al. Porphyromonas gingivalis fimbriae dampen P2X7-dependent IL-1β secretion. J Innate Immun. 2014;6:831–45. doi: 10.1159/000363338. doi: http://dx.doi.org/10.1159/000363338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos-Junior ES, Morandini AC, Almeida-da-Siva CLC, Franco EJ, Potempa J, Nguyen KA, et al. A dual role for P2X7 receptor during Porphyromonas gingivalis infection. J Dent Res. 2015;94:1233–42. doi: 10.1177/0022034515593465. doi: http://dx.doi.org/10.1177/0022034515593465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atanasova KR, Yilmaz Ö. Looking in the Porphyromonas gingivalis ‘Cabinet of Curiosities’: The microbium, the host and cancer association. Mol Oral Microbiol. 2014;29:55–66. doi: 10.1111/omi.12047. doi: http://dx.doi.org/10.1111/omi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atanasova KR, Yilmaz Ö. Prelude to oral microbes and chronic diseases: past, present and future. Microbes Infect. 2015;17:473–83. doi: 10.1016/j.micinf.2015.03.007. doi: http://dx.doi.org/10.1016/j.micinf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung Y-J, Jun H-K, Choi B-K. Contradictory roles of Porphyromonas gingivalis gingipains in caspase-1 activation. Cell Microbiol. 2015;17:1304–19. doi: 10.1111/cmi.12435. doi: http://dx.doi.org/10.1111/cmi.12435. [DOI] [PubMed] [Google Scholar]
- 61.Hung S-C, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, et al. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 2013;8:e70210. doi: 10.1371/journal.pone.0070210. doi: http://dx.doi.org/10.1371/journal.pone.0070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spooner R, DeGuzman J, Lee KL, Yilmaz Ö. Danger signal adenosine via adenosine 2a receptor stimulates growth of P. gingivalis in primary gingival epithelial cells. Mol Oral Microbiol. 2014;29:67–78. doi: 10.1111/omi.12045. doi: http://dx.doi.org/10.1111/omi.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–94. doi: 10.1016/j.bbamcr.2008.01.024. doi: http://dx.doi.org/10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 64.Goth SR, Stephens RS. Rapid, transient phosphatidylserine externalization induced in host cells by infection with Chlamydia spp. Infect Immun. 2001;69:1109–19. doi: 10.1128/IAI.69.2.1109-1119.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang RH, Phillips G, Jr., Medof ME, Mold C. Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. J Clin Invest. 1993;92:1326–35. doi: 10.1172/JCI116706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yilmaz Ö, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis . Infect Immun. 2004;72:3743–51. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yilmaz Ö, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected with Porphyromonas gingivalis . Cell Microbiol. 2010;12:188–98. doi: 10.1111/j.1462-5822.2009.01390.x. doi: http://dx.doi.org/10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bostanci N, Meier A, Guggenheim B, Belibasakis GN. Regulation of NLRP3 and AIM inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell Immunol. 2011;270:88–93. doi: 10.1016/j.cellimm.2011.04.002. doi: http://dx.doi.org/10.1016/j.cellimm.2011.04002. [DOI] [PubMed] [Google Scholar]
- 70.Belibasakis GN, Guggenheim B, Bostanci N. Down-regulation of NLRP3 inflammasome in gingival fibroblasts by subgingival biofilms: involvement of Porphyromonas gingivalis . Innate Immun. 2013;19:3–9. doi: 10.1177/1753425912444767. doi: http://dx.doi.org/10.1177/1753425912444767. [DOI] [PubMed] [Google Scholar]
- 71.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, et al. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol. 2009;11:1587–99. doi: 10.1111/j.1462-5822.2009.01349.x. doi: http://dx.doi.org/10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slocum C, Coats SR, Hua N, Kramer C, Papadopoulos G, Weinberg EO, et al. Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 2014;10:e1004215. doi: 10.1371/journal.ppat.1004215. doi: http://dx.doi.org/10.1371/journal.ppat.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zenobia C, Hajishengallis G. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence. 2015;6:236–43. doi: 10.1080/21505594.2014.999567. doi: http://dx.doi.org/10.1080/21505594.2014.999567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zenobia C, Hasturk H, Nguyen D, Van Dyke TE, Kantarci A, Darveau RP. Porphyromonas gingivalis lipid A phosphatase activity is critical for colonization and increasing the commensal load in the rabbit ligature model. Infect Immun. 2014;82:650–9. doi: 10.1128/IAI.01136-13. doi: http://dx.doi.org/10.1128/IAI.01136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. 2015;7:28788. doi: 10.3402/jom.v7.28788. doi: http://dx.doi.org/10.3402/jom.v7.28788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. doi: http://dx.doi.org/10.1038/nature08938. Erratum in: Nature 2010; 466: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng F, Xing S, Gong Z, Mu W, Xing Q. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm. 2014;2014:507208. doi: 10.1155/2014/507208. doi: http://dx.doi.org/10.1155/2014/507208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamaguchi Y, Kurita-Ochia T, Kobayashi R, Suzuki T, Ando T. Activation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated atherosclerosis. Pathog Dis. 2015;73 doi: 10.1093/femspd/ftv011. pii: ftv011. doi: http://dx.doi.org/10.1093/femspd/ftv011. [DOI] [PubMed] [Google Scholar]
- 79.Hakimi M, Peters A, Becker A, Bockler D, Dihlmann S. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg. 2014;59:794–803. doi: 10.1016/j.jvs.2013.03.048. doi: http://dx.doi.org/10.1016/j.jvs.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 80.Brown PM, Kennedy DJ, Morton RE, Febbraio M. CD36/SR-B2-TLR2 dependent pathways enhance Porphyromonas gingivalis mediated atherosclerosis in the Ldlr Ko mouse model. PLoS One. 2015;10:e0125126. doi: 10.1371/journal.pone.0125126. doi: http://dx.doi.org/10.1371/journal.pone.0125126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nunes-Alves C. Inflammasomes: new LPS receptors discovered. Nat Rev Immunol. 2014;14:582. doi: 10.1038/nri3736. doi: http://dx.doi.org/10.1038/nri3736. [DOI] [PubMed] [Google Scholar]
- 82.Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer's disease? J Oral Microbiol. 2015;7:29143. doi: 10.3402/jom.v7.29143. doi: http://dx.doi.org/10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jamilloux Y, Pierini R, Querenet M, Juruj C, Fauchais AL, Jauberteau MO, et al. Inflammasome activation restricts Legionella pneumophila replication in primary microglial cells through flagellin detection. Glia. 2013;61:539–49. doi: 10.1002/glia.22454. doi: http://dx.doi.org/10.1002/glia.22454. [DOI] [PubMed] [Google Scholar]
- 84.Rapsinski GJ, Wynosky-Dolfi MA, Oppong GO, Tursi SA, Wilson RP, Borodsky IE, et al. Toll-like receptor 2 and NLRP3 cooperate to recognize functional bacterial amyloid, curli. Infect Immun. 2015;83:693–701. doi: 10.1128/IAI.02370-14. doi: http://dx.doi.org/10.1128/IAI.02370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Saecker A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. doi: http://dx.doi.org/10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating the intracellular nucleation from soluble to particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. doi: 10.1038/ni.2639. doi: http://dx.doi.org/10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gold M, Khoury JE. β-amyloid, microglia, and the inflammasome in Alzheimer's disease. Semin Immunopathol. 2015;37:607–11. doi: 10.1007/s00281-015-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furusho H, Miyauchi M, Hyogo H, Inubushi T, Ao M, Ouhara K, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J Gastroenterol. 2013;48:1259–70. doi: 10.1007/s00535-012-0738-1. doi: http://dx.doi.org/10.1007/s00535-012-0738-1. [DOI] [PubMed] [Google Scholar]
- 90.Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–15. doi: 10.4248/IJOS11075. doi: http://dx.doi.org/10.4248/IJOS11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee CH, Chang JS, Syu SH, Wong TS, Chan JY, Tang YC, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230:875–84. doi: 10.1002/jcp.24816. doi: http://dx.doi.org/10.1002/jcp.24816. [DOI] [PubMed] [Google Scholar]
- 92.Joseph R, Rajappan S, Nath SG, Paul BJ. Association between chronic periodontitis and rheumatoid arthritis: a hospital-based case-control study. Rheumatol Int. 2013;33:103–9. doi: 10.1007/s00296-011-2284-1. doi: http://dx.doi.org/10.1007/s00296-011-2284-1. [DOI] [PubMed] [Google Scholar]
- 93.Nesse W, Dijkstra PU, Abbas F, Spijkervet FK, Stijger A, Tromp JA, et al. Increased prevalence of cardiovascular and autoimmune diseases in periodontitis patients: a cross-sectional study. J Periodontol. 2010;81:n1622–8. doi: 10.1902/jop.2010.100058. doi: http://dx.doi.org/10.1902/jop.2010.100058. [DOI] [PubMed] [Google Scholar]
- 94.Erciyas K, Sezer U, Ustün K, Pehlivan Y, Kisacik B, Senyurt SZ, et al. Effects of periodontal therapy on disease activity and systemic inflammation in rheumatoid arthritis patients. Oral Dis. 2013;19:394–400. doi: 10.1111/odi.12017. doi: http://dx.doi.org/10.1111/odi.12017. [DOI] [PubMed] [Google Scholar]
- 95.BIyIkoğlu B, Buduneli N, Aksu K, Nalbantsoy A, Lappin DF, Evrenosoğlu E, et al. Periodontal therapy in chronic periodontitis lowers gingival crevicular fluid interleukin-1beta and DAS28 in rheumatoid arthritis patients. Rheumatol Int. 2013;33:2607–16. doi: 10.1007/s00296-013-2781-5. doi: http://dx.doi.org/10.1007/s00296-013-2781-5. [DOI] [PubMed] [Google Scholar]
- 96.Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006;24:656–63. [PubMed] [Google Scholar]
- 97.Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA, et al. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 2009;157:415–22. doi: 10.1111/j.1365-2249.2009.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]