Abstract
Traditional knowledge can be used as a source for development of new medicines. In the present study, we compare the data on saffron in Razi’s Al-Hawi book with modern scientific studies. A computerized search of published articles was performed using MEDLINE, Scopus as well as native references. The search terms used were saffron, Crocus sativus, crocetin, crocin, safranal, Razi, and Al-Hawi. A variety of properties of saffron including diuretic, analgesic, anti-inflammatory, hepatoprotective, appetite suppressant, hypnotic, antidepressant, and bronchodilator effects were mentioned in Al-Hawi. Modern studies also confirmed most of these characteristics. This review indicates that the pharmacological data on saffron and its constituents are similar to those found in Razi’s Al-Hawi monograph and it can be concluded that ethnobotanical information and ancient sources have precious data about medicinal plants that lead to finding new compounds for treatment of several diseases.
Keywords: Al-Hawi, Crocin, Crocus sativus L, Razi, Saffron, Safranal, Traditional medicine
Introduction
Islamic period is one of the most important historical eras for herbal medicine advancement. Considering religious influence, Muslims made great improvements in the field of medicine, translating and compiling medical texts. Oriental medicine owes considerably to Iranian scientists for progress. A lot of aspects of Islamic medicine in the past is been indicated clinically today and its validity is proven by modern medicine. Most well-known Iranian medical scientists in 3rd to 6th centuries are Avicenna and Razi (1, 2). Their endeavors resulted in changing the science of medicine from theory to practice, regulating and completing Greek medicine, preparing herbal drugs and prescribing them to the patients. Their compilations on describing and treatment of diseases such as rubella, smallpox and their translation to more than 40 languages are firm proofs of their reputation (1).
The aim of the present study is to represent therapeutic uses of saffron mentioned in the book Al-hawi as well as comparing it to the results of recent studies. The positive results of this study show the significance of Islamic medicine and scientists; moreover underlying the discovery and development of new drugs based upon herbal medicine referenced in Islamic medical textbooks.
Zakariya Razi
Abu Bakr Mohammad ibn Zakariya Razi, known as Rhazes (865-925 AD), the famous Iranian physician, chemist and philosopher, wrote about 250 books and treatises (3-5) (Figure 1).
Figure 1.

A Persian stamp with Rhazi`s portrait
He was born in Al-Ray, just south of modern Tehran. His father was a goldsmith, thus in an artisan setting he became involved in the study of alchemy and chemistry.
He studied philosophy under the supervision of the famous Shiite Muslim philosopher Abu Zaid Ahmad Ibn Sahl Al-Balkhi (a Persian Muslim polymath) and later developed his own philosophical system. He studied medicine in Muqtadiri hospital under the supervision of Ali Ibn Rabane in Baghdad. After finishing his apprenticeship, Razi left Baghdad for Ray and founded his hospital in this city (6, 7).
Razi and Avicenna (937 to 1037) are the two greatest physicians in medieval medicine and developed the theory and practice of medicine. Razi, an Iranian physician, followed both Hippocrates and Galen in their procedures and their concepts. He joined the knowledge of textual scholarship with astute clinical observations. The most important aspects of his research deal with immunology and allergic diseases. He was the first physician ever to write articles in this field. Another important study of Razi was on smallpox; the first known description of smallpox was by him. At the end of his life, he became blind as a result of complications of cataract. Razi died in Rey in 925 A.D. at sixty years of age (3, 6, 8).
The most important and main complete book of Razi is “Al-Hawi”, a 25-volume medical encyclopedia. Razi spent 15 years on this book. It comprises his observations and experiments with information that was found in Greek medical books on diseases and their treatments. Faraj ibn Salem (Farragut) translated this book into Latin in 1279. “Al-Hawi” was reissued five times in Europe between 1488 and 1542 (8, 9).
Other main medical books of Razi are: “Man la Yahduruhu al-Tabib (For One without Doctor)”, “Al-Mansouri”, “Al-Jodari wa al-Hasbah (Smallpox and Measles)” and “Al-Morshed (The Guide)” (10, 11).
Saffron
Saffron is a spice obtained from the stigmas of the flower of Crocus sativus L, which is widely cultivated in Iran and other countries such as India and Greece. Saffron cultivation and making use of this plant go back to nearly 3000 years based on current evidence but first records on this plant were made at the Assyrians age. Since then saffron has been used to treat more than 90 diseases (12).
Saffron is a small and perennial plant which grows 10 to 30 cm high. From the center of the bulb, several leaves end in 2 to 3 flowers. The color depends on the level of carotenoid and lycopene inside a 3-branched stigma, and its size varies rather than the branches, which is always 3 (13, 14) (Figure 2).
Figure 2.

Saffron
The chemical components of saffron are 5% fat, 10% moisture, 5% minerals, 12% protein, 5% crude fiber, and 63% sugars (% w/w). More than 150 volatile compounds including terpenes alcohol, terpenes, and their esters are present in saffron stigmas among which, three major bioactive compounds in saffron are crocin, picrocrocin and safranal, which are responsible for saffron’s exclusive color, taste and odor, respectively (12, 15-17).
The bitter taste of saffron is caused by picrocrocin, which leads to safranal in the end. Other active ingredients include zeaxanthin, lycopene, carotene, and vitamins particularly riboflavin and thiamine (17).
Pharmacological activities
Many nations have used saffron to cure numerous diseases for centuries and various pharma- cological activities of saffron and its constituents have been extensively studied (18). These activities include anticonvulsive (19, 20), anti-ischemic (21-23), anti-genotoxic (24, 25), antidote (26, 27), anti- Alzheimer (28), antitussive (29), hypolipidemic (30), antinociceptive, anti-inflammatory, and antioxidant effects (31, 32). Saffron also offers protective effects against, cardiovascular disease (33), diabetes (34), Parkinson’s disease (35), depression (36), cancer and tumor activity (37), atherosclerosis, and other diseases (16, 38) (Figure 3).
Figure 3.
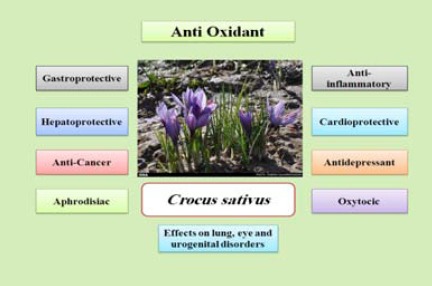
Pharmacological activities of saffron
Traditional uses
Traditional uses of saffron have been noted in many articles. For example in Iranian folk medicine saffron is used as bitter, stimulant, fragrant, tonic, aphrodisiac, stomachic, antispasmodic, emmenagogue, diuretic, anticancer, laxative, galactagogue, and is useful in bronchitis, cephalalgia, pharyngoplasty, vomiting, fever, epilepsy, inflammations, skin diseases, septic inflammations, stimulation of circulation, etc. In Indian folk medicine saffron is used as an adaptogen (13, 39, 40).
Materials and Methods
A search was carried out using Scopus, MEDLINE and Web of Science databases and local references without date restriction. The keywords for the search were: Crocus sativus, saffron, crocin, safranal, crocetin, anticonvulsant, analgesic, neuroprotective, cardiovascular, antidepressant, immune system, Razi, and “Al-Hawi”. In terms of the pharmacological effects of saffron as reported in the literature, recent and related articles were chosen and compared with the plant monograph of Razi’s Al-Hawi book.
Comparative evaluation of saffron in Al-Hawi and in modern medicine
Effects on sexual behavior and genitourinary tract
Razi: ‘It is a diuretic and a stimulant of sexual desire’ (41).
In traditional medicine, saffron is recommended as an aphrodisiac agent (14, 39, 42). In Iranian medicine, it was used as a diuretic and as a purifier of kidney and bladder. It also was used to treat urinary obstruction. The aphrodisiac effect of saffron was approved in many traditional medicines such as Middle Eastern medicine (39). Many experimental and clinical studies show that saffron and its bioactive pigment, crocin, can affect sexual factors by enhancement of erectile function, increasing of libido, amelioration of semen quality and reduction of hesitation time (43, 44). In an animal model, the aphrodisiac activities of C. sativus stigma aqueous extract and its constituents, safranal and crocin in different doses, were compared with sildenafil (60 mg/kg body wt, as a positive control). The results of this study showed that crocin, at all doses, and the extract at high doses increased mount frequency, intromission frequency, erection frequency, and reduced ejaculation latency, intromission latency, and mount latency parameters. Although the aphrodisiac effects of crocin and aqueous extract were shown in this study, safranal did not show aphrodisiac effects at any dose (45). In a clinical study, the effect of saffron was investigated on the semen analysis in 52 men with idiopathic infertility whose problem could not be solved surgically. Saffron, 50 mg, was administered 3 times a week for 3 months. Sperm normal morphology and sperm motility were improved significantly after the study period, but sperm count was not changed significantly (46). Saffron supplementation (30 mg/day for 4 weeks) was efficacious in treating fluoxetine-related erectile dysfunction in married men and depressed women with major depression whose depression had been stabilized on fluoxetine. This study showed that saffron supplementation might lead to improvements in sexual function in men and women with major depression (47, 48). The effect of 200 mg saffron for ten days on parameters of nocturnal penile tumescence and international index of erectile function of men was evaluated. Results showed that saffron can reduce erectile dysfunction and a significant improvement in tip rigidity and tip tumescence, as well as base rigidity and base tumescence, were seen after saffron treatment (43). The effects of hydroalcoholic extract of saffron on sex hormones in female rats that received cyclophosphamide showed that saffron can reduce toxic effects of low dose cyclophosphamide on the pituitary-gonadal axis and lead to estrogen production (49).
The aqueous extracts of saffron exhibited a diuretic effect in rats. Hydrochlorothiazide (10 mg/ kg body wt, intraperitoneally), a potent diuretic as positive control and normal saline solution as placebo for the control group were compared with aqueous extracts of saffron at doses of 60, 120 and 240 mg/kg body wt. The results showed that higher doses of saffron induced a diuretic effect in a dose-dependent manner and urine volume and urine electrolyte concentration of sodium and potassium were increased in the test period (5 hr). The onset of diuretic activity of saffron was rapid and diuretic activity of saffron was 0.86 to hydrochlorotiazide (50).
Crocin showed an antilithiatic effect with doses of 20 and 40 mg/kg in rats fed ethylene glycol 1% in drinking water. Crocin with an antioxidant effect and balancing promoter and inhibitor factors can increase magnesium and citrate levels in urine. Crocin with a dose of 40 mg/kg decreased total protein loss in urine. The number of calcium deposits in the kidney tissue of lithiatic rats was decreased after prophylactic treatment with 20 and 40 mg/kg of crocin (51). Saffron has protective effects against calcium oxalate nephrolithiasis induced by ethylene glycol in rats. At doses of 50, 100 mg/kg it showed prophylactic effects, and at 100 mg/kg curative effects (52).
Effects on delivery and premenstrual symptoms
Razi: ‘Ingestion of 6 to 7 g of saffron induces the delivery. I prescribed it many times and the results were always positive. It was useful for the treatment of female genitourinary system disorders. It is prescribed in hardness, blockage, adhesions, and malignant ulcers of the uterus’ (41).
In traditional medicine saffron has been used as facilitator of difficult delivery, and it was useful in treating uterus pain and in regulation of menstrual cycle as well (39). Antaki has written: “It has been experienced that oral use of 3500 mg of saffron with rose water and sugar can facilitate delivery. The application of a vaginal suppository prepared using 3500 mg of saffron precipitated labor and delivery of the placenta” (53). Ahmadi et al showed that the use of saffron can shorten the delivery process. In this study 30 women were randomly divided into two groups who took saffron capsules (250 mg) and placebo capsules as positive group and control group, respectively. The results showed that administration of saffron capsule at the beginning of the active phase of labor (one capsule every 2 hr for maximum 3 capsules) could shorten the active phase of labor time in saffron consumer group (54). Using more than 10 g of saffron is effective in induction of abortion but this method is accompanied by life-threatening complications (55). Agha hosseini et al showed that saffron was effective in relieving symptoms of premenstrual syndrome (PMS). Saffron (30 mg/day) alleviated the symptoms of PMS in cycles 3 and 4 (Total Premenstrual Daily Symptoms and Hamilton Depression Rating Scale) (56). Saffron has been suggested as an effective means to relieve the emotional symptoms associated with PMS (57).
The specified mechanisms of saffron in alleviating PMS symptoms in women have not been completely studied.
Effects on respiratory tract
Razi: ‘Use of aqueous extract of saffron can facilitate respiration’ (41). Saffron`s aroma and its oil are good for diaphragmitis and pleurisy and have been used in traditional Iranian medicine (39).
Hosseinzadeh et al have shown that ethanolic extract of C. sativus (100–800 mg/kg) and safranal (0.25–0.75 ml/kg) reduced the number of coughs. The extracts or agents were given intraperitoneally 30 min prior to exposing guinea pigs for 10 min to an aerosol of an irritant agent, citric acid. Safranal and the aqueous extract of saffron showed an antitussive property, but crocin did not show this effect (29). The relaxant effects of aqueous ethanolic extracts of C. sativus and safranal, were examined on guinea-pig tracheal chains by Boskabady and Aslani. Results showed that a relatively potent relaxant effect of C. sativus on guinea-pig tracheal chains, which was comparable with theophylline as positive control in this test and the relaxant effect of this plant could be due to b-adrenoceptor stimulatory, muscarinic and/or histamine (H1) receptor inhibitory effect; authors suggested that safranal was responsible for this (58). In another study, it was shown that safranal possibly acts as a histamine H1 receptor competitive antagonist in guinea pig tracheal chains. The effective concentration (EC50) of histamine obtained in the presence of safranal was significantly greater than that of saline and this effect was dose-dependent (59).
In addition, saffron can protect the lungs from pathological changes and can decrease progress of asthma in sensitized guinea pigs. The lowering effects of saffron on the serum histamine level, eosinophil count of lung lavage, total white blood cells, and pathological changes of sensitized animals are perhaps due to its suppressing effect on lung inflammation, and it was comparable with dexamethasone in this study (60). Saffron in combination with honey reduced the course of treatment in children with pneumonia. The recurrence of pneumonia was much less in saffron`s group for six weeks (61).
Effects on liver and gastrointestinal tract
Razi:’Saffron neutralizes gastric acid, cleanses the stomach, increases digestion of food, strengthens liver and stomach and decreases appetite’ (41).
Administration of aqueous extract of saffron (30 min before treatment with agents) can reduce gastric ulcerations induced by pylorus ligation (Shay rats), indomethacin (40 mg/kg) and various necrotizing agents including (80% ethanol, 0.2 M NaOH and 25% NaCl) in rats. The saffron aqueous suspension at doses of 250 and 500 mg/kg exhibited a decrease in basal gastric secretion and ulcer index in Shay rats and indomethacin-treated groups. Saffron suspension significantly prevented the depletion of gastric wall mucus induced by 80% ethanol. Saffron prevented the depletion of gastric non-protein sulfhydryl contents (62).
However, in another study saffron extract increased basal and stimulated gastric acid and pepsin secretions. In this study 100 mg/kg saffron extract was administered orally for 5 days to rats. After this period gastric contents such as basal and stimulated acid and pepsin secretions were increased by saffron. Saffron can improve digestion of proteins with the benefit of lower pH conditions aiding in digestion (63). To evaluate the effects of saffron on appetite, Gout et al took 60 women in a randomized, double-blind, placebo-controlled trial. 31 women consumed 1 capsule of Satiereal (176.5 mg saffron extract per day) and 29 women consumed a placebo. After 8 week Satiereal consumption produced a reduction of snacking and created a satiating effect that could contribute to body weight loss (1 kg) (64).
Crocin exhibited a dual inhibitory activity against the cyclooxygenase-1 (COX1) and cyclooxygenase-2 (COX2) enzymes. Use of 12, 25, and 50 mg/kg crocin to male Kunming and Sprague–Dawley rats prevented damage to the stomach mucosa in a dose-dependent manner (65). Saffron, crocin and safranal showed protective effects against oxidation-induced tissue injuries due to their antioxidant properties. Use of indomethacin (40 mg/kg, orally in non-diabetic rats and 15 mg/kg, orally in diabetic rats) induced oxidative stress, gastric lesions, lipid peroxidation and decrease of glutathione levels. The influence of pretreatment with saffron extracts (25, 100 or 250 mg/kg, p.o.), crocin (2.5, 5 or 10 mg/kg, orally) and safranal (0.25, 2, 5 ml/kg, orally) and omeprazole (30 mg/kg, p.o.) 30 min before administration of indomethacin, revealed that saffron extracts, crocin and safranal reduced ulcer index similar to omeprazole and prevented lipid peroxidation and increased glutathione measures (66).
Treatment with diazinon (DZN) as a hepatotoxic agent, induced reactive oxygen species (ROS) generation and apoptosis pathway activation. Crocin at doses of 12.5, 25 and 50 mg/kg per day, intraperitoneally in association with DZN 15 mg/kg per day, orally after 4 weeks decreased malondialdehyde levels significantly in the group that received DZN plus 25 mg crocin,. However, crocin attenuated the activation of caspases and reduced the Bax/Bcl-2 ratio (67). Administration of ethanolic extract of C. sativus stigma (40 and 80 mg/kg) significantly decreased the levels of serum biomarker of hepatic injury and total bilirubin and elevated the levels of albumin that they induced by rifampin (as a hepatotoxic drug 500 mg/kg/per day in 1 month) in rats. In addition to biochemical results, C. sativus improved the pathological change induced by rifampin (68). Aluminum chloride (AlCl3), as a hepatotoxic substance, increased the cholesterol levels, triglycerides, liver enzymes, lipid peroxidation, and hyperglycemia in the AlCl3 treated rats compared to the control. The mentioned hepatic markers were improved to baseline after treating rats with saffron and honey; the lipid peroxidation rate was alleviated subsequently, as well (69).
Antidepressant effect
Razi: ‘Use of saffron would lead to a high feeling of pleasure which is so close to a psychotic state. (41).
One of the best-known qualities of saffron is its enlivening and antidepressant activity. Saffron is considered an excellent therapeutic plant for treating depression in traditional medicine. Modern scientific firmly supported the beneficial impact of saffron stigma and petal extracts in the treatment of mild to moderate depression (70, 71).
The effects of aqueous or ethanolic extracts of C. sativus stigmas and safranal on CNS have been widely studied and various effects such as: anticonvulsant (20), withdrawal syndrome reduction (72), effect on memory (73), and enhancing spatial cognitive abilities after chronic cerebral hypoperfusion (15), have been shown. The antidepressant effect of saffron was highly evaluated and the Razi`s statement about this quality of saffron was confirmed.
The antidepressant activity of saffron was evaluated by Hosseinzadeh et al via forced swimming test in mice. The ethanolic and aqueous extracts of stigma (0.2–0.8 g/kg), safranal (0.15–0.5 ml/kg) and crocin (50–600 mg/kg) reduced immobility time in mice. Both safranal and crocin extracts increased swimming time in mice (74). Long term treatment of depression affects the level of the brain-derived neurotrophic factor (BDNF) and nerve growth factor (VGF, non-acronymic), whose transcriptions are dependent on cAMP response element binding protein (CREB) and on the other hand, increased levels of CREB with similar behavioral responses to antidepressants occur (75, 76). Crocin (12.5, 25, and 50 mg/kg) compared with imipramine (10 mg/kg; as positive control) and saline (1 ml/kg; as neutral control), which were administered intraperitoneally to male Wistar rats for 21 days. After 21 days crocin significantly reduced the immobility time in the forced swimming test. 25 and 50 mg/kg of crocin increased the levels of CREB and BDNF significantly in a dose-dependent manner in the hippocampus. All doses of crocin increased the VGF levels in the hippocampus in a dose-dependent manner (77). Saffron with doses of 40, 80 and 160 mg/kg/day in rats can significantly increase the protein levels of BDNF, CREB and p-CREB, and transcript levels of BDNF, but the increase in transcript levels of CREB and VGF was not significant (78). The intraperitoneal injection of kaempferol (a constituent of saffron) with doses of 100 and 200 mg/kg in mice and 50 mg/kg in rats reduced immobility time in mice and rats (79). Administration of saffron capsules (30 mg/day) and fluoxetine (20 mg/day) for 6 weeks in randomized and double-blind clinical trial showed that saffron is as effective as fluoxetine in the treatment of mild to moderate depression (80).
Finally, a meta-analysis of published randomized controlled trials examining the effects of saffron supplementation on symptoms of depression among participants with major depressive disorders (MDD) was done. This study showed that saffron supplementation can improve symptoms of depression in adults with MDD (81). Lopresti et al in a systemic review showed that saffron had large enlivening effects and, when compared with antidepressant remedies, had similar antidepressant efficacy, and its ability is due to its serotonergic, antioxidant, anti-inflammatory, neuroendocrine, and neuroprotective effects (82).
Synthetic drugs that are used for treatment of depression have many adverse effects, therefore natural herbal treatments are attractive in treatment methods today. Larger studies are needed to validate the use of saffron for standard treatment of depression.
Cosmetic effects
Razi: ‘Its oral use improves the complexion (41).
In Iranian traditional medicine, saffron can improve complexion and can be used for the treatment of erysipelas. In Greek traditional medicine, it can refresh facial skin and is used to relieve liver from the dominance of bile and to cure acne, skin diseases and wounds. Also, it can make the body look more youthful and brighter (83, 84).
In evaluation of antisolar and moisturizing effects of saffron, Golmohammadzadeh et al showed that lotions with 4% ground saffron in comparison with homosalate (8%) lotion reference, have equivalent sun protection factor values, but saffron 8% has a significantly greater one than homosalate 8%. There were no significant differences in skin moisture due to saffron lotions and the control lotion without saffron during the 7 hr post-application period (85). A study similar to previous work was done by Golmohammadzadeh et al; in this study formulations included homosalate reference, nanoliposomes comprising 0.25, 0.5, 1, 2, 4, and 8% safranal, and empty liposomes were evaluated for antisolar and moisturizing properties, and the results confirmed the previous work (86). To evaluate the moisturizing effect of a cream containing C. sativus extracts, a formulation containing 3% saffron concentrated extracts, and a base containing no extract was formulated. The resulting cream was applied to the skin for 8 weeks and skin parameters were evaluated every week. The increase in skin moisture contents and changes in transepidermal water loss were significant with respect to the base formulation without saffron (87). In addition to antisolar and moisturizing properties of saffron, the skin cancer prevention of saffron due to its antioxidant benefits is also important (88, 89).
In all of the studies mentioned above saffron was used topically and evaluation of oral treatment for its cosmetic effects is still needed.
Anti-inflammatory and antinociceptive effects
Razi: ‘Saffron features include: softening and quenching boils, improving internal organ pain. Rectal suppository form and ointment of saffron are utilized in the pain of the uterus and anus. It is also painted on erysipelas and is useful in hot swellings of the ear. The smell of its oil reduces inflammation of the liver and heart’ (41).
Seven-day treatment with the ethanolic and aqueous extracts (50, 100 and 200 mg/kg) and safranal (0.025, 0.05 and 0.1 mg/kg) attenuated the behavioral symptoms of all kinds of neuropathic pain such as thermal allodynia, thermal hyperalgesia and mechanical allodynia in a dose-dependent manner in rats (90). The aqueous and ethanolic extracts of C. sativus stigma and petals have an antinociceptive effect in different aspects of pain and anti-inflammatory activity in acute and/or chronic inflammation in mice and these effects might be due to the presence of tannins, flavonoids, alkaloids, anthocyanins, and saponins (91). Nasri et al concluded that antinociceptive and anti-inflammatory effect of saffron is caused by safranal. Probable stimulation of opioid, N-Methyl-D-aspartic acid, glutamatergic, and nitric-oxide-dependent pathways leads to pain inhibition in acute and chronic phases. They used the ethanolic extract with a dose of 5 mg/kg for acute phase and with a dose of 10 mg/kg for chronic phase. Naloxone, dextromethorphan and N(G)-nitro-L -arginine methyl ester inhibited the extract action in acute phase as well as chronic phase. Safranal and crocin showed an anti-nociceptive effect in acute and chronic phases, respectively. Saffron extract with a dose of 2.5, 5 and 10 mg/kg controlled the inflammation 30%, 66% and 80%, respectively, but safranal and crocin did not show a similar effect (92). In comparison between saffron ethanolic extract and dexamethasone as a glucocorticoid used in soothing rheumatoid arthritis, Taghizadeh et al showed that injection of saffron ethanolic extract intraperitoneally every day for 12 days (25-600 mg/kg) can reduce significantly, at the higher concentrations, paw and tibiotarsal joint diameters compared with dexamethasone 2 mg/kg (93). The second phase of pain after injection of formalin is due to the inflammatory process, which the ethanolic extract, safranal and crocin remove. This suggests that saffron can inhibit COX enzymes that are liable in inflammation. This thesis was confirmed by Xu et al who showed that crocin inhibits COX1 and COX2 in vitro. In in vivo tests pretreatment with crocin dose-dependently inhibited the xylene-induced ear edema in mice and carrageenan-induced paw edema in rats. Fewer stomach lesions as compared to the number of stomach lesions caused by indomethacin in rats, inhibition of the productions of prostaglandin E2 and prevention of the nuclear translocation of the nuclear factor kappa B, p50, and p65 subunits showed that crocin exhibits obvious anti-inflammatory effects in this study (94).
To investigate the effect of saffron on skin inflammation, Tamaddonfard et al used carrageenan to produce local edema, allodynia, hyperalgesia and neutrophil infiltration in paw tissues of rats. Crocin at doses of 25, 50 and 100 mg/kg and safranal at doses of 0.5, 1 and 2 mg/kg suppressed inflammatory pain responses, attenuated edema and decreased the number of neutrophils (95).
The aqueous and ethanolic extracts of saffron (200 mg/kg) could reduce the neuropathic pain in the chronic constriction injury model (CCI) in rats. Proinflammatory cytokines such as tumor necrosis factor α, interleukin-1β and interleukin 6 and oxidative stress markers such as malondialdehyde were deducted in days 3 and 7 after saffron extracts treatment. The neuropathic pain might be alleviated with anti-inflammatory, antiapoptotic and antioxidant properties of saffron in this study (96).
The antinociceptive and anti-inflammatory effects of saffron have many other advantages such as activation of microglia and help in restoring CNS homeostasis (97), desensitization of lung to inflammatory cytokines (60) and antiarthritic benefits (98).
Antimicrobial and Anticancer effects
Razi: ‘Saffron is useful for treatment of infectious and malignant wounds’ (41).
Various studies have shown the anticancer effect of saffron and its constituents (37, 99). Crocin and crocetin have significant anticancer activities in breast, lung, pancreatic, and leukemic cells (18).
Oral administration of saffron extract (200 mg/kg) inhibited the growth of ascites tumors derived from sarcoma-180, Ehrlich ascites carcinoma, Dalton’s lymphoma ascites in a dose-dependent manner, and significantly elevated (2- to 3-fold) life spans of treated tumor-bearing Swiss albino mice (100). It was reported that saffron and crocetin induced apoptosis in human breast cancer cells (MCF-7) via p53-mediated stimulation of apoptosis (101). The inhibitory effect of saffron on liver cancer in rats was investigated by Amin et al. In this study administration of saffron at doses of 75, 150, and 300 mg/kg/day was started 2 weeks prior to the diethylnitrosamine DEN injection, and was continued for 22 weeks. Saffron counteracted DEN-induced oxidative stress in rats as assessed by restoration of catalase, superoxide dismutase and glutathione-S-transferase levels, diminishing of myeloperoxidase activity, and malondialdehyde and protein carbonyl formation in liver (102). The anticancer effects of saffron are mainly due to crocin. Escribano et al showed that crocin in comparison with crocetin, picrocrocin and safranal has more anticancer potency, and is able to induce apoptosis in human cancer cells in vitro (103).
The antimutagenic and cytotoxic effects of saffron were assessed by using the Ames/Salmonella test system, two well-known mutagens (BP, 2AA), the in vitro colony-forming assay, and four different cultured human normal (CCD-18LU) and malignant (Hela, a-204 and Hepg2) cells by Abdullaeva et al. Saffron displayed a dose-dependent repressive effect only against human malignant cells in in vitro colony-forming test system. All isolated carotenoid ingredients of saffron demonstrated cytotoxic activity against in vitro tumor cells (104). Bathaei et al showed that aqueous extract of saffron (100, 150 and 175 mg/kg/day) by intraperitoneal injection for 50 days in rats could reduce adverse effects of 100 μg/ml orally 1-methyl -3- nitro -1- nitrosoguanidine (as an alkylating agent) such as different stages from hyperplasia to adenoma in stomach in a dose-dependent manner. Saffron increased the antioxidant status in cancerous rats and decreased lactate dehydrogenase and increased apoptosis in cancerous cells. Carotenoids that are abundant in saffron possess anticarcinogenic, antimutagenic and immunomodulating effects. Saffron treatment improved the antioxidant capacity of plasma and preserved tissues such as stomach from injury (105).
The specified mechanisms of anticancer effects of saffron are not clear, but it seems that carotenoids exhibit biological activities as antioxidants, modulation of sigma-1 receptors, affect cell growth regulation, inhibition of topoisomerase II and modulate gene expression and immune response (106, 107).
The antibacterial effect of aqueous and alcoholic extracts from petal of saffron was evaluated by Gandomi et al. The minimum inhibitory concentration (MIC) of ethanolic extracts was 40 mg/ml-1 against Listeria monocytogenes, Staphylococcus aureus and Bacillus cereus, while the MICs for Salmonella typhimurium and Eschrichia coli O157:H7 were estimated > 40 mg/ml (108).
The anti-salmonella activity of safranal (8–16 mg/ml) and crocin (64–128 mg/ml) was also reported by Pintado et al (109).
Hypnotic and anxiolytic effects
Razi: ‘it is a sedative agent’ (41).
Saffron stigma is used for insomnia and anxiety in traditional medicine (40).
Safranal at doses of 0.15 and 0.35 ml/kg showed anxiolytic effects and increased the total sleep time dose dependently in mice (110). It increased the duration of non-rapid eye movement (NREM) sleep, shortened NREM sleep latency, and enhanced the delta power activity of NREM sleep in mice. The hypnotic effects of safranal may be related to the activation of the sleep-promoting neurons in the ventrolateral preoptic nucleus and the simultaneous inhibition of the wakefulness-promoting neurons in the tuberomammillary (111).
Effects on the eyes
Razi: ‘It stops excessive discharges of the eyes when applied with woman’s milk.’ (41).
Saffron has been used traditionally by different nations for different eye diseases such as corneal disease, painful eye, cataracts, and purulent eye infection. It can strengthen eyesight and is useful in day blindness, keratitis and in blue discoloration of the eyes due to some other ailments (112, 113).
Present investigations show that saffron extract with antioxidant, anti-inflammatory and antiapoptotic properties can reduce ocular diseases such as cataracts (114), retinal degeneration (115), light-mediated photoreceptor cell death (116), and enhances ocular blood flow and retinal function (117). Saffron in rabbits with chronic ocular hypertension reduced glutamic acid concentration and reduced retinal damage caused by overproduction of glutamate (118). Also saffron with antioxidant effects reduced overproduction of ROS and reduced retinal damages in rabbits (119). Saffron through acting as a regulator of programmed cell death may protect photoreceptor cells from retinal stress and maintain both morphology and function of these cells (120).
Cardiovascular effects
Razi: ‘It is a heart enlivening agent. It strengthens internal weak organs’ (41).
In Iranian traditional medicine, saffron has been used as a cardiotonic and hypotensive agent. It can provide blood flow and nutrition to the heart. It can prevent coagulation and in large amounts can destroy blood clots (40).
Saffron exerted a significant cardioprotective effect by preserving hemodynamics and left ventricular functions, maintaining structural integrity and augmenting antioxidant status against isoproterenol-induced myocardial injury in rats (121). A similar study showed that muscle creatine kinase and lactate dehydrogenase in rats treated with saffron were significantly lower in comparison with the isoproterenol group. The histological findings of the heart sections confirmed the cardioprotective effect of saffron (122).
Imenshahidi et al showed the hypotensive effects of safranal and crocin. In this study, crocin (50, 100 and 200 mg/kg), safranal (0.25, 0.5 and 1 mg/kg) and the aqueous extract of saffron (2.5, 5 and 10 mg/kg) reduced the arterial blood pressure and heart rate in normotensive and hypertensive anaesthetized rats in a dose-dependent manner (123). In another study crocetin prevented the cardiac hypertrophy induced by norepinephrine in rats. Crocetin increased the levels of the antioxidant enzymes such as myocardial superoxide dismutase, catalase, and glutathione peroxidase; and also significantly improved the myocardial pathological histological changes induced by norepinephrine (124). The chronic administration (5 weeks) of saffron with doses of 10, 20 and 40 mg/kg/day in normotensive and desoxycorticosterone acetate (DOCA)-salt induced hypertensive rats could drop the mean systolic blood pressure in DOCA salt treated rats in a dose-dependent manner, but in normotensive group did not show any antihypertensive effect. The antioxidant, diuretic and vasodilatory effects of saffron are three main mechanisms of antihypertensive features of saffron (125). In a clinical, double-blind, placebo-controlled trial with a limited number of volunteers, administration of saffron at doses of 200 mg and 400 mg via saffron tablets, did not show any coagulative effect after 1 month of study (126), but in Jessi SW et al`s clinical study saffron decreased lipid peroxidation in platelet barrier and reduced platelets sticking in dose-dependent manner (127). The injection of crocetin in rabbits that were fed high cholesterol regimen, reduced serum cholesterol by half and prevented atherosclerosis (128). Crocin reduced the activity of pancreatic lipase and excreted lipids in the feces in rats. As a result, it reduced TG, LDL, VLDL, and total cholesterol (129). Anti-atherosclerotic effect of saffron has been shown by He et al. Crocin reduced the oxidation of LDL and prevented the risk of atherosclerosis in quails (130).
In vitro and in vivo studies confirmed that saffron can increase tissue oxygenation (131). Saffron increased the rate of plasma oxygenation and oxygen supply in capillary endothelial cells in experimental spinal cord injury in dogs (132), in cats with brain injury (133) and in rats with hemorrhagic shock (134).
In addition to cardioprotective and anti-atherosclerotic mechanisms that were mentioned above, insulin sensitizing effects, inhibition of foam cell formation, aortic intima thickening, lipid absorption, and vascular cell adhesion molecule-1 (VCAM-1) expression are other main mechanisms for this effects of saffron (12).
Toxicity
Razi: ‘Three mithqal (13.5 gm) of saffron makes a man so overjoyed that, as a result, high consumption of saffron does not have a good effect on the brain and may be fatal. Abuse of it might cause insane behavior’ (41).
Headaches, nausea, head fullness, dizziness, hypomania, and appetite suppression are major adverse effects of saffron that are mentioned in traditional medical books (135).
The accumulation of saffron in sclera, skin, or mucosa can produce yellowish visage and mimics icteric complaints (53).
In vivo studies have shown that saffron has little toxicity in animals. The orally lethal dose (LD50) of saffron was 20.7 g/kg and higher doses can induce neurotoxicity and nephrotoxicity, causing death. The injection of saffron at doses of 0.1–5 g/kg in mice was not toxic (136). In the Bahmani et al study, administration of saffron at doses of 0.5, 1 or 2 g/kg/day for three weeks for neonate mice, did not show any changes in the histopathology and common markers of liver toxicity such as liver enzymes and bilirubin, but it increased serum urea nitrogen. The histopathological changes were seen in the kidney of neonates. The excitation of mice was seen 4 min after treatment with saffron, after this state sedation was seen (137). The evaluation of acute and sub-acute toxicity of safranal has shown that oral administration to male rats once daily for 21 days leads to a significant decrease in red blood cells counts, hematocrit, hemoglobin, and platelets. The intraperitoneal LD50 values of safranal were 1.48 ml/kg in male mice, 1.88 ml/kg in female mice and 1.50 ml/kg in male rats. The oral LD50 values were 21.42 ml/kg in male mice, 11.42 ml/kg in female mice and 5.53 ml/kg in male rats (138).
Other studies performed on saffron toxicity include its hematopoietic system toxicity. The aqueous extract of stigma (0.16, 0.32 and 0.48 g/kg) and petal (1.2, 2.4 and 3.6 g/kg) for a 2-week period were examined in rats. Results showed that normochromic normocytic anemia was produced in both groups and lung and liver damage was seen in the petal extract group (139), but Modaghegh et al performed a similar study in healthy volunteers and evaluated hematological and biochemical parameters. Alteration of these parameters was observed, but it was in the normal ranges (140). In human studies use of 1.5 g/d of saffron is safe but doses above 10 g/d were accompanied by hematuria, drowsiness, nausea, vomiting, and uterine bleeding; and may lead to abortion. Saffron at doses above 20 g was fatal (13, 53). After 1 month administration of 20 mg/day crocin to 44 healthy volunteers, no major adverse events were seen in biochemical, hematological, hormonal and urinary parameters of precipitants, but a reduction in amylase, mixed white blood cells and PTT was reported. Crocin with a dose of 20 mg/kg was safe in this study (141).
We summarized traditional uses of saffron and compared them with modern pharmacological findings in Table 1. As can be seen, traditional uses of saffron are confirmed by scientific findings today.
Table 1.
Traditional uses and modern pharmacological effects of saffron
| Organ | Traditional uses | Modern pharmacological findings | |||
|---|---|---|---|---|---|
| Immune System & Infections | Antibacterial (39, 40) | Antifungal (39, 40) | Anti-inflammation and analgesic (39) | Increases IgG levels, decreases IgM levels, decreases the percentage of basophils and increases the percentage of monocytes (142) | Antisalmonella activity, potent inhibitory effect against Staphylococcus strain and Escherichia coli (108, 109) |
| Skin | To refresh facial skin (13, 39, 40) | Healing wounds (40) | To treat purpura, eczema and erysipelas (40) | Shining and depigmentation properties due to its antioxidant effects (142) | Anti-itching and antipruritic effects (142) |
| Vision | To cure eye infection and corneal disease (39) | Useful in day blindness (40) | To treat lacrimation (40) | Reduces glutamate concentration and retinal damage due to reduction of ocular diseases such as cataracts with antioxidant properties (114, 118) | Regulates programmed cell death that protects photoreceptor cells from retinal stress (120) |
| Reproductive System | Aphrodisiac (39, 40) | To treat impotence (39, 40) | To treat uterine pain (40) | Improves sperm normal morphology, sperm motility, erectile function, and increases the libido (43, 46) | Reduces uterine pain and emotional symptoms associated with PMS (56, 57) |
| Urinary Tract and Kidney | Diuretic, purifier of kidney and bladder (39) | With honey, facilitates passage of renal stone (40) | To cure infection of urinary tract (13) | Antilithiatic effect and reduces urine protein and number of calcium deposits in the kidney tissue (51, 52) | Diuretic effect (50) |
| Gastrointestinal Tract | Strengthening liver and stomach (39) | To decrease appetite (40) | Carminative (40) | Decreases basal gastric secretion and inhibits COX1 and COX2 enzymes (63, 65) | Improves digestion with lowering pH effect (63) |
| Respiratory System | Strengthening respiratory system (39, 40) | Anti-asthma (40) | To treat cough and sore throat (39) | b-adrenoceptor stimulatory and muscarinic receptor inhibitory effect (58) | Histamine H1 receptors antagonist (58) |
| Cardiovascular System | Cardiotonic (13) | Improving circulation (40) | Preventing coagulation (39) | Cardioprotective against oxidative stress and hypotensive effect (142) | Prevents lipoprotein oxidation and atherosclerosis (142) |
| Central nervous System | To cure Obstructions inside the brain (39) | Induces better memory (39) | Hypnotic agent (40) | Decreases microglial activation, neuroprotective and inhibits hippocampal cell death (143) | Activates sleep-promoting neurons (111) |
| General | To strengthen senses (39, 40) | Anti-poisonous (39) | Anti-diabetic (39) | Decrease in exercise-induced protein oxidation due to its antioxidant and alveolar oxygen transport augmentation properties (142) | Fasting blood glucose lowering effect due to its antioxidant effect (144) |
Conclusion
It can be concluded that the topics that cover saffron in Razi`s book (Al-Hawi) have been well validated in current research on saffron and its constituents such as crocin, crocetin, and safranal. It should be noted that many of the positive effects of saffron have been established using in vitro or in vivo animal studies, but whether these positive effects are identical in humans still remains unclear. Further clinical research to elucidate the potential benefits or detrimental effects in humans is warranted.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Fallah M. Introduction to the Traditional Medicine. http://iran-med.ir/?PageID=5 .
- 2.Najmabadi M. International Institute of Iranian Traditional Medicine Mud. http://maadteb.com/history-of-medicine/
- 3.Tadjbakhsh H. History of Veterinary Medicine in Medicine of Iran. Vol. 2. Tehran: University Publications; 2001. pp. 284–292. [Google Scholar]
- 4.Najmabadi M. Mohammad ibn Zakarya Razi, Iranian Physician, Chemist and Philosopher (in Persian) Tehran: Razi University Publications; 1992. [Google Scholar]
- 5.Safa Z. History of Rational Sciences in lslamic Civilization. Vol. 1. Tehran: University Publications (in Persian); 1997. pp. 165–179. [Google Scholar]
- 6.Richter-Bernberg L. Abū Bakr Muhammad al-Rāzī’s (Rhazes) medical works. Med Secoli. 1994;6:377–392. [PubMed] [Google Scholar]
- 7.Zarshenas MM, Mehdizadeh A, Zargaran A, Mohagheghzadeh A. Rhazes (865-925 AD) J Neurol. 2012;259:1001–1002. doi: 10.1007/s00415-011-6398-x. [DOI] [PubMed] [Google Scholar]
- 8.Ligon Lee B. Rhazes:His Career and His Writings. Seminars in Ped Dis. 2001;12:266–272. [Google Scholar]
- 9.Meyerhof M. Thirty-three clinical observations by Rhazes (circa 900 AD) Isis. 1935;23:32–372. [Google Scholar]
- 10.Daffâ AA. Leaders of Medicine in Islamic Civilization. Al-Resalah Publishers, Beirut (in Arabic) 1998:210–222. [Google Scholar]
- 11.Nadjmabadi M. History of Medicine in Iran During the lslamic Era. Tehran University Publications (in Persian) 1995:324–442. [Google Scholar]
- 12.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world’s most expensive spice:Saffron. Food Res Int. 2010;43:1981–1989. [Google Scholar]
- 13.Kianbakht S. Systematic review of pharmacological properties of saffron and active ingredients of it. Med Plants. 2008;28:1–23. [Google Scholar]
- 14.Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents:a review. Drug Res. 2014 doi: 10.1055/s-0034-1375681. Article in Press. [DOI] [PubMed] [Google Scholar]
- 15.Rezaee R, Hosseinzadeh H. Safranal, from an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16:12–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50:761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 17.Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacog Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin:a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinzadeh H, Sadeghnia HR. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat:Involvement of GABAergic and opioids systems. Phytomed. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharmac Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- 22.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Med Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharmaco Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Sadeghnia HR. Effect of safranal, a constituent of Crocus sativus (saffron), on methyl methanesulfonate (MMS)-induced DNA damage in mouse organs:An alkaline single-cell gel electrophoresis (comet) assay. DNA Cell Biol. 2008;26:841–846. doi: 10.1089/dna.2007.0631. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–664. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 26.Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomed. 2011;18:499–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behavior and long-term potentiation. Phytother Res. 2000;14:149–52. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Hosseinzadeh H, Ghenaat J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia. 2006;77:446–448. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Xu GL, Yu SQ, Gong ZN, Zhang SQ. Study of the effect of crocin on rat experimental hyperlipidemia and the underlying mechanisms. Zhongguo Zhong Yao Za Zhi. 2005;30:369–372. [PubMed] [Google Scholar]
- 31.Vahidi AR, Bashardost N, Akhondi H. The analgesic effect of saffron extract in rats as compared with morphine sulfate. Planta Med. 2007;73:995. [Google Scholar]
- 32.Assimopoulou A N, Sinakos Z, Papageorgiou V P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 33.He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L. Effect of crocin on experimental atherosclerosis in quails and itsmechanisms. Life Sci. 2005;77:907–921. doi: 10.1016/j.lfs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Xi L, Qian ZY, Shen XC, Wen N, Zhang YB. Crocetin prevents dexamethasone-induced insulin resistance in rats. Planta Medica. 2005;71:917–922. doi: 10.1055/s-2005-871248. [DOI] [PubMed] [Google Scholar]
- 35.Purushothuman S, Nandasena C, Peoples CL, El Massri N, Johnstone DM, Mitrofanis J, et al. Saffron pre-treatment offers neuroprotection to nigral and retinal dopaminergic cells of MPTP-treated mice. J Parkinsons Dis. 2013;3:77–83. doi: 10.3233/JPD-130173. [DOI] [PubMed] [Google Scholar]
- 36.Hosseinzadeh H, Motamedshariaty V, Hadizadeh F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline. 2007;2:367–370. [Google Scholar]
- 37.Hosseinzadeh H, Behravan J, Ramezani M, Ajgan Kh. Anti-tumor and cytotoxic evaluation of Crocus sativus L. stigma and petal extracts using brine shrimp and potato disc assays. J Med Plants. 2005;4:59–65. [Google Scholar]
- 38.Razavi BM, Hosseinzadeh H, Movassaghi AR, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem Biol Interact. 2013;203:547–555. doi: 10.1016/j.cbi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Bathaie SZ, Mousavi SZ. Historical uses of saffron:identifying potential new avenues for modern research. Avicenna J Phytomed. 2011;1:57–66. [Google Scholar]
- 40.Hosseinzadeh H, Nassiri-Asl M. Avicenna’s (Ibn Sina) the canon of medicine and saffron (Crocus sativus):A review. Phytother Res. 2013;27:475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 41.Anonymous. Synopsis of Rhazes Al-Hawi “Continens of Rhazes” (Persian Translation) book. 1st edition. Mashhad: Edited by Mashhad University of Medical Sciences; 2009. [Google Scholar]
- 42.Srivastava R, Ahmed H, Dixit RK, Dharamveer, Saraf S. Crocus sativus L. A comprehensive review. Pharmacogn Rev. 2010;4:200–208. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shamsa A, Hosseinzadeh H, Molaei M, Shakeri MT, Rajabi O. Evaluation of Crocus sativus L. (saffron) on male erectile dysfunction:A pilot study. Phytomed. 2009;16:690–693. doi: 10.1016/j.phymed.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Kotta S, Ansari SH, Ali J. Exploring scientifically proven herbal aphrodisiacs. Pharmacogn Rev. 2013;7:1–10. doi: 10.4103/0973-7847.112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomed. 2008;15:491–495. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 46.Heidary M, Nejadi JR, Delfan B, Birjandi M, Kaviani H, Givrad S. Effect of saffron on semen parameters of infertile men. J Urol. 2008;5:255–259. [PubMed] [Google Scholar]
- 47.Modabbernia A, Sohrabi H, Nasehi AA, Raisi F, Saroukhani S, Jamshidi A, et al. Effect of saffron on fluoxetine-induced sexual impairment in me:Randomized double-blind placebo-controlled trial. Psychopharmacology. 2012;223:381–388. doi: 10.1007/s00213-012-2729-6. [DOI] [PubMed] [Google Scholar]
- 48.Kashani L, Raisi F, Saroukhani S, Sohrabi H, Modabbernia A, Nasehi AA, et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women:Randomize double-blind placebo-controlled study. Hum Psychopharmacol Clin Exp. 2012;28:54–60. doi: 10.1002/hup.2282. [DOI] [PubMed] [Google Scholar]
- 49.Vasegh M, Koohpeyma F, Kargar Jahromi H, Bathaee SH, Saberi R, et al. Investigating effects of hydroalcoholic extract of saffron on sex hormones in female rats undergoing chemotherapy with cyclophosphamide. J Comp Clin Pathol. 2014;24:399–402. [Google Scholar]
- 50.Shariatifar N, Shoeibi S, Sani MJ, Jamshidi AH, Zarei A, Mehdizade A, et al. Study on diuretic activity of saffron (stigma of Crocus sativus L.) Aqueous extract in rat. J Adv Pharm Technol Res. 2014;5:17–20. doi: 10.4103/2231-4040.126982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghaeni FA, Amin B, Hariri AT, Meybodi NT, Hosseinzadeh H. Antilithiatic effects of crocin on ethylene glycol-induced lithiasis in rats. Urolithiasis. 2014;42:549–58. doi: 10.1007/s00240-014-0711-y. [DOI] [PubMed] [Google Scholar]
- 52.Amin B, Moghri Feriz H, Timcheh Hariri A, Tayyebi Meybodi N, Hosseinzadeh H. Protective effects of the aqueous extract of Crocus sativus against ethylene glycol induced nephrolithiasis in rat. EXCLI J. 2015;14:411–422. doi: 10.17179/excli2014-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javadi B, Sahebkar AH, Emami SA. A Survey on saffron in major islamic traditional medicine books. Iran J Basic Med Sci. 2013;16:1–11. [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmadi S, Azhari S, Rakhshandeh H, Jaafarzadeh H, Mazlum S. Evaluation of the effect of saffron oral capsules on duration of the active phase of labor first stage. Iran J Reprod Med. 2014;12:44–50. [Google Scholar]
- 55.Schmidt M, Betti G, Hensel A. Saffron in phytotherapy:Pharmacology and clinical uses. Wien Med Wochenschr. 2007;157:315–319. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 56.Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour H, Zarrinara AR, et al. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome:a double-blind, randomised and placebo-controlled trial. J Obstet Gynaecol. 2008;115:515–519. doi: 10.1111/j.1471-0528.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 57.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L. in the treatment of mild to moderate depression:a double-blind, randomized and placebo controlled trial. Phytother Res. 2005;19:25–29. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 58.Boskabady MH, Aslani MR. Relaxant effect of Crocus sativus (saffron) on guinea-pig tracheal chains and its possible mechanisms. J Pharm Pharmacol. 2006;58:1385–1390. doi: 10.1211/jpp.58.10.0012. [DOI] [PubMed] [Google Scholar]
- 59.Boskabady MH, Rahbardar MG, Jafari Z. The effect of safranal on histamine (H(1)) receptors of guinea pig tracheal chains. Fitoterapia. 2011;82:162–167. doi: 10.1016/j.fitote.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Boskabady MH, Tabatabaee A, Byrami G. The effect of the extract of Crocus sativus and its constituent safranal, on lung pathology and lung inflammation of ovalbumin sensitized guinea-pigs. Phytomed. 2012;19:904–911. doi: 10.1016/j.phymed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Mannan A, Ubaidullah, Ahmad R, Khan RA. Therapeutic role of saffron and honey in pneumonia in children. Curr Pediatr Res. 2006;10:25–27. [Google Scholar]
- 62.Al-Mofleh LA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Qureshi S, Rafatullah S. Antigastric ulcer studies on ‘saffron’ Crocus sativus L. in rats. Pak J Biol Sci. 2006;9:1009–1013. [Google Scholar]
- 63.Nabavizadeh F, Salimi E, Sadroleslami Z, Karimian SM, Vahedian J. Saffron (Crocus sativus) increases gastric acid and pepsin secretions in rats:Role of nitric oxide (NO) J Pharm Pharmaco. 2009;3:181–184. [Google Scholar]
- 64.Gout B, Bourges C, Paineau-Dubreuil S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr Res. 2010;30:305–313. doi: 10.1016/j.nutres.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem. 2009;57:8325–8330. doi: 10.1021/jf901752f. [DOI] [PubMed] [Google Scholar]
- 66.Kianbakht S, Mozaffari K. Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J Med Plants. 2009;8:30–38. [Google Scholar]
- 67.Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2013;31:367–376. doi: 10.1177/0748233713475519. [DOI] [PubMed] [Google Scholar]
- 68.Mohajeri D, Doustar Y, Rezaei A, Mesgari-Abbasi M. Hepatoprotective effect of ethanolic extract of Crocus sativus L. (saffron) stigma in comparison with silymarin against rifampin induced hepatotoxicity in rats. Zahedan J Res Med Sci. 2011;12:53–59. [Google Scholar]
- 69.Shati AA, Alamri SA. Role of saffron (Crocus sativus L.) and honey syrup on aluminum-induced hepatotoxicity. Saudi Med J. 2010;31:1106–1113. [PubMed] [Google Scholar]
- 70.Ríos JL, Recio MC, Giner RM, Máñez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–193. [Google Scholar]
- 71.Chaqmini. Small Canon. Tehran: Iran University of Medical Sciences and Health Services; 2006. [Google Scholar]
- 72.Hosseinzadeh H, Jahanian Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother Res. 2010;24:726–730. doi: 10.1002/ptr.3011. [DOI] [PubMed] [Google Scholar]
- 73.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 74.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Horticulturae. 2004;650:435–445. [Google Scholar]
- 75.Vásquez CE, Riener R, Reynolds E, Britton GB. NMDA receptor dysregulation in chronic state:A possible mechanism underlying depression with BDNF downregulation. Neurochem Int. 2014;79:88–97. doi: 10.1016/j.neuint.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 76.Gumuslu E, Mutlu O, Sunnetci D, Ulak G, Celikyurt IK, Cine N, et al. The antidepressant agomelatine improves emory deterioration and upregulates CREB and BDNF gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights. 2014;5:11–21. doi: 10.4137/DTI.S13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;22:16–22. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghasemi T, Abnous K, Vahdati F, Mehri S, Razavi BM, Hosseinzadeh H. Antidepressant Effect of Crocus sativus Aqueous Extract and its Effect on CREB, BDNF, and VGF Transcript and Protein Levels in Rat Hippocampus. Drug Res (Stuttg) 2014;65:337–343. doi: 10.1055/s-0034-1371876. [DOI] [PubMed] [Google Scholar]
- 79.Hosseinzadeh H, Motamedshariaty V, Hadizadeh F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacol. 2007;2:367–370. [Google Scholar]
- 80.Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression:a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Hausenblas HA, Saha D, Dubyak PJ, Anton SD. Saffron (Crocus sativus L.) and major depressive disorder:a meta-analysis of randomized clinical trials. J Integr Med. 2013;11:377–383. doi: 10.3736/jintegrmed2013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lopresti AL, Drummond PD. Saffron (Crocus sativus) for depression a systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum Psychopharmacol. 2014;29:517–527. doi: 10.1002/hup.2434. [DOI] [PubMed] [Google Scholar]
- 83.Zargari A. Medicinal plants. 1st ed. Tehran: Tehran University publication; 1997. [Google Scholar]
- 84.Mir Heidar H. Herbal knowledge:Usage of herbs in prevention and treatment of diseases, with latest research around the world. 2nd ed. Tehran: Daftare Nashre Farhange Eslami; 2004. [Google Scholar]
- 85.Golmohammadzadeh Sh, Jaafari MR, Hosseinzadeh H. Does saffron have antisolar and moisturizing effects? Iran J Pharm Res. 2010;9:133–140. [PMC free article] [PubMed] [Google Scholar]
- 86.Golmohammadzadeh S, Imani F, Hosseinzadeh H, Jaafari MR. Preparation, characterization and evaluation of sun protective and moisturizing effects of nanoliposomes containing safranal. Iran J Basic Med Sci. 2011;14:521–533. [PMC free article] [PubMed] [Google Scholar]
- 87.Akhtar N, Khan HM, Ashraf S, Mohammad IS, Saqib NU, Bashir K. Moisturizing effect of stable cream containing Crocus sativus extracts. Pak J Pharm Sci. 2014;27:1881–1884. [PubMed] [Google Scholar]
- 88.Das I, Chakrabarty RN, Das S. Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice. Asian Pac J Cancer Prev. 2004;5:70–76. [PubMed] [Google Scholar]
- 89.Das I, Das S, Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma:A histopathological study. Acta Histochem. 2010;112:317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 90.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L. and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterap. 2012;83:888–895. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 91.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;15:2–7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasri S, Hosseini SY, Sahraei H, Zardooz H. Inhibition of pain and inflamation induced by formalin in male mice by ethanolic extract of saffron (Crocus sativus) and its constituents;crocin and safranal. Kowsar Med J. 2011;15:189–195. [Google Scholar]
- 93.Taghizadeh R, Sahebari M, Mahmoudi Z, Hosseinzadeh H, Haghmorad D, Tabasi N, et al. Inhibitory effect of Crocus sativus L. ethanol extract on adjuvant-induced arthritis. Food Agricul Immunol. 2014;26:170–180. [Google Scholar]
- 94.Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem. 2009;57:8325–8330. doi: 10.1021/jf901752f. [DOI] [PubMed] [Google Scholar]
- 95.Tamaddonfard E, Farshid AA, Eghdami K, Samadi F, Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol Rep. 2013;65:1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 96.Amin B, Abnous K, Motamedshariaty V, Hosseinzadeh H. Attenuation of oxidative stress, inflammation and apoptosis by ethanolic and aqueous extracts of Crocus sativus L. stigma after chronic constriction injury of rats. An Acad Bras Cienc. 2014;86:1821–1832. doi: 10.1590/0001-3765201420140067. [DOI] [PubMed] [Google Scholar]
- 97.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Hemshekhar M, Sebastin Santhosh M, Sunitha K, Thushara RM, Kemparaju K, Rangappa KS, et al. A dietary colorant crocin mitigates arthritis and associated secondary complications by modulating cartilage deteriorating enzymes, inflammatory mediators and antioxidant status. Biochimie. 2012;94:2723–2733. doi: 10.1016/j.biochi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 99.Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari MR. Antitumor activity of PEGylated nanoliposomes containing crocin in mice bearing C26 colon carcinoma. Planta Med. 2013;79:447–451. doi: 10.1055/s-0032-1328363. [DOI] [PubMed] [Google Scholar]
- 100.Nair SC, Pannikar B, Pannikar KR. Antitumour activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 101.Mousavi SH, Tavakkol-Afshari J, Brook A, Jafari-Anarkooli I. Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food Chem Toxicol. 2009;47:1909–1913. doi: 10.1016/j.fct.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 102.Amin A, Hamza AA, Bajbouj K, Ashraf SS, Daoud S. Saffron:a potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology. 2011;54:857–867. doi: 10.1002/hep.24433. [DOI] [PubMed] [Google Scholar]
- 103.Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 104.Abdullaeva FA, Rivero´n-Negretea L, Caballero-Ortegaa H, Manuel Herna´ndeza J, Pe´rez-Lo´peza I, Pereda-Mirandab R, et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L) Toxicol in Vitro. 2003;17:731–736. doi: 10.1016/s0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 105.Bathaie SZ, Miri H, Mohagheghi MA, Mokhtari-Dizaji M, Shahbazfar AA, Hasanzadeh H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the wistar albino rat. Iran J Basic Med Sci. 2013;16:27–38. [PMC free article] [PubMed] [Google Scholar]
- 106.Magesh VSJ, Selvendiran K, Ekambaram G, Sakthisekaran D. Antitumour activity of crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes and histopathological studies. Mol Cell Biochem. 2006;287:127–135. doi: 10.1007/s11010-005-9088-0. [DOI] [PubMed] [Google Scholar]
- 107.Tavakkol-Afshari J, Brook A, Mousavi SH. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol. 2008;46:3443–3447. doi: 10.1016/j.fct.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 108.Gandomi H, Misaghi A, Abbaszadeh S, Azami L, Shariatifar N, Tayyar N. Antibacterial effect of aqueous and alcoholic extracts from petal of saffron (Crocus sativus) on some foodborne bacterial pathogens. J Med Plants. 2012;11:189–196. [Google Scholar]
- 109.Pintado C, de Miguel A, Acevedo O, Nozal L, Novella JL, Rotger R. Bactericidal effect of saffron (Crocus sativus L.) on Salmonella enterica during storage. Food Control. 2010;22:638–642. [Google Scholar]
- 110.Hosseinzadeh H, Noraei NB. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytothera Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 111.Liu Z, Xu XH, Liu TY, Hong ZY, Urade Y, Huang ZL, et al. Safranal enhances non-rapid eye movement sleep in pentobarbital-treated mice. CNS Neurosci Ther. 2012;18:623–630. doi: 10.1111/j.1755-5949.2012.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferrence SC, Bendersky G. Therapy with saffron and the goddess at Thera. Perspect Biol Med. 2001;47:199–226. doi: 10.1353/pbm.2004.0026. [DOI] [PubMed] [Google Scholar]
- 113.Giaccio M. Crocetin from saffron. An active component of an ancient spice. Critical Rev Food Sci Nutr. 2004;44:155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 114.Makri OE, Ferlemi AV, Lamari FN, Georgakopoulos CD. Saffron administration prevents selenite-induced cataractogenesis. Mol Vis. 2013;30:1188–1197. [PMC free article] [PubMed] [Google Scholar]
- 115.Fernández-Sánchez L, Lax P, Esquiva G, Martín-Nieto J, Pinilla I, Cuenca N. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS One. 2012;7:e43074. doi: 10.1371/journal.pone.0043074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laabich A, Vissvesvaran GP, Lieu KL, Murata K, McGinn TE, Manmoto CC, et al. Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture. Invest Ophthalmol Vis Sci. 2006;47:3156–3163. doi: 10.1167/iovs.05-1621. [DOI] [PubMed] [Google Scholar]
- 117.Xuan B, Zhou YH, Li N, Min ZD, Chiou GC. Effects of crocin analogs on ocular blood flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–152. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 118.Yang XG, Zhang Q, Yu JN, Lian JP, Lei AJ. Effect of Crocus sativus extracti on the concentration of glutamic acid in the vitreous body in rabbits with chronic ocular hypertension. Int J Ophthalmol. 2006;6:1025–1026. [Google Scholar]
- 119.Yang XG, Sun DJ, Wang YW, Jin WL, Wang XJ, Duan XL. Effect of Crocus sativus on SOD and MDA alterations in the retina of rabbits with chronic ocular hypertension. Int J Ophthalmol. 2008;8:47–49. [Google Scholar]
- 120.Maccarone R, Di Marco S, Bisti S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Invest Ophthalmol Visual Sci. 2008;49:1254–1261. doi: 10.1167/iovs.07-0438. [DOI] [PubMed] [Google Scholar]
- 121.Sachdeva J, Tanwar V, Golechha M, Siddiqui KM, Nag TC, Ray R, et al. Crocus sativus L. (saffron) attenuates isoproterenol-induced myocardial injury via preserving cardiac functions and strengthening antioxidant defense system. Exp Toxicol Pathol. 2012;64:557–564. doi: 10.1016/j.etp.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 122.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 123.Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 124.Shen XC, Qian ZY, Chen Q, Wang YJ. Protective effect of crocetin on primary culture of cardiac myocyte treated with noradrenaline in vitro. Yao Xue Xue Bao. 2004;39:787–791. [PubMed] [Google Scholar]
- 125.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. The effect of chronic administration of safranal on systolic blood pressure in rats. Iran J Pharm Res. 2015;14:585–590. [PMC free article] [PubMed] [Google Scholar]
- 126.Ayatollahi H, Javan AO, Khajedaluee M, Shahroodian M, Hosseinzadeh H. Effect of Crocus sativus L. (saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytother Res. 2014;28:539–543. doi: 10.1002/ptr.5021. [DOI] [PubMed] [Google Scholar]
- 127.Jessie SW, Krishnakantha TP. Inhibition of human platelet aggregation and membrane lipid peroxidation by food spice, saffron. Mol Cell Biochem. 2005;278:59–63. doi: 10.1007/s11010-005-5155-9. [DOI] [PubMed] [Google Scholar]
- 128.Gainer JL, Jones JR. The use of crocetin in experimental atherosclerosis. Experientia. 1975;31:548–549. doi: 10.1007/BF01932451. [DOI] [PubMed] [Google Scholar]
- 129.Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats:crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–122. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 130.He SY, Qian ZY, Wen N, Tang FT, Xu GL, Zhou CH. Influence of crocetin on experimental atherosclerosis in hyperlipidemic-diet quails. Eur J Pharmacol. 2007;554:191–195. doi: 10.1016/j.ejphar.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 131.Diluccio RC, Gainer JL. Increasing alveolar oxygen transport. Aviat Space Environ Med. 1980;51:18–20. [PubMed] [Google Scholar]
- 132.Gainer JV. Use of crocetin in experimental spinal cord injury. J Neurosurg. 1977;46:358–380. doi: 10.3171/jns.1977.46.3.0358. [DOI] [PubMed] [Google Scholar]
- 133.Gainer JV, Nugent R. Effect of increasing the plasma oxygen diffusivity on experimental cryogenic edema. J Neurosurg. 1976;45:535–538. doi: 10.3171/jns.1976.45.5.0535. [DOI] [PubMed] [Google Scholar]
- 134.Gainer JL, Rudolph DB, Caraway DL. The effect of crocetin on hemorrhagic shock in rats. Circ Shock. 1993;41:1–7. [PubMed] [Google Scholar]
- 135.Aqili Khorasani MH, Makhzan al-Adwiah., Drug Treasure . Reprinted from a copy which was printed in Calcutta dated in 1844. Tehran: Enqelab-e Eslami Publishing and Educational Organization; 1992. pp. 472–473. [Google Scholar]
- 136.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;247:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 137.Bahmani M, Rafieian M, Baradaran A, Rafieian S, Rafieian-Kopaei M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J Nephropathol. 2014;3:81–85. doi: 10.12860/jnp.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hosseinzadeh H, Shakib SS, Sameni AK, Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharma Res. 2013;12:93–99. [PMC free article] [PubMed] [Google Scholar]
- 139.Karimi Gh, Taiebi N, Hosseinzadeh H, Shirzad F. Evaluation of Subacute Toxicity of Aqueous Extract of Crocus sativus L. Stigma and Petal in Rats. J Med Plants. 2004;3:29–36. [Google Scholar]
- 140.Modaghegh MH, Shahabian M, Esmaeili HA, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomed. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 141.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety Evaluation of Crocin (a constituent of saffron) Tablets in Healthy Volunteers. Iran J Basic Med Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 142.Moshiri M, Vahabzadeh M, Hosseinzadeh H. Clinical applications of saffron (Crocus sativus) and its constituents:A review. Drug Res. 2014;65:287–295. doi: 10.1055/s-0034-1375681. [DOI] [PubMed] [Google Scholar]
- 143.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 144.Bajerska J, Mildner-Szkudlarz S, Podgórski T, Oszmatek-Pruszyíska E. Saffron (Crocus sativus L.) powder as an ingredient of rye bread:an anti-diabetic evaluation. J Med Food. 2013;16:847–856. doi: 10.1089/jmf.2012.0168. [DOI] [PubMed] [Google Scholar]


