Abstract
Objective (s):
The scarcity of articular cartilage defect to repair due to absence of blood vessels and tissue engineering is one of the promising approaches for cartilage regeneration. The objective of this study was to prepare an extracellular matrix derived decellularized bovine articular cartilage scaffold and investigate its interactions with seeded rat bone marrow mesenchymal stem cells (BM-MSCs).
Materials and Methods:
Bovine articular cartilage that was cut into pieces with 2 mm thickness, were decellularized by combination of physical and chemical methods including snap freeze-thaw and treatment with sodium dodecyl sulfate (SDS). The scaffolds were then seeded with 1, 1’-dioctadecyl-3, 3, 3’, 3’-tetramethylindocarbocyanine perchlorate (DiI) labeled BM-MSCs and cultured for up to two weeks.
Results:
Histological studies of decellularized bovine articular cartilage showed that using 5 cycles of snap freeze-thaw in liquid nitrogen and treatment with 2.5% SDS for 4 hr led to the best decellularization, while preserving the articular cartilage structure. Adherence and penetration of seeded BM-MSCs on to the scaffold were displayed by histological and florescence examinations and also confirmed by electron microscopy.
Conclusion:
ECM-derived decellularized articular cartilage scaffold provides a suitable environment to support adhesion and maintenance of cultured BM-MSCs and could be applied to investigate cellular behaviors in this system and may also be useful for studies of cartilage tissue engineering.
Keywords: Articular cartilage, Decellularization Extracellular matrix, Tissue engineering
Introduction
Tissue engineering is a novel approach that applies principles to biology and engineering for the development of biological replacement that strives to maintain or improve tissue function in patients (1). The first and major component of tissue engineering is to provide a three-dimensional structure for the growth of cells, while maintaining their differentiated function is the scaffold (2). Various types of scaffolds including natural and synthetic have been used in tissue engineering both experimentally and in clinical applications. Many research lines have revealed that naturally derived- scaffolds derived from decellularized tissues and organs are functionally superior to synthetic polymers (3, 4). They usually retain their biocompatibility and may evade the immune response, because their xenogenic or allogenic cellular antigens are removed. Furthermore, due to preservation of most of the structural and functional proteins in the scaffold, they provide signals, which facilitate cell attachment (5). (extracellular matrix)ECM[AGA1], which is composed of a rich meshwork of proteins and proteoglycans, is a main communication layer between the environment and cells (6). ECM could be harvested from animal tissues and be used directly as scaffolds for tissue engineering applications. ECMs derived from a variety of tissues have been used as biological scaffolds, including heart valves (7), blood vessels (8), skin (9), skeletal muscle (10), tendons (11), small intestinal submucosa (SIS) (12, 13), urinary bladder (14, 15) and liver (16). In this study, decellularized bovine articular cartilage was prepared and used as a scaffold.
To do so, multiple treatments with sodium dodecyl sulfate (SDS) were applied in order to find the best structural properties, in which decellularization was achieved while collagen and glycosaminogelycans (GAGs) contents were preserved. Self-repair of cartilage lesions is difficult due to the low regenerative capacity and blood supply of cartilage, and tissue engineering as an interdisciplinary field is on the lookout for cartilage repair (2). The main goal of the decellularization protocol is the best elimination of all cellular and nuclear materials, while minimizing any adverse effects on the composition, biological architecture and function of the remaining ECM (17). However, cartilage is a compact tissue, which is difficult to remove its cell components completely (2). SDS is an ionic detergent, which permeabilizes the cytoplasmic and nuclear membranes and has been used to decellularize other musculoskeletal tissues including meniscus, temporomandibular joint, tendon and ligament (11, 18-20).
The source of cells is another important component of tissue engineering strategies. Bone marrow-derived mesenchymal stem cells (BM-MSCs) considered as a promising candidates for cartilage tissue engineering due to their ability of proliferation, multiple differentiation and ease of harvest (5). These multipotent cells can be expanded and differentiated to obtain connective tissue cells like bone, cartilage, muscle, tendon and fat (21-24). The objectives of this study was to produce a decellularized bovine articular cartilage scaffold, which retains the main ECM molecules by a combination of physical and chemical methods and also to investigate the interaction of this scaffold with seeded BM-MSCs.
Materials and Methods
Preparation of ECM-derived cartilage scaffold
Animal experiments were performed according to the Iranian Council for the Use and Care of Animals Guidelines and were approved by the Animal Research Ethical Committee of Ferdowsi University of Mashhad. In this experimental study, articular cartilages of femur bovine bones were harvested from the sacrificed animals immediately after slaughter and were cut into coin-like pieces with 5 mm diameter and 2 mm thickness. To decellularize the articular cartilage tissue, a combination of physical and chemical methods was applied. For physical decellularization, specimens were maintained at -4 °C for a week and then after thawing and washing with normal saline, snap freeze-thaw in liquid nitrogen were performed for 5 cycles. In order to perform chemical decellularization, specimens were treated with 2.5% SDS (Merck, Darmstadt, Germany) for 1, 4 and 8 hr at 37 °C to achieve the best elimination of cells, while preserving the extracellular matrix components. Then, the specimens were rinsed in phosphate-buffered saline (PBS) for 30 min. In the next step, in order to remove SDS and minimize potential contaminations, the decellularized scaffolds were placed in a sterile Buchner funnel and washed with 75% ethanol, sterile distilled water and PBS, respectively.
Evaluation of the decellularized bovine articular cartilage scaffold by histological methods
Paraformaldehyde 4% (Merck, Darmstadt, Germany) was applied to prepare specimens for histological and fluorescence studies. After fixation, specimens were dehydrated through a graded series of ethanol, embedded in paraffin (Lab-O-Wax, Milan, Italy), cross-sectioned at a thickness of 5 µm with a microtome (Leits, Vienna, Austria), deparaffinized, rehydrated and stained with hematoxylin-eosin (H&E) (Merck, Darmstadt, Germany) to determine the construct cellularity. Safranin-O (Merck, Darmstadt, Germany) (25) and toluidine blue (Merck, Darmstadt, Germany) stainings were performed to demonstrate the GAGs content in decellularized ECM. Picrosirius-red (Merck, Darmstadt, Germany) staining was also employed to examine the effects of decellularization on the collagen-rich ECM. Using polarizing microscope (Olympus, IX70, Japan) for observing the sections stained with picrosirius-red is a specific method to identify the collagenous structures (26). DNA contents were stained with 4, 6 diamidino-2-phenylindole (DAPI; Sigma-Aldrich, Taufkirchen, Germany), which is a fluorescent dye that binds to nucleic acids with high affinity for DNA.
Bone marrow-derived mesenchymal stem cells (BM-MSCs) isolation and expansion
To derive rat BM-MSCs, the bone marrow of a one- month old male Wistar rat was collected and the cells were transferred into a cell culture flask filled with 5 ml Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Paisley, Scotland) supplemented with 15% fetal bovine serum, (FBS, Gibco, Scotland) and 100 µl penicillin/streptomycin (Biosera, Sussex, UK). Cells were then incubated at 37 °C and 5% CO2 in air. After the removal of blood and stromal cells, BM-MSCs were subcultured and purified by trypsinization (0.25% trypsin /EDTA (Ethylenediaminetetraacetic acid) solution, Biosera, Sussex, UK) for four times (27, 28).
Cell labeling, seeding and culture method
To confirm the detection of BM-MSCs on the scaffolds, the cells were labeled before seeding. 1, 1’-dioctadecyl-3, 3, 3’, 3’-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen, Karlsruhe, Germany) that stains the cytoplasm of cells was used to label BM-MSCs. To prepare the stock solution of DiI, 50 µg of DiI was dissolved in 50 µl dimethyl-sulfoxide (DMSO, Merck, Darmstadt, Germany), and 1 µl of the stock solution was added to 1 ml PBS. Then the medium was removed and 1 ml of the prepared dye was added to a 25 cm2 flask. The cells were incubated at 37 °C for 5 min and then transferred to -4 °C for 10 to 15 min. Fresh medium was then added to each flask. The cells were incubated overnight and then applied for seeding.
DiI stained BM-MSCs at passages 4 were trypsinized, counted and the scaffolds were seeded with 2×105 cells/scaffold in 2 ml complete medium, and specimens were placed in 24-well plates.
Seeding was performed in a dropwise fashion onto the scaffold. Seeded scaffolds were incubated in a humidified atmosphere at 37°C with 5% CO2 in air. The medium was changed every 2 to 3 days. Some unseeded scaffolds were cultured as controls. Histological analyses were performed after 3, 7 and 14 days of culture.
SEM analysis
In order to prepare samples for electron microscopy, the specimens were fixed with 2.5% glutaraldehyde (TAAB Laboratories Equipment Ltd. Reading, UK) for 24 hr followed by three 15 min washes in 0.1 M sodium cacodylate buffer (pH 7.4, TAAB Laboratories Equipment Ltd. Reading, UK). Then, the specimens were treated with 1% Osmium tetroxide (TAAB Laboratories, UK) for 1 hr and washed in 0.1 M sodium cacodylate buffer. At the next step, the sections were subjected to sequential dehydration with 30%, 50%, 70%, 90%, and absolute ethanol solutions, followed by three washes with absolute ethanol for 15 min. Then the specimens were fixed on metal stubs and coated with gold-palladium by sputtering (Sputter coater, SC7620, Sussex, UK) and examined under a scanning electron microscope (SEM; LEO 1450VP, Oberkochen, Germany).
Results
Characterization of the decellularized articular cartilage by histological studies
All specimens were harvested from bovine articular cartilage, cut into coin-like pieces and subjected to physical decellularization and then chemical treatments with 2.5% SDS for 1, 4 and 8 hr. The obtained decellularized scaffolds were evaluated histologically.
H&E staining of native and decellularized articular cartilage groups revealed that treatment with SDS for 1 hr resulted in incomplete decellularization of articular cartilages, while in other groups treated for longer period, better elimination of cells was obtained. Using safranin-O and toluidine-blue stainings that indicate the distribution of sulfated proteoglycans and GAGs in the ECM, showed an extensive reduction in stained GAGs in specimens treated for 8 hr, which reveals qualitative loss of GAGs after decellularization. Specimens stained by Picrosirius Red also demonstrated the preservation of collagen fibers in decellularized samples treated with SDS for 1 and 4 hr, while collagen content of scaffolds treated for 8 hr displayed extensive decrease after decellularization (Figure 1). Therefore, the specimens treated with 2.5% SDS for 4 hr appeared to be the best group to achieve a decellularized articular cartilage scaffold with the best elimination of cells and most preservation of ECM contents.
Figure 1.
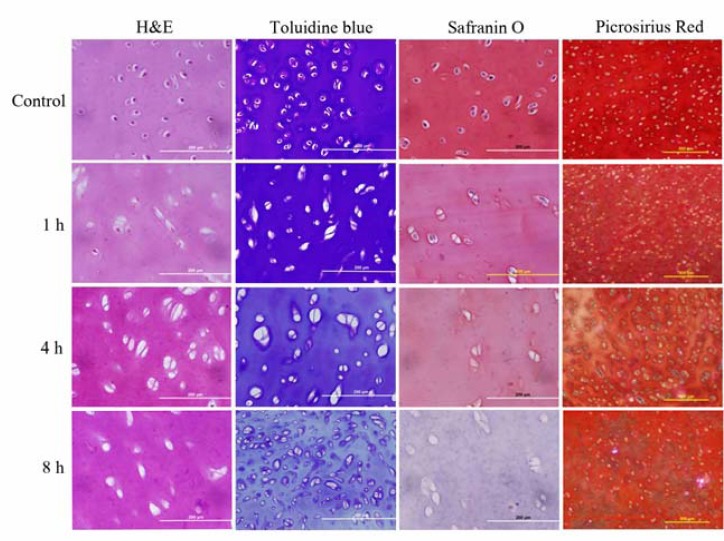
Control and decellularized articular cartilage specimens. Construct cellularity, glycosaminoglycan (GAG) and collagen content have been displayed in various decellularization groups compared with native cartilage. H&E staining demonstrated that treatment with 2.5% SDS for 1 hr has led to incomplete elimination of cellular components while treatment for 4 and 8 hr eliminated cell nuclei and components completely. Toluidine blue and Safranin-O staining demonstrated the decrease of GAG content and Picrosirius Red staining displayed decrease of collagen content in specimens treated for 8 hr. Decellularized specimens treated for 4 hr have shown the best elimination of cellularity while preserving of GAG and collagen contents
Furthermore, DAPI staining also confirmed the elimination of nuclear DNA in obtained decellularized scaffolds as shown in Figure 2. In agreement with results of light and fluorescence microscopy, all cells had been removed, and the decellularized cartilage tissue could be observed in the SEM images (Figure 5).
Figure 2.
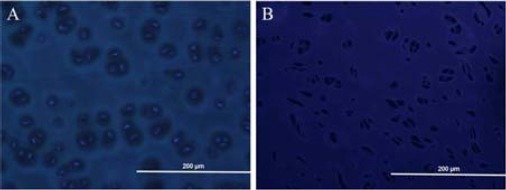
Staining of bovine articular cartilage with 4, 6 diamidino-2-phenylindole (DAPI) before and after decellularization. Native bovine articular cartilage combined with bright nuclei representative of the presence of intact cells in native tissue (A). Elimination of cell nuclei in obtained decellularized scaffold (B) compared with native articular cartilages is displayed, which proved the efficiency of decellularization procedure
Figure 3.
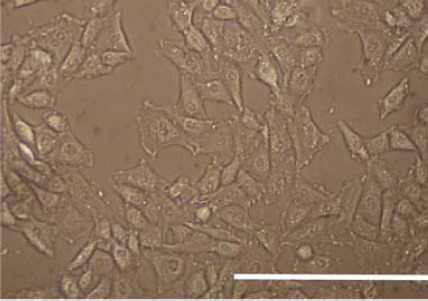
Morphology of the monolayer cultured rat bone marrow derived mesenchymal stem cells at passage 4 (scale bar=200 μm)
Figure 4.
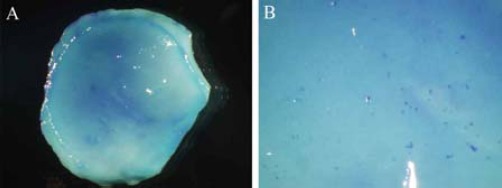
Methylene blue staining of seeded scaffolds after 24 hr of culture (A, ×15 & B, ×70 magnification)
Figure 5.

A) Scanning electron micrographs of the native articular cartilage tissue B) Decellularized articular cartilage scaffold and C) Penetration and adherence of bone marrow mesenchymal stem cells on the scaffold after 48 hr of culture is displayed
Investigation of interactions between BM-MSCs and decellularized articular cartilage scaffold
DiI labeled BM-MSCs at passage 4 were used for seeding onto the decellularized articular cartilage scaffolds (Figure 3).
After 24 hr of seeding, some scaffolds were stained with methylene blue and as it is shown in Figure 4, blue spots on scaffolds verified the viability of seeded cells. SEM images of seeded scaffolds also demonstrated the adherence of BM-MSCs to the scaffolds after culture (Figure 5).
The adhesion and maintenance of seeded cells were also investigated after 3, 7 and 14 days of culture. As shown in Figure 6, H&E staining of cultured scaffolds indicated the attachment and penetration of seeded MSCs into the lacuna of decellularized articular cartilage.
Figure 6.
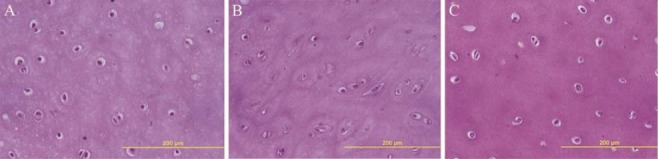
Hematoxylin-eosin staining of seeded scaffolds after 3, 7 and 14 days of culture, which display adherence and maintenance of bone marrow mesenchymal stem cells into lacuna of decellularized scaffold during culture
Fluorescence microscopy of specimens can be observed in Figure 7. Seeded articular cartilage scaffolds with DiI labeled BM-MSCs were examined after DAPI staining at 3, 7 and 14 days of culture. Labeled BM-MSCs were detected as brightly spots located inside the lacuna of decellularized articular cartilage scaffolds.
Figure 7.
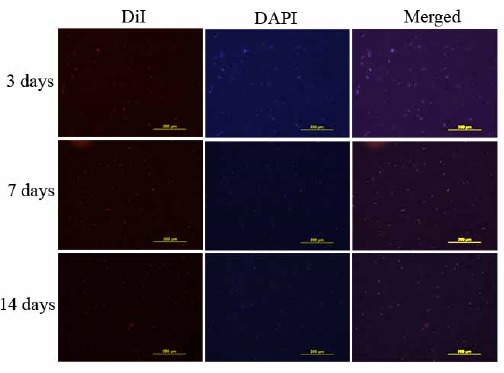
Penetration and adherence of 1, 1’-dioctadecyl-3, 3, 3’, 3’-tetramethylindocarbocyanine perchlorate (DiI) labeled bone marrow mesenchymal stem cells into lacuna of decellularized scaffold were detected after 4, 6 diamidino-2-phenylindole (DAPI) staining at 3, 7 and 14 days after culture by fluorescent microscope
Discussion
In recent decades, the tissue engineering has been progressing rapidly. One of the most important factors in cartilage tissue engineering is the preparation of a scaffold (29). To achieve an optimal scaffold in cartilage tissue engineering, development and application of scaffolds derived from natural and synthetic materials have been performed. So far, various decellularization methods have been suggested to develop extracellular matrix derived scaffolds (30, 31). To prevent the immune response, it is essential that antigenic epitopes are removed from intra and extracellular matrix (32, 33). Several investigations have reported that snap freeze-thaw cycles followed by treatment with various detergents would lead to removal of cellular components from tissues (19). In this study, in order to decellularize the articular cartilage, a combination of both physical and chemical methods was used. Snap freeze-thaws cycling in liquid nitrogen were used for 5 cycles as the physical method. Studies have shown that although the freeze-thaw cycling disrupts the cells and intracellular proteins, some cellular components such as cell nuclei would not be removed by this process and treatment with other reagents is necessary to remove cell components completely (34). [AGA2]Several detergent based methods are developed to decellularize various tissues and organs (35). The combination of detergent treatments with freeze-thaw shows a synergistic effect on removal of cellular components and leads to a greater success in decellularization (5, 19, 36, 37). In this study, treatment with 2.5% SDS for different time periods was employed to achieve the best elimination of cellular components with preservation of extra-cellular contents. [AGA3]Although using 2.5% SDS for 1 hr did not damage the extracellular matrix, many cellular components were remained in the tissue. On the other hand, treatment with 2.5% SDS for 4 hr led to complete omission of cellular components with perseveration of extracellular matrix contents, while 8 hr treatment led to the loss of histochemical characteristics of the ECM.
Studies have demonstrated that removal of DNA is relatively difficult compared to other intracellular components due to its sticky properties and strong tendency to adhere to ECM proteins (17). DNA remaining after decellularization process might be a reason for inflammatory reactions following implantations (38, 39). Thus, it is important to use more specific methods for detection of DNA. DAPI staining demonstrated complete elimination of DNAs from the obtained decellularized cartilage tissue. Furthermore, microstructures of our obtained scaffolds were analyzed and compared with native articular cartilage using electron microscopy and confirmed elimination of cells from the decellularized tissues. Therefore, it can be concluded that a combination of freeze-thaw cycles and treatment with 2.5% SDS for 4 hr would lead to a maximal removal of cells along with relative preservation of GAGs and collagen contents of the cartilage ECM.
In order to assess the ability of obtained ECM derived scaffold to support the attachment and growth of the cells, rat BM-MSCs were seeded onto the scaffolds. Using these cells has several advantages including the presence of non-invading methods for their harvest (especially from adipose tissue), and also they have great potential for differentiation into osteocytes, chondrocytes and adipocytes (40). These cells were initially labeled with a cytoplasmic fluorescent dye and DiI and then were used to seed the scaffolds. Methylene blue staining of scaffolds after 24 hr of culture confirmed the existence of living cells on the scaffolds, which can be observed as blue spots. SEM micrographs showed the adhesion of BM-MSCs onto the scaffold. Maintenance of seeded cells was also demonstrated during longer culture periods. It is interesting that most of the seeded cells were located within the lacuna that might indicate the ability of BM-MSCs to adhere and adapt to the scaffold. These results are in agreement with Yang et al study who demonstrated the adhesion, proliferation and differentiation of seeded MSCs labeled with fluorescent dye PKH26, to chondrocytes on decellularized human joint scaffold (5).
Conclusion
An effective method for decellularization of each tissue and organ depends on several factors such as thickness, density and cellularity of the tissue. Any change in the ECM characteristics due to decellularization process may have effect on behavior of seeded cells. Therefore, more investigations are required to optimize the preparation process of decellularized scaffolds for tissue engineering applications.
Acknowledgment
The authors gratefully acknowledge Mrs M Saeinasab for her great help and support. This work was supported by Ferdowsi University of Mashhad, Mashhad, Iran [grant numbers 33237].
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Gong YY, Xue JX, Zhang WJ, Zhou GD, Liu W, Cao Y. A sandwich model for engineering cartilage with acellular cartilage sheets and chondrocytes. Biomaterials. 2011;32:2265–2273. doi: 10.1016/j.biomaterials.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 3.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834–3842. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Bolland F, Korossis S, Wilshaw SP, Ingham E, Fisher J, Kearney JN, et al. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials. 2007;28:1061–1070. doi: 10.1016/j.biomaterials.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–2387. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Brown BN, Barnes CA, Kasick RT, Michel R, Gilbert TW, Beer-Stolz D, et al. Surface characterization of extracellular matrix scaffolds. Biomaterials. 2010;31:428–437. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenke-Layland K, Vasilevski O, Opitz F, Konig K, Riemann I, Halbhuber KJ, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143:201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt CE, Baier JM. Acellular vascular tissues:natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25:2679–2686. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 10.Borschel GH, Dennis RG, Kuzon WM., Jr Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Reconstr Surg. 2004;113(2):595–602. doi: 10.1097/01.PRS.0000101064.62289.2F. [DOI] [PubMed] [Google Scholar]
- 11.Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–140. doi: 10.1002/(sici)1097-4636(200001)49:1<134::aid-jbm17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 13.Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46:396–400. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Yoo JJ, Atala A. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology. 1999;54:407–410. doi: 10.1016/s0090-4295(99)00179-x. [DOI] [PubMed] [Google Scholar]
- 15.Freytes DO, Badylak SF, Webster TJ, Geddes LA, Rundell AE. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25:2353–2361. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 19.Stapleton TW, Ingram J, Katta J, Knight R, Korossis S, Fisher J, et al. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng Part A. 2008;14:505–518. doi: 10.1089/tea.2007.0233. [DOI] [PubMed] [Google Scholar]
- 20.Lumpkins SB, Pierre N, McFetridge PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4:808–816. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering:the application of stem cell technology. Curr Opin Biotechnol. 2005;16:503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risbud MV, Shapiro IM. Stem cells in craniofacial and dental tissue engineering. Orthod Craniofac Res. 2005;8:54–59. doi: 10.1111/j.1601-6343.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 24.Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Review:application of stem cells for vascular tissue engineering. Tissue Eng. 2005;11:1535–1552. doi: 10.1089/ten.2005.11.1535. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 26.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 27.Ghasroldasht MM, Irfan-Maqsood M, Matin MM, Bidkhori HR, Naderi-Meshkin H, Moradi A, et al. Mesenchymal stem cell based therapy for osteo-diseases. Cell Biol Int. 2014;38:1081–1085. doi: 10.1002/cbin.10293. [DOI] [PubMed] [Google Scholar]
- 28.Neshati Z, Matin MM, Bahrami AR, Moghimi A. Differentiation of mesenchymal stem cells to insulin-producing cells and their impact on type 1 diabetic rats. J Physiol Biochem. 2010;66:181–187. doi: 10.1007/s13105-010-0013-y. [DOI] [PubMed] [Google Scholar]
- 29.Chen WC, Yao CL, Chu IM, Wei YH. Compare the effects of chondrogenesis by culture of human mesenchymal stem cells with various type of the chondroitin sulfate C. J Biosci Bioeng. 2011;111:226–231. doi: 10.1016/j.jbiosc.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Jia S, Liu L, Pan W, Meng G, Duan C, Zhang L, et al. Oriented cartilage extracellular matrix-derived scaffold for cartilage tissue engineering. J Biosci Bioeng. 2012;113:647–653. doi: 10.1016/j.jbiosc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Benders KE, Weeren PR, Badylak SF, Saris DB, Dhert WJ, Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. T Trends Biotechnol. 2013 doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Erdag G, Morgan JR. Allogeneic versus xenogeneic immune reaction to bioengineered skin grafts. Cell Transplant. 2004;13:701–712. doi: 10.3727/000000004783983594. [DOI] [PubMed] [Google Scholar]
- 33.Ross JR, Kirk AD, Ibrahim SE, Howell DN, Baldwin WM, 3rd, Sanfilippo FP. Characterization of human anti-porcine “natural antibodies” recovered from ex vivo perfused hearts--predominance of IgM and IgG2. Transplantation. 1993;55:1144–1150. doi: 10.1097/00007890-199305000-00040. [DOI] [PubMed] [Google Scholar]
- 34.Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62. doi: 10.1016/j.jmbbm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Meezan E, Hjelle JT, Brendel K, Carlson EC. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life sciences. 1975;17:1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- 36.Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells:a novel composite for tendon engineering. J Orthop Res. 2009;27:937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahabipour F, Mahdavi-Shahri N, Matin MM, Tavassoli A, Zebarjad SM. Scaffolds derived from cancellous bovine bone support mesenchymal stem cells’ maintenance and growth. In Vitro Cell Dev Biol Anim. 2013;49:440–448. doi: 10.1007/s11626-013-9591-7. [DOI] [PubMed] [Google Scholar]
- 38.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA:possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 39.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Maeda S, Fujitomo T, Okabe T, Wakitani S, Takagi M. Shrinkage-free preparation of scaffold-free cartilage-like disk-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng. 2011;111:489–492. doi: 10.1016/j.jbiosc.2010.11.022. [DOI] [PubMed] [Google Scholar]


