Abstract
Objective(s):
Sub-health has been described as a chronic condition of unexplained deteriorated physiological function, which falls between health and illness. In the present study, we evaluated the effects of Lycium barbarum polysaccharide (LBP), a polysaccharide fraction purified from Lycium barbarum (L. barbarum) on the sub-health mice.
Materials and Methods:
The sub-health model mice were built through compound factors. The mice were given intragastric administration of LBP at low dose (50 mg•kg-1) and high dose (100 mg•kg-1), respectively. After LBP treatment for 4 weeks, the antioxidant ability, enhancing immune function and anti-fatigue activity were detected.
Results:
The results showed that LBP could enhance antioxidant ability in sub-health mice. LBP could effectively improve immunity of sub-health mice and protect the immune organs, such as thymus. In addition, LBP showed anti-fatigue ability in sub-health mice.
Conclusion:
LBP could improve sub-health state caused from composite factor through three aspects, such as increasing antioxidant ability, promoting T lymphocyte proliferation, inhibiting thymus lymphocyte apoptosis, and alleviating fatigue.
Keywords: Anti-fatigue, Antioxidant, Immunity, Lycium bararum, Polysaccharide, Sub-health
Introduction
Sub-health was defined as a kind of intermediate state between health and disease. The symptoms of sub-health are mainly fatigue, insomnia, increased incidence of infections and decreased immunity (1). Sub-health has become today’s invisible killer for human health; therefore, there is an increasing need for scientifically validated therapies that can effectively and simply treat sub-health symptoms. The prevention and cure for sub-health are the common concern among traditional Chinese and western medicine (2). In this study, the sub-health mice were built by compound factors including swimming exercise, sleep deprivation and clamp tail stimulus to mimic the cause and symptoms of sub-health.
Lycium barbarum has been widely used to promote health and longevity in China. L. barbarum polysaccharide (LBP), the major active ingredient extracted from L. barbarum, has been found bioactivities such as enhancing the body’s immune capacity and lowering blood glucose (3, 4). We had previously shown that LBP was heteropolysaccharide and contained different carbohydrate compositions (5). However, so far little is known about the effects of LBP on sub-health mice. Fatigue is one of the principal manifestations and symptoms of sub-health, and that, recent evidence suggests that oxidative stress and immune alterations are important in sub-health (6, 7). Based on this framework, we investigated the effects of LBP on antioxidant, immunity, and fatigue in sub-health mice and relevant mechanisms. The experimental results may provide comprehensive, scientific evidence for LBP as a suitable dietary natural agent for sub-health improvement.
Materials and Methods
The fruits of L. barbarum were collected in the Ningxia Hui Autonomous Region, which was the Well-known production area of L. barbarum in China, and were authenticated at the agricultural college of Northwest A&F University. A specimen (NO.20110609) was deposited in the herbarium of the Botany Department. Superoxide dismutase (T-SOD), catalase (CAT), malondialdehyde (MDA), serum urea nitrogen and hepatic glycogen kits were purchased from Nanjing Jiancheng Biotechnology Company. MTT and concanavalin A (ConA) were from Sigma Company. Hematoxylin and eosin (H&E) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). All other reagents and chemicals were of the highest purity grade available.
Preparation of polysaccharide (LBP) from Lycium barbarum
Isolation, purification and identification of LBP were based on our previous published work (8). Briefly, the dried fruit samples were refluxed three times to remove lipids with chloroform: methanol solvent (2:1) (v/v). After filtering, the residues were air-dried and then refluxed again with 80% ethanol. The residues were extracted three times in hot water (90 °C) and filtered. The combined filtrate was precipitated with 95% ethanol, 100% ethanol and acetone, respectively. After filtering and centrifuging, the precipitate was collected and vacuum-dried, giving crude polysaccharides (yield was 3.25 %±0.14%, and the purity of LBP was 95.8 %±2.0%). The crude polysaccharides obtained were then passed through a DEAE cellulose column (OH-, ø25 mm×350 mm) and were further purified by Sephadex G-100 column (ø26 mm×400 mm). LBP was identified to be a homogeneous polysaccharide component, which showed a single symmetrical peak following Sephadex G-100 gel chromatography. The molecular weight (MW) of LBP was 33,867 Da, and retention time was 8.257 min by HPLC. In addition, the monosaccharide composition of LBP was analyzed by paper chromatography and revealed the presence of six spots, corresponding to galactose, glucose, rhamnose, arabinose, mannose, and xylose respectively. According to the IR spectrum, the purified LBP displayed a broadly stretched, intense peak at 3,428 cm-1 characteristic of hydroxyl group and a weak C-H peak at around 2,915 cm-1. The relatively strong absorption peak at around 1,710 cm-1 indicated the carbonyl group. The absorbance of polysaccharides in the range 1,000 to 1,200 cm-1 was the C-O-C and C-O-H link band positions. LBP were protein-bound polysaccharides. The backbone of sugar residues chain in LBP contained 1→6 indican bond according to periodate oxidation, and the results of β-elimination reaction indicated that the chain of polysaccharides and protein were connected by O-linked chemical bond (8).
Preparation of sub-health model and treatments
Forty male Kun-ming mice (initial body weight, 18-22g; 4 weeks old) were obtained from medical sciences laboratory animal center of Changchun University. They were housed in an animal room at 22±2 °C and 50±10% relative humidity and had free access to laboratory chow and tap water. The mice were adapted to an inverse 12:12-hr light-dark cycle. The animals were treated according to the National Institute of Health Guide for the Care and Use of Laboratory Animals, and further approval for their experimentation was obtained from the Animal Ethics Committee of the university.
Ten mice were randomly selected as normal control group, and the rest were used for preparing sub-health model. Model mice were built through mimicking the factors of fatigue-type sub-health (9, 10). In brief, the mice were placed in a swimming pool with the diameter of 70 cm and depth of 30 cm, keeping the water temperature at 25 °C. The mice were forced to swim for 50 min at 8 a.m. and 4 p.m. every day. The mice were deprived of sleep at the rest of the time until 8 p.m. Since the third week, the mice were put in tubes for 4 hr a day, for a total of 7 day. Since the fourth week, the mice were clamped tail using hemostatic forceps wrapped gauze at the cutting-edge. The degree of fatigue in mice was assessed by exhaustive swimming experiments (25 °C water temperature; 7% with lead weights on tail; swimming exhaustion standard: mice nasal tip sank underwater for l0 sec). Sub-health mice were randomly assigned to three groups of ten mice each: model group, low dose group (50 mg·kg-1) and high dose group (100 mg·kg-1) (the doses were chosen according to our previous study). Mice were treated by intragastric administration with LBP dissolved in normal saline, and model group mice received normal saline for 4 weeks.
Determination of antioxidant ability
After the last treatment of LBP for 24 hr, the blood of the mice in all the groups from eyeball was obtained under anesthesia with ether. The blood was let stand at 37 °C for 1 hr, and 4 °C overnight. The upper serum was taken after centrifuge at 4000 rpm for 10 min, and the activities of T-SOD, CAT and the level of MDA were measured using commercial kit. The manipulation was progressed strictly according to the kit instruction manual.
Determination of immune function
Effect of LBP on spleen and thymus gland index After the last treatment of LBP for 24 hr, all mice in the groups were executed, and then the thymus and spleen tissue were gathered quickly under aseptic conditions for weighing. The thymus gland and spleen index (mg·10 g-1) was calculated as follows: thymus glands (spleen) weight/body weight×10.
Spleen lymphocyte transformation test of mice induced ConA
Spleens were harvested from all mice groups under sterile conditions and mechanically homogenized into suspensions followed by treatment with an erythrocyte lysis buffer. Cell suspensions were adjusted to 3×106 cells/ml after the cells were washed with Hank ’s liquid and suspended once again with 1640 completely culture. Each spleen cell suspensions were divided into two holes and added in 24 hole [AGA1]culture plate with 1 ml per hole. 75 μl ConA was added in one hole and another hole acted as control. Four hours before the end of incubation in the incubator (37 °C, 5% CO2), 0.7 ml the supernatant of each hole was obtained and 0.7 ml RPMI 1640 culture was added. At the same time, 50 μl MTT was added (5 mg·ml -1) and continued to culture for 4 hr. At the end of culture, 1 ml isopropyl alcohol [AGA2]was added in each hole and shaken to uniformity for making purple crystal dissolve completely. And then the culture was divided into 96 - well culture plate, and three parallel holes were set for each hole [AGA3](75 μl/hole). The optical density (OD) values were measured at 570 nm. The results were indicated using Stimulation Index (SI) (11).
SI = stimulation hole (ConA) OD value/control hole OD value.
Determination of thymus tissue structure
The harvested thymus of all groups were fixed overnight in 4% formalin at 4 °C, embedded in paraffin and cut into 4 μm sections. The sections were stained with H&E staining method, and histological examinations were carried out by light microscopy.
Apoptosis detection of thymus tissue by TUNEL staining
The thymus tissues from the experimental mice were examined by TUNEL assay to detect 3′ free hydroxyl ends (3-OH) of DNA strands created by nucleases in apoptotic cells. Sections of thymus tissues were dewaxed and rehydrated according to standard protocols and treated for 20 min with Proteinase K (20 μg·ml-1 in phosphate-buffered saline (PBS)). After rinsing with PBS for twice, TUNEL reaction mixture was added on each sample, and incubated for 60 min at 37 °C in a humidified atmosphere in the dark, then slide rinsed three times with PBS, and finally the samples were observed under a fluorescence microscope. The cells whose nucleus turned distinct green were considered as positive (apoptotic) cells.
Determination of anti-fatigue activity Weight loading swimming test
After the last treatment of LBP for 30 min, the mice (5% with lead weights on tail) were placed in a swimming pool with the diameter of 70 cm and depth of 30 cm, keeping the water temperature at 25 °C. The time was recorded since mice nasal tip sank underwater for l0 sec.
Measurement of serum urea nitrogen
After the last treatment of LBP for 30 min, the mice (0% with lead weights on tail) were placed in the swimming pool keeping the water temperature at 25 °C for 30 min. After resting for 15 min, eyeball blood was taken, and centrifuged to obtain serum. The content of serum urea nitrogen was tested using serum urea nitrogen Test kits.
Measurement of hepatic glycogen
After the last treatment of LBP for 30 min, the mice were executed immediately, and the livers were obtained. Taking 100 mg liver, the liver glycogen was measured using commercial kit.
Statistical analysis
All the results were expressed as the mean±SD P-values of less than 0.05 were considered to be significant. Statistical analysis was performed by one-way analysis of variance (ANOVA). All the grouped data were statistically evaluated with SPSS 13.0 software. Statistical significance of differences between two groups was determined using the student’s t-test.
Results
Evaluation of sub-health mice model
Compared with normal control group mice, sub-health mice were irritable and biting each other. After two weeks, the model mice showed mental burnout, fur loss and missing luster[a4]. As showed in Table 1, compared with the normal control, the model mice had weight loss, and decreased independent activity and the times of avoiding electric shock stimulation were significantly decreased. Furthermore, the accumulative total immobile time of the mice tail suspension within 5 min was prolonged, but the weight loading swimming exhaustion time was shorten. The above results were consistent with basic features of sub-health.
Table 1.
Behavior evaluation of sub-health mice model (Mean±S.D.)
| Groups | n | Body weight (g) | Autonomic activity times (x) | Escaping darkness times(x) | Accumulative immobile time within 5 min (second) | Exhaustion time (min) |
|---|---|---|---|---|---|---|
| Normal Control | 10 | 28.47±2.16 | 125±11 | 2.3±0.32 | 19.10±5.18 | 27.31±8.32 |
| Model | 40 | 19.35±2.02* | 93±17* | 1.09±0.21** | 65.62±8.32* | 14.55±5.62* |
Compared with control.
P<0.05.
P<0.01.
Effects of LBP on the activities of T-SOD, CAT and the level of MDA in sub-health mice
To investigate whether LBP exhibited improved effects on sub-health mice, we first examined if LBP had antioxidant activity. Compared with control group, the activities of T-SOD and CAT in model group were significantly decreased, while the level of MDA was significantly increased (P<0.01). As showed in Figure 1, compared with the model group, the activities of T-SOD and CAT in high dose group were increased significantly (P<0.01) after LBP treatment for 4 weeks, whereas the content of MDA was decreased significantly (P<0.05).
Figure 1.
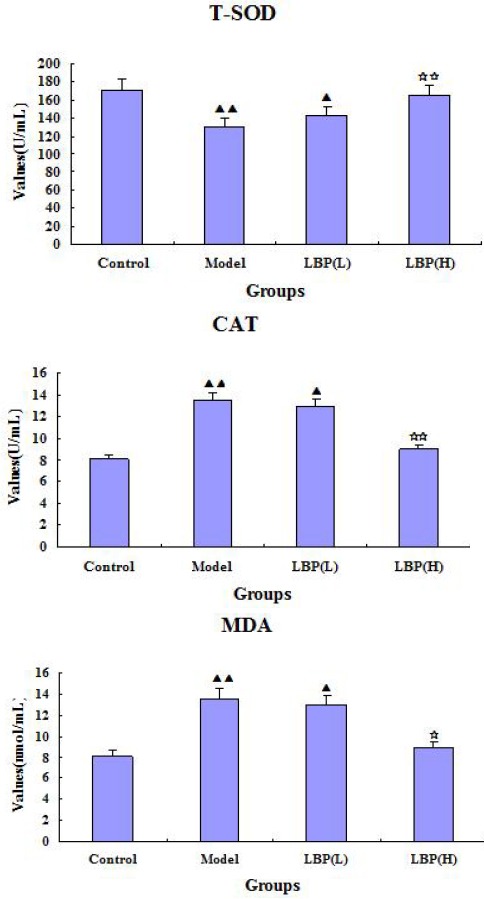
Effect of Lycium bararum polysaccharide (LBP) on activities of superoxide dismutase (T-SOD), catalase (CAT) and level of malondialdehyde (MDA) in serum of sub-health and control mice. Compared with control, ▲P<0.05 ▲▲P<0.01 Compared with model, ☆P<0.05 ☆☆P<0.01. The dose in LBP (L) treated group is 50 mg•kg-1, and the dose in LBP (H) treated group is 100 mg.kg-1
Effects of LBP on the immune function in sub-health mice
To investigate whether LBP exhibited improved effects on sub-health mice, we examined if LBP could enhance immune function. As showed in Table 2, the thymus index, spleen index and spleen lymphocyte transformation ability in model group were significantly lower than that in the control group. Compared with model group, the thymus index and spleen index in the high dose group of LBP were higher (P<0.05), and the spleen lymphocyte transformation ability was increased. These results indicated that LBP treatment could improve cellular immunity in sub-health mice.
Table 2.
Effect of Lycium bararum polysaccharide (LBP) on immunity in sub-health mice (±SD, n=10)
| Groups | Thymus index (mg·10g-1) | Spleen index (mg·10g-1) | Proliferation OD difference |
|---|---|---|---|
| Control | 18.12±2.31 | 60.45±9.67 | 0.363±0.048 |
| Model | 12.97±1.85▲▲ | 49.34±8.71▲▲ | 0.182±0.052▲▲ |
| LBP (50 mg·kg-1) | 12.32±1.35▲▲ | 50.89±6.62▲▲ | 0.193±0.035▲▲ |
| LBP (100 mg·kg-1) | 17.04±3.27* | 58.78±9.25* | 0.357±0.048* |
Compared with control, ▲P<0.05.
P<0.01.
Compared with model.
P<0.05.
LBP protected the thymus against sub-health mice
As showed in Figure 2, histological sections of the thymus revealed extensive melioration in LBP(H) group mice compared with model group mice. In the model group, the disruption of thymic architecture could be observed both in the generalized tissue as well as in the medullary region. Disruption of the medullary region could be vital for T cell development, as it is the principle region where mature thymocytes survive and accumulate. However, this damage was meliorated considerably by the LBP(H) treatment, and the thymus could allow a more stable environment for T cell development.
Figure 2.
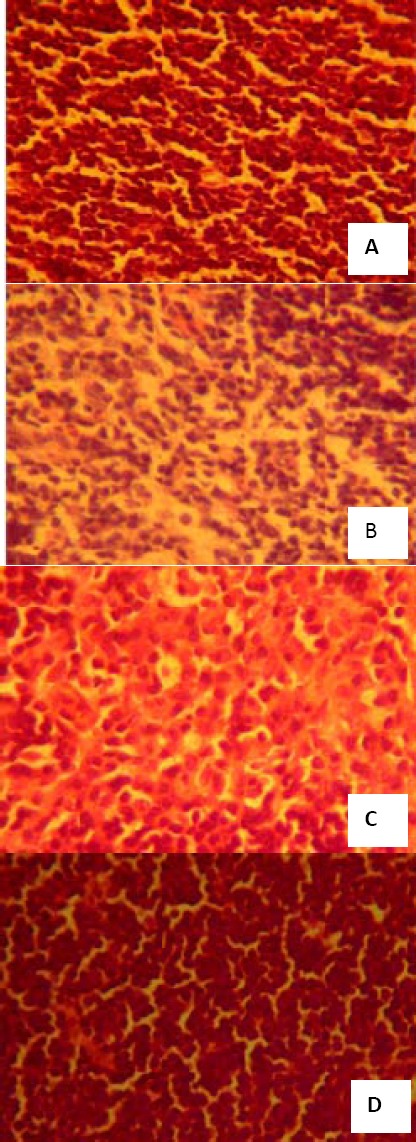
Effect of Lycium bararum polysaccharide (LBP) on thymus structure of sub-health mice (amplificatory rate, 40×10). (A) microstructure of thymus tissue of control group; (B) microstructure of thymus tissue of sub-health group; (C) microstructure of thymus tissue of LBP (L) treated group; (D) microstructure of thymus tissue of LBP (H) treated group. The dose in LBP (L) treated group is 50 mg•kg-1, and the dose in LBP (H) treated group is 100 mg•kg-1
LBP induced thymus lymphocyte survival
To assess the protective role of LBP against thymocytes apoptosis in sub-health mice, we examined the spontaneous apoptosis rate in the thymus tissue of different groups with TUNEL staining. The thymus sections of sub-health group showed that a large number of cells underwent apoptosis, and sections of thymus in LBP(H) treated mice had a significant decrease in the number of positive cells with TUNEL staining(Figure 3). So, LBP could protect thymus tissue by inhibiting apoptosis of thymus lymphocytes.
Figure 3.
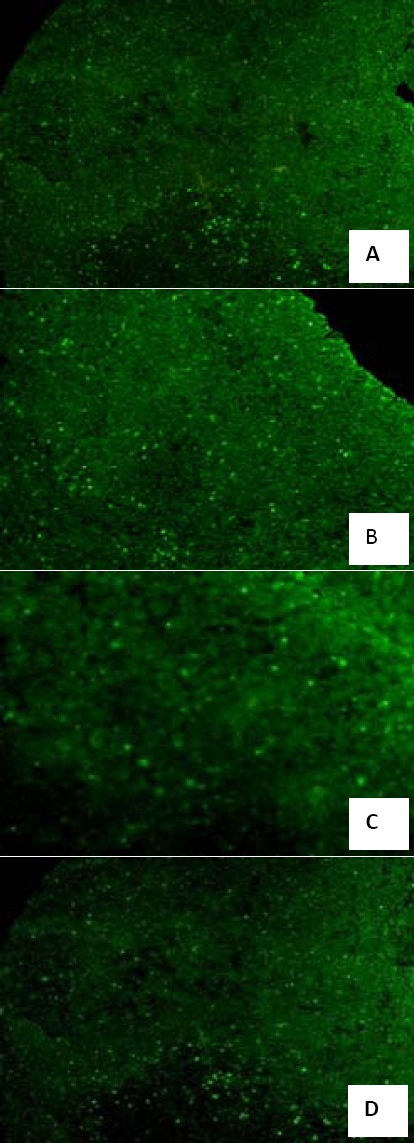
Effect of Lycium bararum polysaccharide (LBP) on apoptosis number of thymus cells in sub-health mice (amplificatory rate, 10×10). (A) TUNEL staining of thymus tissue of control group; (B) TUNEL staining of thymus tissue of sub-health group; (C) TUNEL staining of thymus tissue of LBP (L) treated group; (D)TUNEL staining of thymus tissue of LBP (H) treated group. The dose in LBP (L) treated group is 50 mg•kg-1, and the dose in LBP (H) treated group is 100 mg•kg-1
Effects of LBP on the anti-fatigue function in sub-health mice
Fatigue is one of the main manifestations of sub-health. To further demonstrate the effects of LBP on sub-health, we examined if LBP could relieve fatigue. As showed in Table 3, the weight loading swimming time and the content of hepatic glycogen in model group were significantly lower than that in control group, but the content of serum urea nitrogen was significantly higher than that of control group. Compared with model group, the weight loading swimming time in LBP high dose group had significant difference (P<0.01). The values of urea nitrogen in LBP high dose group were significantly decreased, but the content of hepatic glycogen was significantly increased compared to that in the model group (P<0.05). These results indicated that LBP treatment could extend the weight loading swimming time, and reduce the content of serum urea nitrogen in sub-health mice.
Table 3.
Effect of Lycium bararum polysaccharide (LBP) on swimming time of weight loading and serum urea nitrogen and liver glycogen in sub-health and control mice (Mean±SD, n=10)
| Groups | Swimming time of weight loading (min) | Serum urea nitrogen (mmol·L-1) | Hepatic glycogen (mg·g-1) |
|---|---|---|---|
| Control | 18.37±2.36 | 6.04±1.38 | 6.94±1.35 |
| Model | 9.25±1.31▲▲ | 11.46±3.25▲▲ | 4.95±1.16▲ |
| LBP (50 mg·kg-1) | 9.42±1.26 | 10.96±2.31 | 5.23±1.21 |
| LBP (100 mg·kg-1) | 17.79±2.41** | 7.18±1.44* | 6.75±1.52* |
Compared with control.
P<0.05.
P<0.01.
Compared with model.
P<0.05.
P<0.01.
Discussion
It is pointed out in WHO “Meeting the challenges in the 21st century” that the medical science should not continue with diseases as the main research areas in the 21st century, but rather human health to be as the main research directions. It will become key issues that correct understanding for the connotation of health, maintaining human health status, intervening sub-health state, and reducing the incidence of disease in the future. To date, many drugs have been developed and applied in clinic for sub-health treatment. However, the effect is not satisfactory. L. barbarum has been used as a traditional herbal medicine for thousands of years in China. Its therapeutic activities have been well established in the treatment of conditions such as consumptive disease accompanied by thirst (includes early-onset diabetes and tuberculosis), dizziness, blurred vision, and chronic cough (12, 13). And that, it has a good effect on enhancing immunity, and has anti-aging and anti-tumor properties (14).
In this study, the sub-health mice were built by compound factors including swimming exercise, sleep deprivation and clamping tail stimulus to mimic the cause and symptoms of sub-health. After four weeks, the model mice showed basic features of sub-health, such as mental burnout, memory losing, physical strength degression, and reduced survival desire. The results indicated that the building model methods with compound factors could affect growth and metabolism of mice.
Growing evidence indicates that excessive exercise could produce reactive oxygen species (ROS) (15). Oxidative stress is manifested by an elevation of the lipid peroxidation product such as MDA, and by a reduction of CAT and SOD levels. So, we investigated the potential effects of treatment with LBP against oxidative damage induced on sub-health mice. SOD and CAT are important antioxidant enzymes in the body. The ability for removing free radicals is reflected indirectly by the activity of these enzyme (16, 17). MDA is a degradation product of lipid peroxidation, and its quantity is indicative of free radical-induced cell damage (18). The experimental results showed that the activities of T-SOD and CAT were increased in high dose group of LBP; however, the MDA level in high dose group of LBP was significantly decreased. It indicated that LBP could enhance antioxidant capacity in sub-health mice, and the effect was better in the high dose group.
Sub-health condition in patients is often associated with weakness in spleen and kidney. In addition, the ability of antibody formation in spleen cells is decreased, and leukocyte migration inhibition index is increased, which leads to declining in immune function (19). T lymphocytes are transformed into lymphoblasts after they receive specific antigens stimulation such as ConA. It reflects the level of cellular immune function. Spleen lymphocyte transformation test is a kind of in vitro experiment for detection of cellular immunity function, and the conversion of high or low [a5]can directly reflect the organism cellular immunity function. The experimental results showed that T lymphocyte proliferation was significantly reduced in sub-health mice. It is suggested that cellular immune function is declined in sub-health mice because of psychological stress response caused long-term restrictions on the activities. After LBP treatment, T lymphocyte proliferation was improved in sub-health mice. It indicated that LBP could effectively improve immunity of sub-health mice. To further elucidate the protective effect of LBP on immune function, in the present study, it was found that the thymus of sub-health mice were seriously damaged, and TUNEL staining further revealed that apoptosis was one of the cell death pathway in thymus lymphocytes. These data contributed a new finding that LBP could protect the immune organ—thymus.
The sugar is the main energy material during the long and high-intensity exercise. A lot of glycogen consumption and the accumulation of urea and other metabolites caused the occurrence of fatigue in body (20, 21). At present, the research shows that fatigue is one of the main signs and typical performance for sub-health. Urea nitrogen is the metabolism product of amino acid and protein. Blood urea nitrogen levels will increase significantly when the body is in a state of high movement and high metabolic rates. Furthermore, the body’s blood urea nitrogen increased with the increase of the labor and exercise load. In a word, the body’s adaptability to the load was worse, and the increase of blood urea nitrogen was more obvious in sub-health mice. The experimental results showed that LBP could improve prolong swimming time in mice, and lower the levels of urea nitrogen. The above results suggested that LBP possessed anti-fatigue effect, but the mechanism still needed further research.
Conclusion
To sum up, LBP improved sub-health state in three different ways including increasing antioxidant ability, enhancing the immune function, and relieving fatigue.
Acknowledgment
This research was supported by the China Postdoctoral Science Foundation of China (grant no. 2014M551281). The results described in this paper were part of student thesis.
Conflict of Interest statement
The authors declare that they have no conflict of interest.
References
- 1.Dunstan RH, Sparkes DL, Roberts TK, Crompton MJ, Gottfries J, Dascombe BJ. Development of a complex amino acid supplement, Fatigue Reviva™, for oral ingestion:initial evaluations of product concept and impact on symptoms of sub-health in a group of males. Nutr J. 2013;12:115–123. doi: 10.1186/1475-2891-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Russell A, Yan Y. Global Health Epide-miology Reference Group (GHERG). Traditional Chinese medicine and new concepts of predictive, preventive and personalized medicine in diagnosis and treatment of suboptimal health. EPMA J. 2014;5:4–13. doi: 10.1186/1878-5085-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huyan T, Li Q, Yang H, Jin ML, Zhang MJ, Ye LJ, et al. Protective effect of polysaccharides on simulated microgravity-induced functional inhibition of human NK cells. Carbohydr Polym. 2014;101:819–827. doi: 10.1016/j.carbpol.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Chen JR, Li EQ, Dai CQ, Yu B, Wu XL, Huang CR, et al. The inducible effect of LBP on maturation of dendritic cells and the related immune signaling pathways in hepatocellular carcinoma (HCC) Curr Drug Deliv. 2012;4:414–420. doi: 10.2174/156720112801323107. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Li QW, Li J, Zhang T. Protective effect of Lycium barbarum polysaccharide 4 on kidneys in streptozotocin induced diabetic rats. Can J Physiol Pharmacol. 2009;87:711–719. doi: 10.1139/y09-068. [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Naidu PS, Gupta S, Kulkarni SK. Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome. J Med Food. 2002;5:211–220. doi: 10.1089/109662002763003366. [DOI] [PubMed] [Google Scholar]
- 7.Rajeevan MS, Dimulescu I, Murray J, Falkenberg VR, Unger ER. Pathway-focused genetic evaluation of immune and inflammation related genes with chronicfatigue syndrome. Hum Immunol. 2015;15:00180–9. doi: 10.1016/j.humimm.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhao R, Qiu B, Li Q, Zhang T, Zhao H, Chen Z, et al. LBP-4a improves insulin resistance via translocation and activation of GLUT4 in OLETF rats. Food Funct. 2014;5:811–820. doi: 10.1039/c3fo60602c. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Sun CX, Zheng Y, Pan H, Zhou Y, Fan Y. The effective mechanism of the polysaccharides from Panax ginsengon chronic fatigue syndrome. Arch Pharm Res. 2014;37:530–538. doi: 10.1007/s12272-013-0235-y. [DOI] [PubMed] [Google Scholar]
- 10.Li YP, Huang SH, Jin W. Construction of a sub-health fatigue rat model. Chin J Tissue Engin Res. 2011;7:1225–1228. [Google Scholar]
- 11.Jiang CM, Chen XL, Lu GF, Chen XY, Wang DY, Hu YL, et al. Comparative study on chicken peripheral lymphocyte and spleen lymphocyte proliferation by Chinese herbal medicinal polysaccharides in vitro. Jiangsu J of Agr Sci. 2015;31:106–111. [Google Scholar]
- 12.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 13.Tian DH. Practical thesaurus of Chinese traditional medicine. Beijing: People’s Medical Publishing House; 2002. pp. 1348–1354. [Google Scholar]
- 14.Wang J. Pharmacological action of Lycium barbarum and the analysis of clinical application value. Asia-Pacific Traditional Med. 2014;10:50–51. [Google Scholar]
- 15.Powers SK, Jackson MJ. Exercise-induced oxidative stress:cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekert PG, Vaux DL. The mitochondrial death squad:hardened killers or innocent bystanders? Curr Opin Cell Biol. 2005;6:626–630. doi: 10.1016/j.ceb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CYJ. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;6828:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 18.Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M, et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med. 2000;4:571–580. doi: 10.1084/jem.192.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Jin YJ. Epidemiological surveys of sub-health state and syndrome differentiation of Traditional Chinese Medicine. Chin J Clin Rehabil. 2004;9:1756. [Google Scholar]
- 20.Castro-Marrero J, Cordero MD, Sáez-Francas N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, et al. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013;15:1855–60. doi: 10.1089/ars.2013.5346. [DOI] [PubMed] [Google Scholar]
- 21.Filler K, Lyon D, Bennett J, McCain N, Elswick R, Lukkahatai N, et al. Association of mitochondrial dysfunction and fatigue:a review of the literature. BBA Clin. 2014;1:12–23. doi: 10.1016/j.bbacli.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


