Abstract
Objective(s):
Isoprenoid biosynthesis is a key metabolic pathway to produce a wide variety of biomolecules such as cholesterol and carotenoids, which target cell membranes. On the other hand, it has been reported that statins known as inhibitors of isoprenoid biosynthesis and cholesterol lowering agents, may have a direct antimicrobial effect on the some bacteria. The exact action of statins in microbial metabolism is not clearly understood. It is possible that statins inhibit synthesis or utilization of some sterol precursor necessary for bacterial membrane integrity. Accordingly, this study was designed in order to examine if statins inhibit the production of a compound, which can be used in the membrane, and whether cholesterol would replace it and rescue bacteria from toxic effects of statins.
Materials and Methods:
To examine the possibility we assessed antibacterial effect of statins with different classes; lovastatin, simvastatin, and atorvastatin, alone and in combination with cholesterol on two Gram-positive (Staphylococcus aureus and Enterococcus faecalis) and two Gram-negative (Pseudomonas aeruginosa and Escherichia coli) bacteria using gel diffusion assay.
Results:
Our results showed that all of the statins except for lovastatin had significant antibacterial property in S. aureus, E. coli, and Enter. faecalis. Surprisingly, cholesterol nullified the antimicrobial action of effective statins in statin-sensitive bacteria.
Conclusion:
It is concluded that statins may deprive bacteria from a metabolite responsible for membrane stability, which is effectively substituted by cholesterol.
Keywords: Bacteria, Hydroxymethylglutaryl coenzyme A, Statin, Sterol
Introduction
Isoprenoid biosynthesis is essential for cell survival. More than 35,000 isoprenoid molecules have been identified in three different classes of life; bacteria, archaea and eukaryotes. Membrane-associated isoprenoids molecules are involved in a wide variety of vital biological functions. Protecting pigments (e.g. carotenoids), respiratory electron carriers (e.g. quinones) and cholesterol are some examples of isoprenoids with important biological roles (1, 2). Cholesterol as a product of the isoprenoid pathway has an important role in stabilizing the membrane. It has been reported that some strains of bacteria can integrate cholesterol into their membranes (3, 4). There are growing lines of evidence confirming a relationship between the incorporation of cholesterol to bacterial membrane and membrane stability (3), resistance to environmental stresses (5), host defence (6), and antibiotics (7). In addition to that, the effect of host cholesterol on intracellular pathogens was researched in some other studies. It has been shown in one of these observations that inhibition of cholesterol biosynthesis (by simvastatin) decreases viable Chlamydophila counts in mice infected with Chlamydophila pneumoniae, an important cause of respiratory infection. The ameliorating effect of simvastatin is attributed to the dependence of bacteria on host cholesterol synthesis (which is blocked by statins) for its intracellular multiplication (8). Concluding and mentioning the evidence above, cholesterol may play an important role even in non-cholesterol synthesizing microorganisms. Simvastatin belongs to a class of cholesterol lowering drugs known as statins, which decreases cholesterol levels by competitively inhibiting hydroxymethylglu- taryl coenzyme A (HMG-CoA) reductase activity an important enzyme catalyzing the rate-limiting reaction in isoprenoids and cholesterol biosynthesis in eukaryotic and some prokaryotic cells (9, 10). Recently, direct antimicrobial effect of some statins has been reported (11, 12). Jerwood et al showed a direct antimicrobial effect of simvastatin and fluvastatin against two gram-positive bacteria (11) while Welsh used atorvastatin and rosuvastatin (12). However, a metabolic basis for the antibacterial action of statins is not currently proposed. We hypothesized that if statins block production of a membrane associated biomolecule, bacterial cells may overcome the toxic effect of statins by substitution of cholesterol; an isoprenoid derivative with membrane associated activity. In this study, we examined the impacts of cholesterol on antibacterial activities of three different kinds of statins (lovastatin; a naturally occurring statin and its synthetic derivative simvastatin, which are not active in their native form and a synthetic statin; atorvastatin) on four bacteria with different gram properties; two out of four are pigmentary bacteria.
Materials and Methods
Chemicals
Simvastatin, lovastatin, atorvastatin, antibiotics and cholesterol were purchased from pharmaceutical companies (Poorsina or Osve, Iran). Cholesterol and culture mediums were purchased from Merck (Germany).
Microorganisms
The organisms, including Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa were isolated from patients, who visited the clinical laboratory. The standard method for isolation and identification was followed. The samples were collected carefully, enriched in selective media and subjected to microscopic and biochemical identification and characterization (data can be obtained from the corresponding author).
Antimicrobial susceptibility assay
Antimicrobial activities of statins were checked by agar -gel diffusion method. The cultures were grown in nutrient broth and incubated at 37°C for 24 hr. After incubation time, a suspension of bacteria was made up to turbidity equal to that of a 0.5 McFarland standard with sterile nutrient broth. One-hundred microliters from bacterial suspension were poured into sterile Mueller-Hinton agar petri plate. The wells were bored in seeded agar. A stock solution of each statin with concentration equal to 1 mg/ml was prepared by dissolving the statin in absolute methanol. Fifty micro litters of statin solution were poured into each well. In another set of experiments, each statin was used (1 mg/ml) in combination with cholesterol, final concentration of 5 mg/ml. Standard disk diffusion method was used for assessing the antibiotic susceptibility of all the isolates using standard antibiotic disks; amikacin, cephalexin, gentamicin and ciprofloxacin. A growth control (well filled with distilled water) and a methanol control (well filled with absolute methanol) to determine whether methanol inhibits bacteria growth was included in each set. Plates were incubated at 37°C for 24 hr. After the incubation period the zone of inhibition was measured and recorded (13). Each set of experiments were performed at least three times. The results were taken to statistical analysis (t test) and expressed as Mean±SE. P-value <0.05 was considered to be statistically significant, comparing treatments with growth control.
Results
As shown in Figure 1, simvastatin and atorvastatin exhibited significant antimicrobial activity against S. aureus, E. coli, and Enter. faecalis (P-value 0.0008, 0.0009 and 0.0003 for simvastatin and 0.0001, 0.001 and 0.0001 for atorvastatin respectively). Simvastatin showed mild and atorvastatin no antimicrobial activities against P. aeruginosa. The most potent antimicrobial activity was experienced in the effect of atorvastatin and simvastatin against Enter. faecalis (P-value 0.0001 and 0.0003 respectively). Contrarily and surprisingly, lovastatin did not show any antimicrobial activities on the tested microorganisms. On the other hand, cholesterol decreased antibacterial activity of simvastatin and atorvastatin significantly in S. aureus, E. coli, and Enter. faecalis when cholesterol was applied together with statins (P-value 0.02, 0.03 and 0.0007 respectively for simvastatin and 0.003, 0.01, and 0.01 for atorvastatin) (see Figure 1 for details of inhibitory zones). There was no detectable inhibitory zone for wells filled with methanol alone (solvent control) and wells filled with distilled water (growth control). All microorganisms were susceptible to amikacin, cephalexin, gentamicin and ciprofloxacin except for P. aeruginosa, which was resistant to cephalexin.
Figure 1.
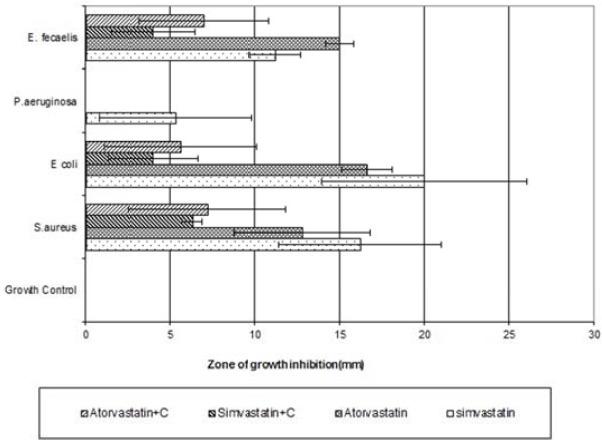
Antimicrobial activity of statins with or without cholesterol against four kinds of bacteria. Antimicrobial activity of simvastatin, atorvastatin, simvastatin plus cholesterol (simvastatin+C), and atorvastatin plus cholesterol (atorvastatin +C) were tested against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Enter. faecalis using agar gel diffusion method. Results are expressed as Mean±SE
Discussion
Statins are competitive inhibitors of HMG CoA reductase, a rate limiting step in the cholesterol biosynthesis pathway in humans (1) and purified bacterial enzyme from S. aureus (9). Recently, the direct antimicrobial effect of some statins has been reported (5, 6). However, it is not clear that how statins interfere in bacterial metabolism. We hypothesized that bacteria may need a membrane associated derivative of isoprenoid the utilization or synthesis of which is blocked by statins. Here we examined in vitro antimicrobial effect of three kinds of statins (simvastatin, atorvastatin, and lovastatin) and the impact of cholesterol in their antimicrobial effects in four bacteria with different kinds; S. aureus and Enter. faecalis as Gram-positive and E. coli and P. aeruginosa as Gram-negative bacteria. Our results showed a direct significant antibacterial effect of statins against all bacteria except for P. aeruginosa. This effect was independent of gram property of bacteria and significantly reduced for all statin sensitive bacteria by adding cholesterol.
Gram-positive bacteria like S. aureus and Enter. faecalis, which were sensitive to statins, employ HMG CoA reductase for their mevalonate dependent biosynthesis of isoprenoids (9, 10). Therefore, a probable target for statins seems to be bacterial HMG CoA reductase. However, unexpectedly, our results showed E. coli a Gram-negative bacterium represents a potent susceptibility to statins (P-value 0.0009 and 0.001 for simvastatin and atorvastatin respectively). Some Gram-negative bacteria synthesize isoprenoids from the mevalonate independent pathway, so they presumably do not need HMG CoA reductase (11, 12). Moreover, lovastatin did not show any antimicrobial activities, even in gram-positive bacteria while its inhibitory effect on purified bacterial enzyme has been postulated previously (13). These evidences show that HMG CoA reductase may not be the only target for statins, especially, when we know that simvastatin and lovastatin need to be activated in the human body to exert their enzyme inhibitory activity. On the other hand, our results showed that cholesterol significantly decreased antimicrobial activity of statins in all statin-sensitive bacteria. Although, inhibition of HMG CoA reductase by statins cannot be excluded, it is also possible that statins deprive bacterial cell membrane of a metabolite with membrane activity that can be substituted by cholesterol. Incorporation of cholesterol into some pathogenic and commensal bacterial cell membranes has been reported (3-5, 14, 15). This effect seems to benefit bacteria through strengthening the bacterial membranes (3, 14) leading to an increment of bacterial resistance to antibiotics (7) and unfavorable environmental conditions (5, 6). On the other side, hopanoids as triterpenoids derivatives with some close structural and biosynthetic similarity to cholesterol are thought to be bacterial surrogates for eukaryotic sterols and possess a membrane-stabilizing function very similar to that of sterols (16, 17). Blockage of hopanoid biosynthesis in bacteria (Burkholderia cenocepacia) results in increased sensitivity to various antibiotics (18). A similar role is presumed for carotenoids. Prevention of carotenoid biosynthesis in Mycoplasm a inhibits growth of steel-nonrecurring species. Surprisingly, this inhibition is reversed by exogenous cholesterol. In other words, cholesterol can effectively replace the end product of polyterpene biosynthesis (19). The evidences clearly show that cholesterol can replace some of membrane stabilizing molecules. In the light of these evidences, we concluded that there is a relationship between the antimicrobial effect of statins and a sterol. Statins may inhibit an intermediate in the isoprenoid biosynthesis pathway necessary for membrane stability, which is substituted by cholesterol and protects bacteria from the toxic effect of statins. Statins may have double clinical benefits, killing bacteria by direct action and by lowering accessible host cholesterol for bacterial growth.
Conclusion
Some bacteria need a sterol-like molecule to survive against environmental stresses. Statins may deprive bacteria from a metabolite responsible for membrane stability, which is effectively substituted by cholesterol. In this manner, bacteria resist the toxic effect of statins. This is a new way to kill bacteria.
Acknowledgment
We acknowledge Mrs Rezaee as research assistant in Booali clinical laboratory, research section where this study was done.
References
- 1.Matsumi R, Atomi H, Driessen AJ, van der Oost J. Isoprenoid biosynthesis in Archaea--biochemical and evolutionary implications. Res Microbiol. 2011;162:39–52. doi: 10.1016/j.resmic.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Heuston S, Begley M, Gahan CG, Hill C. Isoprenoid biosynthesis in bacterial pathogens. Microbiology. 2012;158:1389–1401. doi: 10.1099/mic.0.051599-0. [DOI] [PubMed] [Google Scholar]
- 3.Noh DO, Kim SH, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci. 1997;80:3107–3113. doi: 10.3168/jds.S0022-0302(97)76281-7. [DOI] [PubMed] [Google Scholar]
- 4.Dambekodi PC, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Bifidobacterium longum. J Dairy Sci. 1998;81:1818–1824. doi: 10.3168/jds.S0022-0302(98)75751-0. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt E, McGee DJ. Helicobacter pylori lipopolysaccharide modification, Lewis antigen expression, and gastric colonization are cholesterol-dependent. BMC Microbiol. 2009;9:258. doi: 10.1186/1471-2180-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G. Intestinal probiotics:Interactions with bile salts and reduction of cholesterol. Procedia Environ Sci. 2012;12:1180–186. [Google Scholar]
- 7.McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, Testerman TL. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Antimicrob Agents Chemother. 2011;55:2897–2904. doi: 10.1128/AAC.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erkkilä L, Jauhiainen M, Laitinen K, Haasio K, Tiirola T, Saikku P, et al. Effect of simvastatin, an established lipid-lowering drug, on pulmonary Chlamydia pneumoniae infection in mice. Antimicrob Agents Chemother. 2005;49:3959–3962. doi: 10.1128/AAC.49.9.3959-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stancu C, Sima A. Statins:mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friesen JA, Rodwell VW. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004;5:248. doi: 10.1186/gb-2004-5-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61:362–4. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 12.Welsh AM, Kruger P, Faoagali J. Antimicrobial action of atorvastatin and rosuvastatin. Pathology. 2009;41:689–691. doi: 10.3109/00313020903305860. [DOI] [PubMed] [Google Scholar]
- 13.Biradar YS, Jagatap S, Khandelwal KR, Singhania SS. Exploring of antimicrobial activity of triphala mashi an ayurvedic formulation. Evid Based Complement Alternat Med. 2008;5:107–113. doi: 10.1093/ecam/nem002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Tani H, Sato M, Tsuchiya H, Namikawa I. Cholesterol incorporation into Bacillus megaterium without compositional modification of membrane lipids in response to changes of the membrane functions. Current Microbiol. 1993;26:253–256. [Google Scholar]
- 16.Kannenberg E, Poralla K. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften. 1999;86:168–176. [Google Scholar]
- 17.Siedenburg G, Jendrossek D. Squalene-hopene cyclases. Appl Environ Microbiol. 2011;77:3905–15. doi: 10.1128/AEM.00300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmerk CL, Bernards MA, Valvano MA. Hopanoid production is required for low-pH tolerance, antimicrobial resistance, and motility in Burkholderia cenocepacia. J Bacteriol. 2011;193:6712–6723. doi: 10.1128/JB.05979-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PF, Henrikson CV. Growth inhibition of Mycoplasma by inhibitors of polyterpene biosynthe-sis and its reversal by cholesterol. J Bacteriol. 1966;91:1854–1858. doi: 10.1128/jb.91.5.1854-1858.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


