Abstract
Background
Although commonly utilized interventions, no studies have directly compared the effectiveness of cervical and thoracic manipulation to mobilization and exercise in individuals with cervicogenic headache (CH). The purpose of this study was to compare the effects of manipulation to mobilization and exercise in individuals with CH.
Methods
One hundred and ten participants (n = 110) with CH were randomized to receive both cervical and thoracic manipulation (n = 58) or mobilization and exercise (n = 52). The primary outcome was headache intensity as measured by the Numeric Pain Rating Scale (NPRS). Secondary outcomes included headache frequency, headache duration, disability as measured by the Neck Disability Index (NDI), medication intake, and the Global Rating of Change (GRC). The treatment period was 4 weeks with follow-up assessment at 1 week, 4 weeks, and 3 months after initial treatment session. The primary aim was examined with a 2-way mixed-model analysis of variance (ANOVA), with treatment group (manipulation versus mobilization and exercise) as the between subjects variable and time (baseline, 1 week, 4 weeks and 3 months) as the within subjects variable.
Results
The 2X4 ANOVA demonstrated that individuals with CH who received both cervical and thoracic manipulation experienced significantly greater reductions in headache intensity (p < 0.001) and disability (p < 0.001) than those who received mobilization and exercise at a 3-month follow-up. Individuals in the upper cervical and upper thoracic manipulation group also experienced less frequent headaches and shorter duration of headaches at each follow-up period (p < 0.001 for all). Additionally, patient perceived improvement was significantly greater at 1 and 4-week follow-up periods in favor of the manipulation group (p < 0.001).
Conclusions
Six to eight sessions of upper cervical and upper thoracic manipulation were shown to be more effective than mobilization and exercise in patients with CH, and the effects were maintained at 3 months.
Trial registration
Keywords: Cervicogenic headache, Spinal manipulation, Mobilization, High velocity low amplitude thrust
Background
The International Classification of Headache Disorders defines cervicogenic headache (CH) as, “headache caused by a disorder of the cervical spine and its component bony, disc, and/or soft tissue elements, usually but not invariably accompanied by neck pain.” [1] (p.760) The prevalence of CH has been reported to be between 0.4 and 20 % of the headache population [2, 3], and as high as 53 % in patients with headache after whiplash injury [4]. The dominant features of CH usually include: unilaterality of head pain without side-shift, elicitation of pain with external pressure over the ipsilateral upper neck, limited cervical range of motion, and the triggering of attacks by various awkward or sustained neck movements [4, 5].
Individuals with CH are frequently treated with spinal manipulative therapy including both mobilization and manipulation [6]. Spinal mobilization consists of slow, rhythmical, oscillating techniques whereas manipulation consists of high-velocity low-amplitude thrust techniques. [7] In a recent systematic review, Bronfort and colleagues reported that spinal manipulative therapy (both mobilization and manipulation) were effective in the management of adults with CH [8]. However, they did not report if manipulation resulted in superior outcomes compared to mobilization for the management of this population.
Several studies have investigated the effect of spinal manipulation in the management of CH [9–13]. Haas et al. [10] investigated the effectiveness of cervical manipulation in subjects with CH. Jull et al. [11] demonstrated treatment efficacy for manipulative therapy and/or exercise in the management of CH. However the manipulative therapy group included manipulation and mobilization therefore it cannot be determined if the beneficial effect was a result of the manipulation, mobilization or the combination.
A few studies have examined the benefits of manipulation versus mobilization for the management of mechanical neck pain with or without exercise [14–16]. However, no studies have directly compared the effects of manipulation versus mobilization and exercise in patients with CH. Considering the purported risks of manipulation [17], it is essential to determine if manipulation results in improved outcomes compared to mobilization for the management of patients with CH. Therefore, the purpose of this randomized clinical trial was to compare the effects of manipulation versus mobilization and exercise in patients with CH. We hypothesized that patients receiving manipulation over a 4-week treatment period would experience greater reductions in headache intensity, headache frequency, headache duration, disability, and medication intake at a 3-month follow-up than patients receiving cervical and thoracic mobilization combined with exercise.
Methods
Participants
In this multi-center randomized clinical trial, consecutive patients with CH presenting to 1 of 8 outpatient physical therapy clinics from a variety of geographical locations (Arizona, Georgia, New York, Ohio, Pennsylvania, South Carolina) were recruited over a 29-month period (from April 2012 to August 2014). For patients to be eligible, they had to present with a diagnosis of CH according to the revised diagnostic criteria [5] developed by the Cervicogenic Headache International Study Group (CHISG) [5, 18, 19]. CH was classified according to the “major criteria” (not including confirmatory evidence by diagnostic anesthetic blockades) and “head pain characteristics” of the CHISG. Therefore, in order to be included in the study, patients had to exhibit all of the following criteria: (1) unilaterality of the head pain without sideshift, starting in the upper posterior neck or occipital region, eventually spreading to the oculofrontotemporal area on the symptomatic side, (2) pain triggered by neck movement and/or sustained awkward positions, (3) reduced range of motion in the cervical spine [20] (i.e., less than or equal to 32 ° of right or left passive rotation on the Flexion-Rotation Test [21–23], (4) pain elicited by external pressure over at least one of the upper cervical joints (C0-3), and (5) moderate to severe, non-throbbing and non-lancinating pain. In addition, participants had to have a headache frequency of at least 1 per week for a minimum of 3 months, a minimum headache intensity pain score of two points (0–10 on the NPRS scale), a minimum disability score of 20 % or greater (i.e., 10 points or greater on the 0–50 NDI scale), and be between 18 and 65 years of age.
Patients were excluded if they exhibited other primary headaches (i.e., migraine, TTH), suffered from bilateral headaches, or exhibited any red flags (i.e., tumor, fracture, metabolic diseases, rheumatoid arthritis, osteoporosis, resting blood pressure greater than 140/90 mmHg, prolonged history of steroid use, etc.), presented with two or more positive neurologic signs consistent with nerve root compression (muscle weakness involving a major muscle group of the upper extremity, diminished upper extremity deep tendon reflex, or diminished or absent sensation to pinprick in any upper extremity dermatome), presented with a diagnosis of cervical spinal stenosis, exhibited bilateral upper extremity symptoms, had evidence of central nervous system involvement (hyperreflexia, sensory disturbances in the hand, intrinsic muscle wasting of the hands, unsteadiness during walking, nystagmus, loss of visual acuity, impaired sensation of the face, altered taste, the presence of pathological reflexes), had a history of whiplash injury within the previous 6 weeks, had prior surgery to the head or neck, had received treatment for head or neck pain from any practitioner within the previous month, had received physical therapy or chiropractic treatment for head or neck pain within the previous 3 months, or had pending legal action regarding their head or neck pain.
The most recent literature suggests that pre-manipulative cervical artery testing is unable to identify those individuals at risk of vascular complications from cervical manipulation [24, 25], and any symptoms detected during pre-manipulative testing may be unrelated to changes in blood flow in the vertebral artery [26, 27]. Hence, pre-manipulative cervical artery testing was not performed in this study; however, screening questions for cervical artery disease had to be negative [24, 28, 29]. This study was approved by the Institutional Review Board at Long Island University, Brooklyn, NY. The study was registered at www.clinicaltrials.gov with trial identifier NCT01580280. All patients were informed that they would receive either manipulation or mobilization and exercise and then provided informed consent before their enrollment in the study.
Treating therapists
Twelve physical therapists (mean age 36.6 years, SD 5.62) participated in the delivery of treatment for patients in this study. They had an average of 10.3 (SD 5.66, range 3–20 years) years of clinical experience, and all had completed a 60 h post-graduate certification program that included practical training in manual techniques including the use of cervical and thoracic manipulation. To ensure all examination, outcome assessments, and treatment procedures were standardized, all participating physical therapists were required to study a manual of standard operating procedures and participate in a 4 h training session with the principal investigator.
Examination procedures
All patients provided demographic information, completed the Neck Pain Medical Screening Questionnaire, and completed a number of self-report measures, followed by a standardized history and physical examination at baseline. Self-report measures included headache intensity as measured by the NPRS (0–10), the NDI (0–50), headache frequency (number of days with headache in the last week), headache duration (total hours of headache in the last week), and medication intake (number of times the patient had taken narcotic or over-the-counter pain medication in the past week).
The standardized physical examination was not limited to, but included measurements of C1-2 (atlanto-axial joint) passive right and left rotation ROM using the Flexion-Rotation Test (FRT). The inter-rater reliability for the FRT has been found to be excellent (ICC: 0.93; 95 % CI: 0.87, 0.96) [30].
Outcome measures
The primary outcome measure used in this study was the patient’s headache intensity as measured by the NPRS. Patients were asked to indicate the average intensity of headache pain over the past week using an 11-point scale ranging from 0 (“no pain”) to 10 (“worst pain imaginable”) at baseline, 1-week, 1-month, and 3-months following the initial treatment session [31]. The NPRS is a reliable and valid instrument to assess pain intensity [32–34]. Although no data exists in patients with CH, the MCID for the NPRS has been shown to be 1.3 in patients with mechanical neck pain [32] and 1.74 in patients with a variety of chronic pain conditions [34]. Therefore, we chose to only include patients with an NPRS score of 2 points (20 %) or greater.
Secondary outcome measures included the NDI, the Global Rating of Change (GRC), headache frequency, headache duration, and medication intake. The NDI is the most widely used instrument for assessing self-rated disability in patients with neck pain [35–37]. The NDI is a self-report questionnaire with 10-items rated from 0 (no disability) to five (complete disability) [38]. The numeric responses for each item are summed for a total score ranging between 0 and 50; however, some evaluators have chosen to multiply the raw score by two, and then report the NDI on a 0–100 % scale [36, 39]. Higher scores represent increased levels of disability. The NDI has been found to possess excellent test-retest reliability, strong construct validity, strong internal consistency and good responsiveness in assessing disability in patients with mechanical neck pain [36], cervical radiculopathy [33, 40], whiplash associated disorder [38, 41, 42], and mixed non-specific neck pain [43, 44]. Although no studies have examined the psychometric properties of the NDI in patients with CH, we chose to only include patients with an NDI score of ten points (20 %) or greater, because this cut-off score captures the MCID for the NDI, which has been reported to approximate four, eight, and nine points (0–50) in patients with mixed non-specific neck pain [44], mechanical neck pain [45], and cervical radiculopathy [33], respectively. Headache frequency was measured as the number of days with headache in the last week, ranging from 0 to 7 days. Headache duration was measured as the total hours of headache in the last week, with six possible ranges: (1) 0–5 h, (2) 6–10 h, (3) 11–15 h, (4) 16–20 h, (5) 21–25 h, or (6) 26 or more hours. Medication intake was measured as the number of times the patient had taken prescription or over-the-counter analgesic or anti-inflammatory medication in the past week for their headaches, with five options: (1) not at all, (2) once a week, (3) once every couple of days, (4) once or twice a day, or (5) three or more times a day.
Patients returned for 1-week, 4-weeks, and 3-months follow-ups where the aforementioned outcome measures were again collected. In addition, at the 1-week, 4-weeks and 3-months follow-ups, patients completed a 15-point GRC question based on a scale described by Jaeschke et al. [46] to rate their own perception of improved function. The scale ranges from -7 (a very great deal worse) to zero (about the same) to +7 (a very great deal better). Intermittent descriptors of worsening or improving are assigned values from -1 to -6 and +1 to +6, respectively. The MCID for the GRC has not been specifically reported but scores of +4 and +5 have typically been indicative of moderate changes in patient status [46]. However, it should be noted that recently Schmitt and Abbott reported that the GRC might not correlate with changes in function in a population with hip and ankle injuries [47]. All outcome measures were collected by an assessor blind to group assignment.
On the initial visit patients completed all outcome measures then received the first treatment session. Patients completed 6–8 treatment sessions of either manipulation or mobilization combined with exercise over 4 weeks. Additionally, subjects were asked if they had experienced any “major” adverse events [48, 49] (stroke or permanent neurological deficits) at each follow-up period.
Randomization
Following the baseline examination, patients were randomly assigned to receive either manipulation or mobilization and exercise. Concealed allocation was performed by using a computer-generated randomized table of numbers created by an individual not involved with recruiting patients prior to the beginning of the study. Individual, sequentially numbered index cards with the random assignment were prepared for each of 8 data collection sites. The index cards were folded and placed in sealed opaque envelopes. Blinded to the baseline examination, the treating therapist opened the envelope and proceeded with treatment according to the group assignment. Patients were instructed not to discuss the particular treatment procedure received with the examining therapist. The examining therapist remained blind to the patient’s treatment group assignment at all times; however, based on the nature of the interventions it was not possible to blind patients or treating therapists.
Manipulation group
Manipulations targeting the right and left C1-2 articulations and bilateral T1-2 articulations were performed on at least one of the 6–8 treatment sessions (Figs. 1 and 2). On other treatment sessions, therapists either repeated the C1-2 and/or T1-2 manipulations or targeted other spinal articulations (i.e., C0-1, C2-3, C3-7, T2-9, ribs 1–9) using manipulation. The selection of the spinal segments to target was left to the discretion of the treating therapist and it was based on the combination of patient reports and manual examination. For both the upper cervical and upper thoracic manipulations, if no popping or cracking sound was heard on the first attempt, the therapist repositioned the patient and performed a second manipulation. A maximum of 2 attempts were performed on each patient similar to other studies [14, 50–53]. The clinicians were instructed that the manipulations are likely to be accompanied by multiple audible popping sounds [54–58]. Patients were encouraged to maintain usual activity within the limits of pain; however, mobilization and the prescription of exercises, or any use of other modalities, were not provided to this group.
Fig. 1.
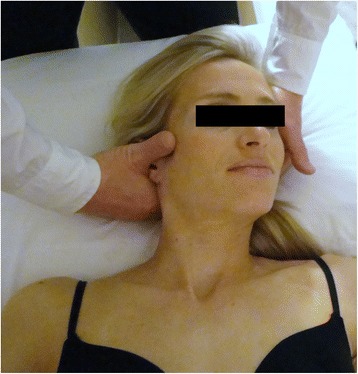
High-velocity low-amplitude thrust manipulation directed to the right C1-2 articulation. The subject provided consent for her image to be used
Fig. 2.
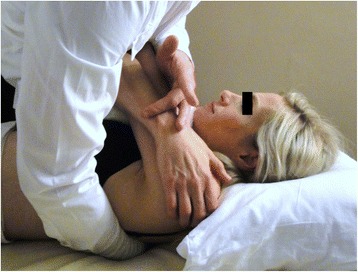
High-velocity low-amplitude thrust manipulation directed bilaterally to the upper thoracic (T1-2) spine. The subject provided consent for her image to be used
The manipulation targeting C1-2 was performed with the patient in supine. For this technique, the patient’s left posterior arch of the atlas was contacted with the lateral aspect of the proximal phalanx of the therapist’s left second finger using a “cradle hold”. To localize the forces to the left C1-2 articulation, the patient was positioned using extension, a posterior-anterior (PA) shift, ipsilateral side-bend and contralateral side-shift. While maintaining this position, the therapist performed a single high-velocity, low-amplitude thrust manipulation to the left atlanto-axial joint using right rotation in an arc toward the underside eye and translation toward the table (Fig. 1). This was repeated using the same procedure but directed to the right C1-2 articulation.
The manipulation targeting T1-2 was performed with the patient in supine. For this technique, the patient held her/his arms and forearms across the chest with the elbows aligned in a superoinferior direction. The therapist contacted the transverse processes of the lower vertebrae of the target motion segment with the thenar eminence and middle phalanx of the third digit. The upper lever was localized to the target motion segment by adding rotation away and side-bend towards the therapist while the underside hand used pronation and radial deviation to achieve rotation toward and side-bend away moments, respectively. The space inferior to the xiphoid process and costochondral margin of the therapist was used as the contact point against the patient’s elbows to deliver a manipulation in an anterior to posterior direction targeting T1-2 bilaterally (Fig. 2).
Mobilization and exercise group
Mobilizations targeting the right and left C1-2 articulations and bilateral T1-2 articulations were performed on at least one of the 6–8 treatment sessions. On other treatment sessions, therapists either repeated the C1-2 and/or T1-2 mobilizations or targeted other spinal articulations (i.e., C0-1, C2/3, C3-7, T2-9, ribs 1–9) using mobilization. The selection of the spinal segments to target was left to the discretion of the treating therapist and it was based on the combination of patient reports and manual examination. However, in order to avoid a “contact” or “attention effect” when compared with the manipulation group, therapists were instructed to mobilize one cervical segment (i.e., right and left) and one thoracic segment or rib articulation on each treatment session.
The mobilization targeting the C1-2 articulation was performed in prone. For this technique, the therapist performed one 30 s bout of left-sided unilateral grade IV PA mobilizations to the C1-2 motion segment as described by Maitland [7]. This same procedure was repeated for one 30 s bout to the right atlanto-axial joint. In addition, and on at least one session, mobilization directed to the upper thoracic (T1-2) spine with the patient prone was performed. For this technique, the therapist performed one 30 s bout of central grade IV PA mobilizations to the T1-2 motion segment as described by Maitland [7]. Therefore, we used 180 (i.e., three 30 s bouts at approximately 2 Hz) end-range oscillations in total on each subject for the mobilization treatment. Notably, there is no high quality evidence to date to suggest that longer durations of mobilization result in greater pain reduction than shorter durations or dosages of mobilization [59, 60].
Cranio-cervical flexion exercises [11, 61–63] were performed with the patient in supine, with the knees bent and the position of the head standardized by placing the craniocervical and cervical spines in a mid-position, such that a line between the subject’s forehead and chin was horizontal, and a horizontal line from the tragus of the ear bisected the neck longitudinally. An air-filled pressure biofeedback unit (Chattanooga Group, Inc., Hixson, TN) was placed suboccipitally behind the patient’s neck and preinflated to a baseline of 20 mmHg [63]. For the staged exercises, patients were required to perform the craniocervical flexion action (“a nod of the head, similar to indicating yes”) [63] and attempt to visually target pressures of 22, 24, 26, 28, and 30 mmHg from a resting baseline of 20 mmHg and to hold the position steady for 10 s [61, 62]. The action of nodding was performed in a gentle and slow manner. A 10 s rest was allowed between trials. If the pressure deviated below the target pressure, the pressure was not held steady, substitution with the superficial flexors (sternocleidomastoid or anterior scalene) occurred, or neck retraction was noticed before the completion of the 10 s isometric hold, it was regarded as a failure [63]. The last successful target pressure was used to determine each patient’s exercise level wherein 3 sets of 10 repetitions with a 10 s isometric hold were performed. In addition to mobilizations and cranio-cervical flexion exercises, patients were required to perform 10 min of progressive resistance exercises (i.e., using Therabands® or free weights) to the muscles of the shoulder girdle during each treatment session, within their own tolerance, and specifically focusing on the lower trapezius and serratus anterior [11].
Sample size
The sample size and power calculations were performed using online software from the MGH Biostatistics Center (Boston, MA). The calculations were based on detecting a 2-point (or 20 %) difference in the NPRS (headache intensity) at the 3 months follow-up, assuming a standard deviation of three points, a 2-tailed test, and an alpha level equal to 0.05. This generated a sample size of 49 patients per group. Allowing for a conservative dropout rate of 10 %, we planned to recruit at least 108 patients into the study. This sample size yielded greater than 90 % power to detect a statistically significant change in the NPRS scores.
Data analysis
Descriptive statistics, including frequency counts for categorical variables and measures of central tendency and dispersion for continuous variables were calculated to summarize the data. The effects of treatment on headache intensity and disability were each examined with a 2-by-4 mixed-model analysis of variance (ANOVA), with treatment group (manipulation versus mobilization and exercise) as the between-subjects variable and time (baseline, 1 week, 4 weeks, and 3 months follow-up) as the within-subjects variable. Separate ANOVAs were performed with the NPRS (headache intensity) and NDI (disability) as the dependent variable. For each ANOVA, the hypothesis of interest was the 2-way interaction (group by time).
An independent t-test was used to determine the between group differences for the percentage change from baseline to 3-month follow-up in both headache intensity and disability. Separate Mann–Whitney U tests were performed with the headache frequency, GRC, headache duration and medication intake as the dependent variable. We performed Little’s Missing Completely at Random (MCAR) test [64] to determine if missing data points associated with dropouts were missing at random or missing for systematic reasons. Intention-to-treat analysis was performed by using Expectation-Maximization whereby missing data are computed using regression equations. Planned pairwise comparisons were performed examining the difference between baseline and follow-up periods between-groups using the Bonferroni correction at an alpha level of .05.
We dichotomized patients as responders at the 3-month follow-up using a cut score of 2 points improvement for headache intensity as measured by the NPRS. Numbers needed to treat (NNT) and 95 % confidence intervals (CI) were also calculated at the 3 months follow-up period using each of these definitions for a successful outcome. Data analysis was performed using SPSS 21.0.
Results
Two hundred and fifty-one patients with a primary complaint of headaches were screened for possible eligibility. The reasons for ineligibility can be found in Fig. 3, the flow diagram of patient recruitment and retention. Of the 251 patients screened, 110 patients, with a mean age of 35.16 years (SD 11.48) and a mean duration of symptoms of 4.56 years (SD 6.27), satisfied the eligibility criteria, agreed to participate, and were randomized into manipulation (n = 58) and mobilization and exercise (n = 52) groups. Baseline variables for each group can be found in Table 1. Twelve therapists from 8 outpatient physical therapy clinics each treated 25, 23, 20, 14, 13, 7, 6 or 2 patients, respectively; furthermore, each of the 12 therapists treated approximately an equal proportion of patients in each group. There was no significant difference (p = 0.227) between the mean number of completed treatment sessions for the manipulation group (7.17, SD 0.96) and the mobilization and exercise group (6.90, SD 1.35). In addition, the mean number of treatment sessions that targeted the C1-2 articulation was 6.41 (SD 1.63) for the manipulation group and 6.52 (SD 2.01) for the mobilization and exercise group, and this was not significantly different (p = 0.762). One hundred seven of the 110 patients completed all outcome measures through 3 months (97 % follow-up). Little’s Missing Completely at Random (MCAR) test was not statistically significant (p = 0.281); therefore, we used the Expectation-Maximization imputation technique to replace missing values with predicted values for the missing 3-month outcomes.
Fig. 3.
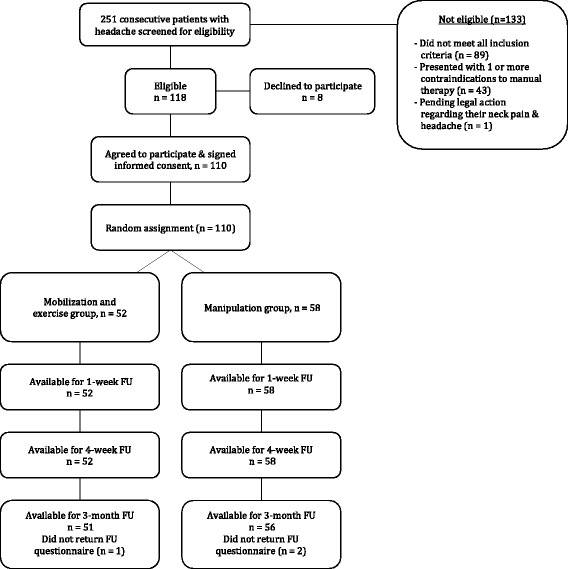
Flow diagram of patient recruitment and retention
Table 1.
Baseline variables: demographics and outcome measures
| Baseline Variable | Manipulation Group (n = 58) | Mobilization & Exercise Group (n = 52) |
|---|---|---|
| Age (years): Mean (SD) | 34.1 (12.6) | 36.4 (10.0) |
| Gender (female): number (%) | 41 (71 %) | 33 (64 %) |
| Duration of symptoms (days): Mean (SD) | 1693.7 (2357.7) | 1633.8 (2229.9) |
| BMI (kg/m2): Mean (SD) | 24.2 (3.8) | 24.0 (3.3) |
| Headache intensity (NPRS 0–10): Mean (SD) | 6.4 (1.6) | 6.0 (2.1) |
| Disability (NDI 0–50): Mean (SD) | 18.1 (7.9) | 19.2 (7.8) |
| Headache frequency (0–7 days): Median | 4 | 4 |
| Headache duration: Median | 3 | 3 |
| Medication intake: Median | 3 | 3 |
NPRS Numeric Pain Rating Scale, 0–10, lower scores indicate less pain; NDI Neck Disability Index, 0–50, lower scores indicate greater function; Headache frequency = number of headache days in the last week, 0–7, higher scores indicate worsening; Headache duration = total headache hours in the last week, 1 = 0–5 h, 2 = 6–10 h, 3 = 11–15 h, 4 = 16–20 h, 5 = 21–25 h, 6 = 26 or more hours, higher scores indicate worsening; Medication intake = frequency of pain medication use in the past week, 1 = not at all, 2 = once a week, 3 = once every couple of days, 4 = once or twice a day, 5 = three or more times a day
The overall group by time interaction for the primary outcome of headache intensity was statistically significant for the NPRS (F(3,106) = 11.196; p < 0.001; partial eta squared = 0.24). Between-group differences revealed that the manipulation group experienced statistically significant greater improvement in the NPRS at both the 1-week (2.1, 95 % CI: 1.2, 2.9), 4-week (2.3, 95 % CI: 1.5, 3.1) and 3-month (2.1, 95 % CI: 1.2, 3.0) follow-up periods (Table 2). In addition, an independent samples t-test revealed the between-group difference in percentage change in headache intensity (36.58 %, 95 % CI: 22.52, 50.64) from baseline to 3-month follow-up was statistically significant (t(108) = 5.156; p < 0.001) in favor of manipulation. See Table 3 for the percentage of subjects gaining 50, 75, and 100 % reduction in headache intensity at 3 months.
Table 2.
Changes in headache intensity (NPRS) and disability (NDI) with 95 % confidence intervals for both groups and between-group differences
| Variable | Manipulation | Mobilization and Exercise | Between-Group Differences |
|---|---|---|---|
| Headache Intensity (NPRS 0–10) | |||
| Baseline: Mean (SD) | 6.4 (1.6) | 6.0 (2.1) | |
| 1-Week: Mean (SD) | 3.1 (1.9) | 4.9 (1.8) | |
| Change Score: Baseline to 1-Week | 3.2 (2.6, 3.8) | 1.2 (0.6, 1.7) | 2.1 (1.2, 2.9); P < 0.001 |
| 4-Week: Mean (SD) | 1.8 (1.6) | 3.8 (2.0) | |
| Change Score: Baseline to 4-Week | 4.5 (4.0, 5.1) | 2.2 (1.7, 2.8) | 2.3 (1.5, 3.1); P < 0.001 |
| 3-Month: Mean (SD) | 2.0 (1.8) | 3.8 (1.9) | |
| Change Score: Baseline to 3-Month | 4.3 (3.7, 4.9) | 2.2 (1.6, 2.9) | 2.1 (1.2, 3.0); P < 0.001 |
| Disability (NDI 0–50) | |||
| Baseline: Mean (SD) | 18.1 (7.9) | 19.2 (7.8) | |
| 1-Week: Mean (SD) | 11.9 (8.5) | 16.1 (7.5) | |
| Change Score: Baseline to 1-Week | 6.2 (4.8, 7.6) | 3.1 (2.0, 4.1) | 3.1 (1.4, 4.9); P < 0.001 |
| 4-Week: Mean (SD) | 6.5 (5.4) | 13.0 (7.5) | |
| Change Score: Baseline to 4-Week | 11.6 (9.7, 13.4) | 6.1 (4.9, 7.4) | 5.4 (3.2, 7.7); P < 0.001 |
| 3-Month: Mean (SD) | 6.3 (5.9) | 13.5 (7.8) | |
| Change Score: Baseline to 3-Month | 11.7 (9.7, 13.8) | 5.7 (4.2, 7.2) | 6.0 (3.5, 8.6); P < 0.001 |
NPRS Numeric Pain Rating Scale, 0–10, lower scores indicate less pain; NDI Neck Disability Index, 0–50, lower scores indicate greater function
Table 3.
Percentage of subjects gaining 50, 75 and 100 % reduction in headache intensity (NPRS) and disability (NDI) as well as the numbers needed to treat at 3 months
| Variable | Manipulation (n = 58) | Mobilization & Exercise (n = 52) |
|---|---|---|
| Headache Intensity (NPRS 0–10) | ||
| 50 % Reduction | 74.1 % | 38.5 % |
| 75 % Reduction | 48.3 % | 13.5 % |
| 100 % Reduction | 29.3 % | 3.8 % |
| Number of individuals achieving at least a 2 point improvement in pain | 53 | 33 |
| Numbers Needed to Treat | 4.0 (95 % CI: 2.3, 7.7) | |
| Disability (NDI 0–50) | ||
| 50 % Reduction | 74.1 % | 23.1 % |
| 75 % Reduction | 43.1 % | 9.6 % |
| 100 % Reduction | 19.0 % | 1.9 % |
For secondary outcomes a significant group by time interaction existed for the NDI (F(3,106) = 8.57; p < 0.001; partial eta squared = 0.20). At each follow-up period the manipulation group had superior outcomes in disability reduction as compared to the mobilization and exercise group. An independent samples t- test revealed the between-group mean percentage change in disability (35.56 %, 95 % CI: 24.95, 46.17) from baseline to 3 months follow-up was statistically significant (t(108) = 6.646, p < 0.001); indicating the manipulation group experienced a significantly greater percentage in disability reduction (Table 3).
Mann–Whitney U tests revealed that patients in the upper cervical and upper thoracic manipulation group experienced less frequent headaches at 1 week (p < 0.001; median 2.0 versus 3.0), 4 weeks (p < 0.001; median 1.0 versus 3.0) and 3 months (p < 0.001; median 1.0 versus 2.5) than patients in the mobilization and exercise group. Headache duration was significantly lower at 1 week (p = 0.005; median 2.0 versus 3.0, 4 weeks (p < 0.001; median 1.0 versus 2.0) and 3 months (p < 0.001; median 1.0 versus 2.0) in the manipulation group. Additionally, patient perceived improvement as measured by the GRC was significantly greater at 1 week (p < 0.001, 4.0 versus 1.0), 4 weeks (p < 0.001, 6.0 versus 3.0) and 3 months (p < 0.001, 6.0 versus 3.0) than patients in the mobilization and exercise group. At 3 months, patients receiving upper cervical and upper thoracic manipulation experienced significantly (p < 0.001) greater reductions in medication intake as compared to the mobilization and exercise group. Based on the cutoff score of 2 points on the NPRS, the NNT was 4.0 (95 % CI: 2.3, 7.7) in favor of the manipulation group at 3-month follow-up.
We did not collect any data on the occurrence of “minor” adverse events [48, 49] (transient neurological symptoms, increased stiffness, radiating pain, fatigue or other); however, no “major” adverse events [48, 49] (stroke or permanent neurological deficits) were reported for either group.
Discussion
Statement of principal findings
To our knowledge, this study is the first randomized clinical trial to directly compare the effectiveness of both cervical and thoracic manipulation to mobilization and exercise in patients with CH. The results suggest 6–8 sessions of manipulation over 4 weeks, directed mainly to both the upper cervical (C1-2) and upper thoracic (T1-2) spines, resulted in greater improvements in headache intensity, disability, headache frequency, headache duration, and medication intake than mobilization combined with exercises. The point estimates for between-group changes in headache intensity (2.1 points) and disability (6.0 points or 12.0 %) exceeded the reported MCIDs for both measures. Although the MCID for the NDI in patients with CH has not yet been investigated, it should however be noted that the lower bound estimate of the 95 % CI for disability (3.5 points) was slightly below (or approximated in two cases) the MCID that has been found to be 3.5 [65], 5 [66], and 7.5 [45] points in patients with mechanical neck pain, 8.5 [33] points in patients with cervical radiculopathy, and 3.5 [44] points in patients with mixed, non-specific neck pain. However, it should be recognized that both groups made clinical improvement. In addition, the NNT suggests for every four patients treated with manipulation, rather than mobilization, one additional patient achieves clinically important pain reduction at 3 months follow-up.
Strengths and weaknesses of the study
The inclusion of 12 treating physical therapists from 8 private clinics in 6 different geographical states enhances the overall generalizability of our findings. Although significant differences were recognized up to 3 months, it is not known if these benefits would have been sustained at long-term. In addition, we used high-velocity, low-amplitude manipulation techniques that employed bidirectional thrusts into rotation and translation simultaneously and Maitland based grade IV PA mobilization techniques; thus, we cannot be certain that these results are generalizable to other kinds of manual therapy techniques. Some might argue that the comparison group might have not received adequate intervention. We sought to balance internal and external validity so standardized treatment for both groups and provided a very explicit description of the techniques used which will also allow for replication. Furthermore, we did not measure minor adverse events and only asked about two potential major adverse events. Another limitation is that we included multiple secondary outcomes. Therapist preferences as to which technique they thought would be superior was not collected and potentially could impact the results.
Strengths and weaknesses in relation to other studies: important differences in results
Jull et al. [11] demonstrated treatment efficacy for manipulative therapy and exercise in the management of CH; however, this treatment package included both mobilization and manipulation. The current study may provide evidence that the management of patients with CH should include some form of manipulation despite the fact it is often suggested that cervical manipulation should be avoided because of the risk of serious adverse events [67, 68]. Furthermore, it has been shown that individuals receiving spinal manipulation for neck pain and headaches are no more likely to experience a vertebrobasilar stroke than if they received treatment by their medical physician [69]. Additionally, after reviewing 134 case reports, Puentedura et al. concluded that with appropriate selection of patients by careful screening of red flags and contraindications, the majority of adverse events associated with cervical manipulation could have been prevented [70].
Meaning of the study: possible explanations and implications for clinicians and policymakers
Based on the results of the current study clinicians should consider incorporating spinal manipulation for individuals with CH. A recent systematic review found both mobilization and manipulation to be effective for the management of patients with CH but was unable to determine which technique was superior [8]. Additionally, clinical guidelines reported that manipulation, mobilization and exercise were all effective for the management of patients with CH; however, the guideline made no suggestions regarding the superiority of either technique. [71] The current results may assist authors of future systematic reviews and clinical guidelines in providing more specific recommendations about the use of spinal manipulation in this population.
Unanswered questions and future research
The underlying mechanisms as to why manipulation may have resulted in greater improvements remains to be elucidated. It has been suggested that high-velocity displacement of vertebrae with impulse durations of less than 200 ms may alter afferent discharge rates [72] by stimulating mechanoreceptors and proprioceptors, thereby changing alpha motorneuron excitability levels and subsequent muscle activity [72–74]. Manipulation might also stimulate receptors in the deep paraspinal musculature, and mobilization might be more likely to facilitate receptors in the superficial muscles [75]. Biomechanical [76, 77], spinal or segmental [78, 79] and central descending inhibitory pain pathway [80–83] models are plausible explanations for the hypoalgesic effects observed following manipulation. Recently, the biomechanical effects of manipulation have been under scientific scrutiny [84], and it is plausible that the clinical benefits found in our study are associated with a neurophysiological response involving temporal sensory summation at the dorsal horn of the spinal cord [78]; however, this proposed model is currently supported only on findings from transient, experimentally induced pain in healthy subjects [85, 86], not patients with CH. Future studies should examine different manual therapy techniques with varying dosages and include a 1-year follow-up. Furthermore, future studies examining the neurophysiological effects of both manipulation and mobilization will be important for determining why there may or may not be a difference in clinical effects between these two treatments.
Conclusion
The results of the current study demonstrated that patients with CH who received cervical and thoracic manipulation experienced significantly greater reductions in headache intensity, disability, headache frequency, headache duration, and medication intake as compared to the group that received mobilization and exercise; furthermore, the effects were maintained at 3 months follow-up. Future studies should examine the effectiveness of different types and dosages of manipulation and include a long-term follow-up.
Acknowledgements
None of the authors received any funding for this study. The authors wish to thank all the participants of the study.
Footnotes
Competing interests
Dr. James Dunning is the President of the American Academy of Manipulative Therapy (AAMT). AAMT provides postgraduate training programs in spinal manipulation, spinal mobilization, dry needling, extremity manipulation, extremity mobilization, instrument-assisted soft-tissue mobilization and therapeutic exercise to licensed physical therapists, osteopaths and medical doctors. Drs. James Dunning, Raymond Butts, Thomas Perreault, and Firas Mourad are senior instructors for AAMT. The other authors declare that they have no competing interests.
Authors’ contributions
JRD participated in the conception, design, data acquisition, statistical analyses and drafting of the manuscript. RB and IY participated in the design, data collection, statistical analyses and revision of the manuscript. FM participated in the design, statistical analyses, data interpretation and revision of the manuscript. MH participated in the conception, design and revision of the manuscript. CF and JC were involved in the statistical analyses, interpretation of data, and critical revision of the manuscript for important intellectual content. TS, JD, DB, and TH were involved in data collection and revision of the manuscript. All authors read and approved the final manuscript.
Contributor Information
James R. Dunning, Email: jamesdunning@hotmail.com
Raymond Butts, Email: buttsraymond@yahoo.com.
Firas Mourad, Email: firas.mourad@me.com.
Ian Young, Email: youngian@spinesport.org.
Cesar Fernandez-de-las Peñas, Email: cesarfdlp@yahoo.es.
Marshall Hagins, Email: marshall.hagins@liu.edu.
Thomas Stanislawski, Email: thomas.stanislawski@gmail.com.
Jonathan Donley, Email: jonathan.donley@palmettohealth.org.
Dustin Buck, Email: dustinbuck@hotmail.com.
Todd R. Hooks, Email: trhooks@hotmail.com
Joshua A. Cleland, Email: joshcleland@comcast.net
References
- 1.The International Classifcation of Headache Disorders: 3rd Edition. Cephalalgia. 2013;33(9):629-808. [DOI] [PubMed]
- 2.Anthony M. Cervicogenic headache: prevalence and response to local steroid therapy. Clin Exp Rheumatol. 2000;18(2 Suppl 19):S59–64. [PubMed] [Google Scholar]
- 3.Nilsson N. The prevalence of cervicogenic headache in a random population sample of 20-59 year olds. Spine (Phila Pa 1976) 1995;20(17):1884–8. doi: 10.1097/00007632-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bogduk N, Govind J. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009;8(10):959–68. doi: 10.1016/S1474-4422(09)70209-1. [DOI] [PubMed] [Google Scholar]
- 5.Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. The Cervicogenic Headache International Study Group. Headache. 1998;38(6):442–5. doi: 10.1046/j.1526-4610.1998.3806442.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cuadrado ML, Pareja JA. Spinal manipulative therapy in the management of cervicogenic headache. Headache. 2005;45(9):1260–3. doi: 10.1111/j.1526-4610.2005.00253_1.x. [DOI] [PubMed] [Google Scholar]
- 7.Maitland GD. Vertebral Manipulation. 5. Oxford: Butterworth-Heinemann; 1986. [Google Scholar]
- 8.Bronfort G, Haas M, Evans R, Leininger B, Triano J. Effectiveness of manual therapies: the UK evidence report. Chiropr Osteopat. 2010;18:3. doi: 10.1186/1746-1340-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas M, Groupp E, Aickin M, Fairweather A, Ganger B, Attwood M, et al. Dose response for chiropractic care of chronic cervicogenic headache and associated neck pain: a randomized pilot study. J Manipulative Physiol Ther. 2004;27(9):547–53. doi: 10.1016/j.jmpt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Haas M, Spegman A, Peterson D, Aickin M, Vavrek D. Dose response and efficacy of spinal manipulation for chronic cervicogenic headache: a pilot randomized controlled trial. Spine J. 2010;10(2):117–28. doi: 10.1016/j.spinee.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jull G, Trott P, Potter H, Zito G, Niere K, Shirley D, et al. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. Spine (Phila Pa 1976) 2002;27(17):1835–43. doi: 10.1097/00007632-200209010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson N. A randomized controlled trial of the effect of spinal manipulation in the treatment of cervicogenic headache. J Manipulative Physiol Ther. 1995;18(7):435–40. [PubMed] [Google Scholar]
- 13.Nilsson N, Christensen HW, Hartvigsen J. The effect of spinal manipulation in the treatment of cervicogenic headache. J Manipulative Physiol Ther. 1997;20(5):326–30. [PubMed] [Google Scholar]
- 14.Dunning JR, Cleland JA, Waldrop MA, Arnot CF, Young IA, Turner M, et al. Upper cervical and upper thoracic thrust manipulation versus nonthrust mobilization in patients with mechanical neck pain: a multicenter randomized clinical trial. J Orthop Sports Phys Ther. 2012;42(1):5–18. doi: 10.2519/jospt.2012.3894. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz EL, Morgenstern H, Harber P, Kominski GF, Yu F, Adams AH. A randomized trial of chiropractic manipulation and mobilization for patients with neck pain: clinical outcomes from the UCLA neck-pain study. Am J Public Health. 2002;92(10):1634–41. doi: 10.2105/AJPH.92.10.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leaver AM, Maher CG, Herbert RD, Latimer J, McAuley JH, Jull G, et al. A randomized controlled trial comparing manipulation with mobilization for recent onset neck pain. Arch Phys Med Rehabil. 2010;91(9):1313–8. doi: 10.1016/j.apmr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Wand BM, Heine PJ, O'Connell NE. Should we abandon cervical spine manipulation for mechanical neck pain? Yes. BMJ. 2012;344:e3679. doi: 10.1136/bmj.e3679. [DOI] [PubMed] [Google Scholar]
- 18.Sjaastad O, Fredriksen TA. Cervicogenic headache: criteria, classification and epidemiology. Clin Exp Rheumatol. 2000;18(2 Suppl 19):S3–6. [PubMed] [Google Scholar]
- 19.Vincent MB, Luna RA. Cervicogenic headache: a comparison with migraine and tension-type headache. Cephalalgia. 1999;19(Suppl 25):11–6. doi: 10.1177/0333102499019S2503. [DOI] [PubMed] [Google Scholar]
- 20.Zwart JA. Neck mobility in different headache disorders. Headache. 1997;37(1):6–11. doi: 10.1046/j.1526-4610.1997.3701006.x. [DOI] [PubMed] [Google Scholar]
- 21.Hall T, Robinson K. The flexion-rotation test and active cervical mobility--a comparative measurement study in cervicogenic headache. Man Ther. 2004;9(4):197–202. doi: 10.1016/j.math.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Hall TM, Briffa K, Hopper D, Robinson KW. The relationship between cervicogenic headache and impairment determined by the flexion-rotation test. J Manipulative Physiol Ther. 2010;33(9):666–71. doi: 10.1016/j.jmpt.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Ogince M, Hall T, Robinson K, Blackmore AM. The diagnostic validity of the cervical flexion-rotation test in C1/2-related cervicogenic headache. Man Ther. 2007;12(3):256–62. doi: 10.1016/j.math.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Hutting N, Verhagen AP, Vijverman V, Keesenberg MD, Dixon G, Scholten-Peeters GG. Diagnostic accuracy of premanipulative vertebrobasilar insufficiency tests: a systematic review. Man Ther. 2013;18(3):177–82. doi: 10.1016/j.math.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Kerry R, Taylor AJ, Mitchell J, McCarthy C. Cervical arterial dysfunction and manual therapy: a critical literature review to inform professional practice. Man Ther. 2008;13(4):278–88. doi: 10.1016/j.math.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Thomas LC, Rivett DA, Bateman G, Stanwell P, Levi CR. Effect of selected manual therapy interventions for mechanical neck pain on vertebral and internal carotid arterial blood flow and cerebral inflow. Phys Ther. 2013;93(11):1563–74. doi: 10.2522/ptj.20120477. [DOI] [PubMed] [Google Scholar]
- 27.Quesnele JJ, Triano JJ, Noseworthy MD, Wells GD. Changes in vertebral artery blood flow following various head positions and cervical spine manipulation. J Manipulative Physiol Ther. 2014;37(1):22–31. doi: 10.1016/j.jmpt.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Kerry R. The 'vertebral artery test'. Man Ther. 2005;10(4):297. doi: 10.1016/j.math.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Kerry R, Taylor AJ, Mitchell J, McCarthy C, Brew J. Manual therapy and cervical arterial dysfunction, directions for the future: a clinical perspective. J Man Manip Ther. 2008;16(1):39–48. doi: 10.1179/106698108790818620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall TM, Robinson KW, Fujinawa O, Akasaka K, Pyne EA. Intertester reliability and diagnostic validity of the cervical flexion-rotation test. J Manipulative Physiol Ther. 2008;31(4):293–300. doi: 10.1016/j.jmpt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 32.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and numeric pain rating scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89(1):69–74. doi: 10.1016/j.apmr.2007.08.126. [DOI] [PubMed] [Google Scholar]
- 33.Young IA, Cleland JA, Michener LA, Brown C. Reliability, construct validity, and responsiveness of the Neck Disability Index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am J Phys Med Rehabil. 2010;89(10):831–9. doi: 10.1097/PHM.0b013e3181ec98e6. [DOI] [PubMed] [Google Scholar]
- 34.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 35.Vernon H. The Neck Disability Index: state-of-the-art, 1991-2008. J Manipulative Physiol Ther. 2008;31(7):491–502. doi: 10.1016/j.jmpt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 36.MacDermid JC, Walton DM, Avery S, Blanchard A, Etruw E, McAlpine C, et al. Measurement properties of the Neck Disability Index: a systematic review. J Orthop Sports Phys Ther. 2009;39(5):400–17. doi: 10.2519/jospt.2009.2930. [DOI] [PubMed] [Google Scholar]
- 37.Pietrobon R, Coeytaux RR, Carey TS, Richardson WJ, DeVellis RF. Standard scales for measurement of functional outcome for cervical pain or dysfunction: a systematic review. Spine (Phila Pa 1976) 2002;27(5):515–22. doi: 10.1097/00007632-200203010-00012. [DOI] [PubMed] [Google Scholar]
- 38.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–15. [PubMed] [Google Scholar]
- 39.Vernon H. The psychometric properties of the Neck Disability Index. Arch Phys Med Rehabil. 2008;89(7):1414–5. doi: 10.1016/j.apmr.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Cleland JA, Fritz JM, Whitman JM, Palmer JA. The reliability and construct validity of the Neck Disability Index and patient specific functional scale in patients with cervical radiculopathy. Spine (Phila Pa 1976) 2006;31(5):598–602. doi: 10.1097/01.brs.0000201241.90914.22. [DOI] [PubMed] [Google Scholar]
- 41.Hoving JL, O'Leary EF, Niere KR, Green S, Buchbinder R. Validity of the neck disability index, Northwick Park neck pain questionnaire, and problem elicitation technique for measuring disability associated with whiplash-associated disorders. Pain. 2003;102(3):273–81. doi: 10.1016/S0304-3959(02)00406-2. [DOI] [PubMed] [Google Scholar]
- 42.Miettinen T, Leino E, Airaksinen O, Lindgren KA. The possibility to use simple validated questionnaires to predict long-term health problems after whiplash injury. Spine (Phila Pa 1976) 2004;29(3):E47–51. doi: 10.1097/01.BRS.0000106496.23202.60. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy MJ, Grevitt MP, Silcocks P, Hobbs G. The reliability of the Vernon and Mior neck disability index, and its validity compared with the short form-36 health survey questionnaire. Eur Spine J. 2007;16(12):2111–7. doi: 10.1007/s00586-007-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pool JJ, Ostelo RW, Hoving JL, Bouter LM, de Vet HC. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine (Phila Pa 1976) 2007;32(26):3047–51. doi: 10.1097/BRS.0b013e31815cf75b. [DOI] [PubMed] [Google Scholar]
- 45.Young BA, Walker MJ, Strunce JB, Boyles RE, Whitman JM, Childs JD. Responsiveness of the Neck Disability Index in patients with mechanical neck disorders. Spine J. 2009;9(10):802–8. doi: 10.1016/j.spinee.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 47.Schmitt J, Abbott JH. Global ratings of change do not accurately reflect functional change over time in clinical practice. J Orthop Sports Phys Ther. 2015;45(2):106–11. doi: 10.2519/jospt.2015.5247. [DOI] [PubMed] [Google Scholar]
- 48.Carlesso L, Macdermid JC, Santaguida L. Standardization of adverse event terminology and reporting in orthopaedic physical therapy - applications to the cervical spine. J Orthop Sports Phys Ther. 2010;40:455–63. doi: 10.2519/jospt.2010.3229. [DOI] [PubMed] [Google Scholar]
- 49.Carlesso LC, Gross AR, Santaguida PL, Burnie S, Voth S, Sadi J. Adverse events associated with the use of cervical manipulation and mobilization for the treatment of neck pain in adults: a systematic review. Man Ther. 2010;15(5):434–44. doi: 10.1016/j.math.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Cleland JA, Glynn P, Whitman JM, Eberhart SL, MacDonald C, Childs JD. Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the thoracic spine in patients with neck pain: a randomized clinical trial. Phys Ther. 2007;87(4):431–40. doi: 10.2522/ptj.20060217. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Iglesias J, Fernandez-de-las-Penas C, Cleland JA, Alburquerque-Sendin F, Palomeque-del-Cerro L, Mendez-Sanchez R. Inclusion of thoracic spine thrust manipulation into an electro-therapy/thermal program for the management of patients with acute mechanical neck pain: a randomized clinical trial. Man Ther. 2009;14(3):306–13. doi: 10.1016/j.math.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Iglesias J, Fernandez-de-las-Penas C, Cleland JA, Gutierrez-Vega MR. Thoracic spine manipulation for the management of patients with neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2009;39(1):20–7. doi: 10.2519/jospt.2009.2914. [DOI] [PubMed] [Google Scholar]
- 53.Lau HM, Wing Chiu TT, Lam TH. The effectiveness of thoracic manipulation on patients with chronic mechanical neck pain - a randomized controlled trial. Man Ther. 2011;16(2):141–7. doi: 10.1016/j.math.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Beffa R, Mathews R. Does the adjustment cavitate the targeted joint? An investigation into the location of cavitation sounds. J Manipulative Physiol Ther. 2004;27(2):e2. doi: 10.1016/j.jmpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Dunning J, Mourad F, Barbero M, Leoni D, Cescon C, Butts R. Bilateral and multiple cavitation sounds during upper cervical thrust manipulation. BMC Musculoskelet Disord. 2013;14:24. doi: 10.1186/1471-2474-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reggars JW. The manipulative crack. Frequency analysis. Australas Chiropr Osteopathy. 1996;5(2):39–44. [PMC free article] [PubMed] [Google Scholar]
- 57.Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine (Phila Pa 1976) 2004;29(13):1452–7. doi: 10.1097/01.BRS.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 58.Evans DW, Lucas N. What is 'manipulation'? A reappraisal. Man Ther. 2010;15(3):286–91. doi: 10.1016/j.math.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Gross A, Miller J, D'Sylva J, Burnie SJ, Goldsmith CH, Graham N, et al. Manipulation or mobilisation for neck pain: a cochrane review. Man Ther. 2010;15(4):315–33. doi: 10.1016/j.math.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther. 2007;12(2):109–18. doi: 10.1016/j.math.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Falla D, Bilenkij G, Jull G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine (Phila Pa 1976) 2004;29(13):1436–40. doi: 10.1097/01.BRS.0000128759.02487.BF. [DOI] [PubMed] [Google Scholar]
- 62.Falla D, Jull G, Dall'Alba P, Rainoldi A, Merletti R. An electromyographic analysis of the deep cervical flexor muscles in performance of craniocervical flexion. Phys Ther. 2003;83(10):899–906. [PubMed] [Google Scholar]
- 63.Jull G. Deep cervical flexor muscle dysfunction in whiplash. Journal of Musculoskeletal Pain. 2000;8:143–54. doi: 10.1300/J094v08n01_12. [DOI] [Google Scholar]
- 64.Rubin LH, Witkiewitz K, Andre JS, Reilly S. Methods for handling missing data in the behavioral neurosciences: Don't throw the baby Rat out with the bath water. J Undergrad Neurosci Educ. 2007;5(2):A71–7. [PMC free article] [PubMed] [Google Scholar]
- 65.Jorritsma W, Dijkstra PU, de Vries GE, Geertzen JH, Reneman MF. Detecting relevant changes and responsiveness of neck pain and disability scale and Neck Disability Index. Eur Spine J. 2012;21(12):2550–7. doi: 10.1007/s00586-012-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stratford PW, Riddle DL, Binkley JM, Spadoni G, Westaway MD, Padfield B. Using the Neck Disability Index to make decisions concerning individual patients. Physiother Can. 1999;51:107–12. [Google Scholar]
- 67.Ernst E. Manipulation of the cervical spine: a systematic review of case reports of serious adverse events, 1995-2001. Med J Aust. 2002;176(8):376–80. doi: 10.5694/j.1326-5377.2002.tb04459.x. [DOI] [PubMed] [Google Scholar]
- 68.Oppenheim JS, Spitzer DE, Segal DH. Nonvascular complications following spinal manipulation. Spine J. 2005;5(6):660–6. doi: 10.1016/j.spinee.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Cassidy JD, Boyle E, Cote P, He Y, Hogg-Johnson S, Silver FL, et al. Risk of vertebrobasilar stroke and chiropractic care: results of a population-based case-control and case-crossover study. Spine (Phila Pa 1976) 2008;33(4 Suppl):S176–83. doi: 10.1097/BRS.0b013e3181644600. [DOI] [PubMed] [Google Scholar]
- 70.Puentedura EJ, March J, Anders J, Perez A, Landers MR, Wallmann HW, et al. Safety of cervical spine manipulation: are adverse events preventable and are manipulations being performed appropriately? A review of 134 case reports. J Man Manip Ther. 2012;20(2):66–74. doi: 10.1179/2042618611Y.0000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Childs JD, Cleland JA, Elliott JM, Teyhen DS, Wainner RS, Whitman JM, et al. Neck pain: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopedic section of the American Physical Therapy Association. J Orthop Sports Phys Ther. 2008;38(9):A1–A34. doi: 10.2519/jospt.2008.0303. [DOI] [PubMed] [Google Scholar]
- 72.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29(1):22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Herzog W, Scheele D, Conway PJ. Electromyographic responses of back and limb muscles associated with spinal manipulative therapy. Spine (Phila Pa 1976) 1999;24(2):146–52. doi: 10.1097/00007632-199901150-00012. [DOI] [PubMed] [Google Scholar]
- 74.Indahl A, Kaigle AM, Reikeras O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine (Phila Pa 1976) 1997;22(24):2834–40. doi: 10.1097/00007632-199712150-00006. [DOI] [PubMed] [Google Scholar]
- 75.Bolton PS, Budgell BS. Spinal manipulation and spinal mobilization influence different axial sensory beds. Med Hypotheses. 2006;66(2):258–62. doi: 10.1016/j.mehy.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 76.Cassidy JD, Lopes AA, Yong-Hing K. The immediate effect of manipulation versus mobilization on pain and range of motion in the cervical spine: a randomized controlled trial. J Manipulative Physiol Ther. 1992;15(9):570–5. [PubMed] [Google Scholar]
- 77.Martinez-Segura R, Fernandez-de-las-Penas C, Ruiz-Saez M, Lopez-Jimenez C, Rodriguez-Blanco C. Immediate effects on neck pain and active range of motion after a single cervical high-velocity low-amplitude manipulation in subjects presenting with mechanical neck pain: a randomized controlled trial. J Manipulative Physiol Ther. 2006;29(7):511–7. doi: 10.1016/j.jmpt.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 78.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14(5):531–8. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunning J, Rushton A. The effects of cervical high-velocity low-amplitude thrust manipulation on resting electromyographic activity of the biceps brachii muscle. Man Ther. 2009;14(5):508–13. doi: 10.1016/j.math.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Haavik-Taylor H, Murphy B. Cervical spine manipulation alters sensorimotor integration: a somatosensory evoked potential study. Clin Neurophysiol. 2007;118(2):391–402. doi: 10.1016/j.clinph.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Millan M. Descending control of pain. Prog Neurobiology. 2002;66:355–74. doi: 10.1016/S0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 82.Skyba D, Radhakrishnan R, Rohlwing J, Wright A, Sluka K. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106:159–68. doi: 10.1016/S0304-3959(03)00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zusman M. Forebrain-mediated sensitization of central pain pathways: "non-specific" pain and a new image for manual therapy. Man Ther. 2002;7:80–8. doi: 10.1054/math.2002.0442. [DOI] [PubMed] [Google Scholar]
- 84.Bialosky JE, George SZ, Bishop MD. How spinal manipulative therapy works: why ask why? J Orthop Sports Phys Ther. 2008;38(6):293–5. doi: 10.2519/jospt.2008.0118. [DOI] [PubMed] [Google Scholar]
- 85.Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine J. 2011;11(5):440–6. doi: 10.1016/j.spinee.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]


