Abstract
OBJECTIVES
Previous animal studies have demonstrated that endothelial adherens-junction molecules are significantly altered in animal myocardium and microvasculature after cardioplegia and cardiopulmonary bypass (CP/CPB). We investigated the effects of diabetes on expression/phosphorylation/localization of vascular endothelial (VE)-cadherin, β- and γ-catenin in human atrial myocardium and coronary vasculature in the setting of CP/CPB.
METHODS
Right atrial tissue was harvested pre- and post-CP/CPB from non-diabetic (ND) [haemoglobin A1c (HbA1c): 5.4 ± 0.15], controlled (CDM) (HbA1c: 6.3 ± 0.14) and uncontrolled diabetic (UDM) (HbA1c: 9.9 ± 0.72) patients (n = 10/group). Expression/phosphorylation/localization of VE-cadherin, β- and γ-catenin were assessed by immunoblotting, immunoprecipitation and immunohistochemistry. In vitro atrial microvascular reactivity was assessed by videomicroscopy in response to the endothelium-dependent vasodilator adenosine 5′-diphosphate (ADP).
RESULTS
There were no significant differences in VE-cadherin protein expression between pre- and post-CP/CPB among groups. There were significant decreases in VE-cadherin densities in vessels of the UDM group versus the ND group at baseline or post-CP/CPB, respectively (P < 0.05). The level of basal phosphorylated VE-cadherin tends to be higher in the UDM compared with the ND group (P < 0.05). CP/CPB induced more phosphorylation of VE-cadherin in all groups (versus pre-CP/CPB; P < 0.05, respectively) and this effect was more pronounced in the UDM group (P < 0.05 versus ND or CDM). The protein levels of both catenins (β and γ) were lower in post-CP/CPB in UDM than ND patients (P < 0.05). There were significant decreases in vasodilatory response to endothelial-dependent vasodilator ADP after CP/CPB (P < 0.05). This alteration was more pronounced in UDM patients (P < 0.05).
CONCLUSIONS
These data suggest that poorly controlled diabetes down-regulates endothelial adherens-junction protein activation/expression/localization in the setting of CP/CPB. The increased tyrosine phosphorylation and deterioration of VE-cadherin indicate the damage of the cell–cell endothelial junctions in the diabetic vessels undergoing CP/CPB and cardiac surgery. These alterations may lead to increase in vascular permeability and endothelial dysfunction and affect outcomes in diabetic patients after cardiac surgery.
Keywords: Cardioplegia, Cardiopulmonary bypass, Coronary artery bypass grafting, Adherens-junction proteins, Diabetes, Vascular permeability
INTRODUCTION
Diabetes mellitus remains an important risk factor for perioperative complications after cardiac surgery. Much investigation has focused on the effects of diabetes on wound healing and infectious complications. We have found that diabetes, especially poorly controlled diabetes, is associated with reduced vasomotor tone manifesting as hypotension, and impaired endothelial function of human coronary and peripheral arterioles in the setting of cardioplegia and cardiopulmonary bypass (CP/CPB) [1–4]. These effects can partially be explained by CP/CPB-initiated systemic inflammation, enhanced expression of vascular endothelial growth factor (VEGF) and vascular permeability factor (VPF) [5, 6]. The resulting increase in microvascular permeability and increased tissue-organ oedema contributes to an increased length of stay and worsened outcomes in patients with poorly controlled diabetes after cardiac surgery [6]. The molecular mechanism underlying CP/CPB-enhanced vascular permeability merits further investigation.
Tyrosine phosphorylation induced by VEGF/VPF and other factors may play an important role in maintaining vascular structure and function [7–11]. The animal studies suggest that the state of tyrosine phosphorylation of proteins involved in cellular integrity [vascular endothelium-cadherins (VE-cadherin), β- and γ-catenins] is closely correlated with increases in vascular permeability [7–11]. Our experimental studies indicate that CP/CPB is associated with down-regulation of adherens-junction proteins, such as VE-cadherin, and β- and γ-catenins, resulting in loss of coronary endothelial integrity in pigs [12, 13]. However, there has been limited investigation into diabetic regulation of adherens-junction proteins in the setting of CP/CPB in human myocardium and coronary vasculature. Thus, we hypothesized that tyrosine phosphorylation and deterioration of VE-cadherin, as well as other components of the adherens junctions would be altered in the poorly controlled diabetic patients when compared with well-controlled, or age-matched, non-diabetic patients. Specifically, this study was designed to compare the effects of diabetes and CP/CPB on the expression/phosphorylation/localization of adherens junctions in human atrial myocardium and coronary vasculature, and to relate these responses to the possible alterations in coronary arteriolar endothelial function.
MATERIALS AND METHODS
Human subjects and tissue harvesting
The method for right atrial tissue harvesting has been previously described in detail [1, 14, 15]. Samples of right atrial appendage were harvested from patients undergoing coronary artery bypass grafting surgery before and after exposure of the heart to blood CP and short-term reperfusion under conditions of CPB [1, 14, 15]. Samples were handled in a non-traumatic fashion. The first sample of the right atrial appendage was harvested prior to the initiation of CP/CPB (pre-CP/CPB). The second sample of atrial tissue was harvested between the purse-strings sutures during removal of the venous cannula after completion of CP/CPB (post-CP/CPB). Sections of atrial samples were immediately frozen in liquid nitrogen (immunoblotting), or placed in 10% formalin (immunohistochemistry) or placed in cold (5–10°C) Krebs–Henseleit buffer (microvascular studies).
Patients were divided into the following three groups: (i) non-diabetic patients [n = 10, normal haemoglobin A1c (HbA1c)]; (ii) well-controlled diabetic patients (n = 10, HbA1c < 7 and (iii) poorly controlled diabetic patients (n = 10, HbA1c > 8.5). All procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients as required by the Institutional Review Board.
Microvessel reactivity
Coronary arterioles (90–150 µm internal diameters) were dissected from harvested pre- and post-CP/CPB right atrial-tissue samples. Microvessel studies were performed by in vitro organ bath videomicroscopy as described previously [1, 14, 15]. After a 60-min equilibration period, the vessel was precontracted with the thromboxane analogue U46619 (10−7 M) to achieve 30–40% of the baseline diameter. The vessel was then treated with adenosine 5′-diphosphate (ADP, 10−5 M). We have previously determined that the human coronary microvascular response to ADP is endothelium-dependent [1, 14, 15].
Immunoblot
The snap-frozen right-atrial-tissue samples (n = 6/group) were dissected and cleaned of fat and connective tissue, and then homogenized on ice in a radioimmunoprecipitation assay buffer (RIPA, Boston BioProducts, Ashland, MA, USA) supplemented with protease and phosphatase inhibitors (Roche, Branford, CT, USA). The total lysate protein concentration was measured spectrophotometrically at a wavelength of 562 nm by using a multimode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) with a BCA protein assay kit (Thermo Scientific, Waltham, MA, US). Total protein was fractionated on 4–20% SDS–PAGE and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA, USA) with a transfer apparatus (BioRad, Hercules, CA, USA). Membranes were then incubated with 5% non-fat dry milk in 50 mmol/l Tris–HCl (pH 8.0), 100 mmol/l NaCl and 0.1% Tween 20 (TBST) buffer for 1 h at room temperature to block non-specific binding. Membranes were incubated overnight at 4°C with primary rabbit monoclonal or polyclonal antibodies with 1:1000 dilutions against VE-cadherin, β-catenin, phosphorylated-β-catenin (Thr41/Ser45) and γ-catenin (all from Cell Signaling, Danvers, MA, USA). After washing with TBST, membranes were incubated for 1 h at room temperature in 2.5% non-fat dry milk in TBST diluted with the appropriated secondary antibody conjugated to horseradish peroxidase. Peroxidase activity was visualized with enhanced chemiluminescence (Thermo Scientific) and the images were captured with a digital camera system (G Box, Syngene, Cambridge, UK). The western blot bands were quantified with densitometry using the ImageJ software (National Institute of Health, Bethesda, MD, USA).
Immunoprecipitation
Harvested atrial tissues were lysed as described above in immunoprecipitation buffer for 30 min on ice. Total tissue lysates (200 µg) in 300 µl RIPA buffer were added to agarose beads (40 µl, Santa Cruz Biotechnology, Dallas, TX, USA) and rabbit polyclonal antibody against VE-cadherin (phosphoY658) (ABCAM, Cambridge, MA, USA) with 1:100 dilution was added to the mixture of tissue lysates and agarose beads and incubated for 3 h at room temperature. The agarose beads were then sedimented by brief centrifugation and washed by resuspending with 100 µL immunoprecipitation buffer (three times). RIPA lysis buffer (25 µl) and Laemmli buffer (10 µl) were added to a 5 µl final agarose pellet and boiled for 10 min. Lysate protein (50 µg) was fractionated on 10% SDS–PAGE and processed for immunoblotting by use of anti-phospho-VE-cadherin antibody (Y658, Cell Signaling).
Immunofluorescence microscopy
The detailed methods have been previously described [2, 4]. After PBS wash, atrial tissue sections were incubated overnight with anti-VE-cadherin antibody and/or anti- smooth muscle α-actin antibody at 4°C (Cell Signaling).
Chemicals
U46619 and ADP were obtained from Sigma-Aldrich (St Louis, MO, USA) and dissolved in ultrapure distilled water on the day of the study.
Data analysis
Data are presented either as the mean or standard error of the mean (SEM). Clinical, western blot data were analysed by Kruskal-Wallis tests followed with Dunn's multiple comparison test (GraphPad Software, Inc., San Diego, CA, USA). Categorical data of patient characteristics were analysed with Fisher's exact test. Microvascular reactivity was analysed using two-way repeated-measures analysis of variance with a post hoc Bonferroni test. To analyse the correlation between pre-CPB HbA1c levels and relaxation response to ADP or phospho-VE-cadherin expression, Pearson correlation was used (GraphPad Software, Inc., San Diego, CA, USA). P values <0.05 were considered significant.
RESULTS
Patient characteristics
The patient characteristics are listed in Table 1. All patients with preoperative hypertension were on anti-hypertensive medication (β-blocker, calcium channel blocker or angiotensin-converting enzyme inhibitor). The preoperative blood HbA1c levels were 5.4 ± 0.15 in the non-diabetic (ND) patients, 6.3 ± 0.14 in the CDM patients and 9.9 ± 0.72 in the UDM patients (P < 0.05). There were significant differences in weight gain between UDM versus ND at post-surgery days 2–4, respectively (Fig. 1A, P < 0.05). The postoperative blood glucose and insulin administration are summarized in Fig 1B and C.
Table 1:
Patient characteristics
| Patient characteristics | Non-diabetes | Controlled diabetes | Uncontrolled diabetes | P-values |
|---|---|---|---|---|
| HbA1c (%)a | 5.4 ± 0.15 | 6.3 ± 0.14 | 9.9. ± 0.72b | 0.001 |
| Age (years)a | 67 ± 7 | 69 ± 9 | 66 ± 9 | 0.7 |
| Male/female (n) | 7/3 | 8/2 | 8/2 | 1.0 |
| Hypertension (n) | 10 | 10 | 10 | 1.0 |
| Hypercholesterolaemia (n) | 10 | 10 | 10 | 1.0 |
| Obesity (BMI > 30) | 0 | 2 | 2 | 0.96 |
| Atrial fibrillation (n) | 0 | 0 | 0 | 1.0 |
| Patient blood glucose (mg/dl, pre-CPB)a | 102 ± 3.6 | 135 ± 10 | 220 ± 15b | 0.0001 |
| Patient blood glucose (mg/dl, during-CPB)a | 152 ± 9.0 | 154 ± 12 | 206 ± 14b | 0.005 |
| Preoperative insulin (n) (u/ha) | 0 (0 ± 0) | 4 (1.6 ± 1.9) | 8 (6 ± 1)b | 0.005 |
| Intraoperative insulin (n) (u/ha) | 2 (1.38 ± 1.31) | 5 (5.5 ± 1.9) | 8 (32.75 ± 7)b | 0.0006 |
| Duration of CPB (min)a | 124 ± 13 | 126 ± 17 | 129 ± 24 | 0.89 |
| Cross-clamp time (min)a | 101 ± 13 | 103 ± 17 | 107 ± 22 | 0.9 |
| CABG only (n) | 8 | 8 | 8 | 1.0 |
| No. of grafts performed (n) | 3 | 3 | 3 | 1.0 |
BMI, body mass index; HbA1c, haemoglobin A1c; CABG, coronary artery bypass grafting.
aData expressed as mean ± SD.
bUncontrolled diabetes versus non-diabetics.
Figure 1:
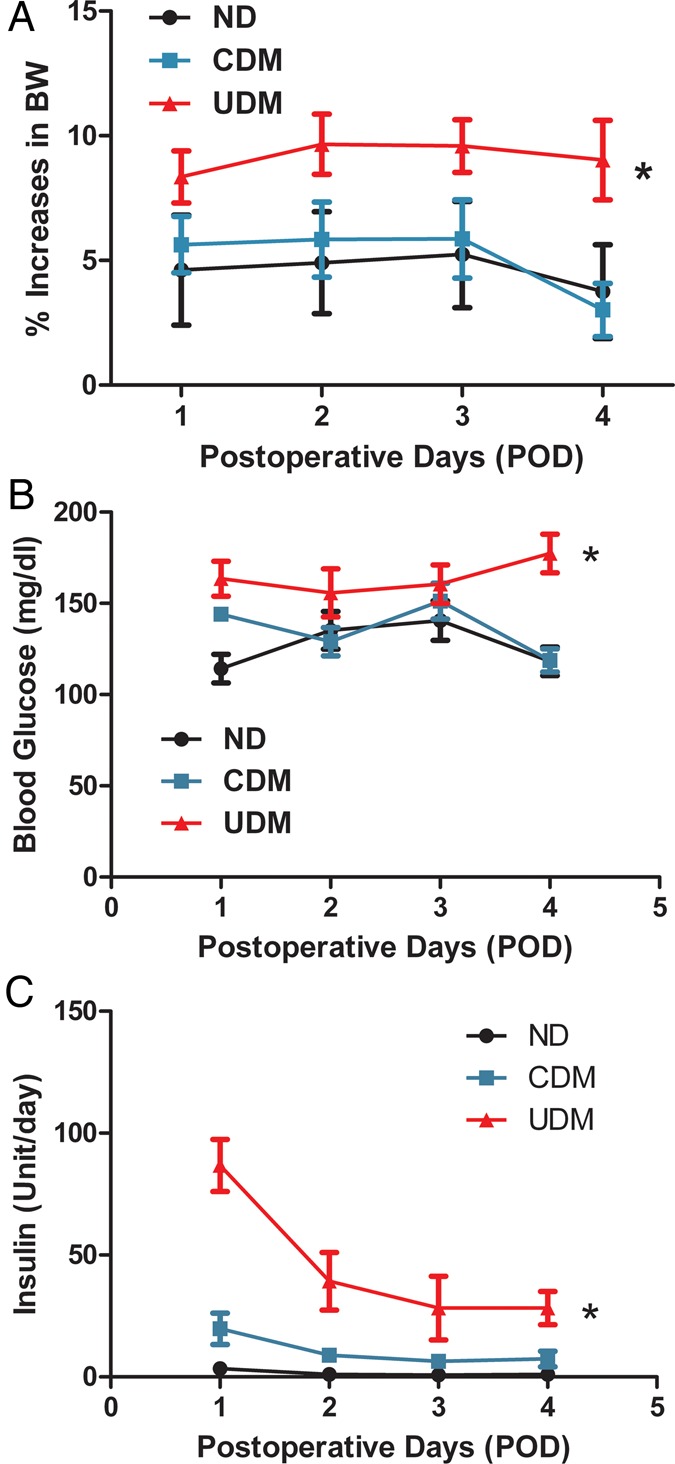
(A) Patients' body weight gain at postoperative days (PODs) 1–4, respectively; P < 0.05 versus ND; (B) patients’ blood glucose levels at PODs 1–4, respectively; (C) patients treated with insulin at PODs 1–4, respectively; n = 10/group. VE: vascular endothelial; CDM: controlled diabetic patients, ND: non-diabetic; UDM: uncontrolled diabetic patients.
Effect of cardioplegia and cardiopulmonary bypass on levels of vascular endothelial-cadherin, phosphor-vascular endothelial-cadherin, β- and γ-catenins
There were no significant differences in basal protein expression of VE-cadherin between UDM and CDM or ND patients. There were no significant differences in total VE-cadherin expression between pre- and post-CP/CPB among groups (Fig. 2A). The level of basal phosphorylated VE-cadherin was higher in the UDM group compared with ND or CDM (P = 0.01 Fig. 2B). CP/CPB induced increased phosphorylation of VE-cadherin in all groups (ND: P = 0.02; CDM: P = 0.01; UDM = 0.04, respectively) and this effect was more pronounced in the UDM group (P < 0.05 versus ND or CDM; Fig. 2B). There were significant direct correlations between VE-cadherin phosphorylation and pre-CP/CPB HgbA1C levels (pre-CP/CPB: r = 0.8306, P = 0.0001; post-CP/CPB: r = 0.7949, P = 0.0001; Fig. 2C and D).
Figure 2:
(A) Total VE-cadherin protein expression in the human myocardium harvested from right atrial tissue samples before and after CP/CPB. There were no significant changes in VE-cadherin between pre- and post-CP/CPB among three groups; (B) phospho-VE-cadherin (Y658) protein expression in the human myocardium harvested from right atrial tissue samples. There were significant increases in phospho-VE-cadherin after CP/CPB among the three groups. These effects were more pronounced in the UDM group compared with the ND or CDM group; *P < 0.05 versus pre-CP/CPB; #P < 0.05 versus ND; @P < 0.05 versus pre-CPB-ND or CDM; mean ± SEM, n = 6-8/group. (C and D) Correlations analysis between VE-cadherin phosphorylation and pre-CP/CPB HgbA1C levels (pre-CP/CPB: r = 0.8306, P = 0.0001; post-CP/CPB: r = 0.7949, P = 0.0001).
There were no significant differences in the total β-catenin and phospho-β-catenin protein expression in the pre-CP/CPB among the three groups (Fig. 3A and B). After CP/CPB, the levels of β-catenin were significantly decreased compared with pre-CP/CPB in all three groups (P < 0.05, respectively) and this effect was more pronounced in the UDM group than the ND (P < 0.05, Fig. 3A). There were no significant changes in the post-CP/CPB levels of phospho-β-catenin protein expression compared with pre-CP/CPB (Fig. 3B). There were no significant differences in basal protein expression of total γ-catenin between UDM and CDM or ND patients. There were significant decreases in γ-catenin protein expression post-CP/CPB in the UDM group when compared with their pre-CP/CPB levels and this effect was also more pronounced in UDM group compared with ND or CDM (Fig. 3C, P < 0.05).
Figure 3:
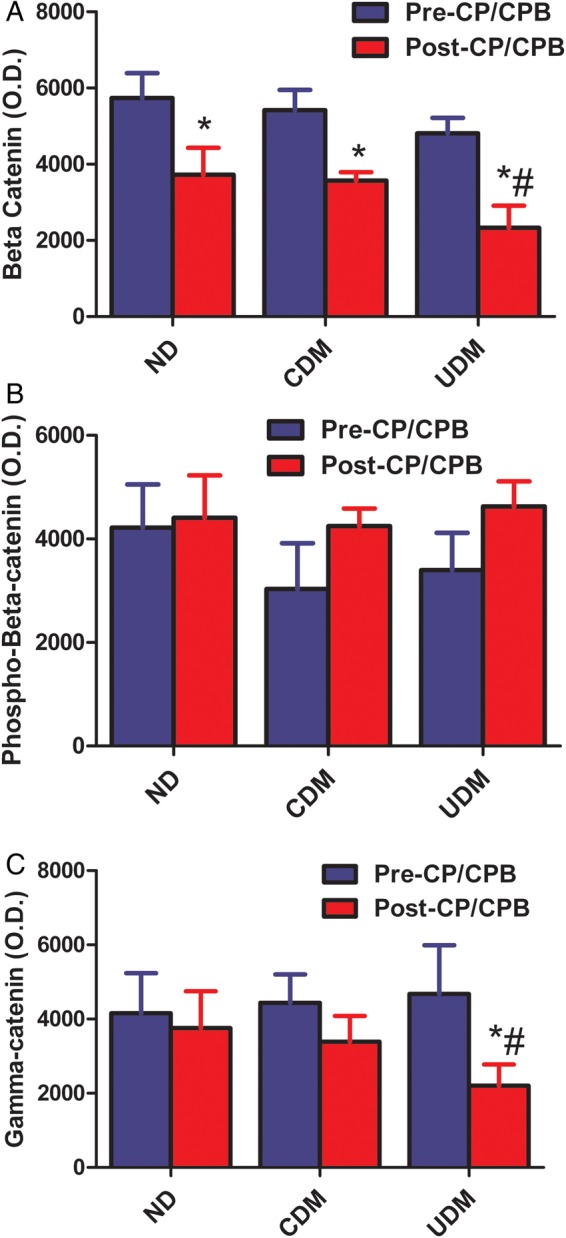
(A) Beta-catenin protein expression in the human myocardium harvested from right atrial tissue samples. There were significant decreases in β-catenin after-CP/CPB among three groups. *P < 0.05 versus pre-CP/CPB; #P < 0.05 versus post-CP/CPB-ND; (B) phospho-β-catenin (Thr41/Ser45) protein expression in the human myocardium harvested from right atrial tissue samples. There were slight increases (not significant) in phospho-β-catenin protein levels after CP/CPB among the CDM and UDM groups; (C) γ-catenin protein expression in the human myocardium harvested from right atrial tissue samples. There were significant decreases in γ-cadherin protein expression after CP/CPB only in the UDM group. *P < 0.05 versus pre-CP/CPB; #P < 0.05 versus ND. Mean ± SEM, n = 8/group.
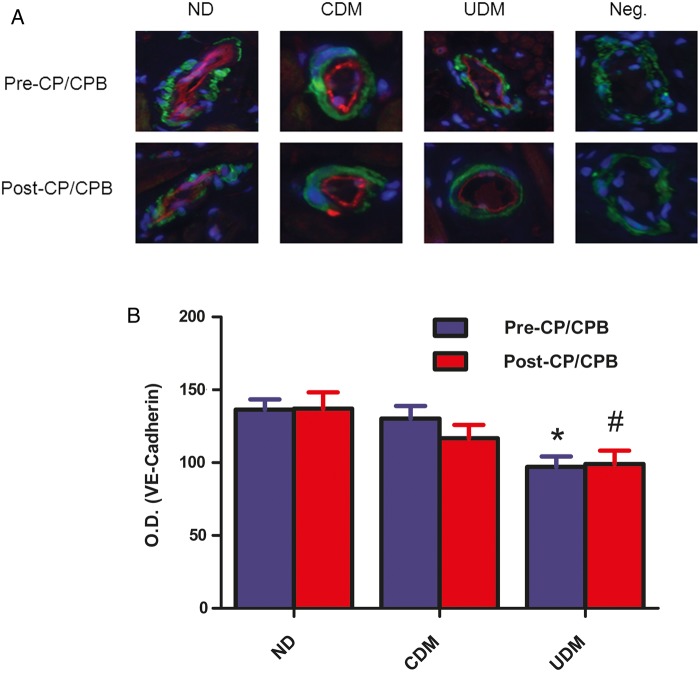
Vascular distribution of vascular endothelial-cadherin
Immunofluorescent double staining demonstrated that VE-cadherin localized to coronary arteriolar endothelial cells (red, Fig. 4A). There were no significant differences in VE-cadherin signals in vessels of pre-CP/CPB or post-CP/CPB between the ND and CDM group. There were no significant differences in VE-cadherin signals in vessels between pre- and post-CP/Rep tissue within each group. There were significant decreases in VE-cadherin densities in vessels of UDM versus ND (pre-CP/CPB: P = 0.008; post-CP/CPB: P = 0.07; Fig. 4B).
Figure 4:
(A) Immunolocalization of VE-cadherin in human atrial myocardium (magnification, 200×). Tissue slices were doubly stained for VE-cadherin (red), and α-actin (green). Negative controls documented a low level of background fluorescence (red), a strong signal of smooth muscle α-actin staining (green) and a signal of nuclear staining (blue); (B) VE-cadherin density analysis; *P = 0.08 versus ND-pre-CP/Rep; #P < 0.05 versus ND-post-CP/Rep; n = 8/group.
Microvascular reactivity
There was no significant difference in the baseline diameter of the microvessels among the three groups (Table 2). The degrees of precontraction by the TXA2 analogue U46619 were 30 ± 2% in the ND group, 32 ± 3% in the CDM group and 29 ± 3% in the UDM group. There were no significant differences in the arteriolar responses to ADP (10−5 M) between the ND and CDM patients before CP/CPB (Fig. 5A). There were significant decreases in the relaxation responses to ADP of the UDM patients compared with those of ND or CDM at baseline (pre-CP/CPB) (P < 0.001, Fig. 5A). The arteriolar response to ADP was significantly decreased post-CP/CPB in all three groups (ND: P < 0.01, CDM: P < 0.01 and UDM: P < 0.05 versus their pre-CP/CPB, respectively). Importantly, absolute values were more pronounced in the UDM group (P < 0.01 versus ND or CDM, respectively; Fig. 5A). There were significant direct correlations between relaxation response to ADP at 10−5 M and pre-CP/CPB HgbA1C levels (pre-CP/CPB: r = −0.639, P = 0.001; post-CP/CPB: r = −0.77; P = 0.0001; Fig. 5B and C).
Table 2:
Microvessel diameter (micrometres)
| Pre-CP/CPB | Post-CP/CPB | |
|---|---|---|
| Non-diabetics | 122 ± 6 | 131 ± 5 |
| Controlled diabetics | 128 ± 7 | 124 ± 5 |
| Uncontrolled diabetics | 120 ± 6 | 126 ± 7 |
Mean ± SEM; n = 8–10/group.
Figure 5:
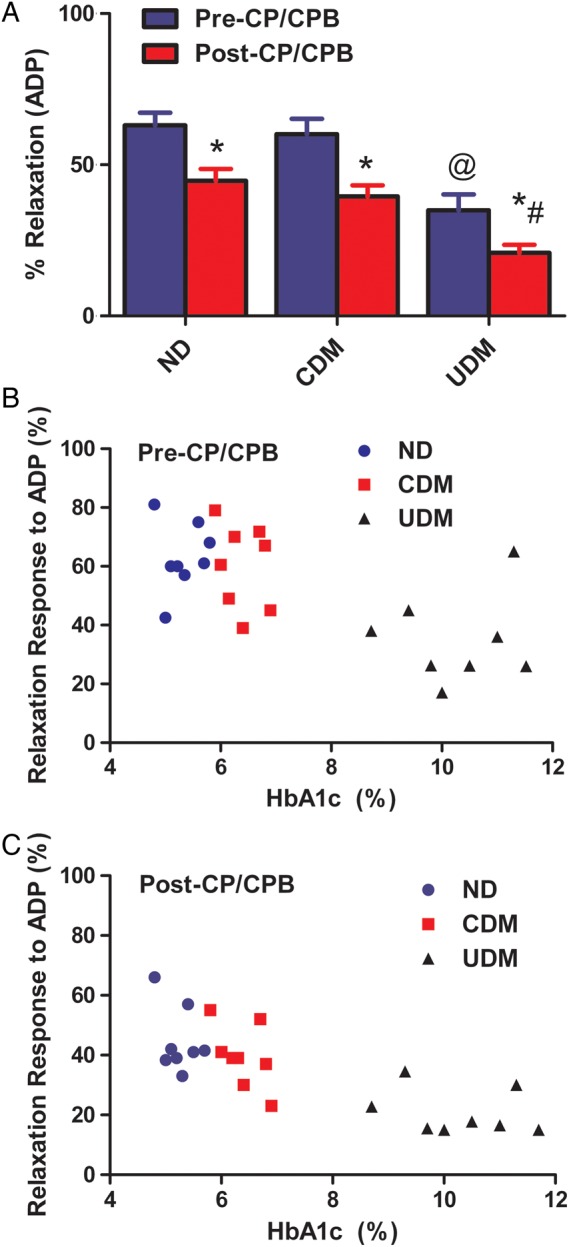
(A) Microvascular vasodilation in response to the endothelium-dependent vasodilator ADP (10−5 M); *P < 0.05 versus pre-CP/CPB; #P < 0.05 versus post-CP/CPB-ND or post-CP/CPB-CDM; @P < 0.05 versus pre-CP/CPB-ND or pre-CP/CPB-CDM; n = 8/group, mean ± SEM; (B) correlation between microvascular relaxation response to ADP before CP/CPB and pre-CP/CPB HbA1c; (C) correlation between microvascular relaxation response to ADP post-CP/CPB and pre-CP/CPB HbA1c.
DISCUSSION
Protein tyrosine phosphorylation plays a critical role in numerous vascular processes including vasomotor regulation and the regulation of vascular permeability mediated through adherens junctions and other endothelial cell–cell contacts [7–11]. Adherens junctions are cellular contacts that are formed by transmembrane and intracellular proteins. They are organized in clusters at cell–cell contacts and connect through their cytoplasmic domain with a complex arrangement of transmembrane proteins known as cadherins. Cadherins are single-chain transmembrane proteins that interact with other related proteins (β-catenin, plakoglobin and p120) to promote linkage to the actin cytoskeleton. In vascular endothelium, the major cadherin, VE-cadherin, is bonded to catenins and the actin cytoskeleton [7–11]. They tend to be dynamic and highly regulated by oxygen-derived free radicals, and cytokines, such as VEGF [16, 17]. Bacterial lipopolysaccharride is known to disrupt endothelial barrier function, in part by protein tyrosine kinase activation. Hence, systemic organ dysfunction during sepsis may in part be due to an altered state of cell–cell junctions [18]. When adherens junctions are stabilized, VE-cadherin loses tyrosine phosphorylation and binds with plakoglobin and actin, while β-catenin is reduced in the adhesion complex with VE-cadherin [11]. The present study demonstrates that CP/CPB increased phosphorylation of VE-cadherin and decreased β- and γ-catenins in patients undergoing CABG surgery. This is consistent with previous studies in pigs, where we found that CP/CPB results in the increase of phosphorylation of VE-cadherin and degradation of β- and γ-catenins in pig myocardium [12, 13]. These findings indicate that CP/CPB has an important effect in endothelial cadherin assembly and integrity of human coronary endothelium.
Diabetes is associated with changes in vascular permeability and vasomotor control as well as with increased morbidity and mortality after surgical procedures [1–3, 5, 19–21]. This is especially true after cardiac surgery involving CP/CPB. Indeed, we have reported that there is a differential up-regulation and down-regulation of specific genes in the myocardium of diabetic and non-diabetic patients after heart surgery [6, 22]. Interestingly, we have recently demonstrated that permeability-modulating proteins such as VEGF/VPF are increased in expression in diabetes and are associated with increased oedema formation and length of hospital stay in diabetic patients after CPB and cardiac surgery [6]. Importantly, in the present study we found that poorly controlled diabetes is associated with increased degradation of VE-cadherin and β/γ-catenins, and phosphorylation of VE-cadherin in human myocardium and coronary arteriolar endothelial cells. Furthermore, poorly controlled diabetes caused more VE-cadherin phosphorylation and β/γ-catenin degradation in the setting of CP/CPB. These findings can partially explain why diabetes is associated with increased vascular permeability and endothelial dysfunction in the human coronary microcirculation in the setting of CP/CPB.
There are several limitations of the current study that deserve mention. Foremost, this work should be considered a pilot study, given the relatively small number of patients and samples. Another limitation is the heterogeneity of the patients. Even though they were reasonably well matched, there were differences in medications and the incidence of coexisting illnesses that may affect the findings. Furthermore, the effects of insulin therapy might have also contributed to the alterations of protein expression in patients with chronic, poorly controlled diabetes.
In conclusion, these data suggest that poorly controlled diabetes down-regulates endothelial adherens-junction protein activation/expression in the setting of CP/CPB. The increased tyrosine phosphorylation and deterioration of VE-cadherin/catenins indicate the damage of the cell–cell endothelial junctions in the diabetic vessels undergoing CP/CPB and cardiac surgery. These alterations may lead to the increase in vascular permeability and endothelial dysfunction and affect outcomes in diabetic patients after cardiac surgery.
Funding
This work was supported by the National Heart, Lung and Blood Institute (NHLBI) at National Institute of Health (NIH) (HL-46716 F.W.S.), and supported in part by Rhode Island Foundation (RIF-20123834 to J.F.), the Institutional Development Award (IDeA) from the National Institute of General Medical Science (NIGMS) of the NIH [5P20-GM103652 (Pilot Project) to J.F.] and NIH Training grant (5T32-HL094300-03 to A.S.).
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We thank all the cardiac surgery operating room nurses, physician assistants, perfusionists at Lifespan Hospitals for collecting tissue samples and the data of patient characteristics. We also thank the nurses and physician assistants at Division of Cardiac Surgery, Lifespan Hospitals for collecting patient consent forms.
REFERENCES
- 1.Feng J, Chu LM, Dobrilovic N, Liu YH, Singh AK, Sellke FW. Decreased coronary microvascular reactivity after cardioplegic arrest in patients with uncontrolled diabetes mellitus. Surgery 2012;152:282–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng J, Liu YH, Chu LM, Singh AK, Dobrilovic N, Fingleton JG et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation 2012;126:S73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng J, Liu YH, Khabbaz K, Hagberg R, Robich MP, Clements RT et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery 2011;149:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, Liu YH, Dobrilovic N, Chu LM, Bianchi C, Singh AK et al. Altered apoptosis-related signaling after cardioplegic arrest in patients with uncontrolled type 2 diabetes. Circulation 2013;128:S144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tofukuji M, Metais C, Li J, Franklin A, Simons M, Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation 1998;98:II-242–46; discussion II-247–48. [PubMed] [Google Scholar]
- 6.Emani S, Ramlawi B, Sodha NR, Li J, Bianchi C, Sellke FW. Increased vascular permeability after cardiopulmonary bypass in patients with diabetes is associated with increased expression of vascular growth factor and hepatocyte growth factor. J Thorac Cardiovasc Surg 2009;138:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V et al. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem 2003;278:19199–208. [DOI] [PubMed] [Google Scholar]
- 8.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004;84:869–901. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Meng FY, Huang Q, Hawker J, Wu HM. Tyrosine phosphorylation of paxillin/pp125FAK and microvascular endothelial barrier function. Am J Physiol 1998;275:H84–93. [DOI] [PubMed] [Google Scholar]
- 10.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest 1996;98:1949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 1995;129:203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi C, Araujo EG, Sato K, Sellke FW. Biochemical and structural evidence for pig myocardium adherens junction disruption by cardiopulmonary bypass. Circulation 2001;104:I319–24. [DOI] [PubMed] [Google Scholar]
- 13.Khan TA, Bianchi C, Araujo E, Voisine P, Xu SH, Feng J et al. Aprotinin preserves coronary cellular junctions and reduces myocardial edema after regional ischemia and cardioplegic arrest. Circulation 2005;112: I196–201. [DOI] [PubMed] [Google Scholar]
- 14.Feng J, Liu YH, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V et al. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegia arrest. Circulation 2008;118:S46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Liu YH, Chu LM, Clements RT, Khabbaz KR, Robich MP et al. Thromboxane induced contractile response of human coronary arterioles is diminished post-cardioplegic arrest. Ann Thorac Surg 2011;92:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong RK, Baldwin AL, Heimark RL. Cadherin-5 redistribution at sites of TNF-alpha and IFN-gamma induced permeability in mesenteric venules. Am J Physiol 1999;276:H736–48. [DOI] [PubMed] [Google Scholar]
- 17.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation 1998;97:99–107. [DOI] [PubMed] [Google Scholar]
- 18.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem 1998;273:35371–80. [DOI] [PubMed] [Google Scholar]
- 19.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg 1999;67:1045–52. [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34. [DOI] [PubMed] [Google Scholar]
- 21.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011;124:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voisine P, Ruel M, Khan TA, Bianchi C, Xu S, Kohane I et al. Differences in gene expression profiles of diabetic and non-diabetic patients undergoing cardiopulmonary bypass and cardioplegic arrest. Circulation 2004;110:II280–II86. [DOI] [PubMed] [Google Scholar]